Abstract
Oxidative stress has been implicated in the triggering of apoptosis in neutrophils. Because red blood cells (RBCs) are well known to scavenge oxidants including H2O2, we tested the hypothesis that RBCs inhibit apoptosis of neutrophils by reducing intracellular oxidative stress. Apoptosis of neutrophils was evaluated by light microscopy and DNA gel electrophoresis. We found that coculture with RBCs protected against neutrophil apoptosis. Neither physical contact between RBCs and neutrophils nor the cellular integrity of RBCs was required to protect against neutrophil apoptosis. Neutrophil apoptosis was promoted by exogenous H2O2 but suppressed by catalase, indicating a role for H2O2 as a mediator of apoptosis. The protective effect of RBCs against apoptosis was due to catalase and glutathione metabolism because blocking of these antioxidant systems in RBCs attenuated the protective effect of RBCs. These results suggest that neutrophils are protected against apoptosis in the circulation.
MATURE NEUTROPHILS are short-lived cells. They have a circulating half-life of less than 10 hours1,2and once they have migrated into tissue they die via apoptosis within several days without the requirement for any apparent inductive stimuli.3,4 This rapid and spontaneous apoptosis in neutrophils is believed to provide a neutrophil clearance mechanism that would act to limit tissue injury and lead to resolution of inflammation.4
Recent studies have suggested that circulating neutrophils can undergo apoptosis in the vasculature.5,6 However, if neutrophils undergo apoptosis rapidly in the vasculature, they would die before responding to inflammatory stimuli and infiltrating tissue. This suggests the need for a mechanism in which neutrophil apoptosis is inhibited in the circulation. Recently, platelets have been shown to inhibit neutrophil apoptosis,7 supporting this view. However, little attention has been paid to interactions between neutrophils and red blood cells (RBCs), which are about 20-fold more abundant than platelets in the circulation.
Several lines of evidence indicate that reactive oxygen species (ROS) are involved in apoptosis of neutrophils. Neutrophil apoptosis is inhibited under hypoxic conditions, which dramatically decreases the generation of ROS.8 Apoptosis is also inhibited in neutrophils obtained from patients with chronic granulomatous disease who carry a mutation that limits ROS generation.9Neutrophil apoptosis is promoted by exogenous H2O2 but delayed by antioxidants including catalase,8 indicating a role for H2O2 as a mediator of neutrophil apoptosis.
In the circulation, RBCs are 1,000-fold more abundant than neutrophils. RBCs have large amounts of antioxidants such as catalase, glutathione, and superoxide dismutase,10-15 and RBC membranes are permeable to H2O2 and O2−,10 suggesting that RBCs can scavenge ROS. RBCs have been shown to protect against H2O2-mediated damage to cells or tissues.11-14 RBCs scavenge H2O2generated by neutrophils16 and therefore block formation of hydroxy radical (·OH) and hypochlorite (HOCl).15 Because H2O2 can freely cross the cell membrane and therefore readily diffuses out of cells if generated intracellularly,17 the reduction of extracellular H2O2 by antioxidants is expected to decrease intracellular H2O2.18
In this report, we show that RBCs inhibit apoptosis of neutrophils by reducing intracellular oxidant stress. Our data suggest that neutrophils may be protected against apoptosis in the circulation.
MATERIALS AND METHODS
Reagents.
All reagents for cell culture were obtained from GIBCO Life Technologies, Inc (Gaithersburg, MD). 3-amino-1,2,4-triazole, 1-chloro-2,4-dinitrobenzene, mercaptosuccinate, and 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS) were purchased from Sigma-Aldrich Japan K.K. (Tokyo, Japan).
Blood samples.
Blood samples were obtained from healthy volunteers by venipuncture of the forearm vein and were immediately anticoagulated with 10 mmol/L EDTA.
Preparation and treatment of RBCs.
RBCs were isolated by centrifugation of the blood samples at 200g for 10 minutes, gentle removal of platelet-rich plasma and leukocytes, washing three times with phosphate-buffered saline (PBS), and resuspension in PBS at 50% hematocrit. For some experiments, RBC antioxidant systems were blocked as previously described. RBC catalase was irreversibly inhibited by incubating a cell suspension with 50 mmol/L aminotriazole (catalase inhibitor) and 0.4 mmol/L H2O2 for 1 hour at 37°C.19 RBC glutathione was blocked by treating a cell suspension with either 2 mmol/L chlorodinitrobenzene (glutathione depleting agent),20 or 0.2 mmol/L mercaptosuccinate (glutathione peroxidase inhibitor)21 for 1 hour at 37°C. If necessary, RBCs were treated simultaneously with aminotriazole, chlorodinitrobenzene, and mercaptosuccinate to inhibit both catalase and glutathione metabolism, which account for the majority of the H2O2-scavenging activity of RBCs. RBC transport of O2− was blocked by incubating a cell suspension with 0.1 mmol/L DIDS for 1 hour at 37°C.10Before use, RBCs treated as above were washed three times in PBS to remove excess reagents. In some experiments, RBCs were lysed by brief sonication with an ultrasonic sonicator.
Preparation of neutrophils.
Human neutrophils were isolated by dextran sedimentation and centrifugation on a Histopaque gradient (without endotoxin; Sigma) as previously described.22 Contaminating erythrocytes were removed by hypotonic water lysis. The isolated neutrophils were washed twice with PBS and resuspended in RPMI1640 containing 5% heat-inactivated fetal calf serum (FCS), 100 U/mL penicillin, and 100 μg/mL streptomycin. To avoid accidental neutrophil activation and clumping, neutrophil preparations were carefully washed by centrifugation at 200g, the supernatant was aspirated, cells were gently resuspended with a pipet, and sudden changes in temperature were avoided. The purity of neutrophil populations was greater than 95% on May-Grünwald-Giemsa stain, and neutrophil viability was greater than 98% as determined by trypan blue dye exclusion. Less than 5% of neutrophil preparations showed a polarized shape, a sensitive marker for neutrophil activation.23
Coculture of neutrophils with RBCs.
Neutrophils (5 × 104) were mixed with autologous RBCs at ratios of 1:10, 1:100, and 1:1000, and cultured in 96-well round-bottom plates (Becton Dickinson, Lincoln Park, NJ) at 37°C in a humidified incubator containing 5% CO2. In some experiments, neutrophils and RBCs were separately cultured in a 24-well plate using the cell culture insert (pore size, 0.4 μm; Becton Dickinson Labwares, Franklin Lakes, NJ). Neutrophils (2.5 × 105) were placed in the lower chamber separated by a membrane from the upper chamber in which RBCs were placed (2.5 × 106).
Apoptosis assay.
Aliquots of neutrophils were cytospun on glass slides and air-dried. Slides were stained with May-Grünwald-Giemsa. Apoptosis was assessed based on nuclear pyknosis or chromatin condensation together with cytoplasmic vacuolation on oil-immersion microscopy.3Three hundred cells were scored in each experiment to determine the percentage of apoptotic cells.
DNA fragmentation assay.
DNA fragmentation was evaluated using Apoptosis Ladder Detection Kit (Wako, Tokyo, Japan) according to the manufacturer’s instructions. Briefly, neutrophils (1 × 104) were resuspended in lysis buffer containing RNase and proteases. Then, DNA was extracted in extraction buffer, precipitated by adding isopropanol, washed in 70% ethanol, air-dried, and resuspended in Tris-EDTA (TE) buffer. DNA was electrophoresed on a 1% agarose gel, stained with SYBR Green I (Molecular Probes, Inc, Eugene, OR), and visualized under ultraviolet light.
Statistics.
Results are presented as mean ± standard error of mean (SEM). Comparisons were made by Student’s t-test or analysis of variance (ANOVA) with Scheffe’s correction as appropriate. P< .05 was accepted as significant.
RESULTS
RBCs inhibit spontaneous apoptosis of neutrophils.
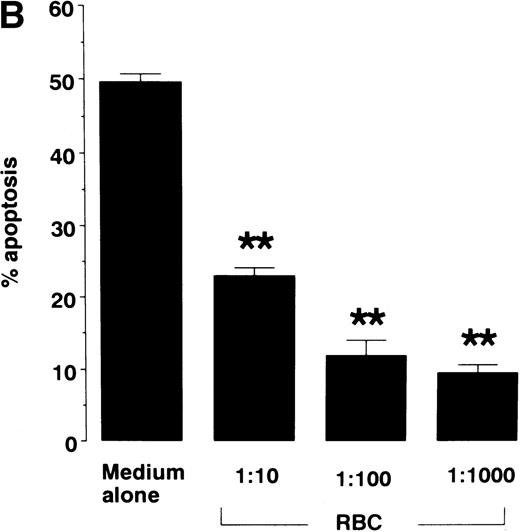
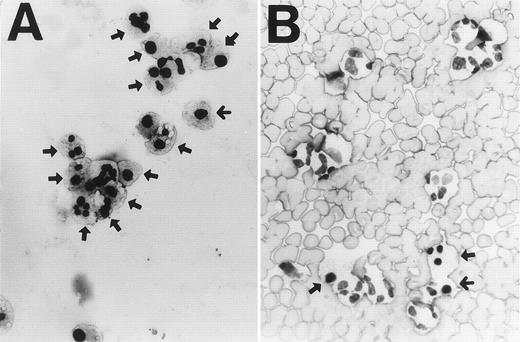
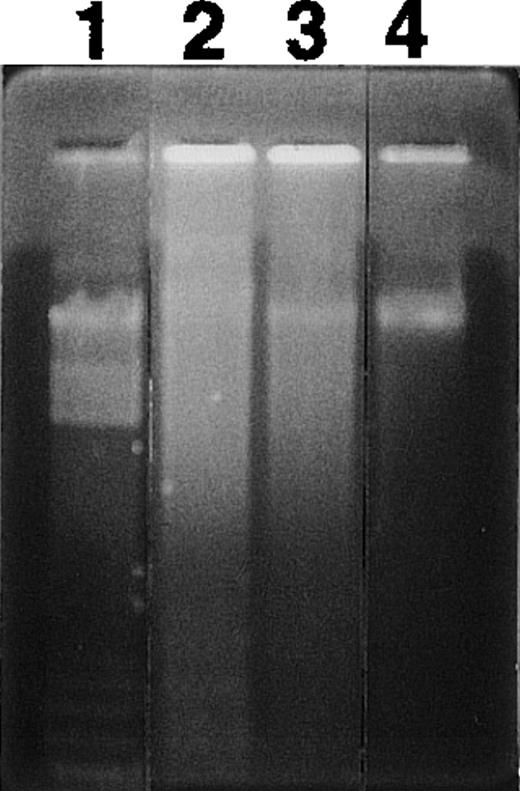
When cultured in RPMI 1640 containing 5% FCS, neutrophils constitutively underwent apoptosis, with 62.8% of cells apoptotic at 24 hours and 81.3% apoptotic by 48 hours as assessed by light microscopy (Fig 1A). The constitutive rate of apoptosis in neutrophils shown here is within the range reported previously.3 9 When neutrophils were cultured with RBCs at a ratio of 1:10, neutrophil apoptosis was significantly inhibited at each time point evaluated (Fig 1A). Inhibition of apoptosis by RBCs was also dose-dependent (Fig 1B). When neutrophils were cultured with RBCs at a ratio of 1:1,000, a physiological ratio in the blood, apoptosis was decreased by about 80%. Figure 2 shows typical micrographs of neutrophils cultured for 24 hours with or without RBCs at a ratio of 1:100. Neutrophil-RBC contact was not required to inhibit apoptosis because apoptosis was inhibited even when neutrophils and RBCs were cultured separately using culture inserts. Percentage of apoptotic neutrophils after 24-hour culture with or without RBCs at a ratio of 1:10 was 85.8% ± 1.0% for no RBCs versus 55.8% ± 1.7% for contact culture and 68.1% ± 2.6% for separate culture (P < .01). The integrity of RBCs was not required because apoptosis was inhibited even when neutrophils were cultured with RBC lysates (data not shown). The protection of neutrophil apoptosis by RBCs was substantiated by the results of DNA gel electrophoresis, which showed that DNA ladder formation was inhibited when neutrophils were cultured with RBCs (Fig 3).
Effect of RBCs on neutrophil apoptosis. Neutrophils were cultured for varying times (A) or 16 hours (B) in RPMI 1640 containing 5% FCS in the presence or absence of 1:10 to 1:1000 RBCs. Apoptotic cells were identified by morphology on light microscopy and % apoptosis was obtained from 300 counts per experiment. Data represents mean ± SEM of 6 experiments. ** P < .01 versus cells in the absence of RBCs.
Effect of RBCs on neutrophil apoptosis. Neutrophils were cultured for varying times (A) or 16 hours (B) in RPMI 1640 containing 5% FCS in the presence or absence of 1:10 to 1:1000 RBCs. Apoptotic cells were identified by morphology on light microscopy and % apoptosis was obtained from 300 counts per experiment. Data represents mean ± SEM of 6 experiments. ** P < .01 versus cells in the absence of RBCs.
Light microscopy of neutrophils cultured for 24 hours without (A) or with (B) RBCs at a ratio of 1:100 (×400). Arrows indicate apoptotic cells showing nuclear pyknosis or chromatin condensation together with cytoplasmic vacuolation. Note apoptosis is inhibited in the presence of RBCs.
Light microscopy of neutrophils cultured for 24 hours without (A) or with (B) RBCs at a ratio of 1:100 (×400). Arrows indicate apoptotic cells showing nuclear pyknosis or chromatin condensation together with cytoplasmic vacuolation. Note apoptosis is inhibited in the presence of RBCs.
Effect of RBCs on nucleosomal DNA fragmentation in neutrophils. Neutrophils were cultured for 24 hours in the presence or absence of 1:1000 RBCs. Cells were harvested and DNA fragmentation was analyzed by agarose gel electrophoresis. Lane 1, molecular weight marker; lane 2, cells cultured without RBCs; lane 3, cells cultured with RBCs; lane 4, cells isolated freshly.
Effect of RBCs on nucleosomal DNA fragmentation in neutrophils. Neutrophils were cultured for 24 hours in the presence or absence of 1:1000 RBCs. Cells were harvested and DNA fragmentation was analyzed by agarose gel electrophoresis. Lane 1, molecular weight marker; lane 2, cells cultured without RBCs; lane 3, cells cultured with RBCs; lane 4, cells isolated freshly.
Role of H2O2 as a mediator of neutrophil apoptosis.
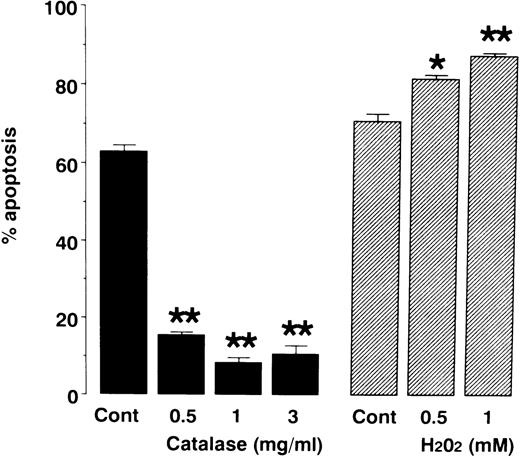
To determine the role of H2O2 in neutrophil apoptosis, we cultured neutrophils in the presence or absence of either catalase or H2O2. As shown in Fig 4, application of catalase inhibited neutrophil apoptosis whereas H2O2 promoted apoptosis. These results are consistent with a previous report9 suggesting that H2O2 is a mediator of neutrophil apoptosis.
Effects of catalase and H2O2 on neutrophil apoptosis. Neutrophils were cultured for 24 hours in the presence or absence of catalase and H2O2. Apoptosis was evaluated as described in Fig 1. To avoid inactivation of H2O2 by serum, serum-free medium was used to assess the effect of H2O2 on neutrophil apoptosis. This probably reflects increased apoptosis in H2O2-untreated control cells (70.8% ± 2.0%) compared with apoptosis in catalase-untreated control cells (62.8% ± 1.7%). Data represents mean ± SEM of 4 experiments. * P < .05, ** P < .01 versus control cells in the absence of catalase or H2O2.
Effects of catalase and H2O2 on neutrophil apoptosis. Neutrophils were cultured for 24 hours in the presence or absence of catalase and H2O2. Apoptosis was evaluated as described in Fig 1. To avoid inactivation of H2O2 by serum, serum-free medium was used to assess the effect of H2O2 on neutrophil apoptosis. This probably reflects increased apoptosis in H2O2-untreated control cells (70.8% ± 2.0%) compared with apoptosis in catalase-untreated control cells (62.8% ± 1.7%). Data represents mean ± SEM of 4 experiments. * P < .05, ** P < .01 versus control cells in the absence of catalase or H2O2.
Catalase and glutathione are responsible for protection of neutrophil apoptosis by RBCs.
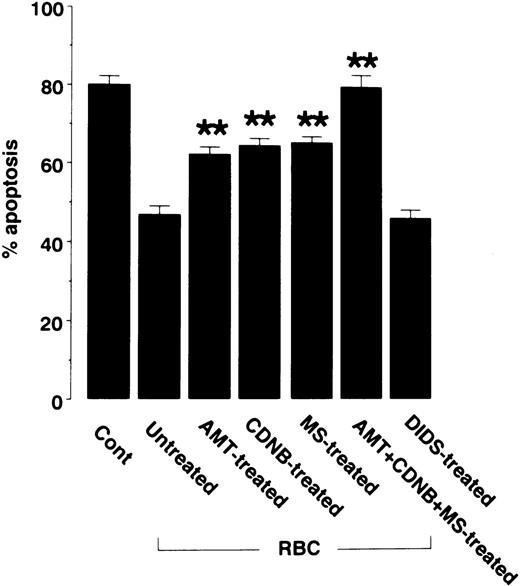
To determine the contribution of each of RBC antioxidant to protection against neutrophil apoptosis, RBC antioxidant systems were pharmacologically blocked before analysis of the protective effect on apoptosis (Fig 5). Inhibition of RBC catalase by pretreatment with aminotriazole partially suppressed the protection against neutrophil apoptosis by RBCs. Likewise, inhibition of glutathione metabolism with chlorodinitrobenzene or mercaptosuccinate partially suppressed the protection against apoptosis. Simultaneous inhibition of catalase and glutathione metabolism, which account for the majority of the H2O2-scavenging activity of RBCs, completely abolished the protection. In contrast, pretreatment of RBCs with the anion channel blocker DIDS, which blocks transport of O2− across RBC membranes, had little effect on the protection against apoptosis by RBCs. These results suggest that the inactivation of H2O2 by catalase and glutathione metabolism is the mechanism by which RBCs protect neutrophils against apoptosis.
Effects of antioxidant inhibitors on the ability of RBCs to inhibit neutrophil apoptosis. RBCs were pretreated with the catalase inhibitor aminotriazole (AMT), the glutathione metabolism inhibitors chlorodonitrobenzene (CDNB) and mercaptosuccinate (MS), a combination of AMT, CDNB, and MS, or the O2− transport inhibitor DIDS. Excess reagents were removed by washing RBCs three times in PBS. Then, RBCs were mixed with neutrophils at a neutrophil/RBC ratio of 1:10. After 48 hours of culture, neutrophil apoptosis was evaluated as described in Fig 1. Data represents mean ± SEM of five experiments. **P < .01 versus untreated RBCs.
Effects of antioxidant inhibitors on the ability of RBCs to inhibit neutrophil apoptosis. RBCs were pretreated with the catalase inhibitor aminotriazole (AMT), the glutathione metabolism inhibitors chlorodonitrobenzene (CDNB) and mercaptosuccinate (MS), a combination of AMT, CDNB, and MS, or the O2− transport inhibitor DIDS. Excess reagents were removed by washing RBCs three times in PBS. Then, RBCs were mixed with neutrophils at a neutrophil/RBC ratio of 1:10. After 48 hours of culture, neutrophil apoptosis was evaluated as described in Fig 1. Data represents mean ± SEM of five experiments. **P < .01 versus untreated RBCs.
DISCUSSION
There is increasing evidence to indicate that ROS play an important role in spontaneous- and stimulus-mediated apoptosis of neutrophils.8,9,24-27 Our data showing that neutrophil apoptosis is promoted by exogenous H2O2 but blocked by catalase confirms a previous study9 indicating H2O2 as a mediator of neutrophil apoptosis. Although cellular sources of H2O2 are numerous and difficult to determine, the major source of endogenous H2O2 is a leak from the mitochondrial electron transport chain. All aerobic cells constantly produce small amounts of O2− via mitochondrial respiratory enzymes. O2− is then dismutated to form H2O2. Many types of cells also generate O2−/H2O2 via various enzyme systems including arachidonic acid metabolism, microsomal cytochrome systems, and DNA synthesis enzymes. Furthermore, phagocytes such as neutrophils are endowed with nicotinamide adenine dinucleotide phosphate (NADPH) oxidase that releases large amounts of O2− and H2O2 on stimulation.28 Thus, the exact source of H2O2-mediating apoptosis of neutrophils remains to be determined. Nevertheless, our data together with others9 indicate that intracellular H2O2, at least in part, functions as a mediator of neutrophil apoptosis.
The major finding in our study is that RBCs inhibit apoptosis of neutrophils. A number of studies have suggested that RBCs can scavenge H2O2 within their microenvironment. For example, RBCs can protect against H2O2-mediated damage to cells and tissues.12,13,15,16 Because H2O2 can freely cross the cell membrane17 and there are 1000-fold more RBCs than neutrophils in the blood, the elimination of extracellular H2O2 by RBCs is expected to decrease H2O2 within neutrophils.
RBCs are endowed with high activities of catalase and glutathione peroxidase, which together probably account for the majority of H2O2-scavenging activity of RBCs.11,13,15 29 Specific inhibition of these RBC antioxidants in the present study showed that both catalase and glutathione metabolism are responsible and sufficient for protection of apoptosis by RBCs. In contrast, inhibition of O2− transport across RBC membranes had no protective effect against apoptosis. These data imply that inactivation of H2O2 by catalase and glutathione within RBCs mediates the protective effect against neutrophil apoptosis.
The fact that RBCs can inhibit neutrophil apoptosis in vitro suggests that neutrophil apoptosis is inhibited in the circulation in vivo. Because blood plasma contains few antioxidant enzymes,30,31RBCs may constitute a major antioxidant system that suppresses neutrophil apoptosis in the blood. However, it should also be noted that although we found that RBCs inhibited neutrophil apoptosis in vitro, this effect was rather small at 6 hours of culture and became apparent after this point of time. Because neutrophils have a circulating half-life of less than 10 hours,1 2extrapolation of our in vitro data to in vivo situations requires caution.
Apoptosis is believed to provide a mechanism of neutrophil clearance that limits tissue injury and leads to resolution of inflammation.4 Recently, apoptosis of neutrophils has also been shown to occur in the circulation.5,6 However, it remains unclear whether apoptosis occurs as rapidly in the circulation as in tissue. If so, a considerable amount of circulating neutrophils would die before reaching their target tissue on inflammatory stimulation. Thus, it seems reasonable to speculate that there is a mechanism by which apoptosis of neutrophils is suppressed in the circulation. A recent study has shown that platelets also inhibit apoptosis of neutrophils although the mechanism is not well defined.7 Taken together, our data suggest that neutrophils are protected against apoptosis in the circulation.
Supported by a Grant-in-Aid for Scientific Research (#30147392) from the Ministry of Education, Science, and Culture, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal