Abstract
We studied integrins involved in the adhesion of resting and activated megakaryocytes (MK) to fibronectin (FN) and fibrinogen (FGN). Guinea pig MK were isolated and in some experiments were activated by thrombin. MK adhering to FN or FGN coated on coverslips were quantitated by a computerized image analysis program. The binding of soluble human FN to MK was detected by Western blotting. Anti-integrin antibodies, disintegrins, and cyclic RGD peptides were used to identify integrins involved in the adhesion of MK to FN or FGN. Resting MK adhered to coverslips with immobilized FN. The adhesion of MK to FN was primarily inhibited by an anti-5 antibody and EMF-10, a distintegrin highly specific for 5β1. However, the adhesion of MK to FN was not blocked by agents that inhibit ΙΙbβ3, vβ3 or 4β1. A β1 activating antibody increased the number of MK bound to FN due to the activation of 5β1. The binding of soluble FN was also primarily inhibited by agents that block 5β1. Resting MK did not adhere to FGN. However, MK activated by thrombin did adhere to FGN. This binding was mediated by ΙΙbβ3, because binding was inhibited by bitistatin, a disintegrin, and a cyclic RGD peptide that are known to block this integrin. The binding of thrombin-activated MK to FN was mediated by both 5β1 and ΙΙbβ3 based on the additive effect of agents that inhibit these integrins. The study indicates that resting MK bind to FN but not to FGN and that 5β1 is the major integrin involved in the binding of MK to FN. Activated MK bind to FGN primarily by IIbβ3. However, the binding of activated MK to FN is due to both 5β1 and IIbβ3. The demonstration that 5β1 and that IIbβ3 are involved in MK adhesion indicates that these integrins may have a role in MK maturation and platelet production.
© 1998 by The American Society of Hematology.
FIBRONECTIN (FN) IS A major component of bone marrow extracellular matrix and is considered to have an important role in hematopoiesis.1,2 Hematopoietic cells are found in areas of bone marrow rich with FN,3 and FN forms septa in bone marrow that may provide anchorage for migrating hematopoietic progenitor cells.4 The attachment of blood cell precursors to FN may modulate the migration and homing of cells to specific bone marrow regions that promote maturation of progenitors and the release of mature blood cells into the circulation.5
A role for FN in megakaryocyte (MK) maturation and platelet production is suggested by the expression of FN in rat fetal liver MK before its expression in fetal hepatocytes.6 We have shown that guinea pig MK can synthesize FN,7 and a previous study has demonstrated FN mRNA in MK.8 The level of endogenous MK FN is 7.5-fold greater than FN in other bone marrow hematopoietic cells. In response to thrombin, FN is secreted and adheres to the surface of MK.7
The purpose of the current study was to identify integrins involved in the binding of FN on the MK surface. α4β1 and α5β1 are the major integrins involved in the binding of FN in hematopoietic cells.2,9,10 αΙΙbβ3 primarily reacts with fibrinogen (FGN) but can also bind FN.11 However, the activation state of these integrins, which determines their ability to bind ligands, can change during cell maturation. There is also evidence that the level of expression of α4β1 and α5β1 regulates the differentiation of erythrocytic and myelocytic precursors.2,9,10 Among the integrins known to bind FN, α5β1, αΙΙbβ3, α4β1, and αvβ3 have been shown to be expressed and to be functional in MK.12-14
We now report information about the binding of resting and activated MK to FN and FGN. We found that resting MK bind to FN but not to FGN and that α5β1 is the major integrin mediating the binding of MK to FN. In contrast, activated MK bind to FGN primarily through αΙΙbβ3 and to FN through both α5β1 and αΙΙbβ3. αvβ3 and α4β1 do not seem to be involved in the binding of isolated recognizable MK to FN or FGN.
MATERIALS AND METHODS
Antibodies, cyclic peptides, and disintegrins.
SAM-1, an α5 blocking antibody (Immunotech, Westbrook, ME); HP2/1, an α4 blocking antibody (Immunotech); LM609, an αvβ3 blocking antibody (Chemicon International, Temecula, CA), monoclonal antibody (MoAb) IST-4 (Sigma, St Louis, MO), which detects human FN; and anti–α-actinin antisera (Sigma) and anti-FN EIIIB antibody prepared by Dr Vickie Bennet,7 which cross-reacts with guinea pig FN, were used. MoAb 8A2 was kindly donated by Dr Nicholas Kovach.15 Whole mouse IgG and isotype-specific IgG were used as controls for the blocking and enhancing antibodies and did not have any effect. All antibodies that were used were antihuman. MK0852, a cyclic RGD peptide, and bitistatin, a disintegrin, were used to demonstrate the activity of αΙΙbβ3. MK0852 was kindly donated by Dr Robert Gould (Merck & Co, Inc, West Point, PA).16,17 Bitistatin was isolated from the venom of Bitis arietans.18Bitistatin selectively inhibits the activity of αΙΙbβ3, because it inhibits ADP-induced human platelet aggregation with an IC50 of 139 nmol/L and FGN binding with an IC50 of 150 nmol/L. However, it does not inhibit α5β1 binding to FN.18 19
EMF10 was used to demonstrate the activity of α5β1. EMF10 is a novel disintegrin isolated from Eristocophis macmahonivenom. It is a heterodimer composed of two subunits A and B that are structurally almost identical to eristocophin I and II, the sequence of which has been previously reported by Siddiqi et al.20 EMF-10 was isolated by reverse-phase high-performance liquid chromatography (HPLC) using a Vydac C18 column and eluted at 41% acetonitrile gradient. EMF10 has been characterized and the molecular mass of this protein, determined by mass spectrometry, was 14,974 Daltons (Marcinkiewicz et al, manuscript in preparation). EMF10 can inhibit the activity of α5β1 and αΙΙbβ3, but α5β1 activity is considerably more sensitive to this agent than is αΙΙbβ3, because inhibition of platelet aggregation required 1,600 nmol/L EMF10. The inhibitory effects of EMF10 on cell adhesion to various ligands were determined in solid phase assays, as shown in Table 1.
Effect of EMF10 on the Adhesion of Cells Expressing Various Integrins to Immobilized Ligands
| Integrin . | Ligand . | IC50 EMF10 . |
|---|---|---|
| αIIbβ3 | Fibrinogen | 500 nmol/L |
| αvβ3 | Vitronectin | >1,000 nmol/L |
| α4β1 | VCAM-1 | >1,000 nmol/L |
| α5β1 | Fibronectin | 20 nmol/L |
| Integrin . | Ligand . | IC50 EMF10 . |
|---|---|---|
| αIIbβ3 | Fibrinogen | 500 nmol/L |
| αvβ3 | Vitronectin | >1,000 nmol/L |
| α4β1 | VCAM-1 | >1,000 nmol/L |
| α5β1 | Fibronectin | 20 nmol/L |
Cultured cells labeled with CMFDA were incubated with ligands immobilized on microplates with various concentrations of EMF10. Cells attached to the plate were lysed by detergent and fluorescence intensity was measured using a microtiter plate reader as previously described.34 CHO cells transfected with αIIbβ3 and with αvβ3 were kindly provided by Dr Mark Ginsberg (Scripps Research Institute, La Jolla, CA). Jurkat cells expressing predominantly α4β1 and K562 cells expressing predominantly α5β1 were purchased from ATCC (Rockville, MD). Recombinant VCAM-1 was a generous gift from Dr Mark Renz (Genentech, San Francisco, CA).
Disintegrins and cyclic peptides can be used to identify integrins involved in binding of FN and other ligands, but higher concentrations of these agents can inhibit several integrins. Antibodies are more specific for integrin subunits, but some anti-integrin antibodies do not cross-react with guinea pig tissues. However, disintegrins and cyclic peptides can be used to inhibit and study integrins in guinea pig MK.
Source of MK.
Guinea pig MK were isolated to approximately 90% purity by cell size and greater than 98% purity by protein content, because MK are considerably larger than other bone marrow cells, as previously described.21 Approximately 1 × 106 cells are isolated by this procedure and their viability is approximately 89%.
Adhesion assays.
In preparation for the adhesion assays, FN- and FGN-coated glass coverslips were prepared by coating the coverslips with a 1% solution of gelatin (Bio-Rad, Melville, NY) for 2 hours at 37°C and then with 50 μg/mL bovine plasma FN (Sigma) or 250 μg/mL purified human plasma FGN (kindly provided by Dr Jose Martinez) overnight at 4°C.
MK were pretreated with varying amounts of antibodies, disintegrins, or peptides in Dulbecco’s phosphate-buffered saline (DPBS) containing Ca2+ and Mg2+ (GIBCO BRL, Grand Island, NY) for 10 minutes at room temperature (RT) before aliquots were placed on the coverslips. Control samples were pretreated with whole IgG or isotype-specific IgG (5 to 20 μg/mL). In some experiments after this 10 minutes of incubation, MK were activated with thrombin (0.5 U/mL) for 3 minutes at RT, followed by the addition of hirudin (0.5 U/mL; Sigma) to stop thrombin activity.
Aliquots (100 μL) containing 1 to 2 × 104 MK were overlayed in triplicate onto FN- or FGN-coated coverslips and incubated at 37°C for 30 minutes. After incubation, the coverslips were rinsed twice with DPBS, followed by fixation with 3.7% formaldehyde in DPBS for 20 minutes at room temperature. After washing, the cells on the coverslips were stained with LeukoStat Solution (Fisher, Pittsburgh, PA), and cell adhesion was quantitated using computerized image analysis (NIH Image 1.60 software; National Institutes of Health, Bethesda, MD).
Immunostaining and confocal microscopy.
MK adhering to FN- or polylysine-coated coverslips were fixed with 3.7% formaldehyde and permeabilized with 0.25 % Triton X-100 for 10 minutes at RT. They were then washed and incubated with goat serum to block nonspecific binding sites. To detect cytoskeletal changes, we used anti-α actinin polyclonal antibody (Sigma) as the primary antibody; to detect human FN, an MoAb, IST-4 (Sigma), and to detect endogenous FN in guinea pig MK, a polyclonal antibody specfic for FN EIIIB.7 The second antibody was goat antirabbit or antimouse IgG-fluorescein isothiocyanate (FITC; Cappel, Durham NC). The specimens were visualized with a Bio-Rad MRC-600 krypton-argon laser confocal microscope (Bio-Rad) adapted to a Zeiss Axiovert 100 inverted microscope equipped with a Zeiss 63XPlan Apo (NA 1.4) lens (Zeiss, Thornwood, NJ).
Binding of soluble FN to MK.
MK were incubated with human plasma FN (40 μg/mL; GIBCO BRL) at 37°C for 2 hours in the presence or absence of anti-integrin antibodies, peptides, or disintegrins. The MK were then washed, lysed, and prepared for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as previously described.7Proteins were transferred to polyvinylidene flouride (PVDF) membrane, and an antihuman FN antibody (IST-4) that does not cross-react with guinea pig FN was used to detect the FN. Bands were visualized by enhanced chemiluminescence (NEN Life Sciences, Boston, MA) and quantitated by densitometry as previously described.7
RESULTS
Unstimulated guinea pig MK adhered to FN coated over gelatin onto glass coverslips. The adherence was maximal in the presence of both calcium (1.0 mmol/L) and magnesium (0.5 mmol/L) and was blocked by EDTA (10 mmol/L). MK did not adhere to gelatin-coated coverslips.
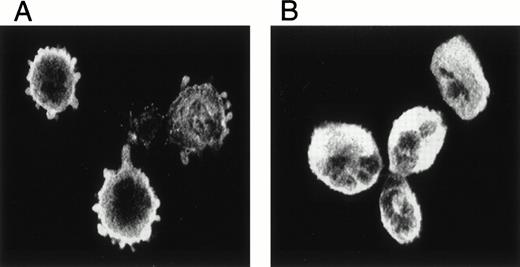
The adhesion of MK to FN-coated coverslips resulted in changes in morphology most likely representing cytoskeletal reorganization. Figure 1 shows that unstimulated MK adhering to FN put out blebs or pseudopods along their entire circumference in contact with the FN demonstrated by the immunostaining of α-actinin and visualization by confocal microscopy. These changes were not seen with the adhesion of MK to polylysine, because MK retained their round shape under these conditions. Thus, these changes were specific to MK adhering to FN.
The adherence of MK to FN. Isolated guinea pig MK were allowed to settle on FN-coated coverslips, immunostained with anti-actinin antibody, and visualized by confocal microscopy. MK attached to polylysine served as a control. MK attached to FN (A) and to polylysine (B).
The adherence of MK to FN. Isolated guinea pig MK were allowed to settle on FN-coated coverslips, immunostained with anti-actinin antibody, and visualized by confocal microscopy. MK attached to polylysine served as a control. MK attached to FN (A) and to polylysine (B).
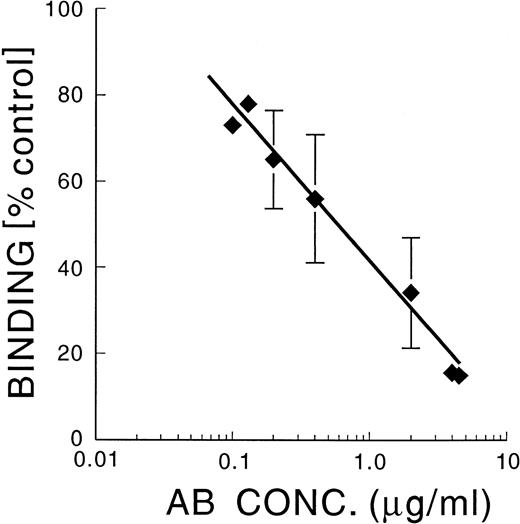
An α5-blocking antibody, SAM-1, inhibited MK adherence on FN in a dose-dependent manner, with an IC50 of 0.6 μg/mL (Fig 2), whereas an α4 blocking antibody, HP2/1, and an αvβ3 blocking antibody, LM609, did not inhibit adhesion at 20 μg/mL (data not shown). Both HP2/1 and LM609 have previously been shown to cross-react with integrins in guinea pig tissues.22,23 We found by flow cytometry that 85% of guinea pig MK stained positively with each of these antibodies (data not shown). This information is similar to the finding that greater than 90% of isolated human MK express α4β1.13 α4β1 was also found to be expressed in human MK grown in culture, but there was less α4β1 in mature than in immature MK.12 Thus, α4β1, α5β1, and αvβ3 are expressed in mature guinea pig MK. Also, HP2/1 (5 μg/mL) caused 83% ± 9% inhibition of the binding of guinea pig MK to vascular cell adhesion molecule (V-CAM), and LM609 (5 μg/mL) caused 61% ± 1% inhibition of binding to vitronectin (mean ± SD; n = 3), indicating that these antibodies are functional against guinea pig integrins.
SAM-1: Inhibition of binding of MK to FN. Guinea pig MK were incubated with varying concentrations of SAM-1 for 10 minutes at RT, The MK were then allowed to adhere to FN-coated coverslips for 30 minutes at 37°C. Adherent cells were fixed, stained, and enumerated by computerized image analysis.
SAM-1: Inhibition of binding of MK to FN. Guinea pig MK were incubated with varying concentrations of SAM-1 for 10 minutes at RT, The MK were then allowed to adhere to FN-coated coverslips for 30 minutes at 37°C. Adherent cells were fixed, stained, and enumerated by computerized image analysis.
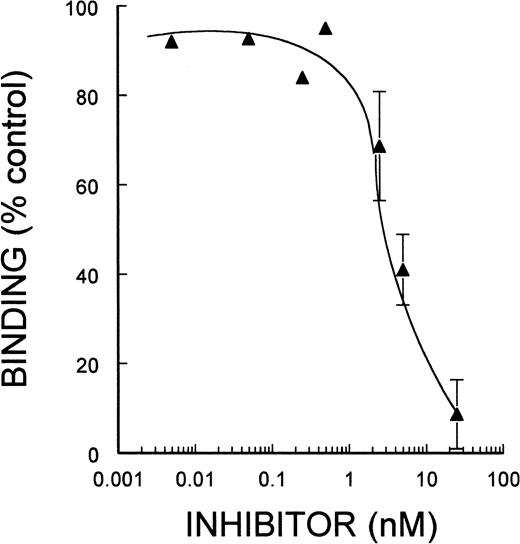
To confirm that α5β1 mediated the adherence of MK to immobilized FN, we tested a newly characterized disintegrin, EMF10. As shown in Fig 3, EMF10 inhibited MK adherence to FN in a dose-dependent manner, with an IC50 of 4.5 nmol/L, which was very similar to its ability to inhibit α5β1-mediated binding of K562 cells to FN, as shown in Table 1. Inhibition of adhesion at this concencentration of EMF10 is not likely due to an effect on αΙΙbβ3, because much higher concentrations are needed to inhibit αΙΙbβ3 mediated binding to FGN, as shown in Table 1.
EMF-10: Inhibition of binding of MK to FN. Guinea pig MK were incubated with varying concentrations of EMF-10. The experimental conditions and the analysis of the data are described in the legend to Fig 2.
EMF-10: Inhibition of binding of MK to FN. Guinea pig MK were incubated with varying concentrations of EMF-10. The experimental conditions and the analysis of the data are described in the legend to Fig 2.
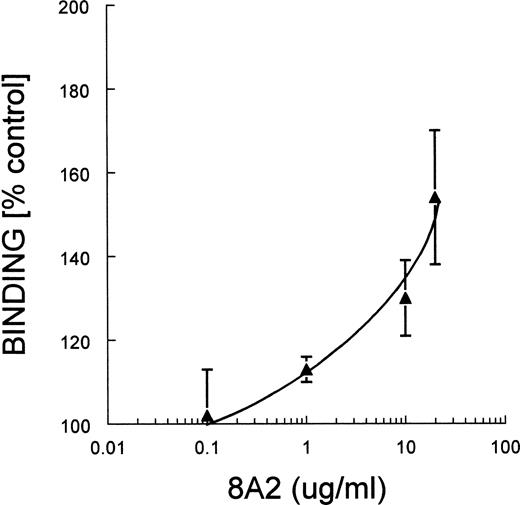
MoAb 8A2, which activates integrins containing the β1 subunit, enhanced the binding of MK to FN, and the binding was concentration dependent, as shown in Fig 4.
MoAb 8A2: Enhancement of binding of MK to FN. Guinea pig MK were incubated with varying concentrations of MoAb 8A2. The experimental conditions and the analysis of the data are described in the legend to Fig 2.
MoAb 8A2: Enhancement of binding of MK to FN. Guinea pig MK were incubated with varying concentrations of MoAb 8A2. The experimental conditions and the analysis of the data are described in the legend to Fig 2.
SAM-1 (5 μg/mL) inhibited the MoAb 8A2-enhanced binding of MK to FN by 62% ± 6% (mean ± SD), whereas HP2/1 (20 μg/mL) alone or in combination with SAM-1 had no effect (n = 3).
HP2/1 (5 μg/mL) did block the MoAb 8A2-enhanced binding of MK to V-CAM by 80% (data not shown). This information indicates that α5β1 was the primary integrin involved in the MoAb 8A2-augmented binding of MK to FN.
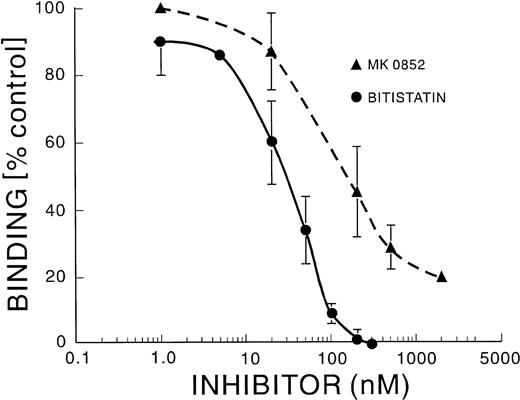
To obtain specific information about the role of αΙΙbβ3, we used bitistatin, a disintegrin, and MK0852, a cyclic RGD peptide. The IC50 for inhibition of MK adherence to FN was greater than 1 μmol/L for bitistatin and greater than 5 μmol/L for MK0852 (data not shown). Thus, αΙΙbβ3 did not appear to mediate the adhesion of resting MK to FN. These agents can inhibit the activity of guinea pig αΙΙbβ3, because we found that they inhibit ADP- and collagen-induced aggregation of guinea pig platelets with an IC50 of 60 and 80 nmol/L, respectively (data not shown).
Thus, α5β1 but not α4β1, αvβ3, or αΙΙbβ3 mediates the adherence of unstimulated MK to immobilized FN.
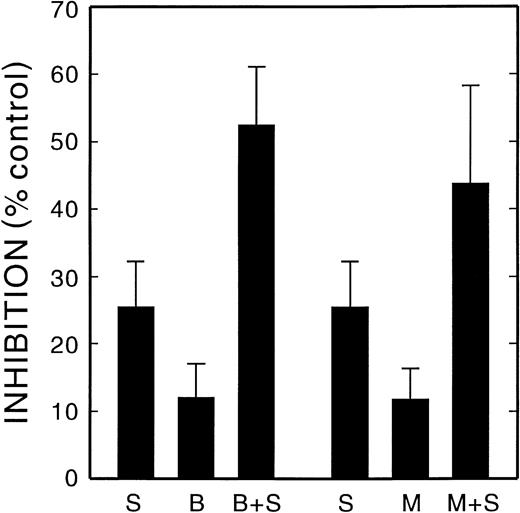
MK did not adhere to immobilized FGN unless they were first activated with thrombin. Bitistatin and MK0852 inhibited the adherence of activated MK to FGN, with an IC50 of 30 and 200 nmol/L, respectively, as shown in Fig 5. The anti-α4 antibody, HP2/1, did not inhibit binding (data not shown). The anti-α5 antibody, SAM-1, did not inhibit binding, and SAM-1 in combination with bitistatin or MK0852 did not cause a greater degree of inhibition over that caused by bitistatin or MK0852 alone (data not shown). Therefore, αΙΙbβ3 mediates the binding of activated MK to FGN, as is true for platelets. However, when thrombin-activated MK were allowed to adhere to FN, concentrations of bitistatin (50 nmol/L) and MK0852 (100 nmol/L) near their IC50 for FGN binding only inhibited FN binding by about 12% (Fig 6). The α5-blocking antibody, SAM-1, at a concentration above its IC50 for inhibiting FN binding by unstimulated MK (2 μg/mL), also showed much less inhibition of activated MK (24%; Fig 6). However, when activated MK were pretreated with either SAM-1 plus bitistatin or SAM-1 plus MK0852, the inhibition of adherence to FN was found to be additive (Fig 6). The binding of activated MK to FN was not blocked by HP2/1 or LM609 either alone or in combination with SAM-1 or bitistatin. A previous study demonstrated that the adherence of thrombopoietin (TPO)-activated MK to FN was mediated by αΙΙbβ3 and to a lesser extent by α5β1 but did not determine whether the effects of inhibiting these integrins was additive.24 These results demonstrate that thrombin-activated MK bind to FN through both α5β1 and αΙΙbβ3, in contrast to resting MK, which use only the α5β1 integrin.
Inhibition of the binding of activated MK to FGN. Guinea pig MK were activated by incubation with thrombin (0.5 U/mL) for 3 minutes at RT in the presence of varying concentrations of MK0852 or bitistatin. This was followed by the addition of hirudin (0.5 U/mL) to stop the reaction. Adhesion to FGN-coated coverslips and the analysis of the data were performed as described in the legend to Fig 2.
Inhibition of the binding of activated MK to FGN. Guinea pig MK were activated by incubation with thrombin (0.5 U/mL) for 3 minutes at RT in the presence of varying concentrations of MK0852 or bitistatin. This was followed by the addition of hirudin (0.5 U/mL) to stop the reaction. Adhesion to FGN-coated coverslips and the analysis of the data were performed as described in the legend to Fig 2.
Inhibition of the binding of activated MK to FN. Guinea pig MK were activated with thrombin as described in the legend to Fig 5in the presence of varying concentrations of SAM-1 (S), Bitistatin (B), MK0852 (M), or combinations of these agents. Adhesion to FN-coated coverslips and the data analysis are described in the legend to Fig2.
Inhibition of the binding of activated MK to FN. Guinea pig MK were activated with thrombin as described in the legend to Fig 5in the presence of varying concentrations of SAM-1 (S), Bitistatin (B), MK0852 (M), or combinations of these agents. Adhesion to FN-coated coverslips and the data analysis are described in the legend to Fig2.
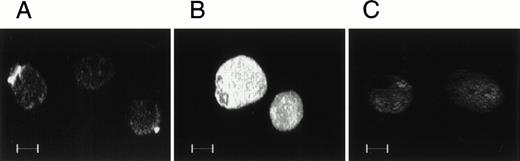
To determine whether unstimulated MK would bind soluble FN with the same integrin that they use to adhere to immobilized FN, we incubated guinea pig MK with human plasma FN in solution. Figure 7A demonstrates that soluble human plasma FN can bind to the surface of guinea pig MK and that endogenous guinea pig FN was not detected by the antihuman FN antibody. Endogenous FN in guinea pig MK was detected with an antibody that we had previously shown to react with FN in this species, as shown in Fig 7B. Endogenous FN was distributed throughout the MK cytoplasm. Control MK incubated with nonimmune normal IgG are shown in Fig 7C.
The binding of soluble human FN to MK. Guinea pig MK were incubated with human plasma FN in solution at 37°C for 2 hours. The MK were then allowed to settle on FN-coated coverslips immunostained with antihuman FN antibody, IST-4, which does not cross-react with guinea pig FN, or an anti-FN EIIIb antibody, which cross-reacts with guinea pig FN. After incubation with an FITC-conjugated second antibody, immunostained MK were visualized by confocal microscopy. The binding of soluble human FN to guinea pig MK was limited to patches on the MK surface as shown in (A). (B) shows endogenous guinea pig FN throughout the MK cytoplasm. (C) is a control in which MK were incubated with nonimmune normal IgG.
The binding of soluble human FN to MK. Guinea pig MK were incubated with human plasma FN in solution at 37°C for 2 hours. The MK were then allowed to settle on FN-coated coverslips immunostained with antihuman FN antibody, IST-4, which does not cross-react with guinea pig FN, or an anti-FN EIIIb antibody, which cross-reacts with guinea pig FN. After incubation with an FITC-conjugated second antibody, immunostained MK were visualized by confocal microscopy. The binding of soluble human FN to guinea pig MK was limited to patches on the MK surface as shown in (A). (B) shows endogenous guinea pig FN throughout the MK cytoplasm. (C) is a control in which MK were incubated with nonimmune normal IgG.
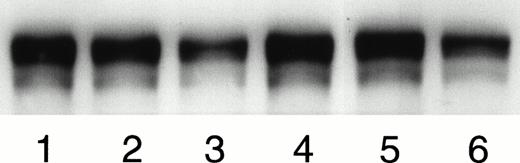
To study the inhibition of binding of soluble FN, MK were incubated with human FN in the presence and absence of integrin blocking agents. After the incubation of MK with human FN, immunoprecipitated FN that had bound to the MK surface was analyzed by Western blotting using the antihuman FN antibody. Figure 8 shows a representative of four experiments in which MK were incubated with MK0852 (lane 1), bitistatin (lane 2), EMF10 (lane 3), control without integrin blockers (lane 4), HP2/1 (lane 5), and SAM-1 (lane 6). Concentrations of these agents are noted in the figure legend. Only SAM-1 and EMF10 significantly inhibited the binding of soluble FN to unstimulated MK, indicating that the α5β1 integrin mediates MK binding of both immobilized and soluble FN. Densitometric analysis showed that EMF10 caused a 55% ± 11.9% and SAM-1 caused a 52% ± 5.2% (mean ± SD of 3 experiments) inhibition of binding compared with the control. However, the densitometric analysis of the MK0852, bitistatin, and HP2/1 bands did not differ significantly from the control. Thus, both the binding of soluble FN to MK and the binding of MK to immobilized FN are mediated primarily by α5β1.
The demonstration of the inhibition of the binding of soluble human FN to MK by Western blotting: Guinea pig MK were incubated with human plasma FN in solution at 37°C for 2 hours. FN bound to the MK surface was extracted, separated by SDS-PAGE, and visualized by ECL on Western blots with an antibody specific to human FN, IST-4. The figure shows a representative of four experiments in which MK were incubated with MK0852 (10 μmol/L; lane 1), bitistatin (2 μmol/L; lane 2), EMF10 (50 nmol/L; lane 3), control without integrin blockers (lane 4), HP2/1 (10μg/mL; lane 5), and SAM-1 (10 μg/mL; lane 6). The densitometric analysis of the three experiments is given in the Results.
The demonstration of the inhibition of the binding of soluble human FN to MK by Western blotting: Guinea pig MK were incubated with human plasma FN in solution at 37°C for 2 hours. FN bound to the MK surface was extracted, separated by SDS-PAGE, and visualized by ECL on Western blots with an antibody specific to human FN, IST-4. The figure shows a representative of four experiments in which MK were incubated with MK0852 (10 μmol/L; lane 1), bitistatin (2 μmol/L; lane 2), EMF10 (50 nmol/L; lane 3), control without integrin blockers (lane 4), HP2/1 (10μg/mL; lane 5), and SAM-1 (10 μg/mL; lane 6). The densitometric analysis of the three experiments is given in the Results.
DISCUSSION
The study demonstrated that α5β1 is primarily responsible for the adherence of resting MK to FN based on the inhibition of binding by SAM-1, an MoAb, and EMF10, a disintegrin, at concentrations that have been shown to block the activity of this integrin in other cells. The binding of MK to FN induced the formation of pseudopods that contained α-actinin, consistent with the ability of ligand-interactions to induce cytoskeletal changes. The binding of resting MK to FN was not mediated by αΙΙbβ3, because neither bitistatin nor MK0852 inhibited the binding at concentrations of 1 and 10 μmol/L, respectively. These concentrations are considerably higher than those shown to block binding to these integrins in other cells.16-19 MoAbs that block α4 activity, HP2/1, or αvβ3 activity, LM609, did not affect the adhesion of MK to FN, suggesting that neither integrin is involved.
The role of α5β1 in the binding of MK to FN is supported by the demonstration that MoAb 8A2 markedly increased the number of MK that were attached to FN, and this binding was only inhibited by the α5 blocking antibody. MoAb 8A2 has been shown to bind to the β1 subunit of integrins, and the antibody amplifies integrin activity.15 25 Therefore, although α5β1 is in an active state in resting MK, its activity can be further heightened.
The binding of soluble human FN to resting MK was also demonstrated, and α5β1 was the primary integrin involved. There were patchy accumulations of human FN on the MK surface, similar to those we detected when endogenous FN was released from thrombin-treated MK.7 There was no evidence of intracellular human FN in these experiments, indicating that exogenous FN was not taken up by guinea pig MK. This is consistent with previous reports that indicate that FN in MK differs from plasma FN and that plasma FN is not taken up by MK.26 α5β1 was identified as the integrin involved in the binding of soluble FN to MK by evidence that the binding was inhibited by SAM-1 and EMF10 but not by anti-αΙΙbβ3 blocking agents (bitistatin and MK0852) or by the anti-α4 antibody (HP2/1). Thus, both the binding of soluble FN to MK and the binding of MK to immobilized FN are mediated primarily by α5β1.
Resting MK did not adhere to FGN, but MK exposed to thrombin did bind to FGN. The adherence of activated MK was mediated by αΙΙbβ3 based on the demonstration that bitistatin and MK0852 inhibited adhesion at concentrations that can inhibit αΙΙbβ3 binding in other cells and that can inhibit platelet aggregation. α4β1 and αvβ1 are not involved in the binding of MK to FGN, because HP2/1 and LM609 did not inhibit the binding of activated MK to FGN. SAM-1, the anti-α5 antibody, did not inhibit the binding, and SAM-1 in combination with bitistatin or MK0852 did not cause a greater degree of inhibition of binding than that caused when only these anti-αΙΙbβ3 agents were used alone. Thus, although αΙΙbβ3 is expressed in MK, it can mediate the binding of MK to FGN only if it is activated, and it appears to be the primary integrin involved in the binding of MK to FGN.
In contrast, the emergence of an activated αΙΙbβ3 integrin influences the role of α5β1 in binding activated MK to FN. The inhibitory effects of the anti-α5 antibody, SAM-1, and the anti-αΙΙbβ3 agents, bitistatin and MK0852, are less than half that demonstrated against resting MK. However, combinations of SAM-1 with bitistatin or with MK0852 are additive. This indicates that both integrins must be blocked to inhibit the adhesion of activated MK to FN; therefore, both are involved in this activity.
Binding to FGN via αΙΙbβ3 is a criteria for activated platelets. By analogy, the absence of binding of freshly isolated guinea pig MK to FGN suggests that they are in a resting state. Although MK can be isolated from bone marrow and remain in a resting state, MK grown in culture in the presence of growth factors would be expected to bind to FGN, because TPO can activate αΙΙbβ3 in MK.24
The demonstration that α5β1 mediates MK binding to FN has several physiological implications in megakaryopoiesis and platelet production. Bone marrow matrix is rich in FN. and FN-α5β1 interactions have a major role in the growth and differentiation of erythrocytes9 and granulocytes.10 α5β1 regulates the adhesion and migration of their precursors and the release of reticulocytes into the circulation27 and, therefore, may also mediate MK maturation.
The binding of FN to α5β1 has also been shown to be an essential step in the formation of assembled FN by fibroblasts, whereas other integrins usually are not involved in this process.28Assembled FN is fibrillar, is usually cross-linked, and is insoluble to deoxycholate. Assembled FN is considerably more active than unassembled FN in cellular biological processes such as adhesion or migration.26 The demonstration that FN is bound primarily to α5β1 on the MK surface indicates that MK may assemble a more biologically active form of FN and thereby regulate the interaction between MK and other bone marrow cells and matrix.
Thrombin-induced amplification of αΙΙbβ3 activity in MK most likely is due to inside-out signaling, a mechanism in which the interaction of agonists or growth factors with a nonintegrin receptor initiates intracellular signaling that results in the activation of an integrin on the cell surface. Inside-out signaling has been extensively studied in platelets in which αΙΙbβ3 is activated in response to agonists such as thrombin, most likely due to conformational changes.29
Inside-out signaling has been demonstrated in MK derived from bone marrow stem cells and in M07e cells, a megakaryocytic cell line that expresses c-MPL and MK markers and responds to TPO.30,31The activity of α4β1 and α5β1 is low in M07e and CD34+ cells.32 However, interleukin-3 (IL-3), stem cell factor (SCF), and granulocyte-macrophage colony-stimulating factor (GM-CSF) can activate α5β1 and α4β1 in M07e cells32,33 and TPO can activate α5β1.24,31 TPO also enhanced αΙΙbβ3-dependent adhesion of MK derived from CD34+ cells by inside-out signaling.24
ACKNOWLEDGMENT
The authors appreciate Drew Lickens’ assistance with the illustrations and Bernice Taylor’s help in the preparation of the manuscript.
Supported in part by National Institutes of Health Grant No. HL51481 and by a grant-in-aid from the American Diabetes Association.
Address reprint requests to P.K. Schick, MD, Cardeza Foundation, Jefferson Medical College, 1015 Walnut St, Philadelphia, PA 19107-5099.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal