Abstract
Self-renewal is considered to be the essential defining property of a stem cell. Retroviral marking, in vitro amplification, and serial transplantation of human cells that can sustain long-term lymphomyelopoiesis in vivo have provided evidence that human hematopoietic stem cell self-renewal occurs both in vitro and in vivo. To investigate whether this process can be manipulated by cytokines, we administered two different combinations of human growth factors to sublethally irradiated nonobese diabetic/severe combined immunodeficient (SCID) mice transplanted with 107 light-density human cord blood cells and then performed secondary transplants to compare the number of transplantable human lymphomyeloid reconstituting cells present 4 to 6 weeks post-transplant. A 2-week course of Steel factor + interleukin (IL)-3 + granulocyte-macrophage colony-stimulating factor + erythropoietin (3 times per week just before sacrifice) specifically and significantly enhanced the numbers of transplantable human lymphomyeloid stem cells detectable in the primary mice (by a factor of 10). Steel factor + Flt3-ligand + IL-6 (using either the same schedule or administered daily until sacrifice 4 weeks post-transplant) gave a threefold enhancement of this population. These effects were obtained at a time when the regenerating human progenitor populations in such primary mice are known to be maximally cycling even in the absence of growth factor administration suggesting that the underlying mechanism may reflect an ability of these growth factors to alter the probability of differentiation of stem cells stimulated to proliferate in vivo.
DISCOVERY OF THE ABILITY OF normal human cells to engraft highly immunocompromised xenogeneic recipients at experimentally useful efficiencies1-4 has paved the way for the development of assays for quantitating transplantable human hematopoietic stem cell frequencies using limiting-dilution analysis. This is based on previous extensive validation of the use of this approach for measuring murine stem cells in which the availability of congenic donors and recipients allows sensitive, precise, and specific measurements of long-term lymphomyeloid repopulating cell frequencies in a variety of test populations.5-9 Because of the way such transplantable hematopoietic stem cells are identified, we have proposed the term competitive repopulating unit (CRU) for their designation. A biologically similar type of human hematopoietic cell (CRU) can be detected by its ability to generate both lymphoid and myeloid progeny in the marrow of sublethally irradiated (350 cGy) nonobese diabetic-scid/scid (NOD/SCID) mice after their intravenous injection 6 to 8 weeks previously. We chose this period to assess engraftment of the mice in our initial studies based on the finding that maximum numbers of regenerated human lymphoid and myeloid cells are detected at that time post-transplant.3,10 Both DNA-11 and fluorescence-activated cell sorting (FACS)–based detection methods12 have been used to identify positive mice containing detectable populations of engrafted human cells with similar sensitivities. However, FACS offers the additional capability of identifying the different lineages of human cells produced. This ensures the specificity of the assay by eliminating the detection of in vivo repopulating cells that have acquired a distorted or constrained differentiation potential, a situation that has been shown to occur under certain circumstances; for example, when murine stem cells are serially transplanted.13
Both retroviral marking14 and limiting dilution analysis approaches12 indicate that single human CRU can generate large numbers of lymphoid and myeloid progeny in immunocompromised mice, even in the absence of injections of exogenous human cytokines. In vitro, these same cells can also proliferate and amplify their numbers but require stimulation by known growth factors.12,15 Thus, at least some human stem cells can execute self-renewal divisions in vitro. Recent studies indicate that this can also occur in NOD/SCID and SCID mice transplanted with human hematopoietic cells.10,16,17 However, the factors that may influence this process in vivo are questions that are just beginning to be addressed. Our initial results with secondary transplants suggested that primary mice administered repeated injections of a combination of human steel factor (SF), interleukin-3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF), and erythropoietin (Ep) in the 2 weeks before their sacrifice had greater (human) secondary repopulating activity than parallel mice administered no human growth factors. Moreover, this was observed even though there was no apparent growth factor effect on the total numbers of human cells, or the numbers of other types of human hematopoietic progenitors found in the primary mice.10 We now report the results of additional experiments designed to test the reproducibility of this preliminary observation and to quantify the magnitude of the growth factor effects observed.
MATERIALS AND METHODS
Preparation of cord blood cells.
Cord blood cells were obtained with informed consent from mothers undergoing normal full-term cesarean deliveries. The cells were collected in tubes containing heparin and low-density (< 1.077 g/mL) cells and were then isolated by centrifugation over Ficoll-Paque (Pharmacia, Piscataway, NJ). After two washes in phosphate-buffered saline (PBS), the cells were resuspended in fetal calf serum (FCS; StemCell Technologies, Vancouver, BC, Canada) and DMSO (Sigma, St Louis, MO.) added to a final concentration of 10% just before aliquoting and cryopreservation at −135°C. As required, vials of frozen cells, usually from two to three donors, were rapidly thawed at 37°C and the suspensions then diluted slowly with Iscove’s medium containing 10% FCS (StemCell) and 0.25 mg/mL deoxyribonuclease (type II-S, D4513; Sigma). These cells were then washed twice in Iscove’s medium + 10% FCS, counted, and the majority resuspended in PBS for injection into irradiated NOD/SCID mice as described below. The remainder were washed in Hanks’ HEPES-buffered salt solution containing 2% FCS for subsequent antibody staining for phenotype analyses and progenitor assays.
Animals.
NOD/LtSz-scid/scid were bred and maintained in microisolators in the animal facility of the British Columbia Cancer Research Center (Vancouver, BC, Canada) from breeding pairs originally obtained from Dr L. Schultz (The Jackson Laboratory, Bar Harbor ME). All animals were kept and handled under sterile conditions, and provided with acidified water (pH = 3), and sterilized food ad libitum. Six- to 8-week-old mice were transplanted by intravenous injection within 24 hours after being administered a sublethal dose of whole-body irradiation (350 cGy of137Cs γ-rays at a dose rate of ∼1 cGy per minute). Human recombinant growth factors, when administered, consisted of a combination of either 10 U/mouse/injection of Ep (StemCell) +10 μg/mouse/injection of SF (Amgen, Thousand Oaks, CA) + 6 μg/mouse/injection each of IL-3 and GM-CSF (Novartis, Basel, Switzerland), or 10 μg/mouse/injection of FL, (Immunex, Seattle, WA) + 10 μg/mouse/injection of SF and 2 μg/mouse/injection of IL-6 (Cangene, Mississauga, ON) intraperitoneally in a final volume of less than 0.5 mL three times per week for the 2 weeks immediately preceding sacrifice, or FL + SF + IL-6 at a 10-fold lower dose injected daily from day 1 post-transplant until the mice were sacrificed 4 weeks later.
Secondary transplants.
Both tibias and femurs of each primary recipient mouse were removed and the total marrow content then flushed out using a syringe and a 26-gauge needle. After gently resuspending the cells in 2% FCS in Hanks’ balanced salt solution (HBSS; StemCell), in most cases, an aliquot was removed for FACS analyses and progenitor assays. The remainder were then pooled and equal aliquots (67% or 100% of the equivalent of two femurs plus two tibias = 18% or 25%, respectively of the equivalent of the total bone marrow of a mouse) injected into secondary irradiated (350 cGy) recipients (usually three or four mice per group). In a few experiments, the FACS analyses and progenitor assays were performed on the pooled suspension.
Flow cytometry.
The initial cord blood cells and the cells harvested from the marrows of both primary and secondary mice were stained at 4°C with various murine antihuman monoclonal antibodies (MoAb) directly conjugated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE) as described10 at ≤107 cells/mL after lysis of the red cells and pretreatment of the suspension with human serum and an antimouse IgG receptor antibody (2.4G218) to block human and mouse Fc receptors. After staining, the cells were washed once in 2% HBSS and then once again in the same medium containing 2 μg/mL propidium iodide (PI) to identify nonviable cells. Cells were analyzed and sorted on a FACStar Plus (Becton Dickinson) also as described.3 Controls consisted of staining additional aliquots of the same cells with irrelevant isotype-matched control antibodies directly labeled with the identical fluorochromes. When mouse-human cell mixtures were being analyzed, marrow cells from untreated NOD/SCID mice were stained with the same antibodies in parallel, to ensure nonreactivity of the antibodies used with murine cells. A minimum of five positive events from a viable (PI−) population of 5000 cells at settings excluding greater than 99.9% of all negative controls was adopted as the minimum criterion for deriving positive values for any particular human phenotype. Total numbers of human cells were determined as those staining positive with a combination of anti-CD45 and anti-CD71 antibodies, human B cells were identified as CD34−CD19+ cells, and human myeloid cells were identified as CD19−CD15+ cells. From the CD45/71+ population, the CD34+ subset was also sorted and used to initiate colony-forming cell (CFC) and long-term culture-initiating cell (LTC-IC) assays, as described below.
Progenitor assays.
Myeloid (CFU-GM), erythroid (BFU-E and CFU-E), and multilineage (CFU-GEMM) progenitor numbers were determined by plating cells in 0.8% methylcellulose medium containing 30% FCS, 1% bovine serum albumin (BSA), 10−4 mol/L β-mercaptoethanol in Iscove’s medium (Methocult 4435; StemCell), supplemented with 3 U/mL human Ep, 50 ng/mL SF, and 20 ng/mL each of IL-3, IL-6, GM-CSF, and G-CSF (Terry Fox Laboratory, Vancouver, BC, Canada), and counting the colonies obtained after 16 days of incubation at 37°C.19
LTC-IC numbers were determined by plating another aliquot of cells in long-term culture medium (Myelocult; StemCell), to which 10−6 mol/L of freshly dissolved hydrocortisone sodium hemisuccinate (Sigma) was added just before use, on top of preformed feeder layers of murine fibroblasts previously engineered to produce SF, G-CSF, and IL-3.19 These cultures were maintained for 6 weeks at 37°C with weekly half-medium changes and were then harvested and assayed for their CFC content. LTC-IC numbers were calculated from the CFC numbers detected in these assays assuming each LTC-IC (of cord blood origin) will produce, on average, ∼30 CFC.19
General experimental design.
The effect of injecting different combinations (and schedules) of human growth factors on the ability of human cells from primary NOD/SCID mice to engraft secondary NOD/SCID recipients was studied in a total of six experiments. (Table 1, experiments one to four; Table 2, experiments one and two.) In each experiment, groups of from 4 to 12 primary, sublethally irradiated NOD/SCID mice were injected intravenously with 107light-density cells from a pool of several human cord blood collections and then half of the mice were injected with growth factors (and the rest not), as indicated. All primary mice in each experiment were sacrificed at the same time between 4 and 6 weeks post-transplant (when human LTC-IC numbers have been shown to reach maximum and subsequently stable values in this model10) and their marrow cells were then isolated and injected into groups of secondary recipients as described above. The total level of repopulation of the marrow of the primary mice with human (CD45/71+) cells in these six experiments was 42% ± 4%, which gave similar absolute numbers of human cells as reported previously.10
Increased Numbers of Human Progenitors Detected in Secondary Recipients of Cells from Primary Recipients Administered Human SF, IL-3, GM-CSF, and Ep
| Exp. . | No. of 1° Recipients per Group . | Proportion of BM Cells from 1° Recipients Injected into 2° Recipients . | Time of Assessment of 1° Recipients (wks posttransplant) . | Human Cell Type Evaluated in 2° Recipients . | 1° Recipients Administered no GF . | 1° Recipients Administered 4 GF × 6 . | ||
|---|---|---|---|---|---|---|---|---|
| No. of Positive 2° Recipients . | No. of Cells per 2° Recipients* . | No. of Positive 2° Recipients . | No. of Cells per 2° Recipients* . | |||||
| 1† | 2 | 18% | 6 | CFC | 1/2 | 4200 | 2/2 | 850, 4600 |
| LTC-IC | 0/2 | 1/2 | 12 | |||||
| 2 | 3 | 25% | 6 | CFC | 0/3 | 2/3 | 870, 1600 | |
| 3 | 3 | 18% | 4 | CFC | 0/4 | 4/4 | 700, 480, 1700, 3300 | |
| 4 | 4 | 18% | 6 | CFC | 1/4 | 150 | 4/4 | 970, 20000, 490, 620 |
| LTC-IC | 0/4 | 4/4 | 4, 70, 1, 12 | |||||
| Exp. . | No. of 1° Recipients per Group . | Proportion of BM Cells from 1° Recipients Injected into 2° Recipients . | Time of Assessment of 1° Recipients (wks posttransplant) . | Human Cell Type Evaluated in 2° Recipients . | 1° Recipients Administered no GF . | 1° Recipients Administered 4 GF × 6 . | ||
|---|---|---|---|---|---|---|---|---|
| No. of Positive 2° Recipients . | No. of Cells per 2° Recipients* . | No. of Positive 2° Recipients . | No. of Cells per 2° Recipients* . | |||||
| 1† | 2 | 18% | 6 | CFC | 1/2 | 4200 | 2/2 | 850, 4600 |
| LTC-IC | 0/2 | 1/2 | 12 | |||||
| 2 | 3 | 25% | 6 | CFC | 0/3 | 2/3 | 870, 1600 | |
| 3 | 3 | 18% | 4 | CFC | 0/4 | 4/4 | 700, 480, 1700, 3300 | |
| 4 | 4 | 18% | 6 | CFC | 1/4 | 150 | 4/4 | 970, 20000, 490, 620 |
| LTC-IC | 0/4 | 4/4 | 4, 70, 1, 12 | |||||
Total CFC or LTC-IC per mouse BM (individual values for each positive 2° recipient).
The results of this first experiment are taken from 10.
Increased Numbers of Human Progenitors Detected in Secondary Recipients of Cells From Primary Recipients Adminsitered Human FL, SF, and IL-6
| Exp. . | No. of 1° Recipients per Group . | Proportion of BM Cells from 1° Recipients Injected into 2° Recipients . | Time of Assessment of 1° Recipients (wks posttransplant) . | Human Cell Type Evaluated in 2° Recipients . | 1° Recipients Administered no GF . | 1° Recipients Administered 3 GF . | ||
|---|---|---|---|---|---|---|---|---|
| No. of Positive 2° Recipients . | No. of Progenitors per 2° Recipients* . | No. of Positive 2° Recipients . | No. of Progenitors per 2° Recipients* . | |||||
| 1† | 4 | 25% | 4 | CFC | 1/4 | 3400 | 3/4 | 3400, 6100, 5100 |
| LTC-IC | 1/4 | 50 | 3/4 | 100, 280, 180 | ||||
| 2† | 3 | 25% | 4 | CFC | 0/4 | 1/3 | 14000 | |
| LTC-IC | 0/4 | 1/3 | 800 | |||||
| 2‡ | 3 | 25% | 4 | CFC | 3/4 | 9900, 16900, 3100 | ||
| LTC-IC | 2/4 | 400, 290 | ||||||
| Exp. . | No. of 1° Recipients per Group . | Proportion of BM Cells from 1° Recipients Injected into 2° Recipients . | Time of Assessment of 1° Recipients (wks posttransplant) . | Human Cell Type Evaluated in 2° Recipients . | 1° Recipients Administered no GF . | 1° Recipients Administered 3 GF . | ||
|---|---|---|---|---|---|---|---|---|
| No. of Positive 2° Recipients . | No. of Progenitors per 2° Recipients* . | No. of Positive 2° Recipients . | No. of Progenitors per 2° Recipients* . | |||||
| 1† | 4 | 25% | 4 | CFC | 1/4 | 3400 | 3/4 | 3400, 6100, 5100 |
| LTC-IC | 1/4 | 50 | 3/4 | 100, 280, 180 | ||||
| 2† | 3 | 25% | 4 | CFC | 0/4 | 1/3 | 14000 | |
| LTC-IC | 0/4 | 1/3 | 800 | |||||
| 2‡ | 3 | 25% | 4 | CFC | 3/4 | 9900, 16900, 3100 | ||
| LTC-IC | 2/4 | 400, 290 | ||||||
Total CFC or LTC-IC per mouse BM (individual values for each positive 2° recipient).
Daily—1 μg FL, 1 μg SF, 0.25 μg IL-6 per injection, every day for 1 month before sacrifice.
3 × wk—10 μg FL, 10 μg SF, 2 μg IL-6 per injection, 3 × week for 2 weeks before sacrifice. Note that the controls for this arm of the experiment are the same as for 2.
RESULTS
Enhanced engraftment of secondary NOD/SCID mice with cells from primary mice administered human SF, IL-3, GM-CSF, and Ep.
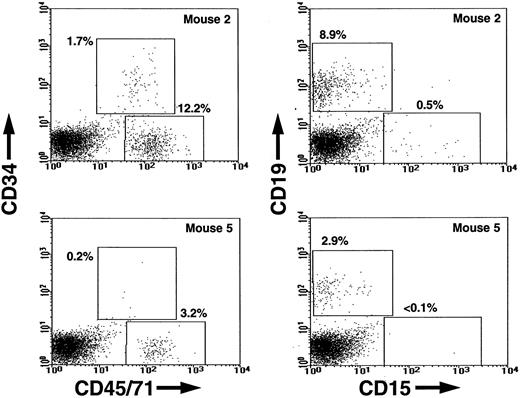
Figure 1 shows a comparison of the FACS profiles obtained from the marrows of individual secondary mice injected 6 weeks previously with marrow cells from primary mice that had been administered either no growth factors or a 2-week course of six injections of SF, IL-3, GM-CSF, and Ep during the 2-week period just before sacrifice. To illustrate the enhancing effect of the growth factor treatment of the primary mice, what is shown in Fig 1 is the result obtained in the secondary mouse that contained the highest frequency of human cells in each of the two groups of secondary mice in a representative experiment. In the experiment shown (Experiment 4 in Table 1), all four of the secondary recipients of cells from the growth factor-injected primary mice contained human lymphoid (CD34−CD19+) cells. Three also contained human myeloid (CD19−CD15+) cells and all four contained CD34+ CFC. In contrast, only three of the four secondary recipients of an equivalent portion of the marrow from the corresponding control primary mice contained detectable numbers of human lymphoid cells and only one contained detectable numbers of human myeloid cells or progenitors.
FACS dot plots showing the frequencies of human CD45/71+ (total) and human CD34+(primitive) hematopoietic cells (left panels) and the frequencies of human CD19+ (B-lymphoid) and CD15+(granulopoietic) cells (right panels) in the most-highly engrafted secondary mice injected with equivalent transplants of cells obtained from primary mice engrafted with human cord blood cells and administered a 2-week course of SF, IL-3, GM-CSF, and Ep (Mouse 2) or not (Mouse 5) in a representative experiment.
FACS dot plots showing the frequencies of human CD45/71+ (total) and human CD34+(primitive) hematopoietic cells (left panels) and the frequencies of human CD19+ (B-lymphoid) and CD15+(granulopoietic) cells (right panels) in the most-highly engrafted secondary mice injected with equivalent transplants of cells obtained from primary mice engrafted with human cord blood cells and administered a 2-week course of SF, IL-3, GM-CSF, and Ep (Mouse 2) or not (Mouse 5) in a representative experiment.
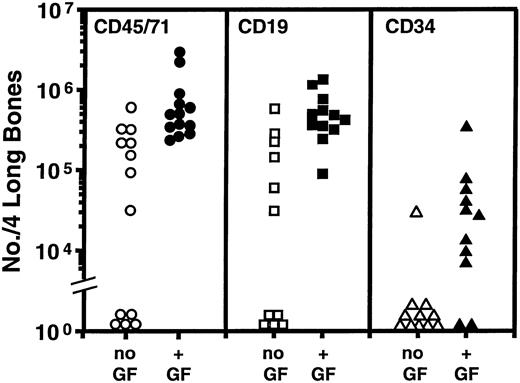
Similar results were obtained in another three experiments in which the effect of this course of growth-factor treatment of the primary mice was investigated. The combined data from all four experiments are summarized in Fig2. Table 1 shows the numbers of human CFC and LTC-IC detected in the CD34+ cell populations isolated from the marrow of the same secondary mice. CD34+ CFC were found in all but 1 of the 13 mice transplanted with marrow from the growth factor-injected primary mice. In 5 of these, LTC-IC were also readily detected. In contrast, none of the 13 secondary recipients of marrow from the control group of primary mice contained detectable numbers of human LTC-IC and in only 2 of these were human CFC identified. Interestingly, the growth factor enhancement of the secondary transplants was not associated with any difference in the total cellularity of the bone marrow of the primary recipients: (1.8 ± 0.2) × 107 versus (2.3 ± 0.3) × 107 cells per four long bones were measured in the two groups. There was also no difference per primary mouse in the total numbers of human CD45+ CD71+ cells, or human B cells, or CD34+ cells, or CFC or LTC-IC present (data not shown), as expected.10
Comparison of the total number of human cells of the phenotypes shown found to be present in the tibias and femurs of mice transplanted with cells obtained from the marrow of primary mice transplanted with human cord blood cells and administered a 2-week course of SF, IL-3, GM-CSF, and Ep (solid symbols) or not (open symbols). Data pooled from the four experiments described in Table 1. Each symbol denotes an individual secondary mouse. The corresponding % human (CD45/71+) cell repopulation values for the marrows of these secondary mice that contained greater than 0.1% human cells ranged from 0.3% to 12%.
Comparison of the total number of human cells of the phenotypes shown found to be present in the tibias and femurs of mice transplanted with cells obtained from the marrow of primary mice transplanted with human cord blood cells and administered a 2-week course of SF, IL-3, GM-CSF, and Ep (solid symbols) or not (open symbols). Data pooled from the four experiments described in Table 1. Each symbol denotes an individual secondary mouse. The corresponding % human (CD45/71+) cell repopulation values for the marrows of these secondary mice that contained greater than 0.1% human cells ranged from 0.3% to 12%.
Enhanced engraftment of secondary NOD/SCID mice with cells from primary mice administered human FL, SF, and IL-6.
Two additional experiments were performed to investigate the potential effect of a different combination of growth factors (ie, FL, SF, and IL-6) on the same endpoints. These cytokines were administered either according to the same 2-week schedule of three injections per week (n = 1) or daily throughout the post-transplant period at a 10-fold lower dose per injection (n = 2). In these experiments, the primary mice were sacrificed after 4 weeks, by which time they had received approximately half the overall dose of growth factors injected into the mice that were administered during the 2-week course of injections. This daily schedule was explored based on the observation that serum levels of human SF, IL-3, and GM-CSF (particularly the latter two) measured in the previous experiments, were found to decline precipitously after injection and, in all cases, to less than 5 ng/mL within 24 hours (Table 3).
Levels of Human Growth Factors Present in the Plasma of Cord Blood Transplanted Mice at Varying Intervals After Injection
| Time After Injection (hours) . | SF (ng/mL) . | IL-3 (ng/mL) . | GM-CSF (ng/mL) . |
|---|---|---|---|
| 1 | 250 ± 120 | 180 ± 120 | 130 ± 40 |
| 6 | 200 ± 70 | 2 ± 2 | 16 ± 7 |
| 24 | 3 ± 1 | 0 ± 0 | .06 ± .03 |
| Time After Injection (hours) . | SF (ng/mL) . | IL-3 (ng/mL) . | GM-CSF (ng/mL) . |
|---|---|---|---|
| 1 | 250 ± 120 | 180 ± 120 | 130 ± 40 |
| 6 | 200 ± 70 | 2 ± 2 | 16 ± 7 |
| 24 | 3 ± 1 | 0 ± 0 | .06 ± .03 |
Growth factors (10 μg SCF, 6 μg IL-3, 6 μg GM-CSF, 10 U Ep) injected 3 × week for 2 weeks before sacrifice.
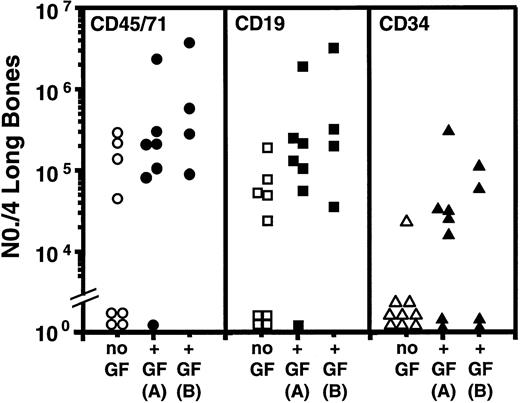
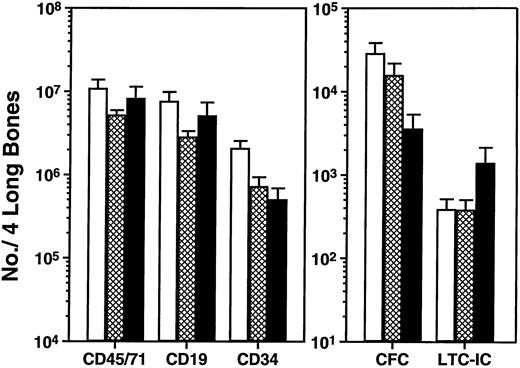
An increased level of repopulation of every type of human hematopoietic cell examined was consistently observed in the secondary recipients of cells from all primary mice administered FL, SF, and IL-6, regardless of the dose administered or schedule used (Fig 3 and Table 2). As was found with the other growth factor combination, there was not a comparable difference (P > .05, Student’s t-test) in the total number of human cells or human CD34−CD19+(B-lineage) cells observed in the marrows of the primary mice from which the secondary transplants were obtained. On the other hand, in this case the total number of CD34+ cells in the primary mice administered FL, SF, and IL-6 was consistently lower (P< .05) than in the controls administered no human growth factors, and the primary mice administered the higher dose of FL, SF, and IL-6 over a 2-week period contained fewer human CFC (∼fivefold reduced,P < .05) and more human LTC-IC (∼fourfold higher than the primary mice administered no growth factors [P = .3], Fig4). However, because these represent data from a single experiment, additional studies will be required to confirm their reproducibility. Comparison of the total numbers of human cells, or human B cells or CD34+ cells regenerated in secondary recipients of cells from mice administered the two different cytokine combinations (Fig 2 versus Fig 3) suggests that the combination of FL, SF, and IL-6 may be slightly less effective (given that the cells from the control groups gave similar outcomes). However, comparison of the CFC and LTC-IC populations regenerated in the same groups of secondary mice suggests that injected human FL, SF, and IL-6 injections may selectively enhance the subsequent output of these earlier cell types.
Comparison of the total number of human cells of the phenotypes shown found to be present in the tibias and femurs of mice transplanted with cells obtained from the marrow of primary mice transplanted with human cord blood cells and administered either no further treatment (open symbols) or FL, SF, and IL-6 (solid symbols) at a low dose, daily (A), or at a high dose three times per week for 2 weeks (B). Data pooled from the two experiments described in Table 2. Each symbol denotes an individual secondary mouse. The corresponding % human (CD45/71+) cell repopulation values for the marrows of these secondary mice that contained greater than 0.1% human cells ranged from 0.1% to 12%.
Comparison of the total number of human cells of the phenotypes shown found to be present in the tibias and femurs of mice transplanted with cells obtained from the marrow of primary mice transplanted with human cord blood cells and administered either no further treatment (open symbols) or FL, SF, and IL-6 (solid symbols) at a low dose, daily (A), or at a high dose three times per week for 2 weeks (B). Data pooled from the two experiments described in Table 2. Each symbol denotes an individual secondary mouse. The corresponding % human (CD45/71+) cell repopulation values for the marrows of these secondary mice that contained greater than 0.1% human cells ranged from 0.1% to 12%.
Comparison of the number of different phenotypically and functionally defined human hematopoietic cells detected in the tibias and femurs of mice 4 weeks after being transplanted with 107 light-density human cord blood cells and then administered no further treatment (□) or FL, SF, and IL-6 injected daily at a low dose (▩), or three times per week for the 2 weeks before sacrifice at a high dose (▪). Values are the mean ± standard error of mean (SEM) of results obtained from three to seven individual mice in the three experiments described in Table 2.
Comparison of the number of different phenotypically and functionally defined human hematopoietic cells detected in the tibias and femurs of mice 4 weeks after being transplanted with 107 light-density human cord blood cells and then administered no further treatment (□) or FL, SF, and IL-6 injected daily at a low dose (▩), or three times per week for the 2 weeks before sacrifice at a high dose (▪). Values are the mean ± standard error of mean (SEM) of results obtained from three to seven individual mice in the three experiments described in Table 2.
Limiting dilution analysis shows a significant effect of human growth factors in vivo on the numbers of transplantable human lymphomyeloid repopulating cells regenerated in primary NOD/SCID mice.
Most of the groups of secondary recipients analyzed in these experiments included some mice that did not contain any detectable (ie, < 0.1%) human cells plus others that did. Therefore it was possible to use these data to derive repopulating cell frequencies by applying Poisson statistics.20 To determine the incidence of transplantable human cells with lymphomyeloid repopulating ability, ie, CRU,12 the same endpoints as previously validated for quantifying these cells in freshly isolated suspensions of human cord blood were adopted. These require that both human CD34+ CFC (either BFU-E and/or CFU-GM and/or CFU-GEMM) and human CD34−CD19+ (B-lymphoid) cells (> 5 per 5000 viable cells analyzed) be present for a mouse to be considered as positive.12 Using these criteria, human CRU frequencies and, hence, total human CRU numbers in the marrow of the various groups of primary mice evaluated in the present study could be calculated. As indicated in Table 4, the marrow of mice administered a 2-week course of SF, IL-3, GM-CSF, and Ep had a 10-fold higher content of human CRU than those administered no growth factors (P < .001). The effect of the FL, SF, and IL-6 injections (assuming no effect of the delivery protocol) on human CRU output was less (threefold increase) and did not quite reach statistical significance (P = .1).
Frequency of Human CRU in Secondary Recipients of Marrow from Control and Growth Factor–Injected Primary Mice
| Growth Factors Injected into Primary Mice . | CRU Frequency (per 108 Murine Marrow Cells) . |
|---|---|
| Control | 1 (0.6 to 1.6) |
| SF, IL-3, GM-CSF, Ep | 10 (7 to 14) |
| FL, SF, IL-6 | 3 (2 to 4) |
| Growth Factors Injected into Primary Mice . | CRU Frequency (per 108 Murine Marrow Cells) . |
|---|---|
| Control | 1 (0.6 to 1.6) |
| SF, IL-3, GM-CSF, Ep | 10 (7 to 14) |
| FL, SF, IL-6 | 3 (2 to 4) |
Values shown are for CRU with the range defined by ± SE shown in parentheses. SF, IL-3, GM-CSF, and Ep at 10 μg, 6 μg, 6 μg, and 10 U per injection, respectively, 3 × week for 2 weeks before sacrifice. FL, SF, and IL-6 daily at 1 μg, 1 μg, and 0.25 μg per injection, respectively, for 1 month before sacrifice or 10 μg, 10 μg, and 2 μg per injection, respectively, 3 × week for 2 weeks before sacrifice.
DISCUSSION
Current investigations of the molecular mechanisms regulating human hematopoietic stem cell populations face three challenges. The first is the development of quantitative assays for the cells of interest with sufficient sensitivity, specificity, precision, and convenience to be experimentally useful. Available evidence from the murine studies suggest that the CRU assay, originally developed for determining murine stem cell frequencies5,21 meets this first requirement. This assay has recently been adapted for human cells using sublethally irradiated NOD/SCID mice11,12 or C12MDP-liposome–treated SCID mice17 as recipients. Nevertheless, improved sensitivity of these procedures and further characterization of the cellular phenotypes they detect4 22is still needed.
A second challenge is to identify those external factors that can influence (either positively or negatively) the viability and/or proliferative activity of human hematopoietic cells with stem cell properties. Again, such studies are well advanced in the murine system in which retroviral marking of highly purified CRU populations has provided the most convincing evidence of their proliferation in response to specific growth factors in vitro,23,24 although factors capable of either amplifying9 or inhibiting25 murine CRU in cultures of highly purified input populations have also been reported. Similar retroviral marking data now exists for human cells.14 26-29 However, such approaches are not well suited to systematic analyses of the roles of specific factors (or factor combinations) on human stem cell proliferation and self-renewal.
A third and major challenge is the delineation of the intracellular targets and sequence of events that influence whether a hematopoietic stem cell will begin to differentiate (irreversibly) or not. Ultimately, this process is believed to involve a complex series of changes in gene transcription, whose regulation in turn depends on key transcription factors and their activities. Although some of these have now been identified,30,31 very little is as yet known about the types of interactions they engage in or how these are regulated. However, it is interesting to note that the types and concentrations of growth factors to which a stem cell is exposed can influence its probability of self-renewal independent of its mitogenic stimulation.32 On the other hand, no set of in vitro conditions has yet been found to reproduce the extent of hematopoietic stem cell expansion achieved in vivo either during ontogeny,33 or after the transplantation of stem cells into irradiated recipients.6 34-36 Thus, delineation of in vivo mechanisms that sustain hematopoietic stem cell proliferation and self-renewal remain of great interest.
The present studies have confirmed that this process can take place when human hematopoietic stem cells (identified as CRU) engraft the marrow of xenogeneic recipients, in this case, irradiated NOD/SCID mice. The number of CRU injected into the primary mice analyzed here would have been approximately 10, based on our previous estimates of CRU numbers in human cord blood.12 This means that, in the absence of human growth factor injections, this number of injected human CRU resulted in the regeneration of at least 10% of the number initially injected (assuming a 100% detection efficiency). With appropriate growth factor injections this number could be increased a further 10-fold (Table 4), ie, to a level at least comparable to the input value. In murine recipients of syngeneic cells, the detection efficiency of CRU can be estimated to be on the order of 10% to 20% because CRU purities of this level can be achieved.13,37-39The frequency of CRU in the CD34+CD38−population isolated from human cord blood, as determined by assaying the cells in NOD/SCID mice, is 0.1% to 0.2%.12,40Although it is unlikely that CD34+CD38−cord blood cells are functionally homogeneous, factors inherent in the assessment of human stem cells in mice probably also reduce considerably their efficiency of detection. We have previously measured the actual proportion of various subpopulations of injected human cells that are present in the marrow of NOD/SCID mice 2 to 3 days post-transplant, including human LTC-IC.10 This value was consistently found to be less than 1%. Thus, the numbers of CRU measured 4 to 6 weeks later are likely to represent a greater than 10- to 100-fold regeneration of the injected CRU population. Secondary transplants performed early after transplantation of primary mice with human cord blood cells would provide more precise information about the seeding efficiency of human CRU in NOD/SCID mice and hence allow the overall expansion that occurs in the 4- to 6-week post-transplant period to be more accurately determined.
Although the present studies clearly show an enhancing effect of certain human growth factors on CRU expansion in vivo (in NOD/SCID mice), they do not address the underlying mechanism or even whether the effects observed are directly or indirectly mediated. Interestingly, Verstegen et al17 have recently reported that the production of human CD34+CD38− cells from CD34+CD38− cells transplanted into a similar mouse model is dependent on the cotransfer of other human CD34+ cells. These authors suggest that the coinjected human CD34+ cells are most likely to act by serving as a source of essential growth factors. In the present studies, potential effects of repeated growth factor injections on the number of human CD34+CD38− cells regenerated in primary recipients of unfractionated human cord blood cells were not investigated. However, it should be noted that the effects observed on CRU numbers did not extend to any other human cell type assessed, including LTC-IC. This reinforces the concept that the CRU and LTC-IC assays do not measure the same functions and hence may not necessarily detect the same cell populations according to their extent of overlap at the single-cell level. The fact that a 2-week course of SF, IL-3, GM-CSF, and Ep seemed to be more effective than the combination of FL, SF, and IL-6 administered according to the same protocol was also not anticipated. A recent analysis of the effects of different growth factor combinations on adult marrow and cord blood LTC-IC amplification41 led us to anticipate that the first combination of growth factors would be suboptimal, and better results were expected with FL, SF, and IL-6. However, the effects of these on human cord blood CRU amplification in vivo were reversed (Table 2). Enhanced regeneration of transplanted murine CRU has also been obtained in recipients of cells transduced with a HOX B4-encoding retroviral vector,35 in mice transplanted with low numbers of normal CRU,6 in mice transplanted with CRU of fetal origin,6,7 and in recipients of normal adult marrow cells followed by repeated injections of SF and IL-11.42Moreover, in none of these situations was the mature blood cell output affected and, in the latter case, the effects obtained with the injections of SF and IL-11 were attributed to a selective ability of these two growth factors to promote stem cell self-renewal. This conclusion was based on evidence that the stem cells were proliferating maximally at the time of assessment in the absence of the growth factor injections. It is, therefore, inviting to speculate that a similar mechanism may underlie the growth factor effects observed here on human stem-cell regeneration in vivo. Further experiments to test this hypothesis are now underway. If confirmed, these would indicate a previously unappreciated and clinically significant use of growth factors to selectively modulate hematopoietic stem cell proliferation and self-renewal in vivo as well as in vitro.32
ACKNOWLEDGMENT
The authors thank Jessyca Maltman and Maya Sinclaire for assistance with the animal work, Gayle Thornbury and Giovanna Cameron for assistance in cell sorting, the staff of the Stem Cell Assay Service for initial processing of the cord bloods, and Bernadine Fox for manuscript preparation. The authors also thank Amgen, Cangene, Immunex, Novartis, StemCell, and Dr Peter Lansdorp (Terry Fox Laboratory) for generous gifts of reagents.
Supported by grants from the National Cancer Institute of Canada (NCIC, with funds from the Terry Fox Run), from the NIH (NHLBI POI-5545), and from Novartis. C.J.E. is a Terry Fox Cancer Research Scientist of the NCIC.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal