Abstract
The role of ceramide as a second messenger is a subject of great interest, particularly since it is implicated in signaling in response to inflammatory cytokines. Ceramide induces apoptosis in both cytokine-dependent MC/9 cells and factor-independent U937 cells. Elevation of cyclic adenosine monophosphate (cAMP) levels inhibits apoptosis induced by ceramide and several other treatments. One target of cAMP-mediated signaling is the transcription factor CREB (cAMP response element binding protein), and recently CREB phosphorylation at an activating site has been shown to also be mediated by a cascade involving p38 mitogen-activated protein kinase (MAPK), one of the stress-activated MAP kinases. Because no role for p38 MAPK in apoptosis has been firmly established, we examined the relationship between p38 MAPK and CREB phosphorylation under various conditions. Ceramide, or sphingomyelinase, like tumor necrosis factor- (TNF-) or the hematopoietic growth factor, interleukin-3 (IL-3), was shown to activate p38 MAPK, which in turn activated MAPKAP kinase-2. Each of these treatments led to phosphorylation of CREB (and the related factor ATF-1). A selective p38 MAPK inhibitor, SB203580, blocked TNF-– or ceramide-induced CREB phosphorylation, but had no effect on the induction of apoptosis mediated by these agents. The protective agents cAMP and IL-3 also led to CREB phosphorylation, but this effect was independent of p38 MAPK, even though IL-3 was shown to activate both p38 MAPK and MAPKAP kinase-2. Therefore, the opposing effects on apoptosis observed with cAMP and IL-3, compared with ceramide and TNF-, could not be explained on the basis of phosphorylation of CREB. In addition, because SB203580 had no effect of TNF- or ceramide-induced apoptosis, our results strongly argue against a role for p38 MAPK in the induction of TNF-– or ceramide-induced apoptosis.
CERAMIDE IS GENERATED intracellularly by sphingomyelinase (SMase)-mediated breakdown of sphingomyelin, and has gained attention as a potential second messenger in response to various extracellular stimuli.1,2Intracellular levels of ceramide have been shown to increase in response to inflammatory cytokines3 and cellular stress,4 and its actions have been reported to affect both differentiation5,6 and apoptosis1,4 in target cells. Ceramide may act via the activation of specific kinases7,8 and/or phosphatases,9,10although not until recently was a cDNA clone identified that corresponded to a ceramide-activated kinase.11 Ceramide has been shown to induce activation of a stress-activated protein kinase (SAPK), and the activation of SAPK has been proposed to be a necessary event in the induction of apoptosis.12 While several downstream events regulated by ceramide have been characterized, potential nuclear events affected by ceramide that might contribute to its biologic effect have not been as well described.
Another second messenger that has been suggested to regulate apoptosis, both positively and negatively, is cyclic adenosine monophosphate (cAMP). For example, cAMP has been shown to induce apoptosis in thymocytes,13,14 but inhibit apoptosis in PC12 cells, in which cAMP has the additional property of inducing differentiation.15 Lanotte et al16 found that cAMP analogs, cholera toxin, and prostaglandins could lead to apoptosis in a rat myelocytic leukemic cell line.
In our recent work, we have studied the role of key enzymes in signaling pathways that are important in regulation of apoptosis in myeloid cells. We17 and others18,19 have shown that activation of phosphatidylinositol (PI) 3-kinase is important, since use of PI 3-kinase inhibitors can cause apoptosis in many, although not all, conditions. Recent studies have also implicated activation of the protein kinase PKB/AKT, downstream of PI 3-kinase as a key event for inhibiting apoptosis.20 21
We have found that elevation of cAMP levels is able to inhibit apoptosis induced by several means, including ceramide treatment, in myeloid cells. This study was designed to characterize downstream phosphorylation events regulated in response to ceramide and cAMP, as well as other agonists known to promote or inhibit apoptosis. We focused our efforts on p38 mitogen-activated protein kinase (MAPK),22 since this kinase has been demonstrated to be an upstream signaling component of one pathway leading to cAMP response element binding protein (CREB) phosphorylation.27Additionally, conflicting results have been published regarding a role for p38 MAPK in the execution of apoptosis in hematopoietic cells. We were therefore interested in the activation state of p38 MAPK in response to each of the conditions that lead to either apoptosis or survival.
Treatment of cells with exogenous C2-ceramide, natural endogenous ceramide generated by bacterial sphingomyelinase, or tumor necrosis factor-α (TNF-α), all led to activation p38 MAPK, and subsequent activation of MAPKAP kinase-2, as well as inducing phosphorylation at activating sites of CREB and a related transcription factor, ATF-1. CREB and ATF-1 phosphorylation in cells with elevated ceramide or following treatment with TNF-α could be blocked by a selective inhibitor of p38 MAPK, SB203580. In contrast, phosphorylation of CREB and ATF-1 induced by interleukin-3 (IL-3) treatment, or by elevated cAMP levels, was not blocked by SB203580, although IL-3 also activated MAPKAP kinase-2 via a p38 MAPK-dependent mechanism. CREB and ATF-1 phosphorylation can therefore be considered downstream targets following p38 MAPK activation in response to elevation of ceramide, but IL-3–induced CREB phosphorylation is independent of p38 MAP kinase. It is not clear at present what the potential physiologic role of CREB and ATF-1 may be in myeloid cells, but our studies show that the contrasting effects of various agents on apoptosis cannot be explained solely by their effects either on a p38 MAPK pathway, or on phosphorylation of CREB and ATF-1.
MATERIALS AND METHODS
Cell culture and reagents.
MC/9 and U937 cells were obtained from the American Type Culture Collection (Rockville, MD). Both cell lines were maintained in RPMI media supplemented with 10% fetal bovine serum at 37°C in a humidified atmosphere containing 5% CO2. Additionally, WEHI-3–conditioned media (10% vol/vol) was added to MC/9 cultures as a source of IL-3. Before experiments, cells were washed several times with phosphate-buffered saline (PBS) and resuspended in RPMI containing 20 mmol/L HEPES-HCl, pH 7.4, and incubated for a minimum of 1 hour at 37°C.
C2-ceramide and dihydro-C2-ceramide were obtained from Calbiochem (La Jolla, CA); Staphylococcus aureus SMase and forskolin were from Sigma (St Louis, MO), and TNF-α from R & D Systems (Minneapolis, MN). Antibodies to MAPKAP kinase-2, CREB, and phospho-CREB were from Upstate Biotechnology (Lake Placid, NY). ATF-2 (1-96) and Hsp25 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
C2-ceramide and sphingomyelinase treatments.
MC/9 or U937 cells (2 to 10 × 106) suspended in 500 μL RPMI media supplemented with 20 mmol/L HEPES-HCl, pH 7.4, were either treated with 2 μL dimethyl sulfoxide (DMSO) or an equal volume of DMSO containing SB203580 for a final concentration of 1 μmol/L, for 20 minutes. Cells were then treated for various times with bacterial sphingomyelinase (100 mU/mL), C2-ceramide (25 to 50 μmol/L), or dihydro-C2-ceramide (50 μmol/L). In some experiments, U937 cells were stimulated with recombinant human TNF-α (10 ng/mL) or forskolin (40 μmol/L). In addition, MC/9 cells were treated with 10 μg/mL synthetic IL-3, previously shown to give maximal activation of tyrosine phosphorylation, or forskolin (40 μmol/L), for various times.
cAMP determinations.
cAMP was measured using an enzyme linked immunosorbent assay kit from Amersham (Montreal, Canada; RPM-225). Cells treated with various stimuli were lysed in ice-cold ethanol:H2O (65:35), dried under nitrogen, and resuspended in assay dilution buffer according to the manufacturer’s protocol. cAMP measured in each unknown was quantitated using a standard curve generated using known quantities of cAMP.
Cell extract preparation and immunoblot analysis.
For analysis of phospho-CREB levels, cells were pelleted following treatments and solubilized in 200 μL of sodium dodecyl sulfate (SDS) sample buffer containing 6 mol/L urea, followed by boiling for 5 minutes. Lysates were then briefly probe-sonicated to shear DNA. Samples were separated by SDS-polyacrylamide gel electrophoresis (PAGE), transferred by semidry blotting to nitrocellulose membranes, and blocked for several hours with 3% skim milk in Tris-buffered saline. Membranes were probed overnight with anti–phospho-CREB (UBI) and detected by chemiluminesence (ECL) according to the manufacturer (Amersham). In some cases, membranes were incubated in stripping buffer (60 mmol/L Tris-HCl, pH 6.6, 2% SDS, and 100 mmol/L 2-mercaptoethanol) for 20 minutes at 50°C, reblocked, and reprobed with anti-CREB (UBI).
For analysis of poly(ADP-ribose) polymerase (PARP) cleavage, cells were harvested following treatments with C2-ceramide for various times, in the presence or absence of forskolin (10 μmol/L) and isobutylmethylxanthine (IBMX; 50 μmol/L). Cell lysates were prepared as for CREB analysis, fractionated by SDS-PAGE (10% 30:1 acrylamide:bisacrylamide), transferred to nitrocellulose, and immunoblotted with an antibody raised against full-length PARP (#422; Enzyme System Products, La Jolla, CA) to detect the unprocessed 118-kD form and the processed 86-kD fragment.
p38 MAPK and MAPKAP kinase-2 in vitro kinase assays.
For kinase assays, cells were pelleted and lysed with ice-cold solubilization buffer (50 mmol/L Tris-HCl, pH 7.4, 10% [vol/vol] glycerol, 1% [vol/vol] Triton X-100, 100 mmol/L NaCl, 25 mmol/L β-glycerophosphate, 10 mmol/L NaF, 1 mmol/L sodium molybdate, 0.2 mmol/L sodium vanadate, 40 μg/mL phenylmethylsulfonylfluoride, 1 μmol/L pepstatin, 0.5 μg/mL leupeptin, and 10 μg/mL soybean trypsin inhibitor), and nuclei removed by brief centrifugation at 4°C. Supernatants were incubated with either anti-p38 antibody and 10 μL of protein A-Sepharose beads or anti-MAPKAP kinase-2 antibody with 10 μL protein G-Sepharose beads for 1 hour at 4°C with continual mixing. Beads were washed twice with solubilization buffer and twice with kinase buffer (20 mmol/L HEPES-HCl, pH 7.4, 25 mmol/L MgCl2, 25 mmol/L β-glycerophosphate, 1 mmol/L dithiothreitol [DTT], and the same quantities of phosphatase and protease inhibitors as in the solubilization buffer). Beads were incubated with 25 μL of kinase buffer containing 50 μmol/L adenosine triphosphate (ATP), 2 μg/sample ATF-2 peptide (corresponding to amino acids 1-96; for p38 assays), 2 μg/sample Hsp25 (for MAPKAP kinase-2 assays), and 10 μCi/sample γ-[32P]-ATP at 30°C for 15 minutes. Reactions were stopped with 6 μL 5× SDS sample buffer and boiling for 5 minutes. Samples were separated by SDS-PAGE, transferred to nitrocellulose membranes, and visualized by autoradiography. Membranes were then blocked with 3% (wt/vol) bovine serum albumin (BSA) in Tris-buffered saline for several hours and probed with antiphosphotyrosine antibody (4G10). Immunoblot analysis of the stripped membranes was performed with anti-p38 antibody to verify equal amounts of immunoprecipitated protein in each sample.
DNA fragmentation assay.
Cell extracts were prepared and DNA fragments were separated by agarose gel electrophoresis exactly as described previously.17
Surface phosphatidylserine measurements.
For quantitation of apoptosis, phosphatidylserine exposure on cells under various conditions was quantitated using annexin-V-FITC (Pharmingen, Mississauga, Ontario, Canada) according to the manufacturer’s protocol. Data reported here are cells that stain positive for annexin-V-FITC, but exclude PI, indicative of early apoptosis.
RESULTS
cAMP protects cells from apoptosis induced under a variety of conditions.
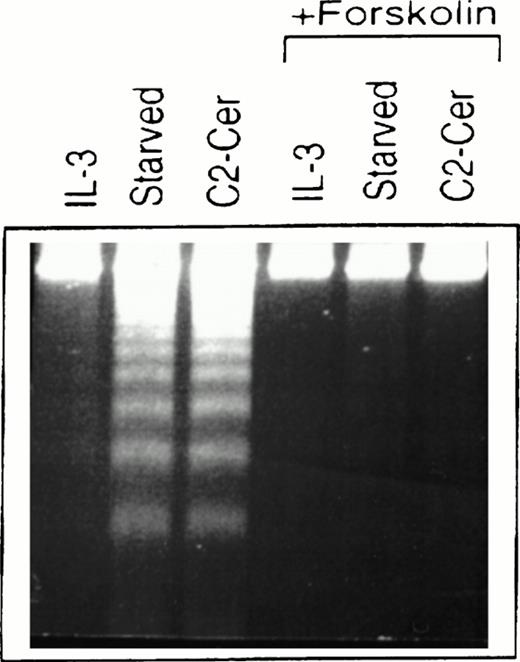
Treatment of cells of the murine IL-3–dependent mast cell line, MC/9, grown in the presence of IL-3, with 50 μmol/LN-acetylsphingosine (hereby referred to as C2-ceramide) for 6 hours resulted in DNA fragmentation typical of cells undergoing apoptosis and similar to cells deprived of cytokine (Fig1). We investigated the ability of elevated cAMP levels to promote survival of these cells and, as shown in Fig 1, cells that were simultaneously incubated with forskolin to activate endogenous adenylate cyclase, along with ceramide or removal of cytokine, showed much less DNA fragmentation. The cells in cAMP also appeared normal morphologically compared with the cells undergoing apoptosis. Although not shown here, similar effects were observed by using a cAMP analog, CPT-cAMP, and elevation of cAMP levels could also inhibit apoptosis observed in the presence of PI 3-kinase inhibitors (M.P.S. and V.D., manuscript in preparation).
Forskolin rescues myeloid cells from ceramide-induced apoptosis. MC/9 cells were treated with IL-3 or left unstimulated (“starved”). Forskolin (20 μmol/L) and C2-ceramide (50 μmol/L) were then added to cells where indicated. Cells treated with C2-ceramide were also incubated in the presence of IL-3. After 6 hours at 37°C, cells were isolated, lysed, and DNA fragments resolved on 2% agarose gels and visualized by ethidium bromide staining.
Forskolin rescues myeloid cells from ceramide-induced apoptosis. MC/9 cells were treated with IL-3 or left unstimulated (“starved”). Forskolin (20 μmol/L) and C2-ceramide (50 μmol/L) were then added to cells where indicated. Cells treated with C2-ceramide were also incubated in the presence of IL-3. After 6 hours at 37°C, cells were isolated, lysed, and DNA fragments resolved on 2% agarose gels and visualized by ethidium bromide staining.
Ceramide in these cells also induced cleavage of PARP, characteristic of caspase activation (Fig 2). Treatment with cAMP-elevating agents inhibited this processing, with only a modest degradation of the 118-kD full-length PARP occurring between 11 and 14 hours after the addition of C2-ceramide. This contrasts with a rapid degradation of PARP as early as 3 hours following exposure to C2-ceramide in the absence of cAMP elevating agents. We found that a signature 86-kD fragment of PARP was visible early during this treatment and quickly disappeared, suggesting further processing by proteases.
PARP cleavage induced by ceramide is prevented by elevation of cAMP. MC/9 cells grown in IL-3 were washed and resuspended in RPMI-1640 supplemented with 10% fetal calf serum. C2-ceramide (50 μmol/L) and forskolin and IBMX (10 μmol/L and 50 μmol/L, respectively) or vehicle (DMSO) were added. At the hours indicated, an aliquot of cells were harvested and lysed in sample buffer containing 6 mol/L urea, followed by sonication and boiling. Samples were fractionated by SDS-PAGE (10%) and transferred to nitrocellulose. Immunoblot analysis was performed to detect PARP (118 kD) and the corresponding 86-kD fragment. This lower fragment appeared to be cleaved further at later time points.
PARP cleavage induced by ceramide is prevented by elevation of cAMP. MC/9 cells grown in IL-3 were washed and resuspended in RPMI-1640 supplemented with 10% fetal calf serum. C2-ceramide (50 μmol/L) and forskolin and IBMX (10 μmol/L and 50 μmol/L, respectively) or vehicle (DMSO) were added. At the hours indicated, an aliquot of cells were harvested and lysed in sample buffer containing 6 mol/L urea, followed by sonication and boiling. Samples were fractionated by SDS-PAGE (10%) and transferred to nitrocellulose. Immunoblot analysis was performed to detect PARP (118 kD) and the corresponding 86-kD fragment. This lower fragment appeared to be cleaved further at later time points.
As an additional measure of apoptosis, the presence of phosphatidylserine at the cell surface, an early event in cells undergoing apoptosis, was detected by binding of fluorescein-conjugated annexin-V. Figure 3 shows the annexin-V staining of MC/9 cells treated with ceramide or ceramide along with cAMP-elevating agents. The data demonstrate that cAMP-elevating agents prevent the symmetrical distribution of phosphatidylserine induced by ceramide.
Phosphatidylserine exposure on myeloid cells induced by ceramide is prevented by cAMP-elevating agents. MC/9 cells growing in IL-3 were treated with vehicle (DMSO; □), C2-ceramide (50 μmol/L; •), C2-ceramide plus forskolin and IBMX (10 μmol/L and 50 μmol/L, respectively; ○), or only forskolin and IBMX (▪). At the times indicated, cells were harvested, washed twice with PBS, and resuspended in buffer containing annexin-V-FITC and PI. Cells stained with annexin-V-FITC but excluding PI were quantitated by flow cytometry. Representative experiment of 3 is shown, with each point being the mean of duplicate determinations, and with error bars representing the range.
Phosphatidylserine exposure on myeloid cells induced by ceramide is prevented by cAMP-elevating agents. MC/9 cells growing in IL-3 were treated with vehicle (DMSO; □), C2-ceramide (50 μmol/L; •), C2-ceramide plus forskolin and IBMX (10 μmol/L and 50 μmol/L, respectively; ○), or only forskolin and IBMX (▪). At the times indicated, cells were harvested, washed twice with PBS, and resuspended in buffer containing annexin-V-FITC and PI. Cells stained with annexin-V-FITC but excluding PI were quantitated by flow cytometry. Representative experiment of 3 is shown, with each point being the mean of duplicate determinations, and with error bars representing the range.
Ceramide and IL-3 activation of p38 MAPK.
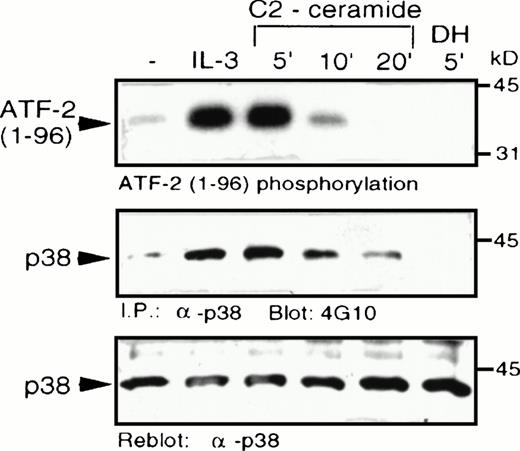
C2-ceramide was added to MC/9 cells and it caused a marked elevation of p38 MAPK activity, comparable to the level of stimulation induced by IL-3 (Fig 4). Activation of p38 MAPK by ceramide was rapid and transient, with activity returning to unstimulated levels within 20 minutes. An inactive ceramide analog,N-acetyl-dihydro-sphingosine (DH-C2-ceramide) was without effect on p38 MAPK. C2-ceramide, as well as IL-3, also induced tyrosine phosphorylation of p38 MAPK, which is characteristic of its activation by dual-specificity kinases such as MKK3/MKK6,23-25 which act upstream of MAPK family members. An effect of ceramide on p38 MAPK activation was also observed independently in U937 human monoblastic leukemia cells or in hepatocytes from a liver regeneration model system.25a,25b Our recent studies have shown that several cytokines, including IL-3, are able to activate p38 MAPK in MC/9 cells.26
C2-ceramide stimulates p38 MAPK activity . MC/9 cells were treated with either synthetic IL-3 (10 μg/mL) for 5 minutes, C2-dihydroceramide (DH; 50 μmol/L for 5 minutes, or C2-ceramide (50 μmol/L) for the indicated times. Cells were then detergent-solubilized and p38 MAPK was immunoprecipitated from the lysates with -p38 MAPK antibody bound to protein A-Sepharose beads. (A) Activity of the washed immunoprecipitates was determined by32P incorporation into a peptide corresponding to amino acids 1-96 of ATF-2. Immunoblot analysis was performed to detect phosphotyrosine (4G10; B) and with -p38 MAPK (C) to confirm equal amounts of p38 MAPK in the immunoprecipitates.
C2-ceramide stimulates p38 MAPK activity . MC/9 cells were treated with either synthetic IL-3 (10 μg/mL) for 5 minutes, C2-dihydroceramide (DH; 50 μmol/L for 5 minutes, or C2-ceramide (50 μmol/L) for the indicated times. Cells were then detergent-solubilized and p38 MAPK was immunoprecipitated from the lysates with -p38 MAPK antibody bound to protein A-Sepharose beads. (A) Activity of the washed immunoprecipitates was determined by32P incorporation into a peptide corresponding to amino acids 1-96 of ATF-2. Immunoblot analysis was performed to detect phosphotyrosine (4G10; B) and with -p38 MAPK (C) to confirm equal amounts of p38 MAPK in the immunoprecipitates.
CREB phosphorylation induced by ceramide, IL-3, and cAMP.
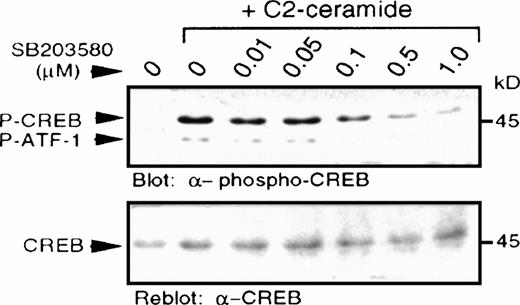
In light of a recent report describing p38 MAPK-dependent phosphorylation of CREB,27 we examined CREB phosphorylation at serine 133, as determined by immunoblotting with a specific antibody recognizing CREB phosphorylated at this site.28,29Increased CREB phosphorylation was observed following treatment with C2-ceramide (Fig 5), but not with dihydro-C2-ceramide (results not shown). Pretreatment with a selective p38 MAPK inhibitor, SB203580, at concentrations as low as 0.1 μmol/L, resulted in partial inhibition of CREB phosphorylation, with almost complete inhibition between 0.5 and 1.0 μmol/L, consistent with the reported potency of SB203580 on inhibition of p38 MAPK activation.22 As reported elsewhere,27 the anti–phospho-CREB antibody also detected phosphorylation of the CREB-related transcription factor, ATF-1, due to similarity in the sequence surrounding the serine phosphorylation site. In all cases, changes in phosphorylation of CREB were mirrored by changes in phosphorylation of ATF-1.
C2-ceramide stimulates CREB phosphorylation. MC/9 cells treated with the indicated concentrations of SB203580 for 20 minutes were stimulated with C2-ceramide (50 μmol/L) or vehicle alone for 5 minutes. Whole-cell lysates were separated on SDS-PAGE and immunoblot analysis was performed to detect phospho-CREB (A) and reprobed to detect CREB (B).
C2-ceramide stimulates CREB phosphorylation. MC/9 cells treated with the indicated concentrations of SB203580 for 20 minutes were stimulated with C2-ceramide (50 μmol/L) or vehicle alone for 5 minutes. Whole-cell lysates were separated on SDS-PAGE and immunoblot analysis was performed to detect phospho-CREB (A) and reprobed to detect CREB (B).
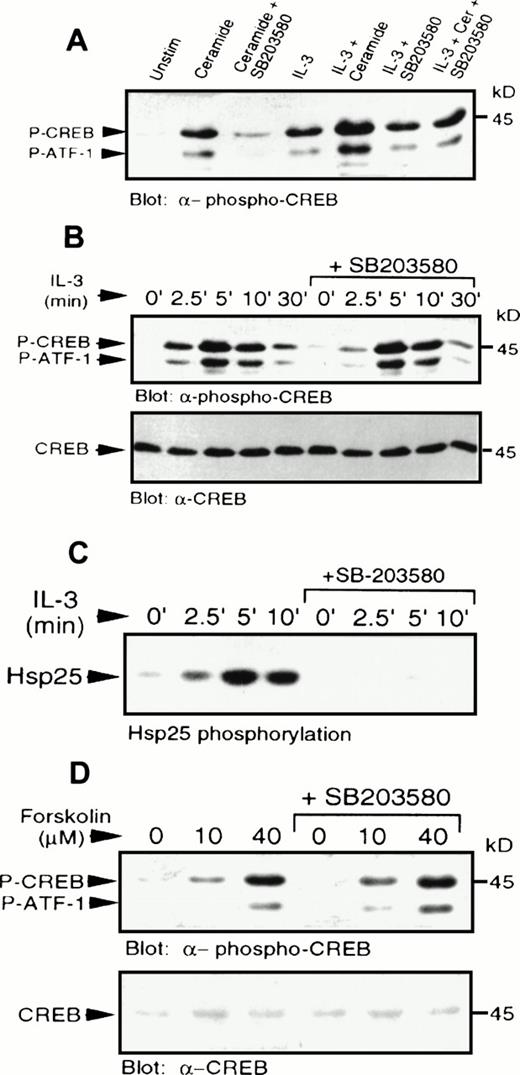
IL-3 also induced phosphorylation of CREB and ATF-1 (Fig 4). The levels of CREB phosphorylation in response to IL-3 or C2-ceramide were comparable. However, pretreatment with SB203580 had little or no effect on IL-3–induced CREB phosphorylation, demonstrating that IL-3–induced activation of p38 MAPK was not a necessary event for CREB phosphorylation, or that there was an alternate pathway activated by IL-3 independent of p38 MAPK. Addition of IL-3 and C2-ceramide together resulted in an additive effect on CREB phosphorylation (Fig6A), which could be reduced to IL-3–stimulated levels by SB203580 pretreatment. Phosphorylation of CREB induced by suboptimal doses of IL-3 (data not shown), or by IL-3 treatment for various times (Fig 6B), was also not affected significantly by SB203580 pretreatment.
IL-3 or forskolin stimulated phosphorylation of CREB occurs independently of p38 MAPK and MAPKAP kinase-2. (A) MC/9 cells were treated with SB203580 (1 μmol/L) or vehicle alone for 20 minutes, followed by treatments with either 10 μg/mL synthetic IL-3, 50 μmol/L C2-ceramide, or both, for 5 minutes. Whole-cell lysates were separated on SDS-PAGE and immunoblot analysis was performed to detect phospho-CREB. (B) MC/9 cells were treated with 10 μg/mL synthetic IL-3 for the times indicated following pretreatment with SB203580 or vehicle alone for 20 minutes. Immunoblot analysis was performed to detect phospho-CREB (top) and CREB (bottom). (C) MC/9 cells were treated with 10 μg/mL IL-3 for the times indicated, following treatment with SB203580 (1 μmol/L) or vehicle alone for 20 minutes. Lysates were incubated with 1 μg anti-MAPKAP kinase-2 antibody and protein G-Sepharose beads, and kinase activity was determined from the washed immunoprecipitates by measuring the32P transferred to Hsp25. (D) Cells were treated with the indicated concentrations of forskolin for 10 minutes following a 20-minute treatment with SB203580 (1 μmol/L) or vehicle alone. Immunoblot analysis was performed to detect phospho-CREB (top) and CREB (bottom).
IL-3 or forskolin stimulated phosphorylation of CREB occurs independently of p38 MAPK and MAPKAP kinase-2. (A) MC/9 cells were treated with SB203580 (1 μmol/L) or vehicle alone for 20 minutes, followed by treatments with either 10 μg/mL synthetic IL-3, 50 μmol/L C2-ceramide, or both, for 5 minutes. Whole-cell lysates were separated on SDS-PAGE and immunoblot analysis was performed to detect phospho-CREB. (B) MC/9 cells were treated with 10 μg/mL synthetic IL-3 for the times indicated following pretreatment with SB203580 or vehicle alone for 20 minutes. Immunoblot analysis was performed to detect phospho-CREB (top) and CREB (bottom). (C) MC/9 cells were treated with 10 μg/mL IL-3 for the times indicated, following treatment with SB203580 (1 μmol/L) or vehicle alone for 20 minutes. Lysates were incubated with 1 μg anti-MAPKAP kinase-2 antibody and protein G-Sepharose beads, and kinase activity was determined from the washed immunoprecipitates by measuring the32P transferred to Hsp25. (D) Cells were treated with the indicated concentrations of forskolin for 10 minutes following a 20-minute treatment with SB203580 (1 μmol/L) or vehicle alone. Immunoblot analysis was performed to detect phospho-CREB (top) and CREB (bottom).
IL-3 was also shown to activate MAPKAP kinase-2 activity (Fig 6C), which was blocked in the presence of SB203580. This result shows that the inability of the inhibitor to block CREB phosphorylation was not due to activation of MAPKAP kinase-2 via a p38 MAPK-independent pathway, and confirms that the compound was able to block some IL-3–dependent responses. As expected, direct activation of PKA by elevation of cAMP using forskolin, a direct activator of adenylate cyclase, or addition of a cAMP analog, CPT-AMP (data not shown) also resulted in phosphorylation of CREB (Fig 6D). The cAMP-mediated activation was also independent of p38 MAPK (Fig 6D), since SB203580 had no effect on phosphorylation of CREB. It was also shown that cAMP elevation had no effect on activation of p38 MAPK (data not shown).
We also tested whether IL-3 may be mediating phosphorylation of CREB through cAMP elevation, thus bypassing the need for p38 MAPK activation by using a cAMP-dependent pathway. In experiments in which cells were treated with IL-3 or cAMP-elevating agents, we found that IL-3 did not significantly change the level of cAMP compared with unstimulated cells, whereas forskolin/IBMX induced a 35- to 40-fold increase of cAMP.
Ceramide and TNF-α effects on p38 MAPK and CREB phosphorylation.
We next examined a human monoblastic leukemia cell line, U937, in which TNF-α has also been shown to induce apoptosis (data not shown). TNF-α has been reported to generate ceramide in these cells as part of the signaling pathway downstream of its receptor.3Elevation of endogenous ceramide levels in U937 cells was achieved by treatment of cells with bacterial SMase, which catalyses the hydrolysis of sphingomyelin to ceramide. Sphingomyelinase and TNF-α treatment each induced rapid elevation of p38 MAPK activity and increased tyrosine phosphorylation of the enzyme (Fig7A). In addition, these treatments also activated MAPKAP kinase-2 (Fig 7B). Sphingomyelinase (Fig8) and TNF-α (Fig9A) also rapidly induced CREB and ATF-1 phosphorylation. Preincubation with SB203580 blocked the effects of ceramide and TNF-α on CREB and ATF-1, suggesting a p38 MAPK-dependent pathway leading to CREB and ATF-1 phosphorylation in response to both of these treatments. As expected, phosphorylation of CREB following direct activation of PKA by cAMP, generated following treatment of cells with forskolin, was unaffected by SB203580 treatment (Fig 9B). Therefore, in this cell system, a cytokine agonist known to elevate endogenous ceramide levels, as well as artificial elevation of ceramide levels, activated the same signaling pathways leading to CREB phosphorylation.
Sphingomyelinase and TNF- stimulate p38 MAPK and MAPKAP kinase-2 activity. (A) U937 cells were stimulated with 10 ng/mL TNF- (T) for 5 minutes or 100 mU/mL bacterial sphingomyelinase for the indicated duration. Detergent-solubilized cell lysates were incubated with -p38 MAPK and protein A-Sepharose beads. Activity (top) of the washed immunoprecipitates were determined by32P incorporation into a peptide corresponding to amino acids 1-96 of ATF-2. Immunoblot analysis was performed to detect phosphotyrosine (4G10; middle) and with -p38 MAPK (bottom) to confirm equal amounts of p38 MAPK in the immunoprecipitates. (B) U937 cells were preincubated with SB203580 (1 μmol/L) or vehicle alone for 20 minutes and then stimulated with either TNF- (10 ng/mL; T) or bacterial sphingomyelinase (100 mU/mL) for the indicated duration and detergent-solubilized. Lysates were incubated with 1 μg -MAPKAP kinase-2 antibody and protein G-Sepharose beads, and kinase activity was determined from the washed immunoprecipitates by measuring the32P transferred to Hsp25.
Sphingomyelinase and TNF- stimulate p38 MAPK and MAPKAP kinase-2 activity. (A) U937 cells were stimulated with 10 ng/mL TNF- (T) for 5 minutes or 100 mU/mL bacterial sphingomyelinase for the indicated duration. Detergent-solubilized cell lysates were incubated with -p38 MAPK and protein A-Sepharose beads. Activity (top) of the washed immunoprecipitates were determined by32P incorporation into a peptide corresponding to amino acids 1-96 of ATF-2. Immunoblot analysis was performed to detect phosphotyrosine (4G10; middle) and with -p38 MAPK (bottom) to confirm equal amounts of p38 MAPK in the immunoprecipitates. (B) U937 cells were preincubated with SB203580 (1 μmol/L) or vehicle alone for 20 minutes and then stimulated with either TNF- (10 ng/mL; T) or bacterial sphingomyelinase (100 mU/mL) for the indicated duration and detergent-solubilized. Lysates were incubated with 1 μg -MAPKAP kinase-2 antibody and protein G-Sepharose beads, and kinase activity was determined from the washed immunoprecipitates by measuring the32P transferred to Hsp25.
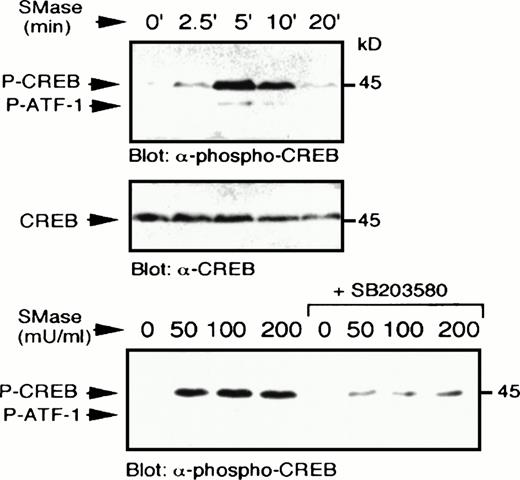
Sphingomyelinase stimulates p38 MAPK-dependent CREB phosphorylation. U937 cells were treated with bacterial sphingomyelinase (100 mU/mL) for the indicated times, lysed in sample buffer, and immunoblot analysis performed to detect phospho-CREB (top) and reprobed to detect CREB (middle). In a separate experiment, U937 cells were preincubated with SB203580 (1 μmol/L) for 20 minutes, which significantly blocked the ability of several concentrations of bacterial sphingomyelinase (50 to 200 mU/mL for 5 minutes) to stimulate CREB phosphorylation (bottom).
Sphingomyelinase stimulates p38 MAPK-dependent CREB phosphorylation. U937 cells were treated with bacterial sphingomyelinase (100 mU/mL) for the indicated times, lysed in sample buffer, and immunoblot analysis performed to detect phospho-CREB (top) and reprobed to detect CREB (middle). In a separate experiment, U937 cells were preincubated with SB203580 (1 μmol/L) for 20 minutes, which significantly blocked the ability of several concentrations of bacterial sphingomyelinase (50 to 200 mU/mL for 5 minutes) to stimulate CREB phosphorylation (bottom).
TNF- stimulates p38 MAPK-dependent CREB phosphorylation. (A) U937 cells were treated with SB203580 or vehicle for 20 minutes and then stimulated with TNF- (10 μg/mL) for the indicated times. Whole-cell lysates were separated by SDS-PAGE and immunoblot analysis to detect phospho-CREB (top) was performed and reprobed to detect CREB (bottom). (B) Cells were treated with SB203580 (1 μmol/L) or vehicle alone for 20 minutes and stimulated with either TNF- (10 μg/mL; T) for 5 minutes or forskolin (40 μmol/L) for 10 minutes.
TNF- stimulates p38 MAPK-dependent CREB phosphorylation. (A) U937 cells were treated with SB203580 or vehicle for 20 minutes and then stimulated with TNF- (10 μg/mL) for the indicated times. Whole-cell lysates were separated by SDS-PAGE and immunoblot analysis to detect phospho-CREB (top) was performed and reprobed to detect CREB (bottom). (B) Cells were treated with SB203580 (1 μmol/L) or vehicle alone for 20 minutes and stimulated with either TNF- (10 μg/mL; T) for 5 minutes or forskolin (40 μmol/L) for 10 minutes.
Effect of SB203580 on cell responses.
Ceramide-induced cell death in MC/9 was not effected by p38 MAPK inhibition, nor was the ability of IL-3 to suppress apoptosis (Fig10). In similar experiments with TNF-α on U937 cells, SB203580 again was unable to prevent apoptosis (data not shown).
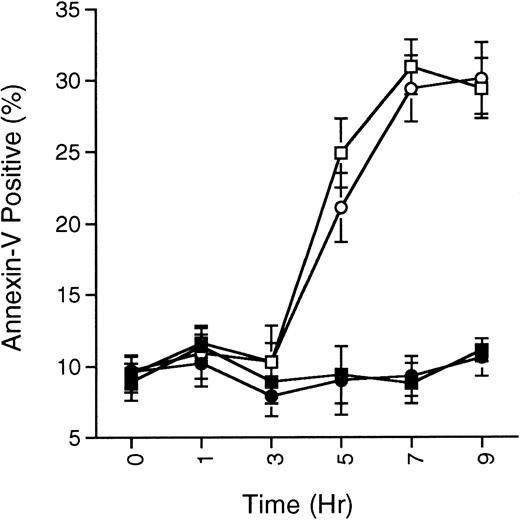
SB203580 does not prevent ceramide-induced apoptosis. MC/9 cells growing in IL-3 were treated with SB203580 (1 μmol/L; •) or vehicle (▪) for 15 minutes, and then treated with C2-ceramide (50 μmol/L; ○ and □, respectively). At the indicated times, cells were removed and the presence of phosphatidylserine on the cell surface was quantitated by annexin-V-FITC staining as described in the Methods. Cells staining positive for PI were not included in the analysis. The percentage of annexin-V-FITC–positive/PI-negative staining cells never surpassed 35%, although total cell death (annexin-V-FITC– and PI-positive cells) steadily climbed and reached about 60% by 9 hours in the C2-ceramide or C2-ceramide plus SB203580 treated cells. Representative experiment of 3 is shown, with each point being the mean of duplicate determinations, and with error bars representing the range.
SB203580 does not prevent ceramide-induced apoptosis. MC/9 cells growing in IL-3 were treated with SB203580 (1 μmol/L; •) or vehicle (▪) for 15 minutes, and then treated with C2-ceramide (50 μmol/L; ○ and □, respectively). At the indicated times, cells were removed and the presence of phosphatidylserine on the cell surface was quantitated by annexin-V-FITC staining as described in the Methods. Cells staining positive for PI were not included in the analysis. The percentage of annexin-V-FITC–positive/PI-negative staining cells never surpassed 35%, although total cell death (annexin-V-FITC– and PI-positive cells) steadily climbed and reached about 60% by 9 hours in the C2-ceramide or C2-ceramide plus SB203580 treated cells. Representative experiment of 3 is shown, with each point being the mean of duplicate determinations, and with error bars representing the range.
DISCUSSION
The study of signal transduction events important in both induction and inhibition of apoptosis in various cell types is currently of intense interest. It is clear that a better understanding of these events will help explain the regulation of cell numbers attributable to regulation of apoptosis. In the case of myeloid cells, these types of events have obvious importance in various blood disorders resulting in either a lack of or an overabundance of cells, as well as in inflammatory conditions in which the longevity of inflammatory cells may cause side effects that result from the persistence of normally short-lived cells.
In our studies, we focused on the signal transduction pathways involved in either induction or inhibition of apoptosis. In previous work, we showed that PI 3-kinase is an important enzyme in the pathways downstream of cytokines that inhibit apoptosis.17 However, that same study showed that one cytokine, granulocyte-macrophage colony-stimulating factor (GM-CSF), was able to bypass a need for PI 3-kinase, thereby inhibiting apoptosis even in the presence of PI 3-kinase inhibitors. We have speculated that GM-CSF (and other cytokines whose antiapoptosis effect is not affected by PI 3-kinase inhibitors) can activate a signaling pathway that is independent of PI 3-kinase. Recent studies have shown the importance of PKB/akt as a downstream kinase activated by PI 3-kinase that is important for inhibition of apoptosis in several systems.20 21 The PI 3-kinase–independent inhibition of apoptosis could therefore be mediated by activation of a kinase that phosphorylates the same target(s) as PKB/akt. The search for alternate pathways that inhibit apoptosis led us to the finding that cAMP elevation in our model systems could inhibit apoptosis as determined by three different criteria: DNA fragmentation (Fig 1), PARP cleavage by caspases (Fig 2), and phosphatidylserine exposure on the plasma membrane (Fig 3), thereby implicating cAMP-dependent protein kinase (PKA) as one possible element in a pathway that is blocking apoptosis.
In contrast, it is also important to delineate the signaling pathways used downstream of potential mediators of apoptosis such as ceramide. We have demonstrated in this study that elevation of intracellular ceramide can lead to activation of p38 MAPK, although as discussed later, we have shown that blocking p38 MAPK does not block the ability of ceramide to induce apoptosis. In addition, treatment of cells with TNF- α or IL-3 under conditions where they induce or inhibit apoptosis, respectively, also activates the same stress-activated kinase. Furthermore, in the case of ceramide or TNF-α , but not IL-3, activity of p38 MAPK was required for subsequent phosphorylation of potentially important nuclear targets, the CREB and ATF-1 transcription factors. Phosphorylation of CREB and ATF-1 on Ser 133 and Ser 63, respectively, was also observed in response to elevated cAMP in both MC/9 and U937 cells, as expected. Our results with TNF-α and ceramide are consistent with the report implicating p38 MAPK and MAPKAP kinase-2 upstream of CREB and ATF-1 phosphorylation, with MAPKAP kinase-2 having been identified as the most likely CREB kinase in response to FGF.27
Consistent with the study of Tan et al,27 we also found the CREB and ATF-1 phosphorylation in response to TNF-α or SMase treatment was sensitive to a selective inhibitor of p38 MAPK, SB203580. The cAMP-mediated phosphorylation of CREB was insensitive to the p38 MAPK inhibitor and cAMP had no effect on p38 MAPK activity. The inhibitor was also unable to block IL-3–mediated CREB phosphorylation, even though it did block IL-3–mediated p38 MAPK and MAPKAP kinase-2 activation, so it is clear that the ability of IL-3 to stimulate CREB and ATF-1 phosphorylation functions independently of the p38 MAPK pathway. We found also that IL-3 could not induce elevation of cAMP at multiple time points after IL-3 treatment, thereby showing that CREB phosphorylation can be mediated by at least three different signaling pathways in myeloid cells, one via p38 MAPK and MAPKAP kinase-2, one via cAMP-dependent protein kinase, and a third unidentified pathway in response to IL-3 stimulation. In the case of EGF treatment of PC12 cells, it was recently shown that the CREB kinase was rsk-2,30 which is activated downstream of erk’s. Because IL-3 activates both erk and p38 MAPK pathways, it is likely that IL-3 may be working preferentially via the erk pathway to stimulate CREB phosphorylation. However, the question of why blocking p38 MAPK has little or no effect on IL-3–mediated CREB and ATF-1 phosphorylation is intriguing.
In contrast to our results, a recent study showed that in endothelial cells, TNF-α activated p38 MAPK, while treatment of the cells with SMase to generate endogenous ceramide was not able to mimic this effect, thereby suggesting that only some actions of TNF-α are ceramide-dependent.31 Our results in hematopoietic cells contrast with those in the latter study, since the activation of p38 MAPK by TNF-α correlates with the ability of ceramide to activate this enzyme in U937 cells. There may be multiple pathways of varying importance leading to activation of the p38 MAPK and SAPK pathways, which could vary among different cell types in response to different agonists.
One question that arises from these studies is whether activation of p38 MAPK in a ceramide-dependent fashion is involved in pathways leading to apoptosis. Treatment of the cells used in this study with C2-ceramide, SMase, or TNF-α can lead to apoptosis. Invariably, addition of concentrations of SB203580 that blocked p38 MAPK activity, did not block apoptosis (Fig 10). In addition, the observation that a growth-promoting cytokine, IL-3, also induces p38 MAPK activation (this study)26 also supports the suggestion that p38 MAPK activation is not involved in pathways leading to apoptosis. Furthermore, addition of SB203580 had no effect on the ability of IL-3 to stimulate growth and survival of MC/9 cells. Together, these results suggest that p38 MAPK activation does not play an active part in the execution of apoptosis, although one must also consider that other, parallel pathways may be operating under the conditions tested. It is possible that p38 MAPK activation could play a role in apoptosis in the case of ceramide and TNF-α, but when inhibited by SB203580, apoptosis could still occur as a result of the activation of other pathways. Likewise, prosurvival pathways activated downstream of growth-promoting agonists may overcome the proapoptotic function of p38 MAPK. For example, PI 3-kinase activation has been demonstrated to provide antiapoptotic signaling, and activation of PI 3-kinase by IL-3 may nullify the proapoptotic activity of p38 MAPK. This would be consistent with the finding of Berra et al, who showed that in the absence of serum-stimulated PI3K activity, p38 MAPK inhibition led to increased apoptosis.32 Activation of p38 MAPK may normally be a redundant event with respect to both apoptosis (via ceramide) and survival (via IL-3), but it could play a role under very specialized conditions.
A role for another stress-activated kinase, SAPK, in mediating apoptosis has been demonstrated recently in U937 cells and bovine aortic endothelial cells.12 However, in a different model system examining apoptosis of murine thymocytes, the upstream activator of SAPK, SEK1, was found to protect from apoptosis.33 It is interesting that these studies suggest that the SAPK pathway can have opposite effects in myeloid versus lymphoid cells. We have also observed an opposite effect of cAMP in myeloid compared with lymphoid cells, since we find that in the former cells, cAMP can inhibit apoptosis, while others have shown that in lymphoid cells it can promote apoptosis.13 14
The role of SAPK activation in response to TNF receptor 1 (p55) activation was also dissociated from effects on apoptosis in a recent study.34 These studies were performed by a cotransfection assay of various forms of the receptor and the appropriate signaling molecules in MCF-7 cells. The ability of TNF to induce apoptosis was found to require the signal transducer FADD binding to the receptor death domain, independently of SAPK activation. Another recent study identified a novel protein, FAN, that couples the TNF receptor to neutral sphingomyelinase, using a discrete domain of the receptor that is distinct from the death domain.35 However, activation of acid SMase requires a domain of the TNF receptor that at least partially overlaps with the death domain of the receptor.36 Other recent studies have also shown a clear requirement for acid SMase in induction of apoptosis, because loss of the gene for acid SMase results in defective radiation-induced apoptosis and a normal response is reconstituted by transfection of acid SMase cDNA.37 At present, the potential linkage between ceramide generation in response to TNF and induction of apoptosis remains unresolved. However, we can postulate that activation of the p38 MAPK by TNF may be dependent on ceramide production, either via acid or neutral SMase activation.
In summary, we have demonstrated that ceramide, as well as TNF-α and cytokines such as IL-3, can all activate p38 MAPK and the downstream kinase, MAPKAP kinase-2. Each of these treatments led to phosphorylation of CREB and ATF-1 at activating sites, but IL-3–mediated CREB phosphorylation, unlike the other agents, was not affected by a p38 MAPK inhibitor. Not unexpectedly, cAMP also stimulated CREB and ATF-1 phosphorylation, and this was not affected by the p38 MAPK inhibitor. Since cAMP and IL-3 are shown to mediate cell survival, while TNF-α and ceramide cause apoptosis in the myeloid cells we have been studying, we can conclude that the phosphorylation of CREB and ATF-1 alone are not able to explain the biologic effects of cAMP and ceramide. However, we cannot rule out a role for CREB or ATF-1 transcriptional activity, since it is now clear that phosphorylation of these factors is not sufficient for their activity, and association with CREB binding protein (CBP) family members will determine the ultimate response. In addition, phosphorylation of the transcription factors at other sites could contribute to regulation of their activity. Future experiments will have to address the effect of the second messengers in controlling CRE-driven gene expression and in inducing phosphorylation of CREB and ATF-1 at other sites, to determine whether opposite effects can be observed at that level of regulation. A role for the stress-activated kinase, p38 MAPK, in inducing apoptosis might be proposed based on the fact that this enzyme is activated in response to ceramide and other stresses known to induce apoptosis, including TNF-α treatment. However, the fact that the selective inhibitor of p38 MAPK has neither a positive or negative effect on apoptosis, coupled with the fact that numerous growth-promoting cytokines can also activate the enzyme, argues against a specific role for p38 MAPK as an inducer of apoptosis.
Supported by grants from the Medical Research Council (MRC) of Canada and the British Columbia (BC) Health Research Foundation. M.P.S. was supported by a Cancer Research Society studentship and I.N.F. was supported by a Medical Research Council of Canada studentship. V.D. was the recipient of a MRC Canada/BC Lung Association Scholarship.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Vincent Duronio, PhD, Department of Medicine, University of British Columbia, The Jack Bell Research Centre, 2660 Oak St, Vancouver, BC, V6H 3Z6 Canada.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal