Abstract
The chemotactic and growth-stimulatory effect of insulin-like growth factor 1 (IGF-1) was investigated in the experimental mouse 5T2 multiple myeloma (MM) model. Chemotaxis was analyzed by classical checkerboard analysis. Bone marrow fibroblasts–conditioned medium exhibited a chemotactic effect on 5T2 MM cells that could be neutralized by adding a blocking antibody to IGF-1. On the other hand, exogenously added IGF-1 also had a chemotactic effect on the 5T2 MM cells. Moreover, in vitro analysis demonstrated that transmigrated 5T2 MM cells have a higher expression of IGF-1 receptor, both in bone marrow–conditioned medium and in IGF-1–induced chemotaxis, in comparison to cells before migration. When analyzed in vivo, 18 hours after injection of the heterogeneous 5T2 MM population, 5T2 MM cells present in the bone marrow show a higher expression of the IGF-1 receptor than their counterparts before injection. When the proliferative effect of IGF-1 was analyzed, no stimulation was observed, which is in contrast to the influence of bone marrow–conditioned medium and interleukin-6. Our results suggest a causal relationship between the presence of IGF-1 in the bone marrow and the chemotaxis of MM cells to and their subsequent presence in the bone marrow.
MULTIPLE MYELOMA (MM) is a B-cell malignancy characterized by the monoclonal expansion of plasma cells in the bone marrow, secreting high concentrations of monoclonal immunoglobulin (Ig) in the serum, and by the activation of osteoclasts, leading to osteolytic lesions. Our group1 has demonstrated the postgerminal origin of MM cells. In the bone marrow microenvironment, the contact between MM and bone marrow stromal cells and their exchange of cytokines (like interleukin-6) are essential for the survival and growth of MM cells.2-8 This implies homing of myeloma cells by extravasation from the intravascular to the extravascular compartment of the bone marrow and anchoring to the stromal cells and extracellular matrix proteins. Such a homing process has been described for lymphocytes in general.9 This process includes several independent steps starting with the initial, reversible contact with the endothelial cells that surround the sinusoids, followed by an activation-dependent arrest, and finally by a transendothelial migration to the extravascular compartment. The combination of the specificities of each step makes this process highly specific. The last two steps of this process involve the action of chemokines. Chemokines are small polypeptides that act mainly as chemoattractants by altering the cytoskeleton assembly of the cells in a concentration-dependent way. Differential chemokine expression in tissues may be responsible for the selective accumulation of specific leukocyte subsets.
In this work, the chemoattractant activity of insulin-like growth factor-1 (IGF-1) was analyzed in the 5T2 experimental mouse MM model. This myeloma cell line originated spontaneously in aging C57BL/KaLwRij mice10 and has since been propagated in vivo by intravenous transfer of the tumoral cells (isolated from the bone marrow) in young syngeneic mice. The 5T2 MM model has been compared with the human MM situation10-12: its spontaneous origin in old age, the bone marrow involvement, the occurrence of serum monoclonal Ig, and the development of osteolysis are similar to that in the human situation and thus suitable for the study of the homing processes of MM. We here demonstrate that the bone marrow microenvironment has a chemotactic activity on 5T2 MM cells that can be mimicked by IGF-1 alone and can be blocked by anti–IGF-1 antibodies.
MATERIALS AND METHODS
Animals
C57BL/KaLwRijHsd mice were purchased from Harlan CPB (Horst, The Netherlands). Male mice were 6 to 10 weeks old when used. They were housed under conventional conditions and had free access to tap water and food. They were killed by carbon dioxide asphyxiation (license no. LA1230281).
Cell Lines
The 5T2 MM originated spontaneously in aging C57BL/KaLwRij mice10 and has since been propagated in vivo by intravenous transfer of the diseased marrow in young syngeneic mice. The tumor take was followed up by protein electrophoresis of the serum samples. At a serum concentration of 10 mg/mL, mice were killed and bone marrow was flushed from femurs, tibiae, and humeri. The isolated cells were suspended in Dulbecco’s modified essential medium (DMEM; GIBCO, Life Technologies, Gent, Belgium) supplemented with penicillin-streptomycin, glutamine, and MEM NEAA-pyruvate (GIBCO). Mononuclear cells were subsequently prepared by LympholyteM (Cedarlane, Hornby, Canada) gradient centrifugation at 450g for 20 minutes. After triple washing steps, the cells were further purified on a Percoll (Pharmacia, Uppsala, Sweden) 60% isoosmotic gradient centrifugation. The cell band floating on top of the gradient, containing enriched 5T2 MM cells, was removed and cell number and viability (by trypan blue exclusion) was assessed. Cells could be transplanted at a dose of 2 × 106 cells/mouse or used in experiments. The purity of the tumor cells was assessed by idiotype-staining in FACS12 and reached at least 90%.
Flow Cytometry
The expression of IGF-1 receptor α- and β-chain on the surface of 5T2 MM cells was determined by flow cytometry. A quantity of 0.2 × 106 cells per sample was incubated (30 minutes, 4°C) with biotinylated anti–5T2-idiotype specific antibodies12 and with rabbit anti-human IGF-1 receptor α- and β-chain antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). Cells were then washed three times with cold phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) and 0.02% sodium azide. Subsequently, cells were incubated with streptavidin-phycoerythrin (Pharmingen, San Diego, CA) and donkey anti-rabbit coupled to fluorescein isothiocyanate (FITC; Cructon Bioproducts, Brussels, Belgium). After incubation, cells were washed and fixed with 2% paraformaldehyde in PBS and analyzed (FACstar; Becton Dickinson, Mountain View, CA). Isotype-matched irrelevant antibodies were used as a control.
Migration Assays
Migration of the MM cells was determined in classical checkerboard analysis. In this assay, the chemoattractant is present in the lower chamber, which is separated from the upper chamber by a polycarbonate pore membrane. Preliminary experiments (not illustrated) demonstrated that the 5T2 MM cells (with an average diameter of 10 μm, as checked by electron microscopy) showed a migration through pores with a size of 5 μm. Hereby, 0.2 × 105 5T2 MM-enriched bone marrow cells were added in the upper chamber of a Transwell system (Costar, Elscolab, Belgium), and after establishment of a concentration gradient by diffusion, cells migrated through the membrane to the lower compartment. When labeled with 51Cr, 2 hours was the time point with the maximum migration and the minimum spontaneous release. A checkerboard was designed whereby on the one hand the upper compartments (200 μL) contained increasing amounts of conditioned medium or purified proteins, which was combined with increasing concentrations in the bottom compartment (300 μL). After 2 hours incubation at 37°C and 5% CO2 in a humidified atmosphere, the upper compartments were removed and the cells of the bottom compartment were harvested. The amount of radioactivity was assessed in a γ-counter and the amount of migrated cells per total cells added was calculated. For nonradioactive assays, cells were harvested and counted, and FACS staining and analysis were performed. For assays with bone marrow–conditioned medium, 10× concentrated (see further), 5×, 2.5×, and control medium were used. For neutralizing assays, anti-human IGF-1 (PreproTech, Rocky Hill, NJ) was used at a concentration of 10 μg/mL in both the upper and bottom compartments. For assays with IGF-1 (Sigma, St Louis, MO) and interleukin-6 (PreproTech), dilutions of 10 ng/mL were used.
Labeling of Cells With 51Cr
5T2 MM cells were prepared as described and incubated for 80 minutes (37°C in a humidified atmosphere of 5% CO2 in air) with 500 μCi 51Cr (Amersham, Gent, Belgium) for 2 × 106 cells. After triple washing, cell viability was assessed by trypan-blue exclusion. In addition to the checkerboard analysis, samples were kept apart for quantification of the maximal release.
Fibroblast Cultures (Bone Marrow)
Adherent layers were established by flushing out the content of the femurs of C57BL/KaLwRij mice in DMEM followed by preparation of a mononuclear cell suspension by Lympholyte M gradient centrifugation. The mononuclear cells were plated out at a concentration of 1 × 10 6 cells/mL in DMEM supplemented with 12.5% bovine serum (Fetal Clone I; Hyclone, Logan, UT), 12.5% horse serum (Globe Pharm, Surrey, UK), 10-6 mol/L hydrocortisone (Sigma), penicillin-streptomycin, glutamine, and MEM. The cells were incubated at 37°C, 5% CO2. After two trypsinizations, cells were cultured until confluence was reached after 2 to 4 weeks. The medium was replaced at weekly intervals. To obtain bone marrow–conditioned medium, medium was removed and cells washed with serum-free DMEM medium; after 48 hours in DMEM serum-free condition, medium was harvested and concentrated 10× by Centriprep-3 (Amicon, Beverly, MA).
Fibroblast Cultures (Skin)
Skin fibroblasts were isolated from skin explants. Hereby, C57BL/KaLwRij mice were killed, skin disinfected with 70% ethanol, and the abdominal part shaved. Thin longitudinal sections of the skin were cut into fragments of 1 mm3 in a 100 × 15 mm polystyrene Petri dish (Vel, Leuven, Belgium). The fragments were allowed to adhere to the Petri dish for approximately 5 minutes. Subsequently, small amounts of DMEM containing 10% fetal calf serum were carefully added without allowing the explants to float. Skin fibroblasts grew out the fragments after a few days. After 2 weeks, the skin fragments were removed and cells further grown until confluency was reached. Cells were trypsinized and cultured in culture flasks (Falcon, Vel). Medium was replaced weekly.
Cocultures and Thymidine Incorporation Assay
Confluent bone marrow stromal cells at state of confluence in 96-well plates were irradiated with 1,500 rad. A quantity of 0.05 × 106 isolated 5T2 MM cells was added on top of this monolayer. In addition, 5T2 MM cells were also incubated in medium (10% fetal calf serum [FCS]) only and in the presence of different concentrations of IGF-1 and interleukin-6. Sixteen hours before harvesting, cells were pulsed with 1 μCi (methyl-3H) thymidine (Amersham, Buckinghamshire, UK). Cells were harvested by a cell harvester (Inotech, Wohlen, Switzerland) on glass filters (Filtermat A; Wallac, Turku, Finland). Filters were dried for 1 hour in a 60°C oven and sealed in sample bags (Wallac) containing 4 mL Optiscint Scintillation Liquid (Wallac). Radioactivity was counted using a 1450 Microbeta Liquid Scintillation Counter (Wallac). Results are expressed as the total number of counts. In case of cocultures, this number was corrected for the counts generated by bone marrow stromal cells alone.
Scanning Electron Microscopy
Cells incubated for 2 hours in the presence of 5 ng/mL IGF-1 were spun on cover slips, rinsed twice with PBS, and fixed with 2% glutaraldehyde in Na-cacodylate buffer (0.1 mol/L sucrose) for 12 hours. They were subsequently treated with filtered 1% tannic acid in 0.15 mol/L Na-cacodylate for 1 hour. Scanning electron microscopy (SEM) samples were dehydrated in graded ethanol series, dried with hexamethyldisilazane,13 and sputter-coated with 10 nm gold. The samples were examined with Philips SEM 505 (Philips, Eindhoven, The Netherlands) at an accelerating voltage of 30 kV.
RESULTS
Expression of the IGF-1 Receptor
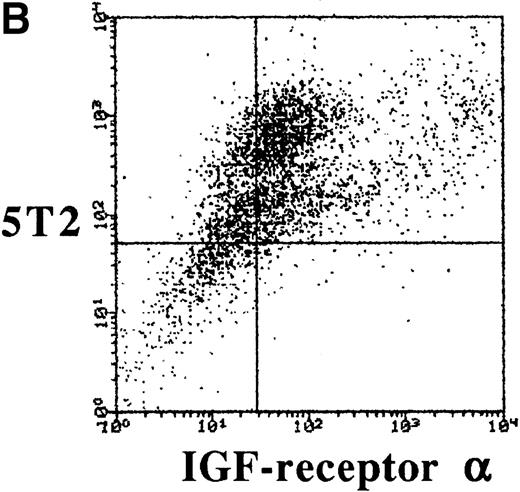
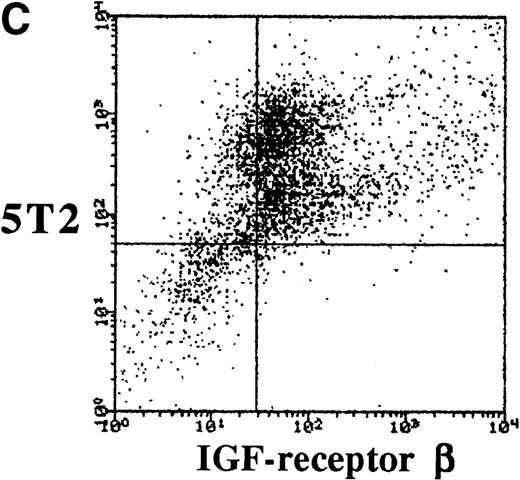
To characterize the expression of IGF-1 receptor on 5T2 MM cells, double stainings were performed and analyzed by FACS. 5T2 MM cells were stained with anti-idiotype monoclonal antibodies,12 while IGF-1 receptor was stained by either anti–α- or anti–β-chain (extracellular) antibodies. Approximately 70% of the 5T2 MM idiotype-positive cells were positive for the α- or β-chain of IGF-1 receptor (Fig 1).
Dot-plots showing the isotype controls (A) and expression of the - (B) and β-chain (C) by the 5T2 MM cells. This experiment is representative of three independent experiments.
Dot-plots showing the isotype controls (A) and expression of the - (B) and β-chain (C) by the 5T2 MM cells. This experiment is representative of three independent experiments.
Migration of the MM Cells
A total of 0.2 × 10551Cr-labeled 5T2 MM enriched cells was added in the upper compartment of the transmigration system. After 2 hours, the quantity of transmigrated cells was assessed by radioactivity and compared with the total radioactivity of the cells added in the upper compartment. In addition, purity of 5T2 MM cells was assessed by flow cytometry. Since the starting 5T2 MM-enriched population was approximately 90% pure, flow cytometry could indicate which cells transmigrated. The purity of the transmigrated cells remained the same or was enhanced, but never decreased in the chemokinetic and chemotactic conditions of the checkerboard analysis (results not illustrated).The combination of the quantity of migrated cells with the purity clearly indicated a chemokinetic (diagonal effect, with increasing total concentrations) and chemotactic effect (vertical, increasing concentrations in the bottom compartment, see arrow) of bone marrow–conditioned medium and exogenously added IGF-1 alone on 5T2 MM cells (Table 1). Longer incubation times could enhance the number of transmigrated cells, but also enhanced the spontaneous release of 51Cr. Blocking anti–IGF-1 (10 μg/mL) antibodies abolished the chemokinetic and chemotactic effects of bone marrow–conditioned medium and exogenously added IGF-1 (results not illustrated) on 5T2 MM cells, while isotype-matched irrelevant control antibodies did not affect the migration of the cells. Conditioned medium of skin fibroblasts and interleukin-6 showed no chemotactic effect.
Checkerboard Analysis of Purified 5T2 MM Cells
| IGF-1 (bottom compartment) . | Upper Compartment . | ||
|---|---|---|---|
| 0 ng/mL . | 5 ng/mL . | 10 ng/mL . | |
| 0 ng/mL | BV | 60 | 10 |
| 5 ng/mL | 33 | 70 | 50 |
| 10 ng/mL | 50 | 82 | 64 |
| Bone Marrow– Conditioned Medium (bottom compartment) | Upper Compartment | ||
| 0× | 5× | 10× | |
| 0× | BV | 52 | 42 |
| 5× | 47 | 76 | 52 |
| 10× | 61 | 95 | 66 |
| Bone Marrow– Conditioned Medium + 10 μg/mL Anti–IGF-1 (bottom compartment) | Upper Compartment | ||
| 0× | 5× | 10× | |
| 0× | BV | 1 | 1 |
| 5× | 1 | 1 | 1 |
| 10× | 1 | 1 | 1 |
| IL-6 (bottom compartment) | Upper Compartment | ||
| 0 ng/mL | 5 ng/mL | 10 ng/mL | |
| 0 ng/mL | BV | 1 | 1 |
| 5 ng/mL | 1 | 1 | 1 |
| 10 ng/mL | 1 | 1 | 2 |
| Skin Fibroblast– Conditioned Medium (bottom compartment) | Upper Compartment | ||
| 0× | 5× | 10× | |
| 0× | BV | 1 | 22 |
| 5× | 1 | 3 | 26 |
| 10× | 1 | 1 | 22 |
| IGF-1 (bottom compartment) . | Upper Compartment . | ||
|---|---|---|---|
| 0 ng/mL . | 5 ng/mL . | 10 ng/mL . | |
| 0 ng/mL | BV | 60 | 10 |
| 5 ng/mL | 33 | 70 | 50 |
| 10 ng/mL | 50 | 82 | 64 |
| Bone Marrow– Conditioned Medium (bottom compartment) | Upper Compartment | ||
| 0× | 5× | 10× | |
| 0× | BV | 52 | 42 |
| 5× | 47 | 76 | 52 |
| 10× | 61 | 95 | 66 |
| Bone Marrow– Conditioned Medium + 10 μg/mL Anti–IGF-1 (bottom compartment) | Upper Compartment | ||
| 0× | 5× | 10× | |
| 0× | BV | 1 | 1 |
| 5× | 1 | 1 | 1 |
| 10× | 1 | 1 | 1 |
| IL-6 (bottom compartment) | Upper Compartment | ||
| 0 ng/mL | 5 ng/mL | 10 ng/mL | |
| 0 ng/mL | BV | 1 | 1 |
| 5 ng/mL | 1 | 1 | 1 |
| 10 ng/mL | 1 | 1 | 2 |
| Skin Fibroblast– Conditioned Medium (bottom compartment) | Upper Compartment | ||
| 0× | 5× | 10× | |
| 0× | BV | 1 | 22 |
| 5× | 1 | 3 | 26 |
| 10× | 1 | 1 | 22 |
Each analysis is representative for 3 independent experiments. For bone marrow– and skin fibroblast–conditioned medium, dilutions of the 10× concentrated medium are shown. For IGF-1 and IL-6, 5 and 10 ng/mL are used. In the assay combining bone marrow–conditioned medium and anti–IGF-1 blocking antibodies, dilutions of 10× concentrated medium are combined with a constant concentration of 10 μg/mL anti–IGF-1. BV (basic value) represents the spontaneous migration of the MM cells without any added stimulus. The other values are the % increase of this basic value. Vertical arrows indicate the chemotactic effect.
Characterization of the Migrated Cells
In vitro.
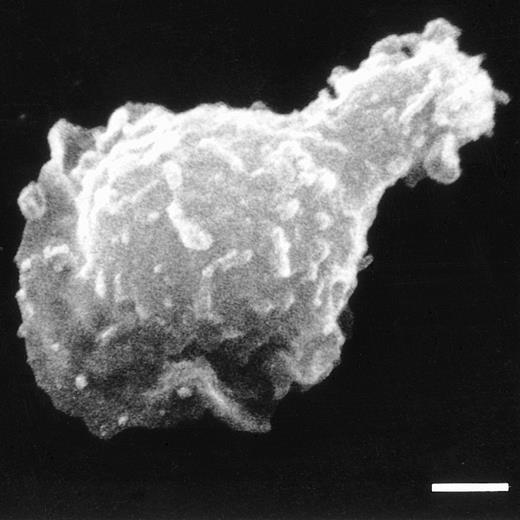
A total of 0.2 × 105 5T2 MM (in 200 μL) enriched bone marrow cells was added in the upper chamber of a transmigration assay with 300 μL 5 ng/mL IGF-1 in the bottom compartment. Two hours later, the nonmigrated and transmigrated cells were harvested and analyzed. During FACS analysis, 5T2 MM-positive cells were gated and the IGF-1 receptor expression analyzed. Flow cytometry demonstrated a 2.4 increase in mean log fluorescence of the IGF-1 receptor expression of the in vitro–transmigrated 5T2 MM cells when compared with the cells before migration (Fig 2A). When the total 5T2 MM population was incubated with IGF-1 alone, without migration, no alteration in the FACS profile was observed, indicating no upregulation of the receptor in the presence of IGF-1 (results not demonstrated). Figure 3 demonstrates an SEM photograph of a 5T2 MM cell in locomotion, with a leading protopod.
Overlay demonstrating the expression of the IGF-1 receptor expression by the 5T2 MM cells. (A) 5T2 MM cells transmigrated in vitro under influence of 5 ng/mL IGF-1 (filled curve) show a clear shift toward a higher expression of IGF-1 receptor when compared with the cells before migration (open curve). (B) 5T2 MM cells transmigrated in vivo. The expression of the IGF-1 receptor was analyzed before injection (open curve) and 18 hours after injection (filled curve). The majority of 5T2 MM cells express the IGF-1 receptor after injection. These experiments are representative for three independent experiments.
Overlay demonstrating the expression of the IGF-1 receptor expression by the 5T2 MM cells. (A) 5T2 MM cells transmigrated in vitro under influence of 5 ng/mL IGF-1 (filled curve) show a clear shift toward a higher expression of IGF-1 receptor when compared with the cells before migration (open curve). (B) 5T2 MM cells transmigrated in vivo. The expression of the IGF-1 receptor was analyzed before injection (open curve) and 18 hours after injection (filled curve). The majority of 5T2 MM cells express the IGF-1 receptor after injection. These experiments are representative for three independent experiments.
SEM of a 5T2 MM cell with a polarized morphology, typical for locomotory activity. Bar = 2 μm.
SEM of a 5T2 MM cell with a polarized morphology, typical for locomotory activity. Bar = 2 μm.
In vivo.
A total of 2 × 106 5T2 MM-enriched bone marrow cells was injected into naive syngeneic mice. Eighteen hours later, bone marrow was removed and stained for flow cytometry. 5T2 MM-positive cells were gated out and the IGF-1 expression of the cells in the bone marrow demonstrated a 2.6 increase in mean log fluorescence when compared with the cells before injection. These double stainings (Fig 2B) thus showed that almost all 5T2 MM cells present in the bone marrow were IGF-1 receptor-positive, which is in contrast to the starting population, which was heterogeneous for IGF-1 receptor expression.
Proliferative Effect as Compared With Bone Marrow–Conditioned Medium
DNA synthesis was measured using 3H-thymidine incorporation. Coculture and conditioned medium of bone marrow stromal cells could stimulate DNA synthesis in analogy with exogenous added interleukin-6 and coculture with skin fibroblasts, while IGF-1 alone had no proliferative effect at all (Fig 4). On the other hand, IGF-1 has a synergistic effect on the IL-6–induced proliferation.
The proliferation of 5T2 MM cells as quantified by3H-thymidine incorporation is expressed as CPM (counts per minute). 5T2 cells were incubated alone, on bone marrow fibroblasts, in bone marrow fibroblasts–conditioned medium, in the presence of 10 ng/mL interleukin-6 or IGF-1, and on skin fibroblasts (SK). Results represent the mean ± SD of quadruplets. This experiment is representative of three independent experiments.
The proliferation of 5T2 MM cells as quantified by3H-thymidine incorporation is expressed as CPM (counts per minute). 5T2 cells were incubated alone, on bone marrow fibroblasts, in bone marrow fibroblasts–conditioned medium, in the presence of 10 ng/mL interleukin-6 or IGF-1, and on skin fibroblasts (SK). Results represent the mean ± SD of quadruplets. This experiment is representative of three independent experiments.
DISCUSSION
The results presented here demonstrate the in vitro and in vivo role of IGF-1 on murine 5T2 MM cells. IGF-1 appears to be at least one of the factors responsible for the attraction and/or entrance of the 5T2 MM cells from the intravascular to the extravascular compartment of the bone marrow.
In vivo, bone marrow endothelial cells act as gate-keepers separating the bone marrow from the sinusoidal lumen and may therefore play an important role in the trafficking of MM cells. Studies have already shown an important role of these endothelial cells in the trafficking of hematopoietic progenitors from the bone marrow to the circulation.14 However, mechanisms involved in the recruitment of the MM cells to the bone marrow are not yet understood. After their extramedullar origin, MM cells home specifically to the bone marrow, since in the initial stages of the disease, no extramedullar growth is observed. In the 5T2 mouse MM model, this entrance is mimicked by intravenous transfer of freshly isolated bone marrow–containing tumoral cells. In our previous work,12we demonstrated that in diseased mice only the bone marrow and part of the spleen (a hematopoietic organ in the mouse) are involved, a situation analogous to that of the human pathology. When analyzed 18 hours after injection, viable 5T2 MM cells are observed in the bone marrow and spleen (manuscript in preparation), indicating a selective entry.
The bone marrow microenvironment is known to contain high local concentrations of IGF-1, a polypeptide growth factor produced by osteoblasts,15-17 bone marrow stromal cells,18,19 and bone endothelial cells.20 IGF-1 generally acts on stimulation of cell proliferation, cell differentiation, and insulin-like metabolic responses.21IGF-1 also appears to have pleiotropic effects on MM cells. Under certain circumstances IGF-1 stimulates the survival and proliferation of certain MM cell lines,22,23 while others report on interleukin-6–responsive human cell lines that could not be induced to proliferate under the influence of IGF-1 alone.24 In addition, an anti-apoptotic effect of IGF-1 on MM cells was also described.25 IGF-1 binds to specific cell-surface receptors, predominantly on B lymphocytes, in contrast to the binding of insulin, which appears to be predominantly to monocytes.26
The expression of functional IGF-1 receptors was higher on MM cells than on B-lymphoblastoid cell lines,23 suggesting that the late-stage maturation within the B lineage is associated with higher IGF-1 receptor expression. The binding of IGF-1 on its receptor stimulated receptor autophosphorylation and substrate phosphorylation, phosphoinositide-3 (PI) kinase activation, DNA synthesis, and glucose metabolism.27 All human (U266 and RPMI8226) and mouse (Ag 8.653) MM lines tested by these investigators23 seemed to be insulin- and IGF-1–responsive.
On the other hand, IGF-1 appears to stimulate the in vitro migration of preosteoclasts across bone endothelial cells.28 Chemotaxis, a directed cell locomotion towards a soluble extracellular chemical gradient, is one of the steps necessary for migration of cells. Leukocytes use this chemotaxis to accumulate at sites of injury or infection.29 After binding of the chemokine to its receptor, various intracellular signals are generated, leading to alterations of the cytoskeleton, which is involved in the motile response. This reconfiguration of the cytoskeleton can be visualized by the typical polarized form of the cells,30 which could also be demonstrated for the 5T2 MM cells under influence of IGF-1. Among many potential signaling events, polyphosphoinositides have received attention. It has been suggested that activation of PI-3-kinase could be the signal responsible for cytoskeletal changes in neutrophils.31 As mentioned earlier, this activation of PI-3-kinase has been described in MM cells after binding of IGF-1 on its receptor.27 In this work, we were interested in the possible chemotactic effect of IGF-1 on 5T2 MM cells. The expression of the IGF-1 receptor on 5T2 MM cells seemed to be heterogeneous, with only part of the cells being positive. The IGF-1 receptor is a heterotetrameric protein composed of two α and two β subunits, which are connected by interchain disulfide bonds. The α subunits contain the binding sites for IGF, while the β subunits contain a transmembrane domain, an adenosine triphosphate (ATP)-binding site, and a tyrosine kinase domain. Both α and β subunits are encoded by a single gene (in humans, located on chromosome 15). The subunits are generated by a cleavage of an Arg-Lys-Arg-Arg sequence at positions 701-710.32 In the literature, no evidence was found to confirm the presence of only one of the chains. When the migration of the myeloma cells was analyzed by classical checkerboard analysis, chemokinesis and chemotaxis could be observed for bone marrow–conditioned medium and IGF-1 alone. Moreover, anti–IGF-1 antibodies could block the chemokinetic and chemotactic effect of the conditioned medium and exogenously added IGF-1. As a control, the chemokinetic and chemotactic effect of interleukin-6 was also checked. Interleukin-6 is known to be one of the major growth factors of myeloma cells in the bone marrow.3-6 In addition, conditioned medium of skin fibroblasts was also analyzed to investigate whether this chemotactic effect is a general property of fibroblasts. For both interleukin-6 and skin fibroblasts, no chemotactic effect could be observed. When the phenotype of the transmigrated 5T2 MM cells was analyzed, both in vitro and in vivo, the majority of the cells were positive for the receptor. Since in vitro there was no upregulation of the IGF-1 receptor under the influence of IGF-1, a selective migration of the IGF-1 receptor-positive myeloma cells is suggested. Since in some human MM lines IGF-1 acts as a growth factor, the proliferation of the 5T2 MM cells under influence of IGF-1 was also analyzed. Apparently, in the 5T2 MM model, IGF-1 has no proliferative effect, in contrast to bone marrow–conditioned medium or IL-6 alone. This result is not contradictory to the human situation, since in some lines no proliferation was observed.24 The results presented here clearly demonstrate that chemoattraction and induction of proliferation are two distinct phenomena: bone marrow fibroblast–conditioned medium and IGF-1 alone can attract the MM cells, while this conditioned medium, skin fibroblast–conditioned medium, and interleukin-6 alone can induce proliferation in vitro, in contrast to IGF-1. The combination of interleukin-6 and IGF-1 has a synergistic effect on the induction of proliferation, which is analogous to the results obtained with human MM cell lines.33
The findings of our study suggest that IGF-1 is one of the main factors responsible for the selective recruitment of 5T2 MM cells to the bone marrow. Since the homing of lymphocytes is known to be a multistep process whereby the high selectivity is a result of the combination of different specific processes,9 IGF-1 might be one of the parameters making this homing of MM cells to the bone marrow highly specific. Other processes like adhesion to and migration through the endothelial lining and the subsequent adhesion to bone marrow stroma, mediated by the interaction of various adhesion molecules and cytokines, might be as important in regulating this highly specific process.
Supported by the Onderzoeksraad-Vrije Universiteit Brussel (OZR-VUB), Fonds Voor Wetenschappelijk Onderzoek (FWO), and Vlaamse Kankerliga. K.V. is a postdoctoral fellow of the “Fonds voor Wetenschappelijk Onderzoek.”
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Karin Vanderkerken, PhD, Free University Brussels, Department HEIM, Laarbeeklaan 103, 1090 Brussels, Belgium.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal