Abstract
Tumor necrosis factor- (TNF-) exerts two separate effects on neutrophils, stimulating effector functions while simultaneously inducing apoptosis. We examined here the involvement of caspases in neutrophil apoptosis and the effect of TNF-–induced apoptosis on reactive oxygen production. Immunoblotting and affinity labeling showed activation of caspase-8, caspase-3, and a caspase with a large subunit of 18 kD (T18) in TNF-–treated neutrophils. Active caspase-6 and -7 were not detectable in this cell type. Caspase-8 activated caspase-3 and T18 in neutrophil cytoplasmic extracts. zVAD-fmk blocked neutrophil apoptosis, in parallel with the inhibition of caspase activation. TNF-–induced caspase activation was accompanied by a decrease in the ability of neutrophils to release superoxide anion. Conversely, TNF- treatment in the presence of zVAD-fmk caused a prolonged augmentation of superoxide release. Granulocyte-macrophage colony-stimulating factor inhibited TNF-–induced caspase activation and apoptosis, while reversing the diminution in superoxide release. These observations not only suggest that a caspase cascade mediates apoptotic events and downregulates oxygen radical production in TNF-–treated neutrophils, but also raise the possibility that suppression of caspase activation with enhanced proinflammatory actions of TNF- may underlie the pathogenesis of inflammatory diseases.
TUMOR NECROSIS factor-α (TNF-α) has been implicated as a proinflammatory cytokine that plays critical roles in the pathophysiology of inflammatory diseases, including bacterial sepsis, rheumatoid arthritis, Behçet’s disease, and adult respiratory distress syndrome (ARDS).1-4TNF-α can initiate cytokine cascades involving other downstream proinflammatory cytokines such as interleukin-1 (IL-1), IL-6, and granulocyte-macrophage colony-stimulating factor (GM-CSF).1,2 However, recent studies suggest that TNF-α also plays a pivotal role in the resolution of inflammatory responses.5 Mice deficient in TNF-α suffer from progressive inflammatory processes after bacterial infections.6 The cellular and molecular basis by which TNF-α can mediate both proinflammatory and anti-inflammatory effects remains unclear.
Anti-inflammatory actions of TNF-α can be ascribed, at least in part, to the ability of this cytokine to induce apoptotic cell death of inflammatory effectors such as neutrophils.7 However, information about the intracellular processes in apoptotic neutrophils is limited to the descriptions of the characteristic morphological and biochemical changes such as chromatin condensation,8coalescence of nuclear lobes,9 condensation and shrinkage of cytoplasm, internucleosomal DNA fragmentation,8 cell surface exposure of phosphatidylserine (PS),10 and shedding of CD16.11 The biochemical basis for these changes is largely unknown.
It is known that neutrophils have two receptors for TNF-α: 55-kD TNF receptor 1 (TNF-R1) and 75-kD TNF receptor 2 (TNF-R2). TNF-R1 initiates the TNF-α–induced death signal, and TNF-R2 facilitates the death effect of TNF-R1 in neutrophils.12 TNF-R1 has a cytoplasmic death domain homologous to Fas, another apoptosis-inducing death receptor.13 In other cell types, binding of ligands to TNF-R1 and Fas can induce the formation of signaling complexes, TNF-R1-TRADD-FADD-pro–caspase-8 and Fas-FADD-pro–caspase-8, respectively, with subsequent release of activated protease caspase-8 (MACH/FLICE/Mch5).14 The activation of caspase-8 is thought to result in proteolytic activation of other caspase proteases,15 which in turn mediate characteristic morphological and biochemical changes of death receptor-triggered apoptosis.16,17 The observation that gelsolin is cleaved in neutrophils treated with TNF-α plus cycloheximide18suggests that caspases have been activated in this cell type as well. Nonetheless, it is unclear how many caspases are activated and whether those active caspase(s) play a critical role in the execution of TNF-α–induced apoptosis.
Although resolution of inflammation can be facilitated by recognition and phagocytosis of apoptotic neutrophils by macrophages,8it also remains to be determined whether neutrophil functions are compromised by TNF-α–induced apoptotic processes before neutrophil phagocytosis by macrophages. TNF-α can stimulate or enhance functions of neutrophils including phagocytosis, degranulation,19 and production of reactive oxygen species.20 A previous report demonstrated the loss of various functional abilities of neutrophils undergoing spontaneous apoptosis ex vivo.21 However, culture of neutrophils for 24 hours in vitro might result in multiple functional changes in addition to apoptotic processes. Thus, the effects of TNF-α–associated apoptosis per se on neutrophil functions remain incompletely understood.
In the present study, we have examined the involvement of caspases in TNF-α–induced neutrophil apoptosis. Because TNF-α activates not only death signals, but also survival signals that are mediated by the activation of NF-κB transcription factors,22 23 we used cycloheximide to block survival signals mediated through protein synthesis. We show that treatment of neutrophils with TNF-α plus cycloheximide results in activation of caspases and concomitant loss of the ability to produce superoxide anion. Inhibition of caspase activation not only rescued the functional capacity of neutrophils, but also resulted in prolonged enhancement of superoxide release. Pretreatment of neutrophils with GM-CSF inhibited both TNF-α–induced activation of caspases and the downregulation of reactive oxygen production. These results are discussed with regard to the possibility that TNF-α–induced apoptotic signaling leading to caspase activation may work as a switch between proinflammatory and anti-inflammatory actions of TNF-α.
MATERIALS AND METHODS
Reagents.
N-(Nα-benzyloxycarbonylglutamyl-Nε-biotinyllysyl)-aspartic acid [(2,6-dimethylbenzoyl)oxy] methyl ketone (zEK(bio)D-aomk) (Peptide Institute, Osaka, Japan) and Acetyl-Asp-Gln-Thr-Asp-aldehyde (DQTD-CHO) (Peptide Institute) were dissolved in dimethylsulfoxide (DMSO) at 10 mmol/L and stored at −80°C. Stock solutions of Acetyl-Asp-Glu-Val-Asp-aminomethylcoumarin (DEVD-MCA), Acetyl-Tyr-Val-Ala-Asp-aminomethylcoumarin (YVAD-MCA), Acetyl-Tyr-Val-Ala-Asp-aldehyde (YVAD-CHO; Peptide Institute), Benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluoromethylketone (zVAD-fmk; Enzyme Systems, Dublin, CA), propidium iodide (Calbiochem, La Jolla, CA), and 3, 3′-dihexyloxacarbocyanine iodide [DiOC6(3); Molecular Probes, Eugene, OR] were prepared and stored as previously described.17 24 Cycloheximide (Research Organics, Cleveland, OH) was dissolved at 1 mg/mL in water and stored at 4°C. Recombinant human TNF-α and GM-CSF were kindly provided by Dainippon Pharmaceutical (Osaka, Japan) and Schering-Plough Research Institute (Kenilworth, NJ), respectively.
Analysis of neutrophil apoptosis.
Human neutrophils were isolated from peripheral blood of healthy adult volunteers by sedimentation through two-step Percoll (Pharmacia, Uppsala, Sweden) gradients, as previously described.25Freshly purified cells were resuspended in RPMI-1640 supplemented with 10% heat-inactivated fetal bovine serum, preincubated with or without inhibitors such as zVAD-fmk or GM-CSF for 1 hour at 37°C, and exposed to TNF-α (10 U/mL) plus cycloheximide (10 μg/mL) for the indicated time period at 37°C in a humidified atmosphere containing 5% CO2. Flow cytometric analyses of neutrophil apoptosis were performed using propidium iodide (PI)-staining of ethanol-permeabilized cells for DNA fragmentation, staining with phycoerythrin-conjugated annexin V (R&D Systems, Minneapolis, MN) for PS externalization, and staining with DiOC6(3) for mitochondrial permeability transition, as previously described.17
Fluorometric analysis of caspase activities.
For preparation of cytoplasmic extracts, neutrophils (1 × 107) were lysed in 20 μL lysis buffer (50 mmol/L KCl, 50 mmol/L PIPES, pH 7.0, 10 mmol/L EGTA, 2 mmol/L MgCl2, 1 mmol/L dithiothreitol, 20 μmol/L cytochalasin B, 100 μmol/L phenylmethyl sulfonyl fluoride [PMSF], and 1 mg/mL each of chymostatin, leupeptin, antipain, and pepstatin) as previously described.24 For real time recording of caspase activity, lysates were mixed with 100 μL of reaction buffer24 and the activity was measured by the release of 7-amino-4-methyl-coumarin (AMC) from 100 μmol/L DEVD-MCA using a fluorometric microplate reader (Fluoroskan Ascent; Labsystems, Helsinki, Finland) with excitation and emission wavelengths of 355 nm and 460 nm, respectively.
Affinity labeling of active caspases.
Extracts (140 μg total protein) were incubated with 1 μmol/L zEK(bio)D-aomk for 5 minutes at 37°C, resolved in a 16% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, and transferred to a nitrocellulose membrane (Hybond-ECL; Amersham, Arlington Heights, IL). Blots were stained with streptavidin-conjugated horseradish peroxidase (HRP; Amersham; 1:300 dilution) for 3.5 hours at room temperature. Signals were detected by enhanced chemiluminescence (ECL) kit (Amersham) as recommended by the manufacturer.
Cell-free activation of caspases.
Cytosolic extracts (140 μg total protein) were incubated either with 1 μg recombinant active caspase-8 purified as described24or with 1 μmol/L horse heart cytochrome c (Sigma, St Louis, MO) plus 1 mmol/L dATP (Sigma) for 30 minutes at 37°C in a reaction volume of 10 μL. Samples were assayed for caspase activation by affinity labeling with zEK(bio)D-aomk or by fluorometric analysis using 100 μmol/L DEVD-MCA.
Western blotting.
Whole cell extracts were obtained by boiling neutrophil pellets in SDS sample buffer17 for 3 minutes. Proteins were resolved on SDS-PAGE gels, transferred to nitrocellulose membranes, and visualized by ECL. Antibodies used were 1:1,000 dilution of monoclonal antibody against gelsolin (Sigma), 1:20 dilution of affinity-purified anti–caspase-3 antibodies,24 26 1:2,000 dilution of a rabbit polyclonal antiserum against caspase-7 (kindly provided by Dr Gerald Cohen, University of Leicester, Leicester, UK), 1:1,000 dilution of monoclonal antibody against caspase-8 (MBL, Nagoya, Japan), and 1:1,000 dilution of monoclonal antibody against caspase-3 (Transduction Lab, Lexington, KY).
Release of superoxide anion from neutrophils.
RESULTS
Apoptotic cell death of neutrophils induced by TNF-α plus cycloheximide.
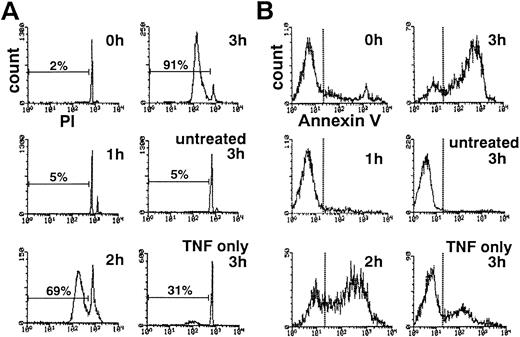
The addition of cycloheximide markedly potentiated TNF-α–induced apoptosis as previously described.7 TNF-α alone induced DNA fragmentation characteristic of apoptosis in approximately 30% of neutrophils within 3 hours (Fig 1A). After 3 hours of treatment with TNF-α and cycloheximide, greater than 90% of neutrophils showed hypodiploid DNA content (Fig 1A). Without TNF-α, cycloheximide alone induced minimal DNA fragmentation within 3 hours (data not shown).
Time course of DNA fragmentation (A) and cell surface exposure of phosphatidylserine (B) in neutrophils exposed to TNF- plus cycloheximide. PI-stained, ethanol-permeabilized neutrophils (A) or neutrophils stained with phycoerythrin-conjugated annexin V (B) were subjected to flow cytometric analyses.
Time course of DNA fragmentation (A) and cell surface exposure of phosphatidylserine (B) in neutrophils exposed to TNF- plus cycloheximide. PI-stained, ethanol-permeabilized neutrophils (A) or neutrophils stained with phycoerythrin-conjugated annexin V (B) were subjected to flow cytometric analyses.
Loss of plasma membrane asymmetry, resulting in cell surface exposure of PS, is another biochemical hallmark of neutrophil apoptosis.10 Flow cytometric analysis using phycoerythrin-labeled annexin V, which specifically binds to PS on the cell surface, showed that PS is exposed on the surface of neutrophils treated for 2 to 3 hours with TNF-α plus cycloheximide (Fig 1B). Despite these changes, approximately 100% of neutrophils maintained plasma membrane integrity at 3 hours in TNF-α plus cycloheximide as determined by dye exclusion test (data not shown), ruling out the possibility of necrotic cell death. These observations confirmed that neutrophils treated with TNF-α plus cycloheximide rapidly undergo apoptotic cell death.
Activation of DEVD-cleaving caspase(s).
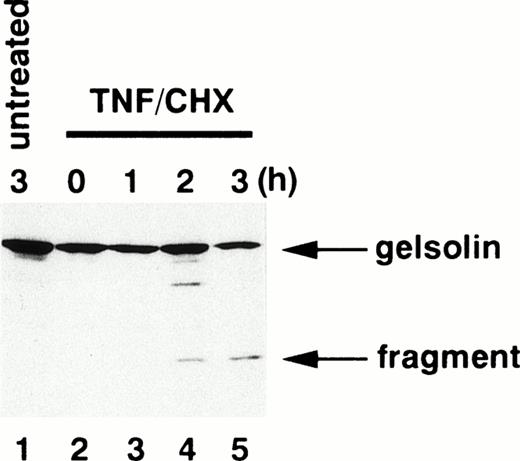
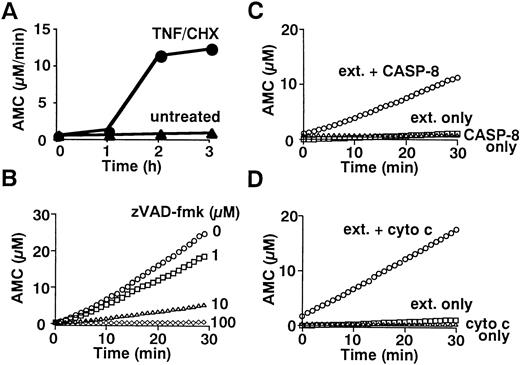
To determine whether caspases are activated in neutrophils during TNF-α–induced cell death, we examined the fate of gelsolin, an actin-binding protein essential for efficient neutrophil locomotion.29 As previously described,18blotting with a monoclonal anti-gelsolin antibody showed that this caspase substrate was cleaved to a 41-kD fragment during TNF-α/cycloheximide-induced apoptosis (Fig 2). Concomitant with this cleavage, a protease activity capable of digesting DEVD-MCA, a fluorogenic caspase substrate that is preferentially cleaved by caspase-3 (CPP32/Yama/apopain) and caspase-7 (Mch3/ICE-LAP3/ CMH-1),30 was detected in neutrophil cytoplasmic extracts. Lysates of untreated neutrophils displayed low level DEVD-MCA cleavage (Fig 3A), possibly reflecting spontaneous apoptotic cell death.8 The activity increased markedly 2 hours after addition of TNF-α and reached a plateau at 3 hours (Fig 3A), in parallel with DNA fragmentation (Fig 1A) and PS externalization (Fig 1B). Although the mature form of caspase-1 (IL-1β–converting enzyme) has been demonstrated in neutrophils by immunoblotting,31 protease activity cleaving YVAD-MCA, a substrate relatively specific for caspase-1,30 was not detected in TNF-α–treated or untreated neutrophils (data not shown). Preincubation of neutrophils with a caspase inhibitor, zVAD-fmk,32 inhibited the appearance of DEVD-MCA cleaving activity in a dose-dependent manner (Fig 3B).
Cleavage of gelsolin in apoptotic neutrophils. Whole cell extracts obtained from 5 × 105 neutrophils treated with (lanes 2 through 5) or without (lane 1) TNF- plus cycloheximide (TNF/CHX) for the time indicated were subjected to SDS-PAGE (10% gel) and immunoblotting using a monoclonal anti-gelsolin antibody.
Cleavage of gelsolin in apoptotic neutrophils. Whole cell extracts obtained from 5 × 105 neutrophils treated with (lanes 2 through 5) or without (lane 1) TNF- plus cycloheximide (TNF/CHX) for the time indicated were subjected to SDS-PAGE (10% gel) and immunoblotting using a monoclonal anti-gelsolin antibody.
Fluorometric analyses of caspase activation. (A) Time course of DEVD-cleaving activity. Cytoplasmic extracts (70 μg) from 1 × 107 neutrophils stimulated with TNF- plus cycloheximide (TNF/CHX) for the indicated time periods were incubated with 100 μmol/L DEVD-MCA for 30 minutes at 37°C. AMC release was measured by a microplate reader as previously described.24(B) Blockade of caspase activation by zVAD-fmk. Neutrophils were preincubated with indicated concentrations of zVAD-fmk before stimulation with TNF- plus cycloheximide for 3 hours. (C and D) Cell-free activation of DEVD-cleaving caspase(s) by recombinant active caspase-8 (C) or cytochrome c (D). Cytoplasmic extracts (70 μg) from nonapoptotic neutrophils were incubated with 0.5 μg purified recombinant caspase-8 (C) or with 1 μmol/L cytochrome c plus 1 mmol/L dATP (D) for 30 minutes at 37°C. (B, C, and D) DEVD-cleaving protease activities in the extracts were analyzed by real-time recordings of AMC release using a fluorometric microplate reader (Fluoroskan Ascent).
Fluorometric analyses of caspase activation. (A) Time course of DEVD-cleaving activity. Cytoplasmic extracts (70 μg) from 1 × 107 neutrophils stimulated with TNF- plus cycloheximide (TNF/CHX) for the indicated time periods were incubated with 100 μmol/L DEVD-MCA for 30 minutes at 37°C. AMC release was measured by a microplate reader as previously described.24(B) Blockade of caspase activation by zVAD-fmk. Neutrophils were preincubated with indicated concentrations of zVAD-fmk before stimulation with TNF- plus cycloheximide for 3 hours. (C and D) Cell-free activation of DEVD-cleaving caspase(s) by recombinant active caspase-8 (C) or cytochrome c (D). Cytoplasmic extracts (70 μg) from nonapoptotic neutrophils were incubated with 0.5 μg purified recombinant caspase-8 (C) or with 1 μmol/L cytochrome c plus 1 mmol/L dATP (D) for 30 minutes at 37°C. (B, C, and D) DEVD-cleaving protease activities in the extracts were analyzed by real-time recordings of AMC release using a fluorometric microplate reader (Fluoroskan Ascent).
Affinity labeling of active caspases in apoptotic neutrophils.
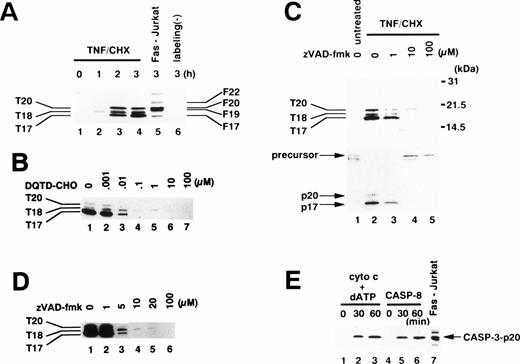
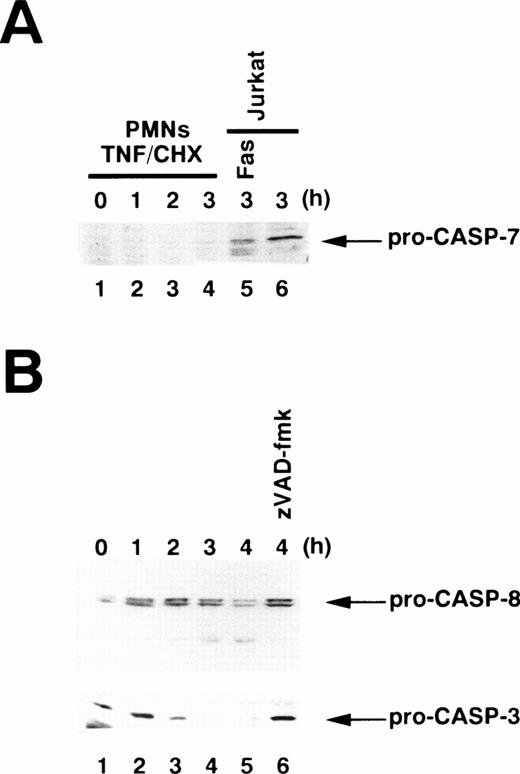
To examine which caspase(s) are activated in TNF-α–induced apoptosis, we used an affinity labeling technique.33Caspase activation involves the proteolytic processing of inactive precursors (pro-caspases) to active proteases that contain two large subunits (molecular weight [Mr] ∼20 kD) and two small subunits (Mr ∼10 kD) in a tetramer.34 zEK(bio)D-amok, an affinity labeling reagent that mimicks the peptide sequences preferred by caspases30 and irreversibly binds to the active site cysteines within the large subunits of most active caspases,35 can distinguish active caspases from one another based on the differences in their apparent molecular weights.24,26 As shown in Fig 4A, 1 μmol/L zEK(bio)D-amok labeled three major polypeptides, designated T20, T18, and T17 according to their apparent molecular mass in kilodaltons, in cytoplasmic extracts from neutrophils treated for 1 to 3 hours with TNF-α plus cycloheximide (lanes 2 through 4). T20 and T17 comigrated with F20 and F17 in Fas-stimulated Jurkat cells (Fig 4A, lane 5), labeled polypeptides corresponding to caspase-3-p20 and caspase-3-p17, respectively.24 Reprobing the same blot with anti–caspase-3 antibodies confirmed that T20 and T17 correspond to caspase-3-p20 and -p17 (data not shown), although we could not rule out the possibility that other active caspases comigrating with caspase-3 might also be present. The identity of T18 remained unclear, although its size is in accord with that of caspase-10 (Mch4/FLICE2; 18.4 kD) predicted from cDNA. The apparent molecular mass of T18 is not consistent with predicted sizes of caspase-1 (21.4 kD), -2 (19.7 kD), -4 (19.9 kD), -5 (22.8 kD), -8 (19.0 kD), or -9 (22.2 or 37.8 kD).36 zEK(bio)D-aomk did not show a band comigrating with F22/caspase-7 (Fig 4A), and immunoblotting using a rabbit antiserum against caspase-7 could not detect pro–caspase-7 in neutrophils (Fig 5A). A polypeptide comigrating with F19/caspase-6 (Mch2) was also absent in apoptotic neutrophils (Fig 4A).
Affinity labeling of active caspases in neutrophils. (A) Time course of caspase activation. Cytoplasmic extracts were obtained from 1 × 107 neutrophils treated with TNF- plus cycloheximide (TNF/CHX) for the indicated time periods (lanes 1 through 4 and 6) or from 5 × 106 Jurkat cells stimulated with anti-Fas antibody (CH-11, 100 ng/mL) for 3 hours (lane 5). Extracts were incubated with (lanes 1 through 5) or without (lane 6) 1 μmol/L zEK(bio)D-aomk for 5 minutes at 37°C. (B) Preferential competition of zEK(bio)D-aomk binding to caspase-3 by DQTD-CHO. Cytoplasmic extracts from 1 × 107 neutrophils treated with TNF- plus cycloheximide for 3 hours were preincubated with DQTD-CHO for 15 minutes at 37°C before labeling with 1 μmol/L zEK(bio)D-aomk. (C) zVAD-fmk inhibition of caspase activation. Neutrophils preincubated for 1 hour with indicated concentrations of zVAD-fmk were stimulated with TNF- plus cycloheximide (lanes 2 through 5) or left untreated (lane 1) for 3 hours. (Upper lanes) zEK(bio)D-aomk–labeled caspases were detected with HRP-conjugated streptavidin. (Lower lanes) The same blot was reprobed with a rabbit anti–caspase-3 antibody. (D) Sensitivities of active caspases to zVAD-fmk. zVAD-fmk at indicated concentrations was added to neutrophils stimulated with TNF- plus cycloheximide for 3 hours. After incubation for 1 hour at 37°C, cytoplasmic extracts were prepared for affinity labeling analysis. (E) Cell-free activation of endogenous caspases in neutrophil extracts by recombinant caspase-8 and by cytochrome c. Cytoplasmic extracts from neutrophils were treated with cytochrome c plus dATP (lanes 1 through 3) or recombinant caspase-8 (lanes 4 through 6) for the indicated time period. Lane 7, cytoplasmic extracts from Fas-stimulated Jurkat cells. All samples were affinity labeled with 1 μmol/L zEK(bio)D-aomk.
Affinity labeling of active caspases in neutrophils. (A) Time course of caspase activation. Cytoplasmic extracts were obtained from 1 × 107 neutrophils treated with TNF- plus cycloheximide (TNF/CHX) for the indicated time periods (lanes 1 through 4 and 6) or from 5 × 106 Jurkat cells stimulated with anti-Fas antibody (CH-11, 100 ng/mL) for 3 hours (lane 5). Extracts were incubated with (lanes 1 through 5) or without (lane 6) 1 μmol/L zEK(bio)D-aomk for 5 minutes at 37°C. (B) Preferential competition of zEK(bio)D-aomk binding to caspase-3 by DQTD-CHO. Cytoplasmic extracts from 1 × 107 neutrophils treated with TNF- plus cycloheximide for 3 hours were preincubated with DQTD-CHO for 15 minutes at 37°C before labeling with 1 μmol/L zEK(bio)D-aomk. (C) zVAD-fmk inhibition of caspase activation. Neutrophils preincubated for 1 hour with indicated concentrations of zVAD-fmk were stimulated with TNF- plus cycloheximide (lanes 2 through 5) or left untreated (lane 1) for 3 hours. (Upper lanes) zEK(bio)D-aomk–labeled caspases were detected with HRP-conjugated streptavidin. (Lower lanes) The same blot was reprobed with a rabbit anti–caspase-3 antibody. (D) Sensitivities of active caspases to zVAD-fmk. zVAD-fmk at indicated concentrations was added to neutrophils stimulated with TNF- plus cycloheximide for 3 hours. After incubation for 1 hour at 37°C, cytoplasmic extracts were prepared for affinity labeling analysis. (E) Cell-free activation of endogenous caspases in neutrophil extracts by recombinant caspase-8 and by cytochrome c. Cytoplasmic extracts from neutrophils were treated with cytochrome c plus dATP (lanes 1 through 3) or recombinant caspase-8 (lanes 4 through 6) for the indicated time period. Lane 7, cytoplasmic extracts from Fas-stimulated Jurkat cells. All samples were affinity labeled with 1 μmol/L zEK(bio)D-aomk.
Immunoblotting for procaspase processing. Whole cell extracts were prepared from 1 × 106 neutrophils (PMNs) stimulated with TNF- plus cycloheximide (TNF/CHX) for the indicated time periods. (A) Pro–caspase-7 is not detectable in neutrophils. Whole cell extracts from 5 × 105 Jurkat cells treated with (lane 5) or without (lane 6) anti-Fas antibody were also analyzed. (B) Processing of pro–caspase-8 (upper panel) and pro–caspase-3 (lower panel) in TNF-–treated neutrophils. Lane 6, neutrophils were preincubated with zVAD-fmk before exposure to TNF- plus cycloheximide. The same blot was sequentially probed with anti–caspase-8 (upper panel) and anti–caspase-3 (lower panel) monoclonal antibodies.
Immunoblotting for procaspase processing. Whole cell extracts were prepared from 1 × 106 neutrophils (PMNs) stimulated with TNF- plus cycloheximide (TNF/CHX) for the indicated time periods. (A) Pro–caspase-7 is not detectable in neutrophils. Whole cell extracts from 5 × 105 Jurkat cells treated with (lane 5) or without (lane 6) anti-Fas antibody were also analyzed. (B) Processing of pro–caspase-8 (upper panel) and pro–caspase-3 (lower panel) in TNF-–treated neutrophils. Lane 6, neutrophils were preincubated with zVAD-fmk before exposure to TNF- plus cycloheximide. The same blot was sequentially probed with anti–caspase-8 (upper panel) and anti–caspase-3 (lower panel) monoclonal antibodies.
To assess which labeled caspase(s) might be responsible for the cleavage of gelsolin (Fig 2), we performed a competition experiment.33 DQTD-CHO was synthesized based on the cleavage site sequence within gelsolin.18 As shown in Fig4B, DQTD-CHO at 10 to 100 nmol/L inhibited the binding of zEK(bio)D-aomk to T20/caspase-3-p20 and T17/caspase-3-p17. In contrast, the labeling of T18 persisted up to 1 μmol/L DQTD-CHO. This indicated that caspase-3 has selective affinity for the gelsolin cleavage site sequence and suggested that caspase-3 is responsible for gelsolin cleavage in TNF-α–treated neutrophils. These results are consistent with the previous claim that gelsolin is a caspase-3 substrate.18 We cannot, of course, rule out the possibility that some other caspase (eg, caspase-8) also contributes to this cleavage, although we note that the DQTD tetrapeptide would be expected to have very low affinity for this caspase.30
Additional affinity labeling experiments were performed to further evaluate the observation that zVAD-fmk suppressed the appearance of DEVD-MCA cleaving activity in TNF-α/cycloheximide-treated neutrophils (Fig 3B). When added before TNF-α and cycloheximide, zVAD-fmk at 10 μmol/L suppressed the appearance of T20/caspase-3-p20, T18, and T17/caspase-3-p17 (Fig 4C, upper panel). Reprobing the same blot with anti–caspase-3 showed that the processing of pro–caspase-3 to mature caspase-3-p20 and caspase-3-p17 was blocked by 10 μmol/L zVAD-fmk (Fig 4C, lower panel). Thus, the TNF-α–induced processing of pro–caspase-3 rather than activity of the processed enzyme was blocked by treating neutrophils with 10 μmol/L zVAD-fmk, as previously reported for Fas-stimulated Jurkat T cells.37 The sensitivities of the labeled caspases to zVAD-fmk were assessed by exposing neutrophils to zVAD-fmk after caspases had been activated by 3 hours of TNF-α plus cycloheximide stimulation. T20/caspase-3-p20 and T17/caspase-3-p17 were suppressed at 5 and 10 μmol/L, respectively, whereas overexposure of the film showed that complete inhibition of T18 requires 20 μmol/L zVAD-fmk (Fig 4D). Thus, 10 μmol/L zVAD-fmk blocked the activation of T18 rather than the protease activity of T18. These observations suggested that a caspase(s) upstream of caspase-3 and T18 is the major target for zVAD-fmk.
Signaling upstream of caspase-3 and T18.
Ligation of TNF-R1 by TNF-α may induce the formation of TNF-R1-TRADD-FADD-pro–caspase-8 complex, resulting in the activation of caspase-8.13 Active caspase-8 tends to escape detection in cell extracts by affinity labeling methods,24 most likely because active caspase-8 at low concentrations is sufficient for apoptotic execution, owing to the amplifying nature of downstream caspase cascades.38 Indeed, immunoblotting demonstrated that pro–caspase-8 is present in neutrophils and is processed during TNF-α–induced apoptosis (Fig 5B). Moreover, addition of active recombinant caspase-8 to neutrophil cytosol resulted in activation of caspase-3-p20 within 30 minutes (Fig 4E, lanes 5 and 6), concomitant with the appearance of DEVD-MCA cleaving activity (Fig 3C). Overexposure of the film showed two additional bands corresponding to caspase-3-p17 and T18 at 1 hour (data not shown). Evidence of caspase-8–induced processing of pro–caspase-3 to caspase-3-p20 before the appearance of T18 argued against the possibility that caspase-3 is indirectly activated by caspase-8 via activation of T18. Instead, these observations suggested that active caspase-8 released from the TNF-R1-TRADD-FADD-pro–caspase-8 complex is responsible for subsequent activation of caspase-3 and T18 in neutrophils. Consistent with this conclusion, we observed that processing of pro–caspase-8 in TNF-α–treated neutrophils was blocked by zVAD-fmk (Fig 5B, lane 6), suggesting that the blockade of pro–caspase-8 autoprocessing by zVAD-fmk39 is responsible for the lack of the downstream caspases, caspase-3 and T18, in human neutrophils.
Caspase-8 is able to stimulate mitochondria, inducing cytochrome c release40 or triggering permeability transition with subsequent release of AIF (apoptosis-inducing factor).41Both cytochrome c and AIF can activate caspase-3.41,42Because mitochondrial permeability transition was minimal in neutrophils treated for 3 hours with TNF-α plus cycloheximide (see below), the present study focused on cytochrome c. The addition of cytochrome c plus dATP to cytoplasm from untreated neutrophils resulted in the activation of caspase-3-p20 within 30 minutes (Fig 4E, lanes 2 and 3), concomitant with the appearance of DEVD-MCA cleaving activity (Fig 3D). This observation indicated that neutrophil cytoplasm contains the machinery43 for the activation of caspase-3 in response to the apoptosis-associated release of cytochrome c from mitochondria.44 45 Thus, it is possible that caspase-8 activates caspase-3 indirectly by inducing mitochondrial release of cytochrome c.
Role for caspase activation in neutrophil apoptosis.
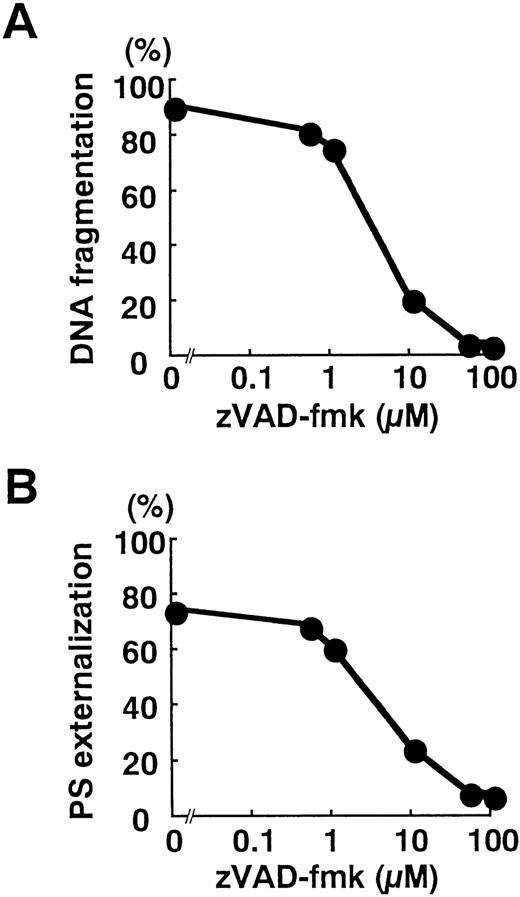
Blockade of caspase activation with zVAD-fmk inhibited apoptotic DNA fragmentation (Fig 6A) and the surface exposure of PS (Fig 6B) in a dose-dependent manner. Despite significant loss of T18 activity at 1 μmol/L zVAD-fmk (Fig 4C), apoptotic changes were minimally interrupted (Fig 6A and B). In contrast, DNA fragmentation and PS externalization was potently suppressed by 10 μmol/L zVAD-fmk (Fig 6A and B), a concentration that significantly blocked the activation of caspase-3 (Fig 4C). These observations implied that caspases activated in response to TNF-α play critical roles in the execution of neutrophil apoptosis.
Blockade of DNA fragmentation (A) and PS externalization (B) by zVAD-fmk. Neutrophils were preincubated for 1 hour with the indicated concentration of zVAD-fmk before stimulation with TNF- plus cycloheximide for 3 hours. The plots represent three independent experiments with essentially identical results.
Blockade of DNA fragmentation (A) and PS externalization (B) by zVAD-fmk. Neutrophils were preincubated for 1 hour with the indicated concentration of zVAD-fmk before stimulation with TNF- plus cycloheximide for 3 hours. The plots represent three independent experiments with essentially identical results.
Negative regulation of superoxide release downstream of caspase activation.
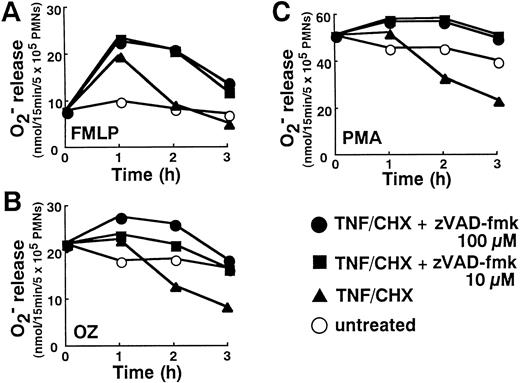
Consistent with previous reports on neutrophil priming by TNF-α,20 neutrophils show increased reactive oxygen production (Fig 7A through C), especially in response to formyl-Met-Leu-Phe (FMLP; Fig 7A), after 1 hour of treatment with TNF-α plus cycloheximide. However, the progression of TNF-α–induced apoptotic processes after 2 hours was associated with a diminution in neutrophil functional capacities. At 2 to 3 hours, the release of superoxide anion from neutrophils stimulated with FMLP (Fig7A), opsonized zymosan (OZ; Fig 7B), and phorbol myristate acetate (PMA; Fig 7C) was suppressed in parallel with the appearance of apoptotic changes (Fig 1).
Downregulation of superoxide release in TNF-–induced neutrophils and reversal by zVAD-fmk. Neutrophils incubated with or without TNF- plus cycloheximide (TNF/CHX) for the indicated time in the absence or presence of indicated concentrations of zVAD-fmk were stimulated with either FMLP (100 nmol/L), OZ (1 mg/mL), or PMA (20 ng/mL) for 15 minutes at 37°C. Superoxide generated during that 15 minute period was determined by cytochrome c reduction. Data shown represent the average values from three separate experiments performed in duplicate.
Downregulation of superoxide release in TNF-–induced neutrophils and reversal by zVAD-fmk. Neutrophils incubated with or without TNF- plus cycloheximide (TNF/CHX) for the indicated time in the absence or presence of indicated concentrations of zVAD-fmk were stimulated with either FMLP (100 nmol/L), OZ (1 mg/mL), or PMA (20 ng/mL) for 15 minutes at 37°C. Superoxide generated during that 15 minute period was determined by cytochrome c reduction. Data shown represent the average values from three separate experiments performed in duplicate.
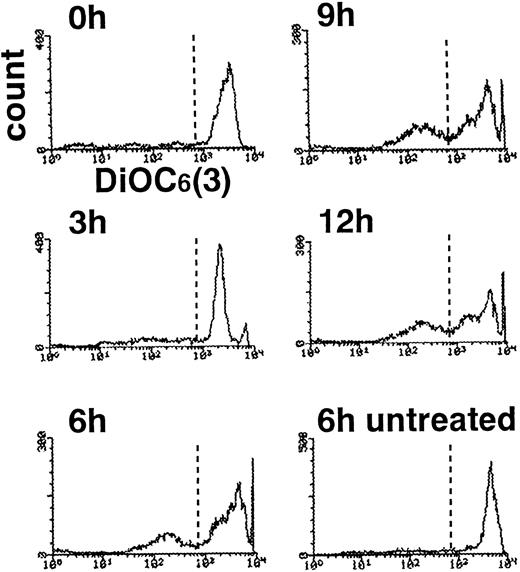
This change might reflect the collapse of mitochondrial membrane potential46 with decline in intracellular ATP level,40 causing a general derangement in neutrophil metabolism, including oxygen radical generation. To assess this possibility, neutrophils were examined by flow cytometry after incubation with DiOC6(3), a fluorescent probe whose sequestration to mitochondria depends on an intact membrane potential.47 Compared with DNA fragmentation and PS externalization, which reached plateaus at 3 hours, loss of mitochondrial membrane potential was a delayed event, as reported in staurosporine-induced apoptosis of HL-6044 and CEM cells40 as well as UVB-induced apoptosis of HeLa cells.40 In our studies, neutrophils with a low mitochondrial potential were first noted at 3 hours and increased over time. Only approximately 40% of cells showed diminished binding of DiOC6(3) after 12 hours in TNF-α plus cycloheximide (Fig 8). Combined with recent studies showing that ATP/ADP ratio is maintained in neutrophils undergoing apoptosis48 and that apoptotic execution requires ATP,49-51 loss of ATP production seemed to be an unlikely explanation for the depressed superoxide production.
Delayed collapse of mitochondrial membrane potential in TNF-–treated neutrophils. Neutrophils treated with TNF- plus cycloheximide for the indicated time periods were incubated with DiOC6(3) and subjected to flow cytometry.
Delayed collapse of mitochondrial membrane potential in TNF-–treated neutrophils. Neutrophils treated with TNF- plus cycloheximide for the indicated time periods were incubated with DiOC6(3) and subjected to flow cytometry.
The ability of neutrophils to release superoxide in response to PMA, FMLP, and OZ was restored by the blockade of apoptotic processes with zVAD-fmk (Fig 7A through C). Interestingly, in the presence of 10 to 100 μmol/L zVAD-fmk, enhanced production of superoxide was not limited to the first hour. Even at 2 and 3 hours after addition of TNF-α plus cycloheximide, superoxide release was significantly higher in neutrophils treated with zVAD-fmk plus TNF-α than neutrophils left untreated (Fig 7A through C). This observation indicated that TNF-α introduces a priming signal for enhanced oxygen radical production that is usually masked by TNF-α–induced apoptotic processes.
Effect of GM-CSF on TNF-α–induced apoptosis, downregulation of respiratory burst, and caspase activation.
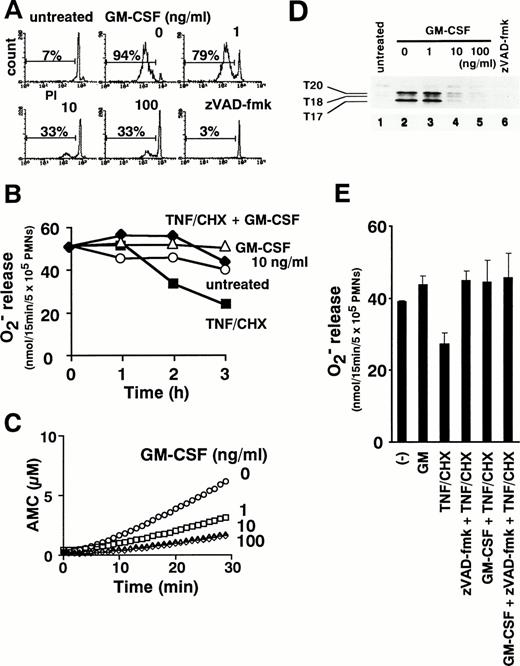
TNF-α can initiate cytokine cascades involving multiple proinflammatory cytokines, including GM-CSF.2 Indeed, lipopolysaccharide-induced production of GM-CSF is depressed in TNF-α knockout mice.6 We therefore examined the ability of combined treatment with GM-CSF and TNF-α to modulate neutrophil functions. GM-CSF simultaneously inhibited TNF-α–induced apoptosis (Fig 9A) and enhanced PMA-triggered release of superoxide from neutrophils treated with TNF-α plus cycloheximide (Fig 9B). As shown in Fig 9C, the activation of caspases, as assessed by DEVD-MCA cleavage, was suppressed in a dose-dependent manner by preincubation of neutrophils with GM-CSF. Affinity labeling with zEK(bio)D-aomk showed that GM-CSF at 10 ng/mL blocked the appearance of active caspase-3-p20, -p17, and T18 (Fig 9D). Thus, GM-CSF inhibits TNF-α–induced apoptotic processes upstream of caspase activation. Interestingly, when TNF-α–induced caspase activation was completely blocked with 100 μmol/L zVAD-fmk, the addition of GM-CSF caused no further augmentation of superoxide production (Fig 9E). This result, which rules out a major contribution by other GM-CSF–triggered signal(s), supports the view that GM-CSF intensifies reactive oxygen generation in TNF-α–stimulated neutrophils mainly by inhibiting caspase activation.
GM-CSF inhibits apoptosis (A), reverses downregulation of superoxide release (B), and suppresses caspase activation (C and D) in TNF-–treated neutrophils. Neutrophils were pretreated with 1, 10, and 100 ng/mL GM-CSF for 1 hour before the addition of TNF- plus cycloheximide. DNA fragmentation (A), superoxide release in response to PMA (B), and the presence of DEVD-cleaving (C) or zEK(bio)D-aomk-binding (D) active caspases were analyzed after incubation for the indicated time period (B) or for 2 hours (A, C, and D). (E) Cells were pretreated with 100 μmol/L zVAD-fmk or 10 ng/mL GM-CSF singly and in combination before addition of TNF-/cycloheximide for 2 hours followed by measurement of PMA-induced superoxide release. Values represent the means + SEM of three independent experiments performed in duplicate.
GM-CSF inhibits apoptosis (A), reverses downregulation of superoxide release (B), and suppresses caspase activation (C and D) in TNF-–treated neutrophils. Neutrophils were pretreated with 1, 10, and 100 ng/mL GM-CSF for 1 hour before the addition of TNF- plus cycloheximide. DNA fragmentation (A), superoxide release in response to PMA (B), and the presence of DEVD-cleaving (C) or zEK(bio)D-aomk-binding (D) active caspases were analyzed after incubation for the indicated time period (B) or for 2 hours (A, C, and D). (E) Cells were pretreated with 100 μmol/L zVAD-fmk or 10 ng/mL GM-CSF singly and in combination before addition of TNF-/cycloheximide for 2 hours followed by measurement of PMA-induced superoxide release. Values represent the means + SEM of three independent experiments performed in duplicate.
DISCUSSION
The present study demonstrated that TNF-α–induced apoptotic cell death of neutrophils is accompanied by activation of multiple caspases. Concomitant with caspase activation, TNF-α–stimulated superoxide generation decreased. Interestingly, inhibition of caspase activation simultaneously suppressed apoptosis and prolonged the TNF-α–induced augmentation of superoxide generation. These findings not only have important implications for understanding the balance between proinflammatory and anti-inflammatory effects of TNF-α, but also might need to be considered in assessing the therapeutic potential of caspase inhibitors.
A combination of affinity labeling and immunoblotting showed the presence of multiple caspases in neutrophils treated with TNF-α and cycloheximide. These included caspase-8, caspase-3, and another caspase with a large subunit of 18 kD (T18), most likely corresponding to caspase-10. In contrast to Fas-stimulated Jurkat T cells,17active caspase-7 and caspase-6 were not detected. The differences in the activated caspases may contribute to the heterogeneity in biochemical and morphological changes, such as the absence of fragmentation into apoptotic bodies in apoptotic neutrophils.10 Moreover, the absence of active caspase-7 in apoptotic neutrophils provides an explanation for the dependence of neutrophil apoptosis on caspase-3. Recently published gene targeting studies showed that neutrophil apoptosis is impaired in caspase-3–deficient mice,52 but did not disclose an explanation for this result. Caspase-7 is highly homologous to caspase-3 and has similar substrate preferences.30 In many cell types, caspase-7 appears to be able to take over when caspase-3 is knocked out.53 Our observation that expression of pro–caspase-7 is very low or absent in neutrophils can explain the inability of this cell type to compensate for the loss of caspase-3.
Our experiments seemed to indicate that TNF-R1 ligation initiates a caspase cascade by inducing autoprocessing of pro–caspase-8 in neutrophils as it does in other cell types.39,54-56 In subsequent experiments, we showed that addition of active caspase-8 to cytosol from nonapoptotic neutrophils was capable of activating caspase-3 and T18 directly. Caspase-8–induced release of cytochrome c from mitochondria may also contribute to the activation of caspase-3. However, these cell-free conditions did not completely reproduce the pattern of activated caspases generated in TNF-α–treated neutrophils. In particular, the autodigestion of caspase-3-p20 to yield caspase-3-p1757 was not efficient in neutrophil cytosol treated with caspase-8, cytochrome c plus dATP, or a combination of these agents. A recent report demonstrating mitochondrial distribution of pro–caspase-358 raises the possibility that mitochondria in intact neutrophils may facilitate the caspase-3 autocatalysis by providing a surface for effective enzyme-substrate interactions.
Apoptosis-associated caspase activation is associated with changes in neutrophil function. Neutrophil functions that are dependent on the integrity of cytoskeleton, eg, shape changes, spreading, chemotaxis, degranulation, and phagocytosis, have previously been shown to decline in aging neutrophils undergoing spontaneous apoptosis.21These changes might be related to the disruption of cytoskeletal networks due to caspase-catalyzed proteolysis of gelsolin,18 catenin,59 and focal adhesion kinase60 as well as the caspase-dependent dephosphorylation of ezrin-radixin-moesin (ERM).61
Spontaneous apoptosis is also accompanied by suppression of superoxide generation in response to the receptor-dependent stimuli FMLP and OZ21 but not the receptor-independent stimulus PMA. In contrast, we observed that TNF-α–induced apoptosis was associated with decreased PMA-triggered superoxide release from neutrophils as well. Although further studies are required to determine the mechanism by which PMA-induced superoxide release is suppressed, current available observations are sufficient to rule out several potential explanations. Neither cytoskeletal disruption nor loss of mitochondrial membrane potential appears to account for this phenomenon. Further, even though ceramide inhibits superoxide release from PMA-stimulated neutrophils,62 a previous report has shown that TNF-α does not stimulate ceramide accumulation in neutrophils.63Several potential explanations for the TNF-α–associated decrease in PMA-induced superoxide release cannot currently be ruled out. One possibility is that decreased amounts of ATP are available for reactive oxygen production due to diversion of most neutrophil ATP stores to apoptotic processes. Alternatively, cytochrome c released from mitochondria might oxidize superoxide to oxygen.64 Finally, proteolysis of D4-GDP-dissociation inhibitor (GDI) by caspase(s)65 may depress superoxide production. D4-GDI, a polypeptide that is homologous to Rho-GDI, is preferentially expressed at high levels in hematopoietic cells, including granulocytes. Because macrophages deficient in D4-GDI show decreased respiratory burst activity,66 it is possible that D4-GDI proteolysis contributes to the suppression of PMA-induced superoxide production.
Whatever the mechanism of decreased PMA-induced superoxide production, it is clear that caspase activation plays a role. Treatment of neutrophils with zVAD-fmk at 10 μmol/L prevented TNF-α–induced DNA fragmentation, PS externalization, and suppression of superoxide production, in parallel with the blockade of pro–caspase-3 processing. Recent studies with other cell types support the idea that caspase-3 mediates DNA fragmentation and PS externalization in apoptotic neutrophils. Caspase-3 can activate caspase-activated DNase (CAD)67 by cleaving the inhibitor of CAD (ICAD).68 Caspase-3 is upstream of PS externalization in Fas-stimulated Jurkat T cells.17 The present results suggest that caspase-3–mediated proteolysis also plays a role in apoptosis-associated suppression of superoxide generation. In addition, the observation that zVAD-fmk enhances and prolongs TNF-α–primed superoxide production raises the possibility that caspase inhibition, a strategy currently being considered for the treatment of a variety of pathological conditions, might be associated with enhanced production of proinflammatory and potentially harmful neutrophil products such as superoxide anions.
GM-CSF, like zVAD-fmk, appears to suppress TNF-α–induced apoptosis upstream of active caspase-3 and T18. Several different GM-CSF–induced biochemical changes might contribute to this suppression of apoptosis. Although GM-CSF downregulates neutrophil TNF receptors, probably by activating a sheddase,69 the downregulation is not complete, with greater than 50% of TNF binding retained after 2 hours of exposure to GM-CSF.70,Lyn, a src family tyrosine kinase, has been reported to play a critical role in the GM-CSF blockade of spontaneous neutrophil apoptosis.71Phosphatidylinositol 3-kinase (PI3K), which has been shown to inhibit apoptosis in other cell types,72 is tyrosine phosphorylated in its 85-kD regulatory subunit and activated in GM-CSF–stimulated neutrophils.73 Bfl-1,74 a survival-promoting Bcl-2 family protein expressed in myeloid cells,75 might be activated downstream of PI3K. ERK2, which has been reported to block apoptosis in other cell types,76 is tyrosine phosphorylated in GM-CSF–stimulated neutrophils.77 Finally, GM-CSF stimulates neutrophils to produce platelet-activating factor (PAF),78 which is able to prevent TNF-α–induced neutrophil apoptosis without downregulating TNF receptors.12 Further study is required to determine the relative contributions of each of these mechanisms to the GM-CSF–induced suppression of neutrophil apoptosis.
TNF-α signaling in vivo occurs in the context of complex cytokine networks and important cell-to-cell interactions. The functional consequences of the GM-CSF–induced block in caspase activation are potentially important in understanding the dual role of TNF-α as a proinflammatory and anti-inflammatory cytokine. Because the present study employed a simplified model system using cycloheximide to focus on the TNF-α death pathway, the results must be interpreted cautiously. Nonetheless, our data imply that TNF-α introduces dual signals to neutrophils with respect to their effector functions: negative apoptotic signal(s) downstream of caspase activation and positive priming signal(s) independent of caspases or protein synthesis. Our data also indicate that other cytokines, eg, GM-CSF can affect the balance between these signals. Differential modulation of diverse TNF-α–induced signals by interactions with other cytokines and inflammatory mediators79 may explain the ability of TNF-α to mediate a wide variety of inflammatory diseases. GM-CSF can suppress apoptotic signals by inhibiting caspase activation and potentiate priming signals for enhanced reactive oxygen production. Such functional interactions between TNF-α and GM-CSF may contribute to neutrophil-mediated tissue damages in inflammatory diseases such as ARDS.80 Caspase cascade(s) in neutrophils provide a point of convergence for complex intracellular signals from multiple exogenous stimuli.81 As a critical regulatory point in apoptotic and anti-inflammatory signals, caspase activation may work as a switch that determines the balance between proinflammatory and anti-inflammatory responses.
ACKNOWLEDGMENT
The authors thank Gerald M. Cohen for anti–caspase-7 antibodies; Akinori Maeda for help in flow cytometric analysis; and Yuri A. Lazebnik, Kohsuke Asagoe, and Katsumi Takada for helpful discussion and thoughtful suggestions.
A.T. is a Research Resident of the Japanese Foundation of Aging and Health. S.H.K. is a Leukemia Society Scholar.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Atsushi Takahashi, MD, First Division, Department of Internal Medicine, Faculty of Medicine, Kyoto University, 54 Shogoin Kawara-cho, Sakyo-ku, Kyoto 606-01, Japan; e-mail:atakahas@kuhp.kyoto-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal