Abstract
If the interplay between caspase proteases and mitochondria decide the fate of the cell during apoptosis, they may constitute useful molecular targets for novel drug design. We have shown that photoactivated merocyanine 540 (pMC540) triggers caspase-mediated apoptosis in HL60 leukemia and M14 melanoma cells. Because pMC540 is a mixture of photoproducts, we set out to purify the biologically active component(s) from this mixture and to investigate their ability to directly activate intracellular caspases and/or trigger mitochondrial events associated with apoptosis. Two photoproducts, namely C1 and C2, purified and characterized by mass spectroscopy and nuclear magnetic resonance (NMR) analysis, effectively induced apoptosis in HL60 and M14 cells. Interestingly, both C1 and C2 induced non–receptor-dependent activation of caspase 8, which was responsible for the downstream activation of caspase 3 and cell death. Both compounds induced the release of cytochrome C from mitochondria of tumor cells and from purified rat liver mitochondria; however, different mechanisms were operative in cytochrome C translocation in response to C1 or C2. C1-induced cytochrome C release was mediated by the mitochondrial permeability transition (MPT) pore and accompanied by a decrease in mitochondrial transmembrane potential (▵ψm), whereas cytochrome C release in response to C2 was independent of MPT pore opening. These findings do not exclude the possibility that changes in mitochondrial ▵ψm are critical for apoptosis in some instances, but support the notion that this may not be a universal step in the apoptotic process. Thus, identification of two novel anticancer agents that directly activate effector components of the apoptotic pathway could have potential implications for the development of newer chemotherapeutic drugs.
AN ALTERNATIVE STRATEGY in cancer management is to design treatments that directly activate effector pathways involved in apoptosis. Thanks to a number of elegant studies, the two most logical candidates to target would be the caspase family of cysteine proteases and the mitochondria.1-19 The role of caspases as orchestrators of apoptotic cell death has been well established and elegantly reviewed by Thornberry and Lazebnik.20 Each caspase is synthesized as an inactive precursor molecule that which is activated in cells undergoing apoptosis by proteolytic cleavage after specific aspartate residues.21 Once activated, caspases lead to proteolysis of a number of cellular substrates,22-25 culminating in apoptotic collapse of the cell. Although caspase 3 (CPP32/apopain/Yama) is the most widely studied caspase in drug-induced apoptosis,26-33 it is becoming clearer that there is a molecular ordering of caspases in the apoptotic program.6,34 Activation of an upstream initiator caspase, such as caspase 8, leads to the subsequent induction of the effector phase of apoptosis via processing of the executioner caspase, such as caspase 3. Caspase 8 activation is classically observed upon triggering of the CD95/CD95L system via induction of the death-inducing signaling complex35; however, two recent reports demonstrate that caspase 8 activation can also be induced by anticancer drugs in the absence of cell surface death receptor-ligand interaction.4,6 Whereas receptor-dependent caspase 8 activation does not essentially require mitochondrial controlled processes, drug-induced caspase 8 activation is completely abolished in the absence of mitochondria,4 suggesting involvement of mitochondrial-derived factors.
Early disruption of mitochondrial function is seen in most cases of drug-induced apoptosis, and the various mechanisms that mediate mitochondrial dysfunction involve changes in cellular redox state via enhanced generation of reactive oxygen species (ROS) or a decrease in their detoxification, depletion of NADPH, or depletion of ATP.36-38 It is also well established that, after an apoptotic stimulus, mitochondria release apoptogenic factors such as apoptosis-inducing factor (AIF), cytochrome C (Cyt.C), and even caspases.1,5,13,18,19,39 The translocation of these factors to the cytosol together with additional factors, namely ATP and Apaf-3, can proteolytically activate caspase 3,7 which, in turn, activates cellular disassembly. Mitochondrial Cyt.C normally resides in the intermembrane space, and its release into the cytosol during apoptosis indicates an enhanced permeability of the outer mitochondrial membrane. One of the mechanisms proposed for the translocation of Cyt.C to the cytosol is a reduction in the mitochondrial transmembrane potential (Δψm) that accompanies some forms of apoptosis.40-43 This reduction in Δψm is thought to be mediated by the opening of the mitochondrial permeability transition (MPT) pore, a dynamic multiprotein complex located at the contact site between the inner and the outer mitochondrial membranes.37,44-49 Opening of the MPT pore has been implicated in cell death induced by ROS, hepatotoxins, calcium, and anoxia, and inhibitors of MPT pore opening, such as cyclosporin A (CsA) and bongkrekic acid, have been shown to inhibit all signs of apoptosis.37,41,44,50-52 Recent observations point to an intricate cross-talk between caspases and mitochondria in the apoptotic process. Several recombinant caspases (caspases 1, 2, 3, 4, and 6) enhance the permeability of PT pore-containing liposomes, and inducers of MPT pore opening, such as atractyloside, trigger the release of caspases 2 and 9 from the mitochondria,13 44 which can activate downstream caspases such as caspase 3, 6, and 7. Activated caspases, in turn, can directly act on the mitochondria, thus engaging in a self-amplifying feedback loop in which changes in mitochondrial permeability lead to caspase activation and vice versa. Thus, if the interplay between caspase proteases and mitochondria decides the fate of the cell during apoptosis, they may constitute useful molecular targets for novel drug design. In this regard, identifying anticancer agents with a predilection for mitochondrial membrane structures, including the MPT pore complex on the one hand and ability to directly activate intracellular caspases on the other, would be an ideal combination to induce effective apoptosis in tumor cells. By targeting the effectors (caspases and mitochondria) of the apoptotic pathway, the need for private signaling mechanisms could be bypassed, which could significantly improve the response of tumor cells to chemotherapy.
We have previously shown that photoactivation of lipophilic agent merocyanine 540 generates a mixture of photoproducts (pMC540) that selectively induce cell death in human leukemia, lymphoma, and a variety of other tumor cell types in vitro and in vivo.53-55 In a recent communication, we investigated the mode of cell death triggered by pMC540 and showed that HL60 leukemia and M14 melanoma cells underwent caspase-mediated cell death with all the characteristic hallmarks of apoptosis.33 In the present study, we isolated and purified three biologically active components from the pMC540 mixture. Two of the purified compounds (C1 and C2) triggered efficient activation of both an initiator caspase, caspase 8, and an executioner caspase, caspase 3, and induced caspase 8-dependent apoptosis in HL60 leukemia and M14 melanoma cells. Both compounds were able to induce Cyt.C release from mitochondria of tumor cells and from purified rat liver mitochondria; however, Cyt.C release in response to C2 occurred independent of MPT pore opening and without a decrease in mitochondrial Δψm.
MATERIALS AND METHODS
Tumor cell lines.
The human promyelocytic leukemia cell line HL60 was obtained from ATCC (Rockville, MD) and maintained in culture in RPMI 1640 supplemented with 10% fetal bovine serum (FBS; GIBCO-BRL, Gaithersburg, MD). M14 human melanoma cell line was a generous gift from Dr Armando Bartolazzi (Oncologia Clinica e Sperimentale, Rome, Italy) and cultured in RPMI/5% FBS.
Purification of photoproducts from pMC540 mixture.
Photoactivation of MC540 was performed as described previously.33 Briefly, 500 mg of MC540 at 1 mg/mL in 70% aqueous ethanol was photoactivated by exposure to a bank of fluorescent lamps (GE Cool White, 40W; General Electric, Cleveland, OH) for 18 hours. After photoactivation, ethanol was removed by freeze drying, and the mixture of photooxidation product was analyzed by thin-layer chromatography (TLC) on aluminum sheets coated with fluorescent silica gel 60F254 (Merck, Darmstadt, Germany) by using the following solvent systems: (1) ethyl acetate/hexane (8:2) and (2) CHCl3/methanol (6:4). UV/VIS absorption spectra were obtained using a spectrophotometer (Biospec 1601, Shimadzu, Japan). Each fraction was then subjected to mass spectrometry (MS) and proton (H1)- and carbon (C13)-nuclear magnetic resonance (NMR) (Bioscience Centre, National University of Singapore, Singapore). Collected fractions were then resuspended in dimethyl sulfoxide (DMSO) at 100 mg/mL and stored at −80°C protected from light.
Determination of caspases 8 and 3 activities.
Activation of intracellular caspase 8 or caspase 3 was assayed by the ApoAlert Fluorescent Assay Kits (Clontech Lab Inc, Palo Alto, CA). HL60 and M14 cells (1 × 106 cells/mL) were exposed to C1, C2, or C5 (50 to 150 μg/mL), washed twice with 1× phosphate-buffered saline (PBS), resuspended in 50 μL of chilled cell lysis buffer (provided by the supplier), and incubated on ice for 10 minutes. Fifty microliters of 2× reaction buffer containing 10 mmol/L dithiothreitol (DTT) and 6 μL of the substrate IETD-AFC (caspase 8) or DEVD-AFC (caspase 3) were added to each sample and incubated at 37°C for 30 minutes. Protease activity was determined by measuring the relative fluorescence intensity at 505 nm after excitation at 400 nm using a spectrofluorimeter (Luminescence Spectrometer LS50B; Perkin Elmer, Buckinghamshire, UK). Results are shown as the fold increase in activity relative to untreated control cells.
Annexin V staining for detection of apoptotic cells.
Externalization of phosphatidylserine, an early marker of apoptosis,was assessed by the ApoAlert-Annexin V Apoptosis Kit (Clontech Lab Inc). Briefly, HL60 and M14 cells (1 × 106/mL) were exposed to 100 μg/mL of C1 or C2 or C5 for 12 hours. Cells were then washed twice with PBS + 1% FBS and resuspended in 200 μL of 1× binding buffer (supplied by the vendor). Annexin V-fluorescein isothiocyanate (FITC) (1 μg/mL) was then added to the cells and left at room temperature for 15 minutes in the dark. After two washes with PBS, cells were resuspended in 0.5 mL of PBS + 1% FBS and analyzed by flow cytometry with the excitation wavelength at 488 nm and the emission set at 525 nm (green). Flow cytometry data were analyzed by the WINMDI software (University of Massachusetts, Amherst, MA).
Detection of cytosolic Cyt.C.
For determination of cytosolic Cyt.C, HL60 cells (30 × 106) were treated with the 100 μg/mL of C1 or C2 for 18 hours and cytosolic fractions were obtained. Briefly, cells were washed twice with ice-cold PBS, pH 7.4, followed by centrifugation at 200g for 5 minutes. The cell pellet was then resuspended in 600 μL of extraction buffer, containing 200 mmol/L mannitol, 68 mmol/L sucrose, 50 mmol/L PIPES-KOH, pH 7.4, 50 mmol/L KCl, 5 mmol/L EGTA, 2 mmol/L MgCl2, 1 mmol/L DTT, and protease inhibitors (Complete Cocktail; Boehringer Mannheim, Mannheim, Germany). After 30 minutes of incubation on ice, cells were homogenized with a dounce homogenizer, the homogenate was spun at 14,000g for 15 minutes, and supernatants were removed and stored at −80°C until analysis by gel electrophoresis for Cyt.C. In a separate set of experiments, M14 cells (2 × 103) were grown on cover slips, exposed to 100 μg/mL of C1 or C2, fixed with methanol:acetone (1:1 vol/vol), and incubated for 2 hours at 37°C with 1:150 dilution (in 3% bovine serum albumin [BSA]) of monoclonal anti-Cyt.C antibody (clone 7H8.2C12; Pharmingen, San Diego, CA). After three washes with 1× PBS + 1% FBS, cells were exposed to a 1:20 dilution of antimouse FITC-conjugated IgG (Pharmingen) for 1 hour, washed twice, and analyzed by confocal microscopy (NUMI Core Facility, NUS, Singapore). Cytosolic Cyt.C was defined as diffuse cytoplasmic staining as compared with punctate mitochondrial Cyt.C staining obtained with nontreated control cells.
Preparation of rat liver mitochondria.
Mitochondria were isolated from rat liver (Albino rats, Wistar strain), as described elsewhere.56 Briefly, liver cells were homogenized in 10 mL of buffer A (0.3 mol/L sucrose, 5 mmol/L TES, 0.2 mmol/L EGTA, pH 7.2, with KOH) and centrifuged at 2,000g for 10 minutes at 4°C. The supernatant (S1) was removed and the pellet was resuspended in 10 mL of buffer A and centrifuged at 2,000g for 10 minutes at 4°C. The supernatant obtained (S2) was then mixed with S1 and centrifuged at 8,000g for 10 minutes at 4°C. The pellet was then resuspended in 1 mL of buffer A, loaded on top of a percoll gradient (60%, 30%, 18%) prepared in buffer A, and centrifuged at 8,000g for 10 minutes at 4°C. Mitochondria were then separated from nonmitochondrial membranes and nonfunctional organelles, collected at the 30%/60% interface, and washed with 10 vol of buffer A at 8,000g for 10 minutes at 4°C to wash off the percoll. Mitochondria were then resuspended in 2 mL of buffer A and kept at 4°C with gentle stirring. All experiments with isolated mitochondria were performed within 4 hours of the preparation.
Mitochondrial swelling and release of Cyt.C.
Large amplitude mitochondrial swelling was determined spectroscopically by the loss of absorbance at 540 nm, as described elsewhere.48 Diethyl pyrocarbonate (DEPC; 200 μmol/L; Sigma, St Louis, MO) was used as a positive control to induce mitochondrial swelling.46 Where indicated, 10 μmol/L CsA (Sigma) was added to the mitochondria before the addition of DEPC, C1, or C2,.
In a separate set of experiments, 0.5 mg of mitochondria was incubated with 100 μg of C1 or C2 for 1 hour at 30°C in the presence and absence of 10 μmol/L CsA, followed by centrifugation at 4,000g for 5 minutes. Control samples received an equal volume of the carrier solvent. The resulting supernatants were used either for caspase activity assays or analyzed by Western blot analysis to detect Cyt.C, as described below.
Determination of mitochondrial Δψm by flow cytometry.
Potential-sensitive probe 3, 3′ dihexyloxacarbocyanine iodide (DiOC6) was used to measure mitochondrial Δψm, as described elsewhere.44 Fifty micrograms purified rat liver mitochondria was exposed to 100 μg/mL of C1 or C2 for 1 hour at 25°C, followed by incubation for 15 minutes at 37°C with 40 nmol/L DiOC6. After two gentle washes with 1× PBS, mitochondria were analyzed in an Epics Profile (Coulter, Hialeah, FL) flow cytometer with the excitation set at 488 nm. At least 10,000 events were collected per sample and data were analyzed by the WINMDI software.
Western blotting for Cyt.C.
Fifty micrograms of cytosolic protein extracts or supernatants from mitochondria treated with C1 or C2 was subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes using a Trans-blot SD semidry system (Bio-Rad Laboratories, Hercules, CA). Membranes were then blocked overnight with 5% dry milk in TBST (50 mmol/L Tris/HCl, pH 7.4, 150 mmol/L NaCl, 0.1% Tween 20). Exposure of blocked membranes to primary anti-Cyt.C antibody (7H8.2C12) was accomplished at room temperature for 1 hour using an antibody dilution of 1:5,000 made in TBS + 0.05% Tween 20 and 1% BSA. After three washes with TBST, the membranes were exposed to 1:5,000 dilution of goat antimouse IgG-horseradish peroxidase (HRP) conjugate supplied as 0.8 mg/mL (Pierce, Rockford, IL) for 1 hour and washed three times with TBST. Chemiluminescence was detected using the SuperSignal Substrate Western Blotting Kit (Pierce).
Cytotoxicity assays.
To determine the sensitivity of tumor cell lines to C1, C2, and C5, HL60 and M14 cells were treated with increasing concentrations of the compounds (25 to 100 μg/mL) for 18 hours. HL60 cells were plated at 1 × 105/well in a 96-well plate and viability was determined by the MTT assay. Ten microliters of 5 mg/mL 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) was added to each well and incubated for 4 hours at 37°C. Elution of the precipitate was performed with 100 μL of DMSO and 10 μL of Tris-Glycine buffer. Cell viability was calculated from the absorption values obtained at 570 nm using an automated enzyme-linked immunosorbent assay (ELISA) reader. M14 cells were plated at 2 × 104/well in RPMI/5% FBS and allowed to attach overnight. Medium was then removed and replaced with fresh medium containing 25 to 100 μg/mL of C1, C2, or C5. After 18 hours of incubation, wells were aspirated and 50 μL of 0.75% crystal violet solution containing 50% ethanol, 0.25% NaCl, and 1.75% formaldehyde was added to each well for 10 minutes. Cells were then washed with water and air-dried, and the dye was eluted with PBS/1% SDS. Cell viability was measured by dye absorbance at 590 nm on an automated ELISA reader.
RESULTS AND DISCUSSION
Purification of three active compounds from pMC540.
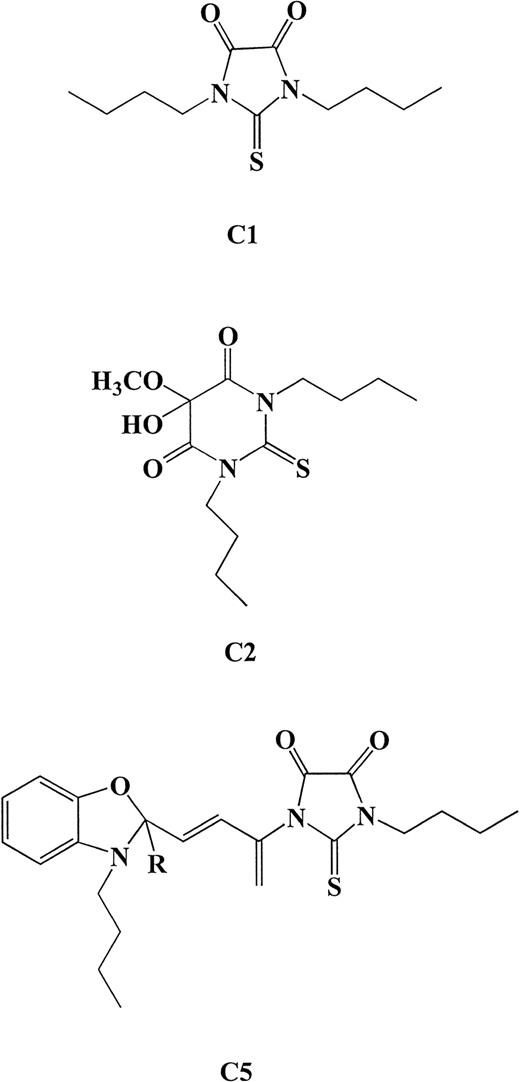
In a previous study, we showed that tumor cells undergo caspase-mediated cell death upon exposure to a mixture of photoproducts generated upon photoactivation of MC540.33 Because pMC540 is a mixture of photoproducts and for it to have potential as a clinical chemotherapeutic agent, the biologically active component(s) in the mixture have to be identified. We report here the purification and characterization of three biologically active components from the photoactivated mixture. The conditions used for photoactivation of MC540 were identical to those described in our in vitro studies demonstrating caspase-dependent antitumor activity of pMC540.33 After photoactivation in ethanol, the solvent was evaporated and the mixture of photo-oxidation product was analyzed by TLC on aluminum sheets coated with fluorescent silica gel. Three major fractions were identified. TLC solvent system 1 yielded two products of Rf 0.83 and 0.65 termed here as C1 and C2, respectively, and solvent system 2 yielded a third product at Rf 0.25 termed C5. Further analysis of the purified fractions by MS and H1- and C13-NMR showed that C1 and C2 were pure compounds with the structural formulae N, N′-Dibutyl-thio-4,5-imidazolindion (molecular weight [MW] = 242) and N, N′-Dibutyl-4,5-dihydro-5-hydroxy-5-ethoxy-4-oxo-2-thiouracil (MW = 316), respectively (Fig 1). These two structures are similar to those previously described upon photo-oxidation of MC540 in methanol and are known as merodantoin and merocil.57 The third compound, named C5, was identified as a highly photosensitive intermediate product with the proposed structure shown in Fig 1. As determined by extraction after analysis on silica-coated TLC plates, the majority of the pMC540 was composed of C5 (∼60%), whereas the percentage yields for C1 and C2 were approximately 16% each.
Structural formulae for the three compounds determined by MS and H1- and C13-NMR analysis of purified fractions. C1, N, N′-Dibutyl-thio-4,5-imidazolindion (MW = 242); C2, N, N′-Dibutyl-4,5-dihydro-5-hydroxy-5-ethoxy-4-oxo-2-thiouracil (MW = 316); C5 was identified as a highly photosensitive intermediate product with the proposed structure shown above.
Structural formulae for the three compounds determined by MS and H1- and C13-NMR analysis of purified fractions. C1, N, N′-Dibutyl-thio-4,5-imidazolindion (MW = 242); C2, N, N′-Dibutyl-4,5-dihydro-5-hydroxy-5-ethoxy-4-oxo-2-thiouracil (MW = 316); C5 was identified as a highly photosensitive intermediate product with the proposed structure shown above.
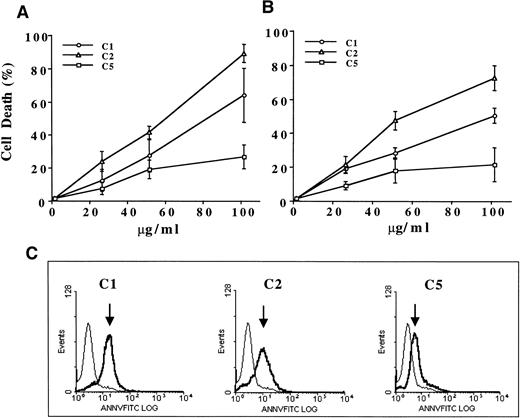
Having identified the major components of pMC540, we set out to determine if the antitumor activity of pMC540 could be attributed to one or more of these photoproducts. Because of our earlier findings with pMC540, we were specifically interested in identifying the compound(s) that could trigger apoptotic cell death in tumor cells by directly activating intracellular caspases and/or trigger mitochondrial events linked to the apoptotic process. Human promyelocytic leukemia cell line HL60 was selected as a model, because our previous studies had shown that human leukemia and lymphoma cells were specifically sensitive to apoptosis induced by pMC540.53,55 In addition, where indicated, similar experiments were performed with M14 human melanoma cells used as a model solid tumor cell line. Although all three compounds (C1, C2, and C5) were able to induce cell death in both tumor cell lines after 18 hours of treatment, C1 and C2 had significantly better activity than C5 (Fig2A and B). Moreover, phenotypic analysis of HL60 cells after exposure to 100 μg/mL of C1 or C2 for 12 hours showed that majority of the cells (>70%) exhibited externalization of inner membrane phosphatidylserine (annexin V positive; Fig 2C), a characteristic morphological change associated with apoptotic cell death.58 Similar to the results obtained with the cell death assays, this morphological change was observed in significantly lesser number (<30%) of C5-treated leukemia cells. These data show that purified compounds C1 and C2 can efficiently kill tumor cells by triggering the apoptotic pathway, as confirmed by PS externalization.
Antitumor activity of C1, C2, and C5 against (A) HL60 and (B) M14 cell lines. A total of 1 × 106 cells/mL were exposed to increasing concentrations (25 to 100 μg/mL) of each compound for 18 hours and cell death was determined by the MTT assay (HL60) or crystal violet assay (M14) as described in Materials and Methods. Data shown are the mean ± SD of three independent experiments performed in triplicate. (C) To assess apoptotic phenotype, HL60 cells (1 × 106) were exposed to 100 μg/mL of C1, C2, or C5 for 12 hours and externalization of inner membrane PS was detected by annexin V-FITC (1 μg/mL) staining and analysis by flow cytometry. At least 10,000 events were analyzed by WINMDI software. Arrows indicate log increase in fluorescence over untreated control cells.
Antitumor activity of C1, C2, and C5 against (A) HL60 and (B) M14 cell lines. A total of 1 × 106 cells/mL were exposed to increasing concentrations (25 to 100 μg/mL) of each compound for 18 hours and cell death was determined by the MTT assay (HL60) or crystal violet assay (M14) as described in Materials and Methods. Data shown are the mean ± SD of three independent experiments performed in triplicate. (C) To assess apoptotic phenotype, HL60 cells (1 × 106) were exposed to 100 μg/mL of C1, C2, or C5 for 12 hours and externalization of inner membrane PS was detected by annexin V-FITC (1 μg/mL) staining and analysis by flow cytometry. At least 10,000 events were analyzed by WINMDI software. Arrows indicate log increase in fluorescence over untreated control cells.
C1 and C2 induce efficient activation of caspase 8 and caspase 3.
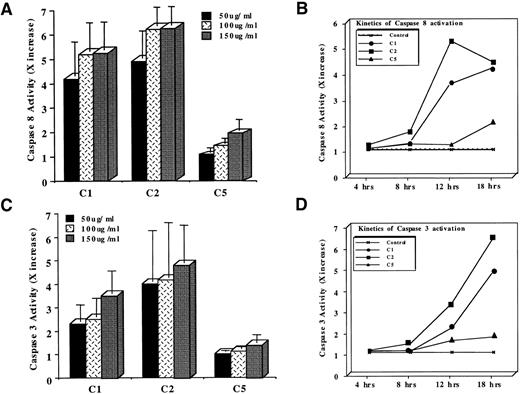
The critical role of intracellular caspases as mediators of all apoptotic cell death has been well established and elegantly reviewed.20 Caspases are not only involved in cell death triggered via cell surface death receptors, but their role in drug-induced apoptosis has also been recently highlighted.29-31,33,59 Whereas caspase 3 is the most extensively studied and the central mediator common to a host of apoptotic triggers, other upstream members of the caspase family, vis a vis caspase 8 (FLICE), have also been shown to be critical for effective apoptosis.4,15,60,61 In our earlier studies, we had shown that tumor cells exposed to pMC540 exhibited efficient activation of caspase 3 and that caspase 3 inhibition was able to block pMC540-mediated apoptosis.33 Stimulated by these findings and by our observation that the three purified components from pMC540 induced apoptosis in tumor cells, we next assessed the ability of the three compounds to induce activation of intracellular caspase proteases. Therefore, HL60 cells were exposed to 50 to 150 μg/mL of C1, C2, or C5 for 12 hours and caspases 8 and 3 activities were determined by fluorimetric assays using tetrapeptide substrates IETD-AFC and DEVD-AFC, respectively. Our results showed that, within 12 hours of treatment, all three compounds (C1, C2, and C5) activated caspases 8 and 3 in a dose-dependent manner, with the maximum activity already obtained with 100 μg/mL for C1 and C2 (Fig 3A). However, C2 was the most efficient (6.2× increase in activity), followed by C1 (5.1× increase in activity) and C5 (2× increase in activity), as shown in Fig 3A. Moreover, a kinetic analysis of caspase 8 activation upon exposure to 100 μg/mL of C1, C2, or C5 for 4 to 18 hours showed that C2 was the earliest (8 hours after treatment) and the most potent (>5× increase in activity at 12 hours) activator of caspase 8 in leukemia cells (Fig 3B). C1 was also efficient in activating caspase 8; however, the peak activity (4× increase) was reached significantly later (18 hours) than with C2 (Fig 3B). On the contrary, C5 did not activate caspase 8 as efficiently as C1 or C2, with the maximum activity (2× over the untreated cells) reached at 18 hours after drug treatment (Fig 3B). Similarly, caspase 3 activation was induced by both C1 and C2, whereas C5-treated cells showed minimal activation by 12 hours that did not significantly increase even after 18 hours of treatment (Fig 3C). Again, the activation kinetics for caspase 3 indicated that C2 was the most potent caspase 3 inducer, with the peak activity (5.1× increase) at 18 hours after drug treatment, followed by C1 (3.5× increase), whereas C5 did not induce significant activation of caspase 3, as shown in Fig 3D. Similar inductions and kinetics of caspases 8 and 3 activities were obtained upon incubation of M14 cells with C1, C2, or C5 (data not shown). In both cell lines, the kinetics of caspase 8 activation always preceded caspase 3 activation, and the ability of each compound to activate caspase proteases, particularly caspase 3, always correlated with the efficiency of HL60 and M14 cell death induced by each purified compound. Because we were interested in the compound(s) that efficiently activated caspase proteases and effectively triggered apoptosis, we subsequently focused our investigations on the mechanism of action of compounds C1 and C2 only.
Determination of (A) caspase 8 or (C) caspase 3 activation by a fluorimetric assay designed to detect cleavage of tetrapeptide substrates. HL60 cells (1 × 106) were treated with 50 to 150 μg/mL of C1, C2, or C5 for 12 hours and lysates were analyzed for IETDase (caspase 8) or DEVDase (caspase 3) activities as described in Materials and Methods. Data shown are the mean ± SD of four independent experiments and are expressed as X increase in activity over the untreated HL60 cells. Kinetics of (B) caspase 8 or (D) caspase 3 activation in HL60 cells exposed to 100 μg/mL of C1 or C2 or 150 μg/mL of C5 for 4 to 18 hours. Data shown are representative of three independent observations.
Determination of (A) caspase 8 or (C) caspase 3 activation by a fluorimetric assay designed to detect cleavage of tetrapeptide substrates. HL60 cells (1 × 106) were treated with 50 to 150 μg/mL of C1, C2, or C5 for 12 hours and lysates were analyzed for IETDase (caspase 8) or DEVDase (caspase 3) activities as described in Materials and Methods. Data shown are the mean ± SD of four independent experiments and are expressed as X increase in activity over the untreated HL60 cells. Kinetics of (B) caspase 8 or (D) caspase 3 activation in HL60 cells exposed to 100 μg/mL of C1 or C2 or 150 μg/mL of C5 for 4 to 18 hours. Data shown are representative of three independent observations.
Apoptosis and caspase 3 activation induced by C1 and C2 are caspase 8-dependent.
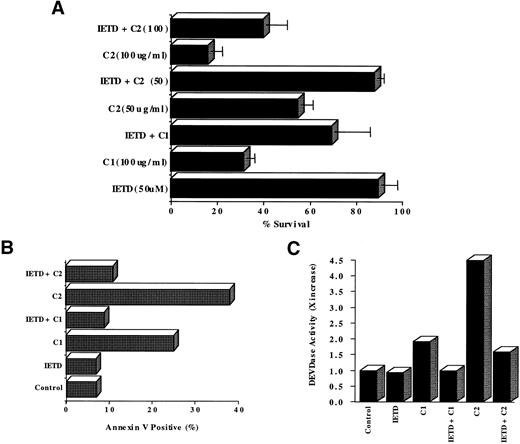
We were intrigued by our observation that two of the three compounds, C1 and C2, efficiently activated caspase 8, an early protease in the caspase cascade that has been better described in cell death induced via the cell surface receptor CD95 (Fas/Apo1).61 Therefore, we first addressed the questions of whether caspase 8 was critical in the process of apoptosis induced by C1 and C2 and if activation of caspase 3 was dependent on caspase 8 activation or if both caspases were independently activated by C1 and/or C2. Using an aldehyde tetrapeptide inhibitor of caspase 8, IETD-CHO (50 μmol/L), we showed a significantly enhanced survival of HL60 cells after exposure to 100 μg/mL of C1 (from 30% to 70%) or 50 μg/mL of C2 (from 50% to 87%), as shown in Fig 4A. Furthermore, this enhanced survival was due to inhibition of apoptosis induced by C1 or C2, as shown by a complete inhibition of annexin V staining in the presence of IETD-CHO as opposed to C1 (100 μg/mL) or C2 (50 μg/mL) alone (Fig 4B). Furthermore, preincubation for 1 hour with IETD-CHO (50 μmol/L) completely inhibited the activation of caspase 3 induced by 12 hours of treatment with either C1 (100 μg/mL) or C2 (100 μg/mL), as shown in Fig 4C. These data demonstrate that caspase 3 activation and cell death induced upon exposure of tumor cells to C1 or C2 were dependent on upstream activation of caspase 8. Whereas activation of downstream executioner caspase 3 has been well documented with a variety of anticancer drugs, the involvement of non–receptor-dependent activation of caspase 8 in drug-induced tumor cell death is a somewhat novel finding. There are two recent reports showing activation of caspase 8 in response to commonly used chemotherapeutic drugs, such as etoposide and doxorubicin, and another using apoptosis inducing agent betulinic acid.4,6,62 Drug-induced caspase 8 activation could be attributed to upregulation of the cell surface CD95/CD95L system. In our present study, caspase 8 activation in response to C1 or C2 was not secondary to induction of CD95-CD95L interaction in HL60 cells. C1 and C2 did not upregulate CD95 or CD95L and the antitumor activity of C1 or C2 was not inhibited in the presence of anti-CD95 or anti-CD95L antibodies (data not shown). To further corroborate these findings obtained in HL60 cells, we performed the same series of experiments with C1 and C2 on M14 melanoma cells. M14 cells have been shown to lack both of the death-inducing receptors, CD95 and TNFR.63 Our results showed that, similar to HL60 cells, both C1 and C2 triggered caspase 8-dependent cell death in CD95-deficient M14 cells (data not shown), thereby ruling out the possibility that caspase 8 activation could be secondary to CD95 receptor aggregation. Thus, the activation of caspase 8 induced by C1 and C2 was due to a direct effect of the drugs and not via mechanisms involving cell surface death receptors. Our findings on caspase 8-dependent activation of caspase 3 by C1 and C2 are in agreement with earlier reports that mature caspase 8 could by itself activate downstream effector caspases, including caspase 3.64 In the light of these findings, we contend that neither C1 nor C2 directly activate caspase 3, but the sequence of events involve activation of an early caspase, caspase 8, that either directly or indirectly triggers activation of the executioner caspase 3.
Blocking caspase 8 activation enhances HL60 cell survival by inhibiting downstream caspase 3 activation and cell death. (A) HL60 cells (1 × 106/mL) were incubated with C1 or C2 (100 μg/mL) in the presence or absence of IETD-CHO (50 μmol/L) for 18 hours and cell survival was determined by MTT assay. Data shown are the mean ± SD of three independent experiments. (B) C1-and C2-treated HL60 cells were assessed for apoptotic phenotype in the presence of IETD-CHO (50 μmol/L) by annexin V-FITC staining by flow cytometry as described in Materials and Methods. Data are shown as the percentage of annexin V positive. (C) Cell lysates obtained from HL60 cells (1 × 106) after exposure to 100 μg/mL of C1 or C2 for 12 hours in the presence or absence of IETD-CHO (50 μmol/L) were analyzed for caspase 3 activation (DEVDase activity). Data are shown as X increase in caspase 3 activity.
Blocking caspase 8 activation enhances HL60 cell survival by inhibiting downstream caspase 3 activation and cell death. (A) HL60 cells (1 × 106/mL) were incubated with C1 or C2 (100 μg/mL) in the presence or absence of IETD-CHO (50 μmol/L) for 18 hours and cell survival was determined by MTT assay. Data shown are the mean ± SD of three independent experiments. (B) C1-and C2-treated HL60 cells were assessed for apoptotic phenotype in the presence of IETD-CHO (50 μmol/L) by annexin V-FITC staining by flow cytometry as described in Materials and Methods. Data are shown as the percentage of annexin V positive. (C) Cell lysates obtained from HL60 cells (1 × 106) after exposure to 100 μg/mL of C1 or C2 for 12 hours in the presence or absence of IETD-CHO (50 μmol/L) were analyzed for caspase 3 activation (DEVDase activity). Data are shown as X increase in caspase 3 activity.
C1 and C2 induce translocation of mitochondrial Cyt.C.
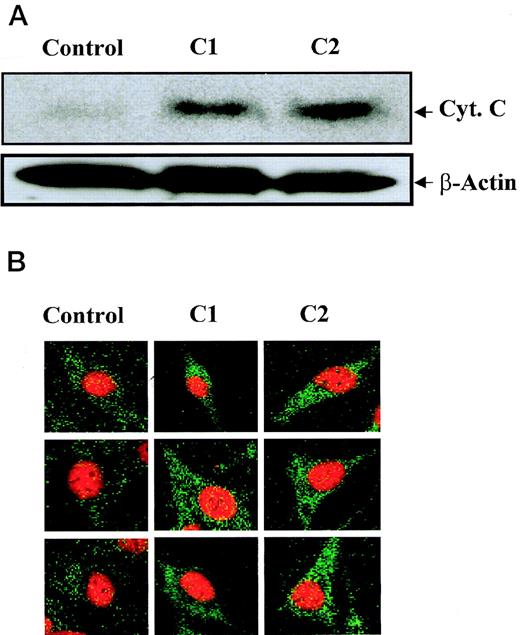
Thus far, we have shown that compounds C1 and C2 trigger caspase 8-dependent apoptosis in HL60 and M14 cells; however, the intracellular events linking activation of caspase 8 by C1 and C2 to downstream caspase 3 activation and death remain to be elucidated. Caspase 8 has been shown to activate downstream caspases, such as caspase 3, via pathways that are either mitochondrial-dependent or independent.4 However, engagement of the mitochondrial-dependent pathway is more efficient than the mitochondrial-independent pathway, because it can be activated by small amounts of caspase 8.15 The mitochondrial-dependent pathway relies on translocation of mitochondrial factors, such as AIF and Cyt.C, which occurs during the early phase of apoptosis induced by a variety of cell death triggers.1,19,65,66 Cyt.C, in concert with other cytosolic factors, then causes the activation of executioner caspases similar to caspase 3,19,65 67 leading to downstream apoptotic events. Having shown that C1 and C2 can efficiently activate caspase 8, we asked if C1 and/or C2 could trigger translocation of mitochondrial Cyt.C to the cytosol of tumor cells. Cytosolic release of Cyt.C was analyzed after exposure of HL60 leukemia cells and M14 melanoma cells with 100 μg/mL of C1 or C2 for 12 hours. Western blot analysis or confocal microscopy was used to detect cytosolic Cyt.C in HL60 and M14 cells, respectively. Western blot analysis of Cyt.C in HL60 cytosolic fractions showed that both C1 and C2 induced translocation of Cyt.C to the cytosol (15-kD band), whereas control cytosols from HL60 cells treated with the carrier solvent did not contain Cyt.C (Fig 5A). Similarly, analysis of M14 cells by confocal microscopy showed a punctate pattern of staining for Cyt.C in untreated control cells, showing mitochondrial localization; however, cells exposed to C1 and C2 for 12 hours exhibited bright green diffuse cytoplasmic staining, indicating translocation of Cyt.C to the cytosol (Fig 5B). Thus, C1 and C2 provoke the cytosolic translocation of Cyt.C, which suggests that both anticancer agents directly or indirectly induce mitochondrial events associated with apoptotic cell death. We propose two possible scenarios, one in which upstream caspase 8 activation induced by both C1 and C2 acts on the mitochondria and induces Cyt.C release and a second in which Cyt.C release could be a result of direct targeting of the mitochondrial membrane structures by C1 and/or C2. We made an attempt to dissect Cyt.C release from caspase 8 activation to decipher the sequence of apoptotic events triggered by C1 and C2. M14 cells were preincubated with caspase 8 inhibitor (IETD-CHO; 100 μmol/L) before the addition of 100 μg/mL of C1 or C2 and analyzed by confocal microscopy for cytosolic Cyt.C. Results showed that preincubation of cells with IETD-CHO did not inhibit the cytosolic translocation of Cyt.C (data not shown), suggesting a possible sequence of events in which C1 and C2 first trigger the release of mitochondrial Cyt.C, which activates caspase 8, leading to downstream activation of executioner caspase 3 and cell death.
C1 and C2 trigger translocation of mitochondrial Cyt.C in HL60 and M14 cells. (A) HL60 (30 × 106 cells) were treated with 100 μg/mL of C1 or C2 for 12 hours and cytosolic fractions were subjected to SDS-PAGE electrophoresis, transferred to PVDF membrane, and subjected to Western blot analysis for Cyt.C as described in Materials and Methods. Anti–β-actin antibody was used to assess equal loading of samples. (B) M14 cells (1 × 103) were grown on cover slips and treated with 100 μg/mL of C1 or C2 for 12 hours, and Cyt.C localization was determined by confocal microscopy using anti-Cyt.C as described in Materials and Methods.
C1 and C2 trigger translocation of mitochondrial Cyt.C in HL60 and M14 cells. (A) HL60 (30 × 106 cells) were treated with 100 μg/mL of C1 or C2 for 12 hours and cytosolic fractions were subjected to SDS-PAGE electrophoresis, transferred to PVDF membrane, and subjected to Western blot analysis for Cyt.C as described in Materials and Methods. Anti–β-actin antibody was used to assess equal loading of samples. (B) M14 cells (1 × 103) were grown on cover slips and treated with 100 μg/mL of C1 or C2 for 12 hours, and Cyt.C localization was determined by confocal microscopy using anti-Cyt.C as described in Materials and Methods.
C1 reduces Δψm and induces MPT-dependent mitochondrial swelling, whereas C2 functions independent of a reduction in Δψm.
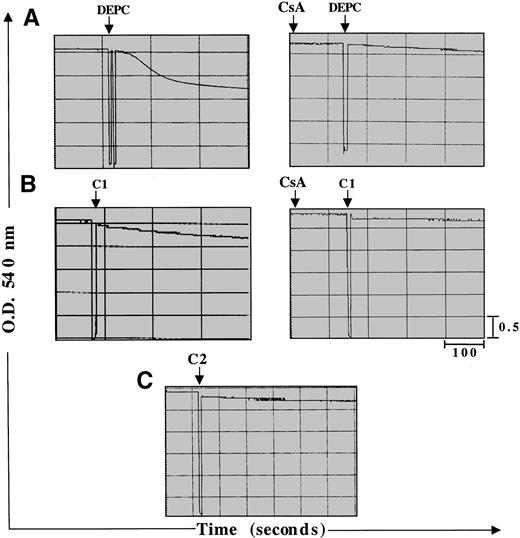
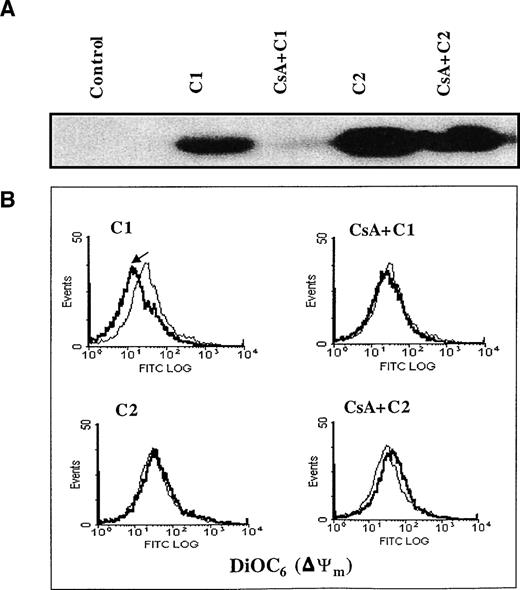
Two major hypotheses have been experimentally suggested for the mechanism of Cyt.C release from the mitochondria during apoptosis: one that is dependent on a decrease in Δψm secondary to opening of the inner membrane MPT pore2 and the other that contends that Cyt.C release is independent of a decrease in Δψm1. MPT pore opening results in volume dysregulation of the mitochondria due to the hyperosmolality of the matrix, causing the matrix space to expand. Inhibitors of MPT pore opening, such as CsA and bongkrekic acid, appear to block apoptosis in some systems,40,50,68 implying a general role. However, some studies have provided evidence that Cyt.C release and caspase activation can occur independently of any detectable loss in mitochondrial Δψm.1 Stimulated by our findings that both C1 and C2 triggered the release of mitochondrial Cyt.C in HL60 and M14 cells, we set out to investigate if this cytosolic translocation of Cyt.C was due to direct effect of the drugs on mitochondrial membrane structures, specifically the MPT pore. To accomplish that, we purified rat liver mitochondria and asked if C1 and/or C2 caused mitochondrial swelling secondary to MPT pore opening and if Cyt.C release was dependent on reduction of mitochondrial Δψm. Freshly isolated mitochondria (0.5 mg) were exposed to 100 μg of C1 or C2 under conditions previously shown to be conducive for swelling induced by activators of MPT, such as Ca2+ and DEPC.46 As expected, the addition of DEPC (0.2 mmol/L) to isolated mitochondria induced rapid swelling that was measured by a decrease in absorbance at 540 nm using a double beam spectrophotometer (Fig 6A). This swelling was completely inhibited by prior addition of CsA (10 μmol/L), an MPT pore inhibitor. Similar to DEPC, the addition of C1 to fresh mitochondria resulted in a loss of absorbance (0.3 OD units) that was completely inhibited by prior addition of CsA (Fig 6B). Unlike C1, C2 had no effect on mitochondrial swelling, as shown in Fig 6C. To further provide impetus to these findings, we directly assessed the ability of C1 and C2 to induce Cyt.C release from isolated mitochondria and to determine if it was dependent on opening of the MPT pore and a decrease in mitochondrial Δψm. Purified mitochondria (0.5 mg) were incubated with 100 μg/mL of C1 or C2 in the presence or absence of MPT pore inhibitor CsA (10 μmol/L). Mitochondria were then gently pelleted and supernatants were used for Western blot analysis of Cyt.C. Our results showed that both C1 and C2 triggered the release of Cyt.C from purified mitochondria; however, the presence of CsA was able to inhibit Cyt.C release only from mitochondria treated with C1 (Fig 7A). Moreover, measurement of mitochondrial Δψm with DiOC6 after incubation for 30 minutes with C1 or C2 showed that C1 induced a decrease in mitochondrial Δψm that was completely inhibited by CsA, whereas C2 had no effect on Δψm, as shown in Fig 7B. These results suggest two different modes of Cyt.C release for C1 and C2, whereby C1 directly targets the MPT pore and induces mitochondrial swelling in a CsA inhibitable manner and contrarily C2 triggers the release of Cyt.C from the mitochondria independently of MPT pore opening.
C1 induces mitochondrial swelling via induction of the MPT pore, whereas C2 has no effect on the pore. Large amplitude mitochondrial swelling was determined spectroscopically by monitoring the loss of absorbance at 540 nm as described in Materials and Methods. Mitochondria (0.5 mg) were treated with (A) 200 μmol/L DEPC as a positive control to induce mitochondrial swelling or (B) C1 (100 μg) or (C) C2 (100 μg) in the absence or presence of MPT pore inhibitor CsA (10 μmol/L).
C1 induces mitochondrial swelling via induction of the MPT pore, whereas C2 has no effect on the pore. Large amplitude mitochondrial swelling was determined spectroscopically by monitoring the loss of absorbance at 540 nm as described in Materials and Methods. Mitochondria (0.5 mg) were treated with (A) 200 μmol/L DEPC as a positive control to induce mitochondrial swelling or (B) C1 (100 μg) or (C) C2 (100 μg) in the absence or presence of MPT pore inhibitor CsA (10 μmol/L).
C1-triggered Cyt.C release and decrease in mitochondrial ▵ψm is dependent on opening of the MPT pore. (A) Purified rat liver mitochondria (0.5 mg) were exposed to 100 μg/mL of C1 or C2 in the presence or absence of CsA (10 μmol/L) for 30 minutes at 30°C. Mitochondria were then pelleted and supernatants were subjected to SDS-PAGE electrophoresis and Western blot analysis for Cyt.C as described in Materials and Methods. (B) Mitochondria were treated with C1 and C2 in the presence or absence of CsA as above and stained with membrane potential-sensitive dye DiOC6 (40 nmol/L) at 37°C for 30 minutes, washed, and analyzed by flow cytometry for ▵ψm.
C1-triggered Cyt.C release and decrease in mitochondrial ▵ψm is dependent on opening of the MPT pore. (A) Purified rat liver mitochondria (0.5 mg) were exposed to 100 μg/mL of C1 or C2 in the presence or absence of CsA (10 μmol/L) for 30 minutes at 30°C. Mitochondria were then pelleted and supernatants were subjected to SDS-PAGE electrophoresis and Western blot analysis for Cyt.C as described in Materials and Methods. (B) Mitochondria were treated with C1 and C2 in the presence or absence of CsA as above and stained with membrane potential-sensitive dye DiOC6 (40 nmol/L) at 37°C for 30 minutes, washed, and analyzed by flow cytometry for ▵ψm.
The release of Cyt.C without a decrease in Δψm seen with C2 suggests that different events control permeability of the inner and outer mitochondrial membranes. A possible mechanism for outer membrane disruption has recently been suggested that involves hyperpolarization,69 rather than hypopolarization of the inner membrane usually associated with pore opening. It is also possible that the release of Cyt.C triggered by C2 may involve disruption of the outer mitochondrial membrane by a hitherto unknown mechanism. Could it be a function of activated caspases directly targeting the mitochondria leading to translocation of Cyt.C or better yet a result of activation of caspases present within the mitochondria? In this regard, recent observations have shown that recombinant caspases (caspase 1, 2, 3, 4, and 6) enhance the permeability of MPT pore-containing liposomes44 and that mitochondria contain pro-caspase 2, 3, and 9, which are liberated into the cytosol during apoptosis.13,70 The possibility that other members of the caspase family reside in the mitochondria and contribute to mitochondrial events during induction of the apoptotic pathway cannot be ruled out. More interestingly, results obtained with C2 are reminiscent of what has been shown with the mammalian cell death protein Bax, which targets the mitochondria and causes release of Cyt.C without MPT pore opening and mitochondrial swelling.71 Our findings do not exclude the possibility that changes in mitochondrial Δψm are critical for apoptosis in some instances, but support the notion that this may not be a universal step in the apoptotic pathway.
Antitumor activity of pMC540 could be reconstituted by simultaneous exposure to C1, C2, and C5.
Having purified the reactive components from the photoactivated mixture pMC540 and highlighted their mechanism(s) of induction of apoptosis, we next asked if the antitumor activity observed in our earlier report with pMC540 could be reconstituted by simultaneous exposure of tumor cells to appropriate concentrations of C1, C2, and C5. As mentioned earlier, the relative yields of C1, C2, and C5 from the pMC540 mixture were approximately 16% each for C1 and C2 and 68% for C5. Keeping these relative yields in mind, HL60 cells were treated with either 150 μg/mL of pMC540 or 25 μg/mL (16%) of C1 or C2 alone or 100 μg/mL of C5 alone, or all possible combinations of the three purified compounds (C1, C2, and C5). All three parameters, vis a vis activation of caspases 8 and 3 (12 and 18 hours after treatment) and actual cell death (18 hours), were determined. A summary of the results is presented in Table 1. It is interesting to note that, whereas all three purified compounds at the respective concentrations were able to activate caspase 8, C2 (25 μg/mL) was the most potent and the earliest caspase 8 activator (2.5× at 12 hours), and this activity correlated with downstream activation of caspase 3 (1.8×) and the percentage of apoptosis assessed by the MTT assay (28%). Moreover, the presence of C2 in the combination experiments (C1+C2 or C2+C5) could explain most of the caspase 8 and 3 activation and tumor cell death observed in these experiments. Furthermore, inclusion of all three compounds (C1+C2+C5) resulted in a much enhanced activation of caspases 8 (6×) and 3 (7×) and significantly increased cell death (50%). We make two conclusions from these data: first, that a combination of purified compounds used at the same ratio as recovered from the pMC540 mixture results in reconstitution of the total pMC540 anti-tumor activity; and second, that C2 is the most active of the three purified compounds. The later deserves special mention due to the fact that C2 is a thiouracil derivative and that one similar compound, 5-fluorouracil (5FU), is a commonly used chemotherapeutic drug. A closer comparison of the antitumor activities of C2 and 5FU is the focus of one of our ongoing studies.
Reconstitution of the Antitumor Activity of pMC540
| Drugs . | Caspase 8 (fold increase) . | Caspase 3 (fold increase) . | Apo. (%) . | ||
|---|---|---|---|---|---|
| 12 h . | 18 h . | 12 h . | 18 h . | ||
| pMC540 (150 μg/mL) | 2.6 | 1.4 | 3 | 1.5 | 40 |
| C1 (25 μg/mL) | 1 | 1.8 | 1 | 1 | 10 |
| C2 (25 μg/mL) | 2.5 | 2.8 | 1.5 | 1.8 | 28* |
| C5 (100 μg/mL) | 1 | 2.1 | 1.23 | 1 | 15 |
| C1 + C2 | 3 | 4.5 | 4 | 2 | 40* |
| C1 + C5 | 1.3 | 2.5 | 1.5 | 1 | 15 |
| C2 + C5 | 2.6 | 4.6 | 3 | 2 | 38* |
| C1 + C2 + C5 | 6 | 4.5 | 7 | 1.5 | 50* |
| Drugs . | Caspase 8 (fold increase) . | Caspase 3 (fold increase) . | Apo. (%) . | ||
|---|---|---|---|---|---|
| 12 h . | 18 h . | 12 h . | 18 h . | ||
| pMC540 (150 μg/mL) | 2.6 | 1.4 | 3 | 1.5 | 40 |
| C1 (25 μg/mL) | 1 | 1.8 | 1 | 1 | 10 |
| C2 (25 μg/mL) | 2.5 | 2.8 | 1.5 | 1.8 | 28* |
| C5 (100 μg/mL) | 1 | 2.1 | 1.23 | 1 | 15 |
| C1 + C2 | 3 | 4.5 | 4 | 2 | 40* |
| C1 + C5 | 1.3 | 2.5 | 1.5 | 1 | 15 |
| C2 + C5 | 2.6 | 4.6 | 3 | 2 | 38* |
| C1 + C2 + C5 | 6 | 4.5 | 7 | 1.5 | 50* |
HL60 cells (1 × 106/mL) were incubated with pMC540, C1, C2, or C5 at the indicated concentrations or combinations of C1 (25 μg/mL) and C2 (25 μg/mL) and C5 (100 μg/mL) for 12 and 18 hours. Caspase 8 and 3 activities were determined by fluorimetric assays as described in Materials and Methods. Data are represented as fold increase in caspase activity over the baseline obtained with untreated cells (1×). The percentage of cell death was determined by the MTT assay 18 hours after drug treatment. Data shown are the mean of three independent observations.
C2 is the most potent of the purified compounds and explains most of the antitumor activity of pMC540.
Conclusion.
Taking these findings together, we have gained insight into the antitumor activity of novel compounds purified from a photoactivated mixture of MC540. In light of these findings, we propose a model for the mode of action of C1 and C2 whereby non–receptor-dependent drug-induced activation of caspase 8 and release of Cyt.C from the mitochondria lead to the activation of downstream executioner caspase 3 and tumor cell death. Whereas tumor cell apoptosis triggered by both C1 and C2 is caspase 8 dependent, the actual tumor cell death correlated better with the amount of caspase 3 activation, further highlighting the executioner role of caspase 3 in the apoptotic process. The release of Cyt.C appears to be a universal event; however, we show that this can occur in the absence of MPT pore opening and independently of a decrease in Δψm, as with C2. On the contrary, the mode of Cyt.C release triggered by C1 seems to suggest a mechanism(s) similar to caspase 8-mediated apoptosis involving cleavage of the proapoptotic protein Bid upon ligation of the CD95 receptor.61,72 73 Our results seem to suggest that drug-induced Bax-like activity is a more efficient activator of the apoptotic pathway than drug-induced Bid-like activity. How does a particular drug influence the recruitment of Bid-like activity or Bax-like mechanism upon activation of caspase 8? Elucidation of this mechanism(s) in drug-induced apoptosis is the focus of our ongoing investigations.
ACKNOWLEDGMENT
The authors thank Dr M. Kini and Dr Dong Hui (Bioscience Center, NUS, Singapore) for their assistance with the purification of the photoproducts and for discussions on the MS and NMR data and thank Muneeza Jhonkar for help with manuscript preparation.
Supported by Grant No. RP3970333 from the National University of Singapore.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Shazib Pervaiz, MD, PhD, Department of Physiology, Faculty of Medicine, National University of Singapore, 10 Kent Ridge Crescent, Singapore 119260; e-mail:phssp@leonis.nus.edu.sg.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal