Abstract
One hypothesis to explain the age-dependent clearance of red blood cells (RBCs) from circulation proposes that denatured/oxidized hemoglobin (hemichromes) arising late during an RBC’s life span induces clustering of the integral membrane protein, band 3. In turn, band 3 clustering generates an epitope on the senescent cell surface leading to autologous IgG binding and consequent phagocytosis. Because dog RBCs have survival characteristics that closely resemble those of human RBCs (ie, low random RBC loss, ≈115-day life span), we decided to test several aspects of the above hypothesis in the canine model, where in vivo aged cells of defined age could be evaluated for biochemical changes. For this purpose, dog RBCs were biotinylated in vivo and retrieved for biochemical analysis at various later dates using avidin-coated magnetic beads. Consistent with the above hypothesis, senescent dog RBCs were found to contain measurably elevated membrane-bound (denatured) globin and a sevenfold enhancement of surface-associated autologous IgG. Interestingly, dog RBCs that were allowed to senesce for 115 days in vivo also suffered from compromised intracellular reducing power, containing only 30% of the reduced glutathione found in unfractionated cells. Although the small quantity of cells of age ≥110 days did not allow direct quantitation of band 3 clustering, it was nevertheless possible to exploit single-cell microdeformation methods to evaluate the fraction of band 3 molecules that had lost their normal skeletal linkages and were free to cluster in response to hemichrome binding. Importantly, band 3 in RBCs ≥112 days old was found to be 25% less restrained by skeletal interactions than band 3 in control cells, indicating that the normal linkages between band 3 and the membrane skeleton had been substantially disrupted. Interestingly, the protein 4.1a/protein 4.1b ratio, commonly assumed to reflect RBC age, was found to be maximal in RBCs isolated only 58 days after labeling, implying that while this marker is useful for identifying very young populations of RBCs, it is not a very sensitive marker for canine senescent RBCs. Taken together, these data argue that several of the readily testable elements of the above hypothesis implicating band 3 in human RBC senescence can be validated in an appropriate canine model.
HUMAN red blood cells (RBCs) have a circulating life span of ≈120 days, after which they are recognized and removed from circulation by macrophages of the mononuclear phagocyte system.1 Although characterization of the mechanisms that permit selective phagocytosis of senescent cells has been undertaken by many,2 progress has been hampered by the lack of a model system that allows isolation of truly senescent cells after their aging in vivo.2 Although density gradient separation methods have allowed the collection of human RBCs that are destined for immediate removal,3 there is still concern that the densest cells may not be truly senescent.4-6Development of hypertransfusion7 and biotinylation methodologies8 to isolate populations of unquestionably senescent RBCs from live animals has successfully generated much information on changes that occur during RBC senescence,4-7,9-17 but these studies may not be relevant to human RBCs because they have used animals (rabbit, rodent) that exhibit high random (age independent) RBC removal and short RBC life spans (≈50 to 70 days).18,19 To circumvent this problem, we have adapted the biotinylation method for application in the dog,20 largely because dog RBC survival characteristics are very similar to human RBCs in exhibiting low random removal18 and a long life span of ≈115 days.18,21 22
Because mature RBCs are unable to synthesize new proteins, the events that trigger removal of senescent cells from circulation must derive from alterations in pre-existing proteins or lipids that lead to recognizable changes at the membrane surface. We and others have proposed that one such alteration is the age-dependent clustering of the membrane-spanning protein, band 3.23-29 According to this hypothesis, band 3 clustering can arise from multiple mechanisms that are active late in an RBC’s life span, including hemoglobin (Hb) denaturation23,25 and protein oxidation.26,27,30 Upon formation of band 3 clusters, there is considerable evidence for an obligatory opsonization of the clusters by autologous antibodies.24,26,27,31-34 Evidence to date suggests that these autologous anti–band 3 antibodies consist predominantly of IgG subclass 2 and 3.31 However, the exact antigenic determinate on band 3 is controversial because both carbohydrate26,35 and protein36 epitopes on the anion transporter have been reported. In addition, anti–band 3 antibodies are able to bind complement component C337 and form C3b-IgG complexes.38 These C3b-IgG complexes can nucleate alternative complement pathway C3 convertases resulting in the deposition of additional complement components on the senescent cell surface.39 Eventually, the RBC becomes coated with sufficient antibody and complement to be recognized and removed from circulation by macrophages of the mononuclear phagocyte system.1,2,24,27 40
The pathway of RBC removal discussed above has been primarily characterized by studying pathologic (sickle and thalassemic),32,34 density separated,33,38 or in vitro–modified RBCs.24,26,27 However, very little evidence supporting the pathway has been obtained from normal in vivo–aged RBCs that are unquestionably senescent and have survival characteristics similar to humans. Our preliminary studies using the biotinylation system in the dog have shown that dog RBCs greater than 104 days old bind significantly more autologous IgG than a random aged population of dog RBCs.41 In this report, we have examined several additional predictions of the clustering hypothesis of RBC senescence in the dog.
MATERIALS AND METHODS
In vivo biotinylation of canine RBCs.
Two dogs (nos. 1630 and 5005) used in these studies were healthy, mature male beagles obtained from a commercial random source vendor. They were housed indoors and maintained according to Purdue University Animal Care and Use Committee regulations. Both dogs were considered healthy as determined by hematology and clinical biochemistry profiles and by parasite (intestinal, blood) testing.
To produce a larger population of biotinylated RBCs for collection at the end of the RBC life span, biotinylation was performed during reticulocytosis in response to an iatrogenic blood loss anemia. Dog no. 5005 was bled three times over a 3-day period, reducing the hematocrit (Hct) to 30% (prebleeding Hct, 49%), and biotinylation was performed 7 days after the first bleeding. Dog no. 1630 was bled three times over a 4-day period, reducing the Hct to 33% (prebleeding Hct, 50%), and biotinylation was performed 13 days after the first bleeding.
Both dogs had 97% to 100% of circulating RBCs biotinylated by intravenous (IV) infusion of N-hydroxysuccinimide biotin (NHS-biotin; Fluka, Milwaukee, WI) dissolved in dimethyl sulfoxide, as described previously.20 Briefly, NHS-biotin was dissolved in medical grade 90% dimethyl sulfoxide (DMSO; Syntex, West Des Moines, IA) and administered very slowly through an indwelling (IV) catheter. Both dogs initially received 35 mg of NHS-biotin per kg body weight over approximately 30 minutes. However, because of a low concentration of biotin per RBC for dog no. 5005, an additional 17 mg NHS-biotin per kg body weight was administered 4 days after the initial biotinylation. Pretreatment of both dogs with atropine sulfate (0.05 mg/kg) was used to counteract cholinergic-like side effects of the DMSO.
Isolation of biotinylated RBCs.
Detailed procedures for the isolation of in vivo–aged biotinylated RBCs by magnetic bead activated cell sorting (MACS) have been described elsewhere.20 Briefly, heparinized whole blood was washed three times in cold PBS-G (125 mmol/L sodium chloride, 20 mmol/L sodium phosphate, pH 7.4, 5 mmol/L EDTA, 0.01% sodium azide, 5 mmol/L glucose), removing the buffy coat and plasma proteins. The percentage of biotinylated RBCs in circulation was determined by incubating an aliquot of RBCs (2% Hct) with avidin-fluorescein isothiocyanate (avidin-FITC) (0.033 mg/mL; Sigma, St Louis, MO, or Becton Dickinson, San Jose, CA) at 37°C for 30 minutes. RBCs were then washed three times with PBS-G and resuspended in BSA solution (1% bovine serum albumin in PBS-G) for analysis using a Coulter Epic Elite series flow cytometer (Coulter, Hialeah, FL). Biotinylated cells in washed, unfractionated samples were labeled for sorting by incubating with avidin-FITC in PBS-G for 10 minutes on ice (0.188 mg avidin-FITC per 0.150 mL packed biotinylated RBCs). The cells were washed twice in PBS-G followed by a 5-minute incubation on ice with biotinylated MACS microbeads (0.0676 mL microbeads per 0.150 mL packed biotinylated RBCs; Miltenyi Biotec, Auburn, CA). In addition to serving as a cross-linker, the avidin-FITC served as an internal fluorescent marker for assessment of purification efficiency.
For sorting, the RBCs were diluted to 2% hematocrit in BSA solution, applied to an MACS separation column (size “D” from Miltenyi Biotec) containing plastic coated steel wool, and placed in the magnetic field of a permanent magnet (providing ≈0.6 T; obtained locally). The biotinylated RBCs (positive fraction) adhered to the steel wool and nonlabeled RBCs (negative fraction) were eluted with BSA solution. Nonretrieved RBC were diluted to 1% Hct with BSA solution, reapplied to the column in the presence of the magnet, and eluted with BSA solution. The column was then extensively washed with BSA solution, removed from the magnet, and biotinylated RBCs were flushed from the column.
Quantitation of intracellular reduced glutathione (GSH).
Reduced glutathione was determined as described by Beutler et al.42 The method was adapted for samples containing 20 μL of 50% Hct RBCs.
Analysis of RBC membranes by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting.
RBC ghost membranes were prepared from isolated cells by hypotonic lysis in 5 mmol/L sodium phosphate, 1 mmol/L EDTA, pH 8.0 in the presence of 40 μg/mL phenylmethyl sulfonyl fluoride (lysis buffer) according to the procedure of Dodge et al.43 The lysate was centrifuged at 12,000g for 40 minutes and the supernatant was removed. The membrane pellets were resuspended and washed three additional times in lysis buffer. Protein content of the membranes was determined by the bicinchoninic acid method (BCA; Pierce, Rockford, IL) and membranes were solubilized in SDS electrophoresis buffer containing 100 mmol/L dithiothreitol and heated 3 minutes in a boiling water bath. For dog no. 5005, an aliquot of each membrane sample was stored overnight at 4°C for analysis by gel electrophoresis the following day. The remaining ghost membranes from dog no. 5005 and all of the membranes from dog no. 1630 were stored at −80°C until completion of the final isolation. Ghost membranes were resolved on 6% to 12% gradient polyacrylamide gels and were either stained for protein with Coomassie brilliant blue (Sigma) or transferred to nitrocellulose (pore size 0.2 μm; Schleicher and Schuell, Keene, NH) using the buffer system of Towbin et al44 and blocked with 5% milk in TBS-T (20 mmol/L Tris, pH 7.5, 500 mmol/L NaCl, 0.05% Tween 20). To develop, the immunoblot was washed with TBS-T and incubated at room temperature for 3 hours in a solution of rabbit anti-dog Hb (Research Plus, Bayonne, NJ) diluted 1/5,000 in TBS-T with 1% milk. After further washing and incubation with peroxidase-labeled goat anti-rabbit IgG (Bio-Rad, Hercules, CA), the immunoblots were developed using an enhanced chemiluminescence (ECL) reagent kit (Amersham, Arlington Heights, IL) followed by exposure to film. To determine if the banding patterns in the SDS-polyacrylamide gels of our ghost membranes were altered during storage at −80°C, an aliquot of each sample from dog no. 5005 was resolved the day immediately after preparation. We found no observable differences between the banding patterns in samples analyzed immediately after preparation and the samples stored at −80°C (results not shown).
Fluorescence-imaged microdeformation assay of dog RBCs.
Dog RBCs were washed two to three times with PBS (PGS-G with no glucose) containing 0.01% BSA (labeling buffer), and band 3 was labeled in situ with eosin-5-maleimide (Molecular Probes, Eugene, OR). The washed RBCs were incubated at room temperature for 45 minutes in 100 μg/mL of eosin-5-maleimide in labeling buffer and then washed three times with labeling buffer. Association of band 3 with the membrane skeletal network through ankyrin was determined by measuring its redistribution in response to mechanical deformation.45 46 Individual cells were partially aspirated into a glass micropipette and a fluorescent image was taken with a liquid nitrogen cooled charge-couple device (CCD) camera (Photometrics, Tucson, AZ). The fluorescence intensity provides a measure of the density of the labeled band 3 along the membrane protrusion extending into the micropipette, and the intensity changes along the aspirated length provide information on the mobility of band 3 relative to the underlying spectrin/actin skeleton.
Iodination of protein A.
Standard procedures were used to radiolabel protein A (Pierce) with125iodine (125I; NEN Life Science Products, Boston, MA) using Iodobeads (Pierce).47 Iodobeads were washed thoroughly in PBS and dried on filter paper before use. To three Iodobeads in a 1.5-mL centrifuge tube was added 0.5 mL of PBS and 0.3 mCi 125I. The mixture was allowed to sit at room temperature for 5 minutes, after which 250 μg of protein A in 0.5 mL of PBS was added. Following incubation at room temperature for 30 minutes, the unreacted free 125I was removed by passage through a desalting column (10DG from Bio-Rad). Protein concentration was determined by the BCA method and radioactivity was measured in a Packard Cobra Gamma counter (Packard Instrument Co, Meriden, CT). Radiolabeled protein A was stored at 4°C and used within 1 month.
125I protein A binding assay: Detection of autologous IgG binding to dog RBCs.
Isolated biotinylated (positive fraction) and non–biotin-labeled (negative fraction) RBCs were suspended at 40% Hct in PBS containing 4% BSA. To determine autologous IgG binding, 25-μL aliquots of the positive and negative fractions were mixed with 0.2 μg of125I-protein A and incubated for 30 minutes at room temperature with gentle shaking. The mixtures were washed five times in PBS buffer containing 2% BSA to remove unbound radiolabel, transferred to new tubes, and counted in a Packard Cobra Gamma counter. The number of RBCs per sample was determined by manual RBC counts (3 replicates per aliquot). The number of protein A molecules per RBC was calculated from these data.
RESULTS
Biotinylation, survival, and magnetic cell sorting.
IV infusion of NHS-biotin labeled greater than 97% of circulating dog RBCs. Both dogs (nos. 1630 and 5005) exhibited normal RBC life span based on previous labeling studies (data not shown).20During the current study, blood was collected from the dogs, and biotinylated (positive) RBCs were isolated by magnetic cell sorting. Nonbiotinylated (negative) RBCs were also collected and used as control cells. In some assays, a presorted RBC sample containing a mixture of positive and negative cells was collected to serve as an additional control. The percentage of biotinylated RBCs in the isolated positive fraction after magnetic cell sorting was always greater than 72% and averaged 88%.
Intracellular reduced GSH in dog senescent RBCs.
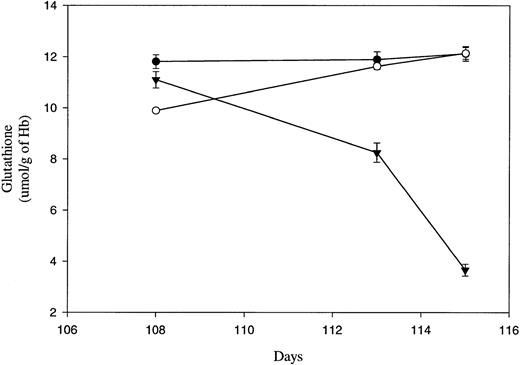
Although the band 3 clustering hypothesis of senescent RBC clearance predicts that senescent cell phagocytosis is triggered by antibody binding to hemichrome-induced aggregates of band 3, little effort has been devoted to identifying the cause of hemichrome formation from oxidized/denatured Hb in late-term erythrocytes. Believing that this latter process might derive from an inability to maintain an adequate reducing power in the cell,2,25 26 we examined the reduced GSH content of the in vivo–aged canine RBCs as a function of cell age. Cellular GSH levels were assayed in control (both presort and negative) and positive (biotinylated) RBC fractions isolated from dog no. 1630 on 3 separate days near the end of the biotinylated RBC’s life span. As shown in Fig 1, unfractionated erythrocytes (solid dots), RBCs not retrieved with avidin-coated beads at day 108 (<108 days old, open circles) and biotinylated RBCs collected on the same day (≥108 days old, solid triangles) all contained 10 to 12 μmol GSH/g Hb in their cells. However, over the next 7 days, as the biotinylated cells reached an age of at least 115 days, their GSH content declined to 1/3 of their value a week earlier, while the nonretrieved cells (<115 days old) as well as the unfractionated cells retained their high (≈12 μmol/g Hb) GSH content. These data suggest that a precipitous change in intracellular reducing power occurs near the end of the RBC’s life span. Although the limited quantities of late-term biotinylated cells prohibited collecting data from multiple dogs, the observation that neither the unfractionated RBCs nor the fractionated RBCs that failed to bind beads (younger cells) from the same dog displayed any decrease in cellular GSH levels suggests that the changes observed in the biotinylated cells are real. However, whether this behavior will prove to be a characteristic of RBC senescence in all dogs will obviously require further scrutiny.
Intracellular reduced glutathione concentrations of in vivo–aged biotinylated dog RBCs. Positive (▾) biotinylated and negative (○) RBCs were isolated from dog no. 1630 at the indicated times post-biotinylation by magnetic cell sorting. In addition, a presort (•) RBC sample was collected from dog no. 1630 before each separation by magnetic cell sorting. Samples were assayed in triplicate for intracellular reduced glutathione and the mean (±SD) is shown.
Intracellular reduced glutathione concentrations of in vivo–aged biotinylated dog RBCs. Positive (▾) biotinylated and negative (○) RBCs were isolated from dog no. 1630 at the indicated times post-biotinylation by magnetic cell sorting. In addition, a presort (•) RBC sample was collected from dog no. 1630 before each separation by magnetic cell sorting. Samples were assayed in triplicate for intracellular reduced glutathione and the mean (±SD) is shown.
Analysis of membrane associated Hb.
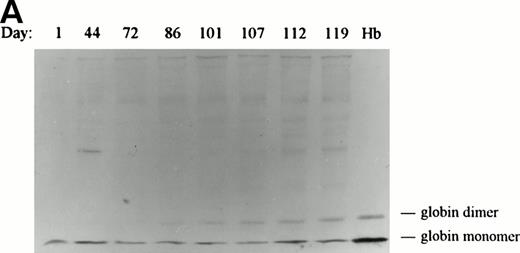
For Hb denaturation to participate in triggering the removal of a senescent erythrocyte from circulation, denaturation/oxidation must be minimal in young and middle-aged erythrocytes, but prominent in cells near the ends of their life span. To determine when Hb denaturation occurs during a RBC’s life span in vivo, membranes were isolated from RBCs retrieved at several time points after biotinylation, separated electrophoretically by polyacrylamide gel electrophoresis, and immunoblotted with an antibody specific for dog Hb. As shown in the immunoblot of Fig 2A, a significant increase in the quantity of membrane-bound globin appears on the membranes of cells isolated 86 days after biotinylation of dog no. 5005 (mean cell age ≤100 days). A similar increase in membrane-bound globin appeared on the membranes of cells isolated from dog no. 1630 (data not shown). The binding of small quantities of Hb to dog membranes prepared from a random aged cell population (lane day 1) was expected and has been shown to also occur in freshly prepared human membranes.48 49 Interestingly, the amount of globin monomer in the immunoblot does not change significantly with cell age. However, the quantity of covalently cross-linked forms of globin are found to increase dramatically as the cell approaches the end of its life (Fig2B), demonstrating that defects in Hb maintenance begin to emerge late in an RBC’s life span.
Western blot analysis of globin deposition on the membranes of in vivo–aged biotinylated dog RBCs. Positive (biotinylated) RBCs were isolated from dog no. 5005 at the indicated times post-biotinylation by magnetic cell sorting. (A) Ghost membranes were prepared as described in the Materials and Methods. At the end of the study, membranes (20 μg) were electrophoresed, transferred to nitrocellulose, and analyzed for globin by immunoblotting as described in Materials and Methods. The lane marked Hb contains 5 μg of soluble Hb obtained from a dog RBC lysate. (B) Results of scanning densitometry of the dimeric globin band seen in the immunoblot in (A). The integrated area of the globin dimer band is plotted as a function of the minimum age of the biotinylated cells. A similar increase in membrane-bound globin appeared on the membranes of cells isolated from dog no. 1630 (data not shown).
Western blot analysis of globin deposition on the membranes of in vivo–aged biotinylated dog RBCs. Positive (biotinylated) RBCs were isolated from dog no. 5005 at the indicated times post-biotinylation by magnetic cell sorting. (A) Ghost membranes were prepared as described in the Materials and Methods. At the end of the study, membranes (20 μg) were electrophoresed, transferred to nitrocellulose, and analyzed for globin by immunoblotting as described in Materials and Methods. The lane marked Hb contains 5 μg of soluble Hb obtained from a dog RBC lysate. (B) Results of scanning densitometry of the dimeric globin band seen in the immunoblot in (A). The integrated area of the globin dimer band is plotted as a function of the minimum age of the biotinylated cells. A similar increase in membrane-bound globin appeared on the membranes of cells isolated from dog no. 1630 (data not shown).
Analysis of band 3 during RBC senescence in vivo.
During the course of these studies, we attempted to examine the aggregation state of band 3 in C12E8 extracted dog ghost membranes by size-exclusion high-performance liquid chromatography.50 However, we found it very difficult to extract the spectrin and actin from dog erythrocyte membranes in preparation for analysis of the cluster size of band 3. It was also problematic to isolate the quantities of senescent cells necessary for the above analyses. Consequently, we elected to look for changes in the properties of band 3 using a methodology that could be readily conducted on small quantities of whole cells.
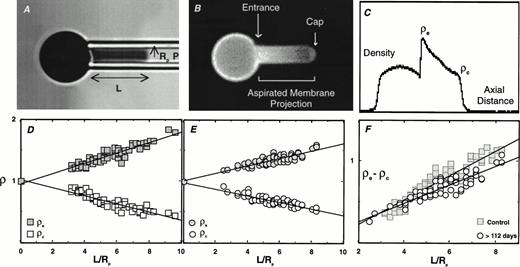
The technique of fluorescence-imaged microdeformation (FIMD) was originally developed to evaluate the distribution of labeled molecular components in a biological membrane during a mechanically induced deformation of that membrane.45,46 Using this technique, Discher et al45 showed that band 3, which is normally ≈30% associated with the membrane skeleton,50 51exhibits a concentration gradient upon deformation that is intermediate between free-flowing lipid and skeletally attached proteins. In the present study, we used FIMD to evaluate the distribution pattern of labeled band 3 in the dog erythrocyte as a function of cell age. Positive (biotinylated) and negative RBCs isolated by magnetic cell sorting were fluorescently labeled with eosin maleimide (specific for band 3) and aspirated into a micropipette. Fluorescence images of deformed cells were collected and analyzed. Figure 3 illustrates the technique used and the results obtained with the positive and the negative cells collected from dog no. 1630 on day 112 post-biotinylation. As shown in Fig 3, entrance density of band 3 (ρe) increases and the cap density of band 3 (ρc) decreases with increasing projection length (L/Rp). However, the extent of increase in ρe and the extent of decrease in ρc was lower for the positive cells compared with the negative cells. This finding is illustrated in the plot of ρe − ρc versus L/Rp. The positive cells had a linear slope of 0.114, while the negative cells gave a steeper slope of 0.150. Similar changes were also noted for positive and negative cells isolated at days 93 and 107 post-biotinylation. Because the slope of this plot is a measure of the “cytoconnectivity” of band 3, it can be concluded that there is a 25% reduction in the number of band 3 molecules attached to the underlying membrane skeleton in senescent dog RBCs compared with the randomly aged RBCs. Importantly, no differences in ρe and ρc values were noted between positive and negative cells isolated at day 58 post-biotinylation. Although the general applicability of these findings to other dogs and humans will require further analysis, the existing data nevertheless imply that band 3 mobility increases at the end of RBC life span.
Fluorescence-imaged microdeformation (A through C) and the comparison of the difference in entrance and cap density for band 3 in positive and negative dog RBCs (D through F). (A through C) A bright field image of a micropipette-aspirated RBC (A), equivalent fluorescent image (B), and integrated fluorescence-density profile (C) used for the analysis. The collected density profiles from the negative (D) and positive RBCs (E) at 112 days post-biotinylation are plotted in (D) and (E), respectively. The composite data of ρe-ρc against L/Rp for positive and negative cells are shown in (F).
Fluorescence-imaged microdeformation (A through C) and the comparison of the difference in entrance and cap density for band 3 in positive and negative dog RBCs (D through F). (A through C) A bright field image of a micropipette-aspirated RBC (A), equivalent fluorescent image (B), and integrated fluorescence-density profile (C) used for the analysis. The collected density profiles from the negative (D) and positive RBCs (E) at 112 days post-biotinylation are plotted in (D) and (E), respectively. The composite data of ρe-ρc against L/Rp for positive and negative cells are shown in (F).
Detection of autologous IgG on in vivo–aged dog RBCs.
Although direct clustering of band 3 in senescent canine erythrocytes could not be evaluated, microdeformation assays showed that impediments to band 3 aggregation disappear near the end of the cell’s life span. Because numerous model studies have shown that hemichromes aggressively cluster mobile band 3 and that these clusters bind autologous IgG,52 we decided to evaluate the time course of autologous IgG binding during RBC senescence.
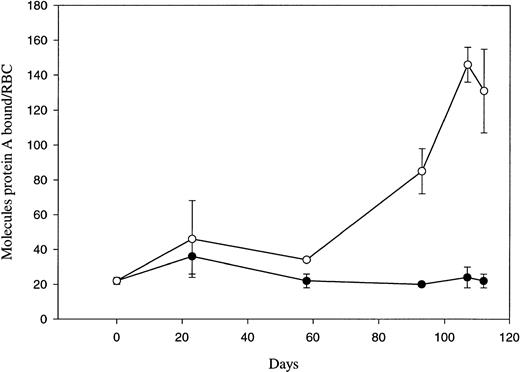
The amount of cell-surface–bound IgG on dog RBCs collected on various days post-biotinylation by magnetic cell sorting was estimated by a125I-protein A binding assay (Fig 4). Each erythrocyte sample was assayed in triplicate on the day the RBCs were isolated, and the displayed data represent the average of the triplicate samples from two dogs. The graph shows that the amount of autologous IgG bound to senescent dog biotinylated RBCs increases dramatically only at the end of the RBC’s life span. By assuming that the binding ratio for protein A to IgG is approximately one to one,53 54 positive (biotinylated) senescent dog RBCs can be calculated to bind ≈150 molecules of IgG at the end of their life span, while negative (control) RBCs can be shown to contain only ≈20 molecules of IgG over the remainder of their life in circulation.
IgG binding to in vivo–aged biotinylated dog RBCs. Positive (○) and negative (•) RBCs were isolated at the indicated times post-biotinylation by magnetic cell sorting. Isolated RBCs were incubated with 125I protein A and then washed five times before determining the number of protein A molecules per RBC. Each fraction was assayed in triplicate, and each point represents the mean ± 1 SD of two dogs. Day 0 data was obtained using RBCs collected just before the in vivo biotinylation.
IgG binding to in vivo–aged biotinylated dog RBCs. Positive (○) and negative (•) RBCs were isolated at the indicated times post-biotinylation by magnetic cell sorting. Isolated RBCs were incubated with 125I protein A and then washed five times before determining the number of protein A molecules per RBC. Each fraction was assayed in triplicate, and each point represents the mean ± 1 SD of two dogs. Day 0 data was obtained using RBCs collected just before the in vivo biotinylation.
Conversion of protein 4.1b to 4.1a during the dog RBC life span.
As with human protein 4.1, dog protein 4.1 is converted from 4.1b to 4.1a by the deamidation of asparagine 502 in a time-dependent manner.55 Interestingly, this simple conversion of asparagine 502 to aspartic acid is accompanied by an increase in the apparent molecular mass of 4.1b (78 kD) to that of 4.1a (80 kD) on SDS-polyacrylamide gels. Figure5 shows the pattern of dog RBC membrane proteins band 3, 4.1, and 4.2 on Coomassie brilliant blue–stained SDS-polyacrylamide gels as a function of cell age. Control membranes, prepared from cells obtained before biotinylation, represent a random aged cell population (mean cell age, ≈55 days) and contain approximately 60% 4.1a and 40% 4.1b (Fig 5, control lanes). Figure 5A shows the banding pattern of protein 4.1 species in cells that did not associate with the avidin-coated beads (negative fraction). Because ≥97% of all erythrocytes were initially biotinylated, these negatively sorted cells are assumed to be no older than the day on which they were isolated. Furthermore, their average age should be only half of this “isolation” age, because new nonbiotinylated cells will have been added to the population daily. As expected, protein 4.1 in the negative RBC fraction was initially enriched in 4.1b (day 23 sample; mean cell age, ≈12 days) and with time, exhibited protein 4.1a and 4.1b concentrations that are representative of a random aged cell population (Fig 5A, day 58 to 112). Figure 5B and C show the conversion of 4.1b to 4.1a in biotinylated (positive) cells as a function of increasing cell age. Surprisingly, there was nearly complete conversion of 4.1b to 4.1a in cells isolated on day 58 post-biotinylation for both dog no. 1630 (Fig5B) and dog no. 5005 (Fig 5C). This deamidation rate for dog protein 4.1b would appear to be significantly faster than what has been previously reported for human protein 4.1 (see Discussion).56
SDS-PAGE analysis of protein 4.1 in age-defined dog RBC membranes. RBCs were isolated from dog no. 1630 or dog no. 5005 at the indicated times post-biotinylation by magnetic cell sorting and ghost membranes were prepared as described in Materials and Methods. Equivalent amounts (20 μg) of membrane protein were electrophoresed on gradient (6% to 12%) polyacrylamide gels and stained for protein with Coomassie brilliant blue. (A) Membranes from nonbiotinylated cells retrieved from dog no. 1630; (B) membranes from biotinylated cells retrieved from dog no. 1630; (C) membranes from biotinylated cells retrieved from dog no. 5005. C denotes control cells not subjected to biotin-avidin sorting.
SDS-PAGE analysis of protein 4.1 in age-defined dog RBC membranes. RBCs were isolated from dog no. 1630 or dog no. 5005 at the indicated times post-biotinylation by magnetic cell sorting and ghost membranes were prepared as described in Materials and Methods. Equivalent amounts (20 μg) of membrane protein were electrophoresed on gradient (6% to 12%) polyacrylamide gels and stained for protein with Coomassie brilliant blue. (A) Membranes from nonbiotinylated cells retrieved from dog no. 1630; (B) membranes from biotinylated cells retrieved from dog no. 1630; (C) membranes from biotinylated cells retrieved from dog no. 5005. C denotes control cells not subjected to biotin-avidin sorting.
DISCUSSION
We and others have proposed a mechanism of senescent cell recognition and removal that depends on the redistribution of the membrane-spanning protein, band 3.23-29 Specifically, we have shown that the oxidative denaturation of Hb leads to formation of band 3 clusters due to the avid association between hemichromes and the cytoplasmic domain of band 3.23,57-59 These band 3 clusters on senescent cells are recognized by the immune system as foreign and become opsonized with autologous IgG and complement.24,26,27,31-34 Upon deposition of sufficient antibodies and complement, the senescent cell is then recognized by macrophages and removed from circulation by phagocytosis.1,39 40 In this report, we have examined this mechanism of RBC removal for the first time using cells of known age that were allowed to senesce by natural mechanisms in vivo.
The globin immunoblot in this report confirms that a pivotal step in the proposed pathway of RBC senescence, ie, the denaturation and deposition of globin on the membrane, occurs at the very end of the RBC’s life span. This result is in agreement with the data of Morrison et al,4 who used a mouse hypertransfusion method to show that RBC membranes from mice bind increased globin only at the very end of their life span. Interestingly, the immunoblot of Fig 2 showed that a majority of the globin which became associated with the membrane at the end of the RBC life span was covalently cross-linked and resistant to reduction with 100 mmol/L dithiothreitol. Therefore, the cross-linked globin species may not be stabilized by disulfide bonds, but rather by nonreducible linkages such as amides or free radical–generated adducts, eg, bityrosine. Similar types of nonreducible cross-linked globin species have been reported upon treatment of Hb in vitro with free radicals.60-62
Because the oxidative denaturation of Hb might be expected to arise in cells suffering from diminished intracellular reducing capacity, we also analyzed the intracellular GSH concentration in several age fractions of circulating dog RBCs. The observed late-term decrease in GSH content of 70% appears to contradict previous studies examining glutathione concentrations in senescent rabbit RBCs10 and dense human RBCs.63 Besides species variation, one possible explanation for the difference between our data and the data obtained from the rabbit is that the dog RBCs deficient in GSH were collected at a significantly older stage of the RBC life span than were the rabbit RBCs. Specifically, the rabbit RBCs were assayed at ≈85% of their total life span (60 days/total ≈70 days),18,19 whereas dog RBCs were essentially at the end of their total life span (≈115 days). However, Piccinini et al63 isolated the most dense 0.4% of human erythrocytes and found no significant decrease in intracellular reduced GSH. This difference between our data and their density separated human RBCs may reflect the different RBC populations sampled by density and biotin-dependent isolation techniques, or it may alternatively reflect variations in the antioxidant systems used by dogs and humans. More specifically, dog RBCs contain approximately 1/40 the catalase activity present in human RBCs.64 This decreased catalase activity may cause an increased reliance on glutathione peroxidase in the dog. Whatever the explanation for this discrepancy between GSH levels in senescent human, rabbit, and canine RBCs, the data appear to indicate that canine RBCs suffer from severe oxidant stress late in their life span. The highly cross-linked nature of the hemichrome-protein aggregates from dense human cells33 would also seem to suggest that oxidative stress is prominent in senescent human cells. Assuming GSH is not diminished by such stress, it will be of interest to learn what antioxidant system is compromised late in the human RBC’s life span.
Based on our fluorescence-imaged microdeformation assay, it was concluded that band 3 is less restricted by membrane-skeleton in senescent RBCs than in younger RBCs. One possibility for this change is that the detached population consists of otherwise normal band 3 molecules that have simply lost their ability to bind ankyrin as a consequence of oxidation of cysteine 201 or cysteine 317.65An alternative explanation is that the newly disjoined band 3 polypeptides comprise the fraction of anion transporters that have already been clustered into microscopic aggregates by hemichromes. Because hemichromes compete aggressively for part of the ankyrin binding site on band 3,25 66 this aggregated population would also be predicted to be free from skeletal constraints.
In the present study, we found that autologous IgG binding increased abruptly at the very end of the dog RBC’s life span. Dog RBCs greater than 107 days old bound ≈150 molecules of IgG, whereas random aged RBCs bound only ≈20 molecules. This sevenfold increase in IgG binding agrees well with our preliminary study where RBCs only ≥104 days old were examined.41 The data are also consistent with the elevated IgG binding seen in human cells isolated by density centrifugation2,33 and in mouse cells collected after serial hypertransfusions.9 However, using the biotinylation system to retrieve rabbit RBCs, Dale and Daniels14 found no increase in the amount of autologous IgG binding during the rabbit RBC life span. As we have discussed earlier,41 the difference in IgG binding between late-term rabbit and dog RBC’s is most likely due to species variations in the mechanisms governing RBC removal.
Although we did not attempt to examine the phagocytosis of senescent dog RBCs, Singer et al9 have shown that RBCs isolated from hypertransfused mice at the end of their life span are phagocytosed four times more aggressively than random-aged mouse RBCs. In addition, the densest fraction of human RBCs, which have been reported to contain anywhere from 100 to 600 molecules of IgG per cell, have been shown to undergo measurably increased phagocytosis in vitro.2Depending on conditions used, it is generally accepted that anywhere from 100 to greater than 4,000 molecules of IgG must bind an RBC to initiate phagocytosis.67-70 Furthermore, IgG subclass, the cell-surface distribution of the antibody (clustered vdispersed), and subsequent degree of opsonization by complement can modulate the phagocytic response.39,52,67 71 Therefore, although we have not performed a phagocytosis assay using our senescent dog RBCs, we predict that the 150 molecules of IgG bound per senescent dog RBC is sufficient to initiate their removal from circulation.
Determination of the exact progression of events responsible for senescent RBC recognition and removal in vivo remains a difficult problem. However, the increase in membrane bound globin chains first observed in cells isolated 86 days after biotinylation (Fig 2) occurs at approximately the same time as the increased IgG binding and decreased attachment of band 3 to the membrane skeleton. These observations strengthen our hypothesis that induction of IgG binding to senescent RBCs is tightly linked to the deposition of globin on the inner membrane surface. Interestingly, whereas increased membrane-bound globin was detected in a cell population ≥86 days old (mean cell age, ≈100 days), we found that intracellular GSH concentrations did not begin to decrease until cells were ≥113 days old. This delayed decrease in cytosolic GSH levels may suggest that its decline is more a consequence than cause of globin deposition on the membrane. Indeed, the oxidative stress accompanying Hb denaturation (for review, see Hebbel and Eaton,72 and Chiu and Lubin73) could potentially amplify a localized redox deficiency into a more global cellular problem.
Although the deamidation of protein 4.1b to 4.1a has no known functional consequences, it often is used to verify that a population of isolated RBCs is reaching senescence.5,74 Meuller et al,5 using a serial hypertransfusion method to isolate age defined mouse RBCs, were the first to show that protein 4.1b is gradually converted to 4.1a over the course of an RBC’s life span. Our analysis of age-defined RBC membranes by gel electrophoresis shows a nearly complete conversion of 4.1b to 4.1a in cells isolated 58 days after in vivo biotinylation. Because virtually all erythrocytes were biotinylated on day 0, and because a partial cohort of young cells was induced by prebiotinylation phlebotomy, a moderate fraction of the biotinylated cells must have been no older than 58 to 62 days (mean cell age, <87 days). Thus, the absence of any residual protein 4.1b in cells isolated on day 58 was not likely a consequence of an inexplicably old sample of biotinylated cells. Curiously, the half-life for deamidation of asparagine 502 in the human has been reported to be ≈41 days.56 Since we see complete conversion of 4.1b to 4.1a during this same time frame, we would suggest that the deamidation rate of asparagine 502 is accelerated in the dog and that dog RBC populations enriched in protein 4.1a are not necessarily composed of old RBCs.
Assuming asparagine 502 deamidation rates indeed differ among mammalian species, what mechanism can be offered to explain this variability? Due to the formation of succinimide ring intermediates,75 it has been shown that asparagine deamidation rates are influenced by the amino acid on the carboxy side of the asparagine residue.75,76 Specifically, the rate of deamidation is greater at asparagine-serine sequences than at asparagine-alanine sequences.76 Inaba et al74 have shown that deamidation of human protein 4.1 is consistent with these criteria in that slow deamidation occurs at asparagine 502-alanine during the human RBC life span. However, a serine immediately follows asparagine 502 in the sequence of dog protein 4.1.55 Thus, according to the rules governing asparagine deamidation, the rate of conversion of dog protein 4.1b to 4.1a would be expected to be faster than the conversion seen in humans.
In conclusion, the dog constitutes a useful animal model of human RBC senescence because of its similar RBC lifespan and strongly age-dependent pathway of RBC removal. Although not all aspects of the band 3 clustering hypothesis of RBC removal could be evaluated in this model, many essential elements of the hypothesis were confirmed for the first time in cells allowed to senesce in vivo to a known age. We conclude that the opsonization of senescent RBCs, presumably at hemichrome-stabilized clusters of band 3,24 31-34 still constitutes a plausible pathway to explain the recognition and removal of senescent RBCs.
Supported in part by National Institutes of Health Grants No. GM24417 and DK 26263.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Philip S. Low, PhD, Department of Chemistry, 1393 Brown Bldg, West Lafayette, IN 47907.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal