Abstract
We have analyzed the effects of interleukin-10 (IL-10) on the entry of quiescent CD4+ T cells into the cell cycle upon stimulation with the superantigen staphylococcal enterotoxin B (SEB). IL-10 arrested cells at G0/G1. IL-10 treatment prevented the downregulation of p27Kip1, an inhibitory protein that controls progression out of the G0 phase of the cell cycle. IL-10 also prevented the upregulation of the G1 cyclins D2 and D3, proteins necessary for entry and progression through the G1 phase of the cell cycle. Associated with the inhibition of the cell cycle, IL-10 suppressed SEB induction of interleukin-2 (IL-2). Addition of exogenous IL-2 to IL-10–treated cells significantly reversed the antiproliferative effects of IL-10. Moreover, IL-10 effects on the early G1proteins p27Kip1 and cyclin D2 were similarly reversed by exogenous IL-2. Although this reversal by IL-2 was pronounced, it was not complete, suggesting that IL-10 may have some effects not directly related to the suppression of IL-2 production. Cell separation experiments suggest that IL-10 can effect purified CD4+ T cells directly, providing functional evidence for the presence of IL-10 receptors on CD4+ T cells. IL-10 also inhibited expression of IL-2 transcriptional regulators c-fos and c-jun, which also inhibit other cell functions. Our studies show that the mechanism of IL-10 regulation of quiescent CD4+ T-cell activation is mainly by blocking induction of IL-2 that is critical to downregulation of p27Kip1 and upregulation of D cyclins in T-cell activation and entry into the cell cycle.
INTERLEUKIN-10 (IL-10) is a multifunctional cytokine produced by B cells, stimulated macrophages, and subsets of CD4+ T cells. It was originally termed cytokine synthesis inhibition factor due to its ability to inhibit production of some cytokines such as interferon γ and interleukin-2 (IL-2).1 It has been shown to have both immunostimulatory and immunosuppressive properties, depending on the target cell type. IL-10 exerts immunostimulatory properties on B cells and mast cells and is a growth factor for immature thymocytes.2 It exerts immunosuppressive effects on cells of the monocyte/macrophage lineage3,4 and also on subsets of activated helper CD4+ T cells,5,6 which are normally found in a quiescent or resting G0 state before activation. However, little is known of the molecular effects of IL-10 on the progression of the target cells through the cell cycle. The IL-10 receptor has been found on macrophages and B cells as well as T cells, including murine CD4+ T-cell clones.7-9 Significant levels of IL-10 receptor mRNA has also been detected in human CD4+T-cell clones.8 The IL-10 receptor is a member of the class II (interferon-like) subgroup of the cytokine receptor family and has been found to have a high affinity for the IL-10 molecule.8 10 In this study, we have specifically examined the effects of IL-10 on the molecular events controlling the cell cycle of CD4+ T cells in peripheral blood after superantigen stimulation.
In the entry of quiescent T cells from the G0 phase into the G1 phase of the cell cycle, two proteins act as mitogenic sensors. These are the cyclin-dependent kinase (cdk) inhibitor p27Kip1 and the D-type cyclins.10 These proteins act in a reciprocal fashion. Mitogenic stimulation causes the destruction of the inhibitor p27Kip1 and the upregulation of cyclins D2 and D3. Cyclin D2 expression occurs early in the G1 phase and is followed later in G1 by the expression of cyclin D3.11 The inhibitor p27Kip1 itself inhibits the kinase activity of complexes formed between the D cyclins and select cdks. These cyclin-cdk complexes are responsible for the phosphorylation of key substrates required for continued G1 progression, an important one being the tumor-suppressor retinoblastoma protein pRB.10 In general, the expression profiles of p27Kip1 and the D-type cyclins are faithful markers of the activation state of T cells. In CD4+ T cells, the downregulation of p27Kip1 and the upregulation of D-type cyclins upon mitogenic stimulation are dependent on the binding of IL-2 to its receptor.12 The importance of p27Kip1 is underscored by the fact that immunosuppressive drugs such as rapamycin that prevent the activation of T cells do so by preventing the downregulation of p27Kip1 that occurs upon mitogenic stimulation.13
In this study, we have used the superantigen staphylococcal enterotoxin B (SEB) as the mitogen. The superantigen SEB is a potent T-cell–specific mitogen that activates the T-cell receptor in a major histocompatibility complex (MHC) class II-restricted manner.14 Thus, SEB more closely resembles conventional antigens in terms of T-cell activation when compared with mitogens such as Concanavalin A (ConA) or phytohemagglutinin (PHA). Activation of T cells by mitogens such as superantigens induces the production of IL-2.15 16
Our data show that IL-10 exerts its immunosuppressive effects on SEB-stimulated CD4+ T cells very early in the cell cycle. IL-10 prevents the activation of quiescent CD4+ T cells from the normal resting G0 state and their entry into the cell cycle. This quiescent state is characterized by high levels of the cell cycle inhibitory protein p27Kip1 and low levels of the early D-type cyclin proteins that are associated with progression into the G1 phase of the cell cycle. These effects occur in good part as a consequence of the inhibition of IL-2 production but may also involve other mechanisms affecting IL-2 receptor signaling.
MATERIALS AND METHODS
Isolation of peripheral blood mononuclear cells (PBMCs) and cell culture.
Human cells were collected from the whole blood of normal healthy volunteers or from leukocyte source packs (Civitan Regional Blood Center, Gainesville, FL). PBMCs were isolated by Histopaque (Sigma, St Louis, MO) density gradient centrifugation17 and viability was determined to be greater than 95% by trypan blue exclusion. For isolation of CD4+ T cells, PBMCs were loaded onto CD4 T Cellect columns (Biotex, Edmonton, Alberta, Canada) as per the manufacturer’s recommendation and cell purity was assessed by flow cytometry. Cells were maintained in RPMI 1640 supplemented with 10% (vol:vol) heat-inactivated fetal bovine serum (FBS), 200 U/mL penicillin, and 200 μg streptomycin in a 5% CO2atmosphere at 37°C and used immediately.
Proliferation assay.
The proliferative response of PBMCs to SEB (Toxin Technology, Sarasota, FL) was determined by the incorporation of [3H]thymidine into DNA. Specifically, PBMCs were added to wells in a 96-well plate at a concentration of 3 × 105 cells/mL and were treated with either IL-10 (Intergen, Purchase, NY) at 10 U/mL or media alone and incubated for 48 hours. Media or SEB was then added at the indicated concentrations and the cultures were incubated for another 48 hours in a final volume of 200 μL/well. Next, 1 μCi of [3H]thymidine (Amersham, Arlington Heights, IL) in media was added per well and the plates were incubated for 8 hours before harvest. All experiments were performed in quadruplicate. The proliferative response of purified CD4+ T cells to SEB was performed in a similar manner, except that cells were added to the plates at a concentration of 2.5 × 106 cells/mL. This was necessary to obtain substantial proliferation, as determined by experimentation. These cells were only incubated with IL-10 for 2 hours before the addition of SEB.
Flow cytometry.
PBMCs were added to 6-well plates at a concentration of 3 × 106 cells/mL and treated with IL-10, SEB, and/or IL-2 (Intergen) at the indicated concentrations in a final volume of 5 mL. Plates were incubated for the time indicated before harvest for flow cytometry analysis. At harvest, cells were gently mixed and cell counts were performed. Cells (1 × 106) were added to a 15-mL tube and washed twice with 12 mL cold standard azide buffer [phosphate-buffered saline (PBS) containing 5% (vol:vol) FBS and 0.1% (wt:vol) sodium azide], centrifuging each time at 500 RPM for 10 minutes at 4°C. After carefully removing the supernatant, the cells were adjusted to 1 × 107 cells/mL by resuspending the pellet in 80 μL cold standard azide buffer and adding 20 μL fluorescein isothiocyanate (FITC)-labeled anti-CD4 antibody (Pharmingen, San Diego, CA) and incubating on ice for 30 minutes in the dark. Cells were then washed twice as described before. For propidium iodide staining, the cells were then resuspended in 1 mL of a 0.112% sodium-citrate buffer containing 50 μg/mL propidium iodide (Sigma) and 100 U/mL RNase A (Sigma) and allowed to stain for up to 1 hour at room temperature.
For intracellular staining of c-fos and c-jun proteins, the cells were harvested as before, washed with cold staining buffer [PBS containing 1% (vol:vol) FBS and 0.1% (wt:vol) sodium azide], and stained with FITC-labeled anti-CD4 antibody in staining buffer as described before. After washing twice with cold staining buffer and once with cold PBS, the cells were fixed with 100 μL of 4% (wt:vol) paraformaldehyde in PBS for 20 minutes at 4°C. The cells were then washed once with cold PBS and twice with cold permeabilization buffer [staining buffer with 0.1% (wt:vol) saponin]. The cells were then resuspended in 100 μL of permeabilization buffer containing 0.7 μg of either c-fos or c-jun specific rabbit antibody (Santa Cruz Biotechnology, Santa Cruz, CA) on ice for 30 minutes. The cells were then washed twice with permeabilization buffer and resuspended in permeabilization buffer containing 0.7 μg of phycoerythrin-labeled antirabbit antibody (Sigma) on ice for 30 minutes. The cells were again washed twice with permeabilization buffer and washed once with staining buffer. Finally, the cells were resuspended in 1 mL of staining buffer.
For all flow cytometry analysis, samples were filtered through 44-μm nylon mesh and data from 30,000 events were acquired with a FACSort (Becton Dickinson Immunocytometry Systems, San Jose, CA) using the LYSIS II software system. Analysis of the cell cycle was performed using CellFIT software (Becton Dickinson, San Jose, CA).
Immunoblotting.
PBMCs were cultured in 6-well plates and harvested as described above. CD4+ T cells were then isolated by passing PBMCs over CD4 T Cellect columns (Biotex, Edmonton, Alberta, Canada) as per the manufacturer’s recommendations. The resulting CD4+ T cells were counted and stored at −70°C.
Cells were lysed at 4°C for 20 minutes in ice-cold lysis buffer consisting of 50 mmol/L Tris-HCl (pH 7.5), 250 mmol/L NaCl, 1% (vol:vol) Triton X-100, 2 mmol/L EDTA, 2 mmol/L EGTA, 50 mmol/L NaF, 20 mmol/L β-glycerophosphate, 2 mmol/L Na-orthovanadate, aprotinin (10 μg/mL), leupeptin (10 μg/mL), pepstatin (10 μg/mL), benzamidine (5 μg/mL), and 1 mmol/L phenylmethanesulfonyl fluoride. Samples were centrifuged at 13,000 RPM for 10 minutes and a BCA protein assay (Pierce, Rockford, IL) was performed on the supernatants. Equal amounts of protein from cell lysates (30 to 70 μg/lane) were subjected to sodium dodecyl sulfate (SDS) gel electrophoresis. After Western transfer, membranes were probed with 1.2 μg of anti-p27Kip1, anti-cyclin D2, or anti-cyclin D3 antibodies (Santa Cruz Biotechnology) and developed using an enhanced chemiluminescence (ECL) detection kit (Amersham). Densitometric analysis of radiographic film using IA-1000 Digital Image Analysis Software (Alpha Innotech Corp, San Leandro, CA) was used to determine fold increase or decrease between band intensities based on total pixel value.
IL-2 assay.
IL-2 levels were determined from cell-free culture supernatants stored at −70°C using IL-2 enzyme-linked immunosorbent assay (ELISA) kits (Intergen). Assay protocol was conducted as described by the manufacturer. All supernatant samples were tested in duplicate.
RESULTS
IL-10 blocks SEB-induced proliferation in PBMCs.
IL-10 was first tested for its ability to inhibit superantigen-induced proliferation of human lymphocytes. Human PBMCs were treated with IL-10 for up to 2 days before the addition of SEB. These cultures were then incubated for up to 5 days, which was the time of optimal proliferation as reflected by [3H]thymidine incorporation. IL-10 significantly inhibited SEB-induced proliferation in a dose-dependent manner (data not shown), and an optimal concentration of 10 U/mL of IL-10 was determined. Furthermore, as shown in Fig 1, 10 U/mL of IL-10 inhibited PBMC proliferation over a wide range of concentrations of SEB. Thus, IL-10 blocked SEB-induced PBMC proliferation.
IL-10 suppression of SEB stimulation: SEB dose-response. PBMCs were added to 96-well plates at 3 × 105 cells/mL along with IL-10 at 10 U/mL or media only. After 2 days of incubation, media alone or SEB at varying concentrations was added and plates were further incubated for another 2 days. Proliferation was measured by [3H]thymidine incorporation. Different PBMC donors are represented in each experiment. Data are expressed as the mean CPM ± SD. Results were determined to be statistically significant by the Student’s t-test (P < .002). Numbers between curves indicate the percentage of suppression by IL-10.
IL-10 suppression of SEB stimulation: SEB dose-response. PBMCs were added to 96-well plates at 3 × 105 cells/mL along with IL-10 at 10 U/mL or media only. After 2 days of incubation, media alone or SEB at varying concentrations was added and plates were further incubated for another 2 days. Proliferation was measured by [3H]thymidine incorporation. Different PBMC donors are represented in each experiment. Data are expressed as the mean CPM ± SD. Results were determined to be statistically significant by the Student’s t-test (P < .002). Numbers between curves indicate the percentage of suppression by IL-10.
IL-10 inhibits SEB-stimulated CD4+ T cells from progressing through the cell cycle.
We next determined the stage of the cell cycle in which IL-10 blocked SEB-induced proliferation, looking specifically at CD4+ T cells, which are one of the primary targets of superantigen stimulation. After stimulation for up to 5 days, CD4+ T cells were examined for cell cycle progression using FITC-labeled anti-CD4 antibodies and propidium iodide staining. Preliminary experiments using SEB showed that CD4+ T cells were entering the S phase between days 4 and 5, although there was some variation among donors with respect to the percentage of cells in S phase at these times. As shown in Table 1, untreated cells were all essentially quiescent (>98% G0/G1). Treatment with SEB induced cells to progress through the G1 phase and into the S phase of the cell cycle. However, IL-10 blocked SEB-induced progression of CD4+ T cells through the G1 phase of the cell cycle. A similar pattern of inhibition was observed with 3 and 30 U/mL of IL-10, with a resulting dose response (Table 2). These data suggest that IL-10 inhibits the early SEB-mediated activation of CD4+ T cells and prevents their progression through the G1 phase of the cell cycle and their subsequent entry into the cell cycle.
Flow Cytometry Analysis of the Effect of IL-10 on SEB-Stimulated CD4+ T Cells
| Treatment . | Cell Cycle Distribution (%)* . | ||
|---|---|---|---|
| G1 . | S . | G2/M . | |
| Day 4 | |||
| None | >98 | <1 | <1 |
| SEB only | 58.80 ± 0.7 | 37.90 ± 0.3 | 3.25 ± 0.4 |
| IL-10 + SEB | 78.95 ± 2.2 | 19.00 ± 1.6 | 2.05 ± 0.6 |
| Day 5 | |||
| None | >98 | <1 | <1 |
| SEB only | 44.10 ± 1.0 | 47.15 ± 0.9 | 8.70 ± 0.1 |
| IL-10 + SEB | 72.95 ± 2.2 | 22.45 ± 1.8 | 4.70 ± 0.4 |
| Treatment . | Cell Cycle Distribution (%)* . | ||
|---|---|---|---|
| G1 . | S . | G2/M . | |
| Day 4 | |||
| None | >98 | <1 | <1 |
| SEB only | 58.80 ± 0.7 | 37.90 ± 0.3 | 3.25 ± 0.4 |
| IL-10 + SEB | 78.95 ± 2.2 | 19.00 ± 1.6 | 2.05 ± 0.6 |
| Day 5 | |||
| None | >98 | <1 | <1 |
| SEB only | 44.10 ± 1.0 | 47.15 ± 0.9 | 8.70 ± 0.1 |
| IL-10 + SEB | 72.95 ± 2.2 | 22.45 ± 1.8 | 4.70 ± 0.4 |
PBMCs were pretreated for 2 days with IL-10 (10 U/mL). SEB (100 pg/mL) was then added to these cultures and the incubation was continued for the indicated number of days before flow cytometry analysis. Data were gated to analyze CD4+ T cells. Results are expressed as the mean of duplicate samples ± SD. All differences between SEB only and IL-10 + SEB treatments were determined to be statistically significant by the Student’s t-test (P < .01).
Flow Cytometry Analysis of the Effect of Increasing Concentrations of IL-10 on SEB-Stimulated CD4+ T Cells
| IL-10 (U/mL) . | SEB . | Cell Cycle Distribution (%)* . | ||
|---|---|---|---|---|
| G1 . | S . | G2/M . | ||
| 0 | − | >98 | <1 | <1 |
| 30 | + | 80.50 | 16.05 | 3.45 |
| 10 | + | 75.10 | 21.00 | 3.85 |
| 3 | + | 73.80 | 21.60 | 4.50 |
| 0 | + | 61.50 | 32.00 | 6.50 |
| IL-10 (U/mL) . | SEB . | Cell Cycle Distribution (%)* . | ||
|---|---|---|---|---|
| G1 . | S . | G2/M . | ||
| 0 | − | >98 | <1 | <1 |
| 30 | + | 80.50 | 16.05 | 3.45 |
| 10 | + | 75.10 | 21.00 | 3.85 |
| 3 | + | 73.80 | 21.60 | 4.50 |
| 0 | + | 61.50 | 32.00 | 6.50 |
PBMCs were pretreated for 2 days with IL-10 at the indicated concentrations and then incubated with SEB (100 pg/mL) for 5.5 days before flow cytometry analysis. Data were gated to analyze CD4+ T cells.
IL-10 inhibition of cell cycle progression in CD4+ T cells stimulated with SEB is dependent on their activation state.
We then examined the inhibitory effect of IL-10 on SEB stimulation of PBMCs when added to cultures at various times relative to superantigen addition. As seen in Table 3, the addition of IL-10 (10 U/mL) 2 days before, 1 day before, or at the time of SEB addition inhibited cell proliferation in the G0/G1 phase of the cell cycle to essentially the same extent. The addition of IL-10 at 1 to 4 days after SEB resulted in progressively less inhibition of cell cycling. The addition of IL-10 4 days after SEB allowed essentially the same percentage of cells to leave the G1 phase and enter the cell cycle as did cells treated with SEB and no IL-10. These data suggest that, once CD4+ T cells have been activated by mitogen and enter the cell cycle, IL-10 has no further effect on these cells. Thus, it appears that IL-10 blocks the activation of quiescent CD4+T cells but not the subsequent proliferation of these cells.
Flow Cytometry Analysis of the Effect of IL-10 Addition at Various Times Relative to SEB Incubation on SEB-Stimulated CD4+ T Cells
| Treatment . | Cell Cycle Distribution (%)3-150 . | ||
|---|---|---|---|
| G1 . | S . | G2/M . | |
| None | >98 | <1 | <1 |
| IL-10 added 2 days before SEB | 84.50 | 11.70 | 3.80 |
| IL-10 added 1 day before SEB | 88.30 | 8.90 | 2.80 |
| IL-10 added same time as SEB | 83.50 | 13.00 | 3.50 |
| IL-10 added 1 day after SEB | 72.10 | 21.90 | 6.00 |
| IL-10 added 2 days after SEB | 70.90 | 22.80 | 6.30 |
| IL-10 added 3 days after SEB | 70.80 | 22.90 | 6.30 |
| IL-10 added 4 days after SEB | 65.80 | 26.80 | 7.40 |
| SEB only | 66.15 | 27.10 | 6.75 |
| Treatment . | Cell Cycle Distribution (%)3-150 . | ||
|---|---|---|---|
| G1 . | S . | G2/M . | |
| None | >98 | <1 | <1 |
| IL-10 added 2 days before SEB | 84.50 | 11.70 | 3.80 |
| IL-10 added 1 day before SEB | 88.30 | 8.90 | 2.80 |
| IL-10 added same time as SEB | 83.50 | 13.00 | 3.50 |
| IL-10 added 1 day after SEB | 72.10 | 21.90 | 6.00 |
| IL-10 added 2 days after SEB | 70.90 | 22.80 | 6.30 |
| IL-10 added 3 days after SEB | 70.80 | 22.90 | 6.30 |
| IL-10 added 4 days after SEB | 65.80 | 26.80 | 7.40 |
| SEB only | 66.15 | 27.10 | 6.75 |
PBMCs were treated with IL-10 (10 U/mL) at the times indicated and incubated with SEB (100 pg/mL). At 5.5 days after the addition of SEB, cells were collected and analyzed by flow cytometry. Data were gated to analyze CD4+ T cells.
IL-10 prevents the elimination of the tumor suppressor protein p27Kip1 in SEB-stimulated CD4+ T cells.
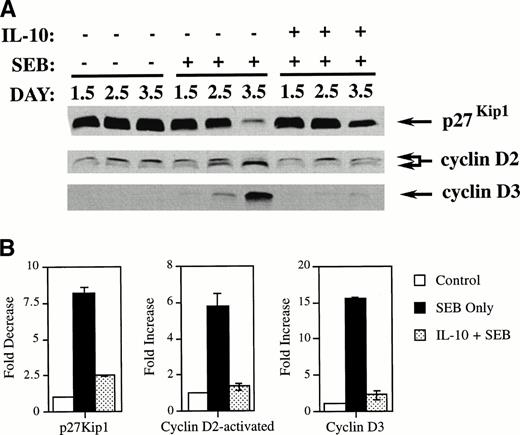
To gain insight into how IL-10 blocked superantigen-induced activation in the G0/G1 phase of the cell cycle, we determined the effects of IL-10 on the tumor suppressor gene protein p27Kip1 and on the cyclins D2 and D3 in CD4+ T cells. p27Kip1 levels are high in quiescent G0cells and are rapidly reduced in cells induced to enter the cell cycle by mitogens,18 whereas cyclin D levels are low in quiescent cells and increase upon mitogenic stimulation.19 In human T cells, cyclin D2 migrates as a doublet, with the faster migrating species representing the activated form.20 PBMCs were incubated with IL-10 (10 U/mL) for 2 days before the addition of SEB. After incubating for the specified number of days, the CD4+T cells were isolated using negative selection chromatography columns and the cell extracts were subjected to Western analysis for p27Kip1 and cyclins D2 and D3. As shown in Fig 2, untreated quiescent cells showed high levels of p27Kip1. Treatment with SEB dramatically reduced the levels of p27Kip1 by day 3.5. By comparison, the p27Kip1 level remained relatively high in cultures treated with IL-10 even in the presence of SEB. The effect of IL-10 on the expression of p27Kip1 in SEB-treated cultures supports the conclusion that IL-10 inhibits cellular proliferation in the early G0/G1 phase of the cell cycle.
IL-10 inhibits the SEB reduction of p27Kip1and prevents SEB-induced upregulation of cyclins D2 and D3 in CD4+ T cells. (A) PBMCs were incubated in 6-well plates at 6 × 105 cells/mL along with IL-10 at 10 U/mL or media only. After 2 days of incubation, media alone or SEB at 100 pg/mL was added. The cells were further incubated until the indicated harvest day. Cells were harvested and CD4+ T cells were separated and collected by negative selection affinity chromatography. Lysates from these cells were subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and Western analysis using antibodies specific for p27Kip1, cyclin D2, and cyclin D3. The lower band in the cyclin D2 doublet represents the faster-migrating activated form of cyclin D2. (B) Quantitation was performed by densitometric analysis of bands from day 3.5. The differences between SEB treatment and treatment with IL-10 and SEB were found to be statistically significant for all immunoblots (P < .001) by the Student’s t-test.
IL-10 inhibits the SEB reduction of p27Kip1and prevents SEB-induced upregulation of cyclins D2 and D3 in CD4+ T cells. (A) PBMCs were incubated in 6-well plates at 6 × 105 cells/mL along with IL-10 at 10 U/mL or media only. After 2 days of incubation, media alone or SEB at 100 pg/mL was added. The cells were further incubated until the indicated harvest day. Cells were harvested and CD4+ T cells were separated and collected by negative selection affinity chromatography. Lysates from these cells were subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and Western analysis using antibodies specific for p27Kip1, cyclin D2, and cyclin D3. The lower band in the cyclin D2 doublet represents the faster-migrating activated form of cyclin D2. (B) Quantitation was performed by densitometric analysis of bands from day 3.5. The differences between SEB treatment and treatment with IL-10 and SEB were found to be statistically significant for all immunoblots (P < .001) by the Student’s t-test.
IL-10 inhibits the upregulation of cyclins D2 and D3 in SEB-stimulated CD4+ T cells.
The expression of cyclins D2 and D3 were also strongly affected by IL-10 treatment of SEB-stimulated CD4+ T cells. Figure 2shows that SEB treatment of quiescent CD4+ T cells increased the levels of cyclin D3 and the activated faster migrating form of cyclin D2 by day 3.5. In contrast, IL-10 prevented the expression of comparable amounts of both cyclin D3 and the faster migrating form of cyclin D2 over the same time period. As mentioned earlier, p27Kip1 and the D-type cyclins behave as sensors for mitogenic stimuli and play a crucial role in the decision of cells to become activated, enter the cell cycle, and subsequently proliferate. Taken together with the effects on p27Kip1, these data demonstrate that IL-10 affects CD4+ T cells very early in the cell cycle by antagonizing the ability of mitogens such as SEB to activate these cells to enter the cell cycle.
IL-10 inhibits SEB-induced IL-2 production.
The potent mitogenic activity of SEB requires the induction and autocrine effects of IL-2 for the activation of CD4+ T cells. IL-2 is the primary cytokine secreted by CD4+ T cells that is responsible for the activation of competent T cells and their subsequent progression through the G1 phase of the cell cycle. IL-10 has also been reported to block induction of IL-2.1 Thus, we next examined the role of IL-2 in this system and its effects on the cell cycle events affected by IL-10. As seen in Table 4, treatment with IL-10 dramatically inhibited the amount of IL-2 produced during the time period of SEB stimulation. These data represent IL-2 levels in supernatants taken from the same cell cultures analyzed in Fig 2. The changes in IL-2 levels are seen to precede changes in the cell cycle proteins. We then measured the amount of IL-2 produced during the first 24 hours after SEB stimulation (Table 5) to study the effect IL-10 has on IL-2 production during the time of initial T-cell activation. IL-2 production was induced as early as 12 hours after the addition of SEB, and IL-10–treated cells showed significantly less IL-2 production than did cells incubated with SEB only. In both cases, similar values were obtained when IL-10 was added 2 days before the addition of SEB and when IL-10 and SEB were added simultaneously (data not shown). Thus, IL-10 blocks induction of IL-2 in the SEB-stimulated cultures.
IL-10 Effects on IL-2 Production by SEB-Stimulated PBMCs
| Days . | IL-10 . | SEB . | IL-24-150 . | Fold Decrease . | |
|---|---|---|---|---|---|
| pg/mL . | U/mL4-151 . | ||||
| 1.5 | − | − | 12 ± 5.0 | 0.12 ± 0.050 | — |
| 2.5 | − | − | <8‡ | <0.08‡ | — |
| 3.5 | − | − | <8 | <0.08 | — |
| 1.5 | − | + | 1,872 ± 185.0 | 18.72 ± 1.850 | — |
| 2.5 | − | + | 2,118 ± 246.0 | 21.18 ± 2.460 | — |
| 3.5 | − | + | 2,277 ± 318.0 | 22.77 ± 3.180 | — |
| 1.5 | + | + | 129 ± 6.0 | 1.29 ± 0.060 | 14.5 |
| 2.5 | + | + | 240 ± 29.0 | 2.40 ± 0.290 | 8.8 |
| 3.5 | + | + | 49 ± 7.0 | 0.49 ± 0.070 | 46.2 |
| Days . | IL-10 . | SEB . | IL-24-150 . | Fold Decrease . | |
|---|---|---|---|---|---|
| pg/mL . | U/mL4-151 . | ||||
| 1.5 | − | − | 12 ± 5.0 | 0.12 ± 0.050 | — |
| 2.5 | − | − | <8‡ | <0.08‡ | — |
| 3.5 | − | − | <8 | <0.08 | — |
| 1.5 | − | + | 1,872 ± 185.0 | 18.72 ± 1.850 | — |
| 2.5 | − | + | 2,118 ± 246.0 | 21.18 ± 2.460 | — |
| 3.5 | − | + | 2,277 ± 318.0 | 22.77 ± 3.180 | — |
| 1.5 | + | + | 129 ± 6.0 | 1.29 ± 0.060 | 14.5 |
| 2.5 | + | + | 240 ± 29.0 | 2.40 ± 0.290 | 8.8 |
| 3.5 | + | + | 49 ± 7.0 | 0.49 ± 0.070 | 46.2 |
IL-2 was measured from supernatants from PBMC cultures treated with media only, SEB (100 pg/mL) only, or IL-10 (10 U/mL) for 2 days before SEB (100 pg/mL). Cell supernatants were then collected after the indicated incubation period. Results were shown to be statistically significant by the Student’s t-test (P < .02).
IL-2 was measured in picograms per milliliter. The specific activity of the IL-2 standard was 1 × 107 U/mg and was used to calculate units per milliliter.
These results were below the detection limit of the assay.
Early IL-10 Effects on IL-2 Production by SEB-Stimulated PBMCs
| Hours . | IL-10 . | SEB . | IL-25-150 . | Fold Decrease . | |
|---|---|---|---|---|---|
| pg/mL . | U/mL5-151 . | ||||
| 6 | − | − | <85-152 | <0.085-152 | — |
| 12 | − | − | <8 | <0.08 | — |
| 18 | − | − | <8 | <0.08 | — |
| 24 | − | − | <8 | <0.08 | — |
| 6 | − | + | <8 | <0.08 | — |
| 12 | − | + | 172 ± 30.8 | 1.72 ± 0.308 | — |
| 18 | − | + | 816 ± 17.0 | 8.16 ± 0.170 | — |
| 24 | − | + | 1,375 ± 18.6 | 13.75 ± 0.186 | — |
| 6 | + | + | <8 | <0.08 | 0 |
| 12 | + | + | 53 ± 6.0 | 0.53 ± 0.060 | 3.2 |
| 18 | + | + | 53 ± 4.7 | 0.53 ± 0.047 | 15.4 |
| 24 | + | + | 96 ± 12.9 | 0.96 ± 0.129 | 14.3 |
| Hours . | IL-10 . | SEB . | IL-25-150 . | Fold Decrease . | |
|---|---|---|---|---|---|
| pg/mL . | U/mL5-151 . | ||||
| 6 | − | − | <85-152 | <0.085-152 | — |
| 12 | − | − | <8 | <0.08 | — |
| 18 | − | − | <8 | <0.08 | — |
| 24 | − | − | <8 | <0.08 | — |
| 6 | − | + | <8 | <0.08 | — |
| 12 | − | + | 172 ± 30.8 | 1.72 ± 0.308 | — |
| 18 | − | + | 816 ± 17.0 | 8.16 ± 0.170 | — |
| 24 | − | + | 1,375 ± 18.6 | 13.75 ± 0.186 | — |
| 6 | + | + | <8 | <0.08 | 0 |
| 12 | + | + | 53 ± 6.0 | 0.53 ± 0.060 | 3.2 |
| 18 | + | + | 53 ± 4.7 | 0.53 ± 0.047 | 15.4 |
| 24 | + | + | 96 ± 12.9 | 0.96 ± 0.129 | 14.3 |
IL-2 was measured from supernatants from PBMC cultures treated with media only, SEB (100 pg/mL) only, or IL-10 (10 U/mL) for 2 days before SEB (100 pg/mL). Cell supernatants were then collected after the indicated incubation period. Results were determined to be statistically significant by the Student’s t-test (P < .05).
IL-2 was measured in picograms per milliliter. The specific activity of the IL-2 standard was 1 × 107 U/mg and was used to calculate units per milliliter.
These results were below the detection limit of the assay.
The addition of IL-2 reverses IL-10’s effects on cell cycle progression in SEB-stimulated CD4+ T cells.
Next, we determined the effect of the addition of IL-2 on the IL-10–induced suppression of SEB stimulation. IL-2 at varying concentrations was added to PBMCs along with IL-10 and SEB. The cells were then incubated for 5 days and the CD4+ T cells were examined for cell cycle progression using FITC-labeled anti-CD4 antibodies and propidium iodide staining. Table 6 shows that the addition of IL-2 overcame the suppressive effect of IL-10 in these cells and allowed them to become activated and progress from the G0/G1 phase into S and G2/M phases of the cell cycle. This reversal of the IL-10 effect by IL-2 occurred in a dose-dependent manner. A similar experiment was performed in which cells were incubated with IL-10 for 2 days before the addition of IL-2 and SEB (Table 7). Results very similar to those in Table 6 were seen in which the addition of IL-2 reversed the G0/G1 block imposed by IL-10 in a dose-dependent manner. In either case, the concentration of IL-2 required to produce an approximately 50% reversal of inhibition was similar to the concentration of IL-2 induced in cultures stimulated by SEB alone (see Table 4). These IL-2 levels probably represent steady-state levels of IL-2 in culture supernatants. These data strongly support the conclusion that one important mechanism by which IL-10 exerts its effects is by downregulating the production of IL-2, especially since pretreatment with IL-10 had no greater effect on the ability of IL-2 to reverse the cell cycle block. In a few donors tested, as shown in Table 7, IL-10 also inhibited cell cycle progression in cells stimulated with exogenous IL-2, indicating that IL-10 may also interfere with the response of CD4+ T cells to IL-2 to varying extents.
Flow Cytometry Analysis of the Effect of IL-2 Addition on Reversing the Effect of IL-10 on SEB-Stimulated CD4+ T Cells: No IL-10 Pretreatment
| IL-2 (U/mL) . | IL-10 . | SEB . | Cell Cycle Distribution (%)6-150 . | ||
|---|---|---|---|---|---|
| G1 . | S . | G2/M . | |||
| 0 | − | − | >98 | <1 | <1 |
| 0 | − | + | 63.80 | 29.10 | 7.10 |
| 100 | + | + | 59.70 | 33.40 | 6.90 |
| 30 | + | + | 60.60 | 31.90 | 7.50 |
| 10 | + | + | 72.30 | 22.10 | 5.60 |
| 3 | + | + | 77.30 | 17.90 | 4.80 |
| 0 | + | + | 80.50 | 15.00 | 4.50 |
| 100 | − | + | 48.60 | 42.70 | 8.70 |
| 3 | − | + | 59.50 | 32.30 | 8.10 |
| 100 | + | − | 94.40 | 5.00 | 0.60 |
| 3 | + | − | 98.40 | 1.40 | 0.20 |
| 100 | − | − | 92.70 | 6.30 | 1.00 |
| 3 | − | − | 97.60 | 2.00 | 0.40 |
| IL-2 (U/mL) . | IL-10 . | SEB . | Cell Cycle Distribution (%)6-150 . | ||
|---|---|---|---|---|---|
| G1 . | S . | G2/M . | |||
| 0 | − | − | >98 | <1 | <1 |
| 0 | − | + | 63.80 | 29.10 | 7.10 |
| 100 | + | + | 59.70 | 33.40 | 6.90 |
| 30 | + | + | 60.60 | 31.90 | 7.50 |
| 10 | + | + | 72.30 | 22.10 | 5.60 |
| 3 | + | + | 77.30 | 17.90 | 4.80 |
| 0 | + | + | 80.50 | 15.00 | 4.50 |
| 100 | − | + | 48.60 | 42.70 | 8.70 |
| 3 | − | + | 59.50 | 32.30 | 8.10 |
| 100 | + | − | 94.40 | 5.00 | 0.60 |
| 3 | + | − | 98.40 | 1.40 | 0.20 |
| 100 | − | − | 92.70 | 6.30 | 1.00 |
| 3 | − | − | 97.60 | 2.00 | 0.40 |
PBMCs were treated simultaneously with IL-10 (10 U/mL), SEB (100 pg/mL), and IL-2 at the indicated concentrations for 5.5 days before flow cytometry analysis. Data were gated to analyze CD4+ T cells.
Flow Cytometry Analysis of the Effect IL-2 Addition on Reversing the Effect of IL-10 on SEB-Stimulated CD4+ T Cells: Two Day IL-10 Pretreatment
| IL-2 (U/mL) . | IL-10 . | SEB . | Cell Cycle Distribution (%)7-150 . | ||
|---|---|---|---|---|---|
| G1 . | S . | G2/M . | |||
| 0 | − | − | >98 | <1 | <1 |
| 0 | − | + | 73.70 | 23.20 | 3.10 |
| 100 | + | + | 61.20 | 33.20 | 5.60 |
| 30 | + | + | 62.10 | 32.60 | 5.30 |
| 10 | + | + | 79.50 | 16.00 | 4.50 |
| 3 | + | + | 88.30 | 9.50 | 2.20 |
| 0 | + | + | 89.20 | 8.70 | 2.00 |
| 100 | − | + | 53.40 | 41.20 | 5.40 |
| 3 | − | + | 70.80 | 24.70 | 4.50 |
| 100 | + | − | 80.80 | 16.80 | 2.40 |
| 3 | + | − | 93.30 | 5.50 | 1.10 |
| 100 | − | − | 65.70 | 29.40 | 4.90 |
| 3 | − | − | 74.00 | 23.80 | 2.20 |
| IL-2 (U/mL) . | IL-10 . | SEB . | Cell Cycle Distribution (%)7-150 . | ||
|---|---|---|---|---|---|
| G1 . | S . | G2/M . | |||
| 0 | − | − | >98 | <1 | <1 |
| 0 | − | + | 73.70 | 23.20 | 3.10 |
| 100 | + | + | 61.20 | 33.20 | 5.60 |
| 30 | + | + | 62.10 | 32.60 | 5.30 |
| 10 | + | + | 79.50 | 16.00 | 4.50 |
| 3 | + | + | 88.30 | 9.50 | 2.20 |
| 0 | + | + | 89.20 | 8.70 | 2.00 |
| 100 | − | + | 53.40 | 41.20 | 5.40 |
| 3 | − | + | 70.80 | 24.70 | 4.50 |
| 100 | + | − | 80.80 | 16.80 | 2.40 |
| 3 | + | − | 93.30 | 5.50 | 1.10 |
| 100 | − | − | 65.70 | 29.40 | 4.90 |
| 3 | − | − | 74.00 | 23.80 | 2.20 |
PBMCs were treated for 2 days with IL-10 (10 U/mL). They were then incubated with SEB (100 pg/mL) and IL-2 at the indicated concentrations for 5.5 days before flow cytometry analysis. Data were gated to analyze CD4+ T cells.
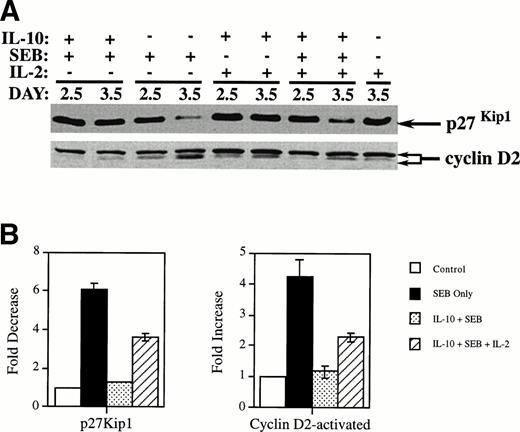
The addition of IL-2 partially reversed IL-10’s effects on cell cycle proteins p27Kip1 and cyclin D2 in SEB-stimulated CD4+ T cells.
We next examined the effect of adding exogenous IL-2 on the levels of the cell cycle proteins p27Kip1 and cyclin D2 in CD4+ T cells. We concentrated on p27Kip1 and cyclin D2, because changes in p27Kip1 and cyclin D2 occur earlier in G1 as compared with cyclin D3. PBMCs were treated simultaneously with IL-10, SEB, and IL-2. The CD4+T cells were then isolated and the cell extracts were subjected to Western analysis as described before. As seen in Fig 3, p27Kip1 levels again decreased in SEB-treated cultures by day 3.5 and remained high in cultures treated with IL-10, as described earlier (see Fig 2). The addition of IL-2 along with the IL-10 and SEB caused the p27Kip1 levels to decrease to nearly those seen in cells treated with SEB only. Figure 3 also shows the effects of adding IL-2 on the level of cyclins D2. The addition of IL-2 to cells treated with SEB and IL-10 significantly restored the expression of cyclin D2. These data thus demonstrate that one mechanism by which IL-10 inhibits entry into the G1 phase of the cell cycle is by the blockage of IL-2 production that is critical to the downregulation of p27Kip1 and the upregulation of early D-type cyclins in T-cell activation. Absence of a complete block of p27Kip1induction and restoration of the active form of cyclin D2, as per Fig3, would suggest other possible IL-2–independent effects of IL-10 on CD4+ T cells.
Addition of IL-2 reverses the effects of IL-10 on the SEB reduction of p27Kip1 and SEB-induced upregulation of cyclin D2 in CD4+ T cells. (A) PBMCs were incubated in 6-well plates at 6 × 105 cells/mL along with IL-10 at 10 U/mL or media only. After 2 days of incubation, media alone, SEB at 100 pg/mL, or SEB and IL-2 (100 U/mL) were added. The cells were further incubated until the indicated harvest day. Cells were harvested and CD4+ T cells were separated and collected by negative selection affinity chromatography. Lysates from these cells were subjected to SDS-PAGE and Western analysis using antibodies specific for p27Kip1 and cyclin D2. The lower band in the cyclin D2 doublet represents the faster-migrating activated form of cyclin D2. (B) Quantitation was performed by densitometric analysis of bands from day 3.5. The differences between SEB treatment and treatment with IL-10 and SEB were found to be statistically significant for both immunoblots (P < .001) by the Student’s t-test. Differences were also statistically significant when comparing IL-10 and SEB treatments with those in which IL-2 was also added: P < .001 for the p27Kip1 blot and P < .002 for the activated form in the cyclin D2.
Addition of IL-2 reverses the effects of IL-10 on the SEB reduction of p27Kip1 and SEB-induced upregulation of cyclin D2 in CD4+ T cells. (A) PBMCs were incubated in 6-well plates at 6 × 105 cells/mL along with IL-10 at 10 U/mL or media only. After 2 days of incubation, media alone, SEB at 100 pg/mL, or SEB and IL-2 (100 U/mL) were added. The cells were further incubated until the indicated harvest day. Cells were harvested and CD4+ T cells were separated and collected by negative selection affinity chromatography. Lysates from these cells were subjected to SDS-PAGE and Western analysis using antibodies specific for p27Kip1 and cyclin D2. The lower band in the cyclin D2 doublet represents the faster-migrating activated form of cyclin D2. (B) Quantitation was performed by densitometric analysis of bands from day 3.5. The differences between SEB treatment and treatment with IL-10 and SEB were found to be statistically significant for both immunoblots (P < .001) by the Student’s t-test. Differences were also statistically significant when comparing IL-10 and SEB treatments with those in which IL-2 was also added: P < .001 for the p27Kip1 blot and P < .002 for the activated form in the cyclin D2.
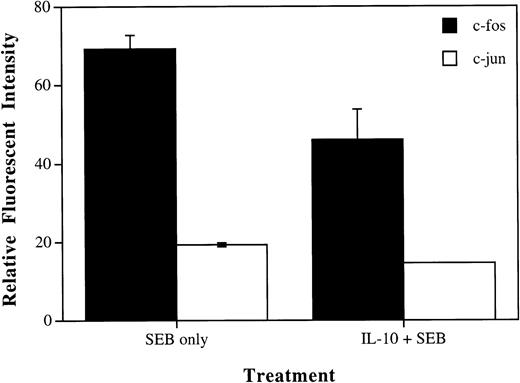
IL-10 reduced the levels of the IL-2 transcriptional regulators c-fos and c-jun in SEB-stimulated CD4+ T cells.
To determine the effects of IL-10 on the production of IL-2 at the molecular level, we performed flow cytometry analysis looking specifically at the transcriptional regulatory proteins for IL-2. AP-1 is an important transcriptional regulator for IL-2 and is a heterodimer composed of the proteins c-fos and c-jun.21 As seen in Fig 4, flow cytometry showed an increase in the expression of both c-fos and c-jun proteins in CD4+ T cells induced by treatment with SEB, consistent with the cells becoming activated. We further show that the addition of IL-10 to these cultures along with SEB caused a decrease in the levels of the c-fos and c-jun proteins. There appeared to be a stronger effect of IL-10 on the expression of c-fos than c-jun, but the reason for this is not known at this time. These results are consistent with our data showing that IL-10 can significantly decrease the amount of IL-2 produced by cells stimulated with SEB and prevents CD4+ T cells from becoming activated and entering the cell cycle.
IL-10 reduces SEB induction of c-fos and c-jun proteins in CD4+ T cells. PBMCs were incubated in 6-well plates at 6 × 105 cells/mL with media alone, SEB (100 pg/mL), or IL-10 (10 U/mL) and SEB. Cells were incubated for 45 minutes, harvested, and subjected to flow cytometry analysis. Data were gated to analyze CD4+ T cells. Results were determined to be statistically significant by the Student’s t-test (P< .01 for c-fos and P < .0005 for c-jun). These values represent net effects after the subtraction of relative fluorescent intensity for untreated cells.
IL-10 reduces SEB induction of c-fos and c-jun proteins in CD4+ T cells. PBMCs were incubated in 6-well plates at 6 × 105 cells/mL with media alone, SEB (100 pg/mL), or IL-10 (10 U/mL) and SEB. Cells were incubated for 45 minutes, harvested, and subjected to flow cytometry analysis. Data were gated to analyze CD4+ T cells. Results were determined to be statistically significant by the Student’s t-test (P< .01 for c-fos and P < .0005 for c-jun). These values represent net effects after the subtraction of relative fluorescent intensity for untreated cells.
IL-10 blocks proliferation of CD4+ T cells.
To investigate the effects of IL-10 on CD4+ T cells in the absence of antigen-presenting cells (APCs), we studied the antiproliferative effects of IL-10 on CD4+ T cells in a system depleted of APCs. CD4+ T cells were isolated from PBMCs and their purity was verified by flow cytometry. These CD4+ T cells were stimulated either with SEB or a mixture of phorbol 12-myristate 13-acetate (PMA) and ionomycin and their proliferation was measured by [3H]thymidine incorporation. SEB is a unique superantigen in that it has been shown to directly activate T cells in the absence of APCs.22Figure 5A shows that SEB did induce CD4+ T cells to proliferate in the absence of APCs and that IL-10 was able to significantly block this proliferation. PHA, whose effects are known to be APC dependent,23 had a negligible effect on proliferation of the CD4+ T-cell population used while also stimulating a population that contained APCs. We also stimulated CD4+ T cells with varying mixtures of PMA and ionomycin, which can also directly activate CD4+ T cells. IL-10 significantly inhibited the proliferation of CD4+ T cells at all of the PMA concentrations tested (Fig 5B) and also at various ionomycin concentrations tested (data not shown). These data show that IL-10 had a direct antiproliferative effect on CD4+ T cells and that these antiproliferative effects are independent of IL-10’s effects on APCs, such as downregulation of MHC class II molecules and downregulation of CD80/86 molecules. Furthermore, these data provide functional evidence for the presence of IL-10 receptors on CD4+ T cells.
IL-10 blocks proliferation of CD4+ T cells. (A) CD4+ T cells were isolated from PBMCs by negative selection chromatography and were added to 96-well plates at 2.5 × 106 cells/mL along with IL-10 at 10 U/mL or media only. After incubating for 2 hours, SEB at varying concentrations was added and plates were incubated for 4 days. Proliferation was measured by [3H]thymidine incorporation. Data are expressed as the mean CPM ± SD. The percentage of suppression by IL-10 and statistical significance, as determined by the Student’s t-test, for each treatment are indicated above the graphs. (B) CD4+ T cells were incubated as indicated above and stimulated with a mixture of ionomycin (100 ng/mL) and varying concentrations of PMA. Proliferation was measured by [3H]thymidine incorporation. Data are expressed as the mean CPM ± SD. The percentage of suppression by IL-10 and statistical significance, as determined by the Student’s t-test, for each treatment are indicated above the graphs. In both (A) and (B), the proliferation of CD4+ T cells incubated with PHA at 5 μg/mL is compared with that of whole PBMCs incubated with 3 μg/mL.
IL-10 blocks proliferation of CD4+ T cells. (A) CD4+ T cells were isolated from PBMCs by negative selection chromatography and were added to 96-well plates at 2.5 × 106 cells/mL along with IL-10 at 10 U/mL or media only. After incubating for 2 hours, SEB at varying concentrations was added and plates were incubated for 4 days. Proliferation was measured by [3H]thymidine incorporation. Data are expressed as the mean CPM ± SD. The percentage of suppression by IL-10 and statistical significance, as determined by the Student’s t-test, for each treatment are indicated above the graphs. (B) CD4+ T cells were incubated as indicated above and stimulated with a mixture of ionomycin (100 ng/mL) and varying concentrations of PMA. Proliferation was measured by [3H]thymidine incorporation. Data are expressed as the mean CPM ± SD. The percentage of suppression by IL-10 and statistical significance, as determined by the Student’s t-test, for each treatment are indicated above the graphs. In both (A) and (B), the proliferation of CD4+ T cells incubated with PHA at 5 μg/mL is compared with that of whole PBMCs incubated with 3 μg/mL.
DISCUSSION
The immunosuppressive effects of IL-10 have been well documented in a number of different systems both in vitro and in vivo.2However, the mechanism of these effects at the level of cell cycle events is currently not known. We showed here that IL-10 blocks SEB-stimulated CD4+ T cells from entering the cell cycle. Specifically, IL-10 treatment of cells prevented the downregulation of p27Kip1, a cell cycle inhibitory protein. Resting cells are characterized by relatively high levels of p27Kip1, which binds to cyclin-cdk complexes, inhibiting phosphorylations that are required for moving cells out of G0 and through the G1 phase of the cell cycle.11,24 IL-10 also prevented upregulation of the G1 cyclins D2 and D3 in SEB-stimulated CD4+ T cells, which are necessary for the cells to enter the cell cycle. Cyclin D2 has been shown to be present at low levels in resting T cells, whereas cyclin D3 is only detected after T-cell activation.19 Thus, IL-10 blocked downregulation of p27Kip1 and upregulation of cyclins D2 and D3, which provides a mechanism for its inhibition of lymphocyte activation.
Related to the inhibitory effects of IL-10 on CD4+ T cells, IL-10 blocked SEB induction of IL-2. It has previously been shown that IL-10 blocked IL-2 induction, but the consequences of this in terms of cell cycle events have not been determined. Addition of IL-2 to cultures treated with SEB in the presence of IL-10 reversed IL-10 inhibition of CD4+ T-cell cycling. This correlated with a downregulation of p27Kip1 and upregulation of cyclin D2. Furthermore, because pretreatment with IL-10 did not significantly affect responses to IL-2, it suggests that events affecting the binding of IL-2 to its receptor are probably not involved in the immediate effects of IL-10. However, the reversal by exogenous IL-2 of the effects on the cell cycle proteins p27Kip1 and cyclin D2 was not complete. Thus, it appears that IL-10 acts by IL-2–independent mechanisms in addition to suppressing induction of IL-2. We have observed in some instances that IL-2 alone can induce a proliferative response, which varied between donors. However, even in these instances the ability of these cells to respond to IL-2 can be suppressed by IL-10. This would suggest that IL-10 could possibly affect signals that do not directly stem from IL-2 but are nonetheless required in conjunction with IL-2–induced signals in mediating a mitogenic response.
IL-10 was examined for its effect on inhibiting upregulation of the AP-1 transcription factors c-fos and c-jun. These proteins are involved in activation of the IL-2 gene as well as a number of other cytokines involved in cell growth.21 Treatment with IL-10 caused a decrease in the amounts of both c-fos and c-jun proteins in SEB-stimulated CD4+ T cells. Thus, the effects of IL-10 on c-fos and c-jun could contribute to the inhibition of IL-2 induction. Given the broad gene-target specificity of c-fos and c-jun, it is probable that IL-10 may also have effects that go beyond the early blockage of IL-2 production.
IL-10 receptors have been shown to be present on murine CD4+ T cells and CD4+ T-cell clones of the TH1 subtype.9 In addition, significant levels of IL-10 receptor mRNA have been detected in human CD4+T-cell clones.8 Consistent with these data, we have demonstrated that IL-10 directly affects the proliferation of purified human CD4+ T cells in response to both the superantigens such as SEB and T-cell mitogens such as PMA and ionomycin. Our data provide further functional evidence for the presence of IL-10 receptors on human CD4+ T cells.
Recent studies have shown that IL-10 induced an anergic state in human CD4+ T cells stimulated with alloantigens or cross-linked with anti-CD3 antibodies.25,26 These studies involved the long-term treatment of cells with IL-10 in which the cells become unresponsive to IL-2. Although we have not directly studied the effect of long-term treatment with IL-10, we found that pretreatment of cells for up to 2 days with IL-10 did not affect the ability of IL-2 to reverse the cell cycle block. Thus, our data, along with those of others,25 would suggest that the effects of IL-10 are twofold. Short exposures to IL-10 produce an immediate inhibition of the activation of CD4+ T-cell entry into the cell cycle that is IL-2 dependent and is reflected by high levels of p27Kip1 and low levels of cyclin D2. This phase is reversible by IL-2. However, continued treatment with IL-10 apparently produces permanent changes in these cells that ultimately makes them unresponsive to IL-2 and renders the cells anergic. The mechanism of this shift to a permanent state of IL-2 unresponsiveness remains unclear.
IL-10 is produced relatively late in the immune response and is thought to be important in the shift from a cell-mediated response to an antibody-mediated response. In this regard, IL-10 functions in a paracrine fashion, affecting neighboring cells. Because IL-10 effectively blocked cell cycling in the presence of SEB, our data suggest that IL-10 can prevent naive CD4+ T cells from being activated even when they are subsequently presented with antigen. This highlights an important use for IL-10 as an immunosuppressive drug. Furthermore, when compared in this fashion, immunosuppressive drugs such as cyclosporin A and FK506 would be expected to resemble IL-10 in their effects on the cell cycle. These drugs act to prevent T-cell activation primarily by blocking IL-2 synthesis27and, thereby, presumably also preventing the cell cycling of target cells in a manner analogous to IL-10. This is in contrast to drugs such as rapamycin that act by blocking signaling after the binding of IL-2 to its receptor.13 Rapamycin, like IL-10, also prevents the downregulation of p27Kip1, but this effect cannot be reversed by exogenous IL-2.13
Overall, our results suggest the importance of IL-10 in regulating the cell cycle proteins necessary for stimulated CD4+ T cells to enter the cell cycle and implicate IL-2 as a key cytokine that mediates the early short-term immunosuppressive effects of IL-10 on the cell cycle. However, failure of IL-2 to completely reverse the IL-10 effects would suggest some direct effects of IL-10 on CD4+T cells.
ACKNOWLEDGMENT
The authors gratefully acknowledge Melissa Chen and Neal Benson at the Flow Cytometry Core Facility, University of Florida, for expert assistance with flow cytometry data acquisition and analysis. This manuscript is Florida Agricultural Experiment Station, Journal Series Number R-06558.
Supported by National Institues of Health Grant No. AI 25904-08 to H.M.J.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to George Q. Perrin, Department of Microbiology and Cell Science, Room 1019, Bldg 981, No Name and Museum Roads, University of Florida, Gainesville, FL 32611; e-mail:gperrin@micro.ifas.ufl.edu.

![Fig. 1. IL-10 suppression of SEB stimulation: SEB dose-response. PBMCs were added to 96-well plates at 3 × 105 cells/mL along with IL-10 at 10 U/mL or media only. After 2 days of incubation, media alone or SEB at varying concentrations was added and plates were further incubated for another 2 days. Proliferation was measured by [3H]thymidine incorporation. Different PBMC donors are represented in each experiment. Data are expressed as the mean CPM ± SD. Results were determined to be statistically significant by the Student’s t-test (P < .002). Numbers between curves indicate the percentage of suppression by IL-10.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/1/10.1182_blood.v93.1.208/5/m_blod40106001x.jpeg?Expires=1766028598&Signature=Baa1F6e-9P2XWvwdGTg6EarY5hbQOIdGyqtweMt5ciKbgpqeVPDu8jS-lgvfqN2abQh56~wJkeEemKgcdqAKuAcaRbj8idvJ2J3afRS8tHzU~q~XYXNyc4X5RR5-iU4jqkyYhX8e0xrTq5p5sbgmmgYYHhSNJPO0jHYRa6uF85dP5juesQM8CVnK7XGBz2ztiAbMhJZVL3fkH1qTKweifdwr9dk~BGJiZIXddQ~5I9U8Z6BOzjdlQuQkL3dL09v-kTP3UFa3OEqCk6b1tDazKsjcNfA1ThUIbVkg7HPCD3PRfjHJ9mghF9HSeVFk1PG8nNHTssdA5EOLBPgfeYDbEw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. IL-10 blocks proliferation of CD4+ T cells. (A) CD4+ T cells were isolated from PBMCs by negative selection chromatography and were added to 96-well plates at 2.5 × 106 cells/mL along with IL-10 at 10 U/mL or media only. After incubating for 2 hours, SEB at varying concentrations was added and plates were incubated for 4 days. Proliferation was measured by [3H]thymidine incorporation. Data are expressed as the mean CPM ± SD. The percentage of suppression by IL-10 and statistical significance, as determined by the Student’s t-test, for each treatment are indicated above the graphs. (B) CD4+ T cells were incubated as indicated above and stimulated with a mixture of ionomycin (100 ng/mL) and varying concentrations of PMA. Proliferation was measured by [3H]thymidine incorporation. Data are expressed as the mean CPM ± SD. The percentage of suppression by IL-10 and statistical significance, as determined by the Student’s t-test, for each treatment are indicated above the graphs. In both (A) and (B), the proliferation of CD4+ T cells incubated with PHA at 5 μg/mL is compared with that of whole PBMCs incubated with 3 μg/mL.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/1/10.1182_blood.v93.1.208/5/m_blod40106005x.jpeg?Expires=1766028598&Signature=YiYC4Xecs9JGkO5tHuXP6muEP0YDMgQfrC~J2rI3aLkSjOvP2-ezEl0jS-ws~Q-HnyAt9QDG3Ni3ZodpLjPeRtbm~sxJwqjrgv7k6juaY8msQ-MKbNxlgj~A5zbHz0tw-SZ3RrUOQpvJ7S8y6to3001l2Om55yrlgPLYi1SK5wYOhof4-CVfkbdvY-ipVg8p9i~tgV-8uU8chlrq6f2JWVBfv39Ny8ghTMr5K5mjjXgQvAxD7y3s0RzFiW8J7zNUuQvSXZ9V--c03xfGnwDdh6VqD0ENhej~MTpU-KgnAzCK~HO-EoC9A2OptFuQH9cK6Ze3TjP5U4qzKKXOHmmlYw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal