Abstract
Despite sufficient levels of HLA class I and class II expression, acute myeloid leukemia (AML) cells usually fail to induce a significant T-cell response in vitro. Therefore, we investigated whether in vitro modifications could enhance the T-cell stimulatory properties of AML cells. AML cells were either cultured with granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-4 (IL-4), and tumor necrosis factor- (TNF-), or transfected with the CD80 (B7.1) gene and used as stimulator cells for primed and unprimed allogeneic T cells. Cytokine treatment increased HLA class I and II expression, but did not induce CD80 on AML cells. Cytokine-treated AML cells efficiently presented nominal and allo-antigens to primed T-cell clones, induced strong T-cell proliferation in HLA mismatched mixed lymphocyte reactions (MLR), but failed to induce primary T-cell responses from an HLA identical bone marrow donor in MLR. In contrast, CD80-transfected AML cells induced T-cell proliferation of HLA-identical bone marrow donor peripheral blood mononuclear cell (PBMC) in primary MLR, allowing the generation of leukemia reactive CD4+ T-cell lines and clones. The majority of the generated oligoclonal (25 of 35) T-cell cultures showed patient specific reactivity that did not discriminate between patient’s leukemic cells and Epstein-Barr virus (EBV)-transformed B cells (EBV-LCL). The remaining 10 oligoclonal T-cell cultures recognized only leukemic cells. One of these latter leukemia reactive oligoclonal T cells was cloned. The majority of the clones (25 of 29) reacted against both leukemic cells and patient’s EBV-LCL. A minority of the T-cell clones with the CD4 phenotype (four of 29) showed strong HLA-DP restricted reactivity against leukemic cells, but not against patient’s EBV-LCL or against HLA-matched nonleukemic cells, indicating that their target antigens are preferentially expressed by leukemic cells. In conclusion, our study shows that the in vitro allogeneic T-cell response induced by CD80-transfected AML cells is mainly directed against patient’s specific minor histocompatibility antigens, while antigens preferentially expressed by leukemic cells can also trigger T-cell responses.
© 1998 by The American Society of Hematology.
ALLOGENEIC BONE MARROW transplantation (BMT) is an effective treatment for acute and chronic forms of leukemia.1-4 Clinical and experimental data indicate that donor-derived T cells play an important role in eliminating the residual leukemic cells after BMT.5-8 In the HLA identical situation, much can be said in favor of donor T cells reactive against the minor histocompatibility antigens (mHag) expressed on patient’s leukemic cells (reviewed in Goulmy9). Donor-derived mHag-specific cytotoxic T cells (CTL) isolated from graft-versus-host disease (GVHD) patients have been shown to effectively lyse leukemic cells and to inhibit the outgrowth of leukemic cell precursors.10,11 However, it is unclear whether leukemic cells express unique antigens that can induce a leukemia cell-specific T-cell response. Addressing this issue has been difficult because leukemic cells rarely induce a significant T-cell response in vitro, which may be caused by the absence of costimulatory molecules such as CD80. Recent studies suggest that the T-cell stimulatory properties of leukemic cells can be upregulated. For instance, upon activation via CD40, B-cell lymphoma or leukemia cells express high levels of HLA and CD80/CD86 molecules and become efficient antigen-presenting cells (APC) for alloreactive T cells.12,13 Moreover, CD40 stimulated pre-B leukemia cells have been succesfully used for the in vitro induction of leukemia reactive autologous CTL from several pre-B leukemia patients.14 Potent APC with dendritic cell phenotype can be also generated from malignant CD34+precursors of chronic myeloid leukemia (CML) cells by culturing with granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-4 (IL-4), and tumor necrosis factor-α (TNF-α).15 In addition, in different mice models, including a leukemia model, low immunogenic tumor cells that were genetically engineered to express CD80 became more immunogenic and were rejected by syngeneic hosts.16-24 In light of these recent data, we searched for in vitro conditions that could potentiate antigen presentation by acute myeloid leukemia (AML) cells. In a previous study, we have shown that AML cells cultured with GM-CSF, IL-4, and TNF-α induced strong T-cell responses in an HLA mismatched combination. This led to the identification of an allo-major histocompatibility complex (MHC)–restricted T-cell clone recognizing leukemic cells and CD34+ early progenitor cells only.25
Here we have compared the T-cell stimulatory capacities of cytokine-treated AML cells and AML cells transfected with CD80 cDNA in HLA identical bone marrow (BM) donor/patient combinations. Cytokine-treated AML cells efficiently presented nominal and allo-antigens to T-cell clones, induced strong T-cell proliferation in HLA mismatched, but not in HLA identical combinations. In contrast, CD80-transfected AML cells were able to induce T-cell proliferation from HLA identical BM donor. This allowed us to generate leukemia reactive CD4+ T-cell lines and clones and to analyze the target cell specificity of these leukemia reactive T cells.
MATERIALS AND METHODS
Cells.
Peripheral blood mononuclear cells (PBMC) or BM cells were obtained by Ficoll-opaque density centrifugation. Patient PBMC that contained morphologically more than 95% malignant cells were assigned as AML cells. PBMC, BM, and AML cells were cryopreserved until usage.
Epstein-Barr virus transformed B cells (EBV-LCL).
PBMC were incubated with EBV for 1.5 hours at 37°C. EBV transformed B cells (EBV-LCL) were maintained in RPMI-1640 plus 10% fetal calf serum (FCS).
T-cell clones.
The HLA-DR15 restricted, hsp65 reactive T-cell clone R2F10 was obtained from Dr T. Ottenhoff (Leiden University Medical Center [LUMC], Leiden, The Netherlands), The HLA-DPB1* 1301 and HLA-DRB1* 1302 specific alloreactive T-cell clones were obtained from Dr S. de Koster (LUMC, Leiden, The Netherlands). The antigen specificities of these clones were described previously.26 27
Cytokine treatment of AML cells.
AML cells were thawed and cultured in the presence of 800 U/mL GM-CSF (kindly provided by Dr S. Osanto, LUMC, Leiden, The Netherlands), 500 U/mL IL-4 (Genzyme, Leuven, Belgium), 50 U/mL TNF-α (Genzyme) in RPMI 1640 (GIBCO, Grand Island, NY) supplemented with 10% FCS, 100 U/mL penicillin, 100 μg/mL streptomycin (GIBCO) during different periods (72 hours to 14 days).
Transfection of AML cells with plasmid vectors.
AML cells were thawed and cultured 16 hours in RPMI supplemented with 10% FCS and antibiotics (penicillin 100 U/mL, streptomycin 100 μg/mL). A total of 6 to 8 × 106 viable AML cells were electroporated at 960 F and 240 V in the presence of 25 mg of plasmid DNA. pCDNA-1 plasmid containing the full-length CD80 (B7.1) cDNA was a gift of Dr S. Schoenberger (LUMC, Leiden, The Netherlands).
Phenotypic analysis.
AML cells were labeled with fluorescein isothiocyanate (FITC) or phycoerythrin (PE)-conjugated monoclonal antibodies (MoAbs) against HLA-DR, CD34 (Becton Dickinson, Mountain View, NY) CD80, CD86 (Ancell, Lâufelfingen, Switzerland), or HLA class I (w6/32, Dr A. Mulder, LUMC, Leiden, The Netherlands) and analyzed by flow cytometry (fluorescence-activated cell sorting [FACS]). All AML cells used in the experiments were >95% CD34+, negative for CD80, and expressed low levels of CD86 before culturing with cytokines or CD80 transfection.
Leukemia reactive T-cell lines and T-cell clones were labeled with FITC or PE-conjugated MoAbs against CD4 and CD8 (both from Beckton Dickinson) and analyzed by flow cytometry (FACS). The T-cell clones, 2.4.1 and 2.4.8, were labeled >95% with anti-CD4 antibodies.
Generation of Leukemia Reactive T-Cell Lines and Clones
Stimulator cells.
Cytokine-treated or CD80-transfected leukemic cells of an AML patient with AML-M1 subclassification were used as stimulator cells. The HLA typing of the stimulator leukemic cells was: HLA-A2, -A24, -B60, -DR13 (DRB1*1302), -DR7 (DRB1*07), -DR52 (Dw26) (-DRB3*0301), -DPB1*0401,*1301.
Responder cells.
PBMC from the HLA identical sibling BM donor of the patient were used as responder cells. Irradiated stimulator cells (3 × 106 cells) were cocultured with an equal amount of responder cells in 5 mL of T-cell culture medium at 37°C and 5% CO2. On day 6, 20 U/mL of r-IL–2 was added. On day 8, the T-cell cultures were tested and restimulated with irradiated untransfected AML cells, and on day 15, leukemia reactive T-cell lines were semicloned by limiting dilution at 5,000 cells/well in 96-well round bottom microtiter plates in the presence of a feeder cell mixture consisting of 30 Gy irradiated PBMC from six random blood donors (1 × 106 c/mL), 30 Gy irradiated leukemic cells (2.5 × 105 cells/mL), 20 U/mL r-IL–2 (Cetus, Emeryville, CA) and 1% Leucoagglutinin-A (Pharmacia, Uppsala, Sweden). IL-2 (20 U/mL) containing medium was added into the cultures every 72 hours. Cloning was performed by limiting dilution at 0.3 cells/well in 96-well round bottom microtiter plates in the presence of the feeder-cell mixture described above. The T-cell clones were expanded in the presence of r-IL–2 and restimulated each week with the feeder-cell mixture.
T-cell proliferation assays.
Responder T cells (104 cells/well) and irradiated (30 Gy) stimulator cells (5 × 104 cells/well) were cocultured in 96-well flat bottom microtiter plates for 88 hours. Antigens were added in the assay. Sixteen hours before harvesting the cells were labeled with 0.5 Ci of 3H-thymidine. The3H-thymidine incorporation was determined by liquid scintillation counting. The results are expressed as the mean of triplicate cultures.
Mixed lymphocyte reactions (MLR).
Irradiated stimulator cells and responder cells (both 105cells/well) were cocultured in 96-well round bottom microtiter plates for 5 days. Sixteen hours before harvesting the cells were labeled with 0.5 Ci of 3H-thymidine. The 3H-thymidine incorporation was determined by liquid scintillation counting. The results are expressed as the mean of triplicate cultures. The standard error of the mean (SEM) of the results never exceeded 15%.
Cytokine measurements.
T cells (4 × 104 cells/well) were stimulated with irradiated stimulator cells (1.5 × 105 cells/well) or with phorbol myristate acetate (PMA) (1 ng/mL) plus iIonomycine (1 pg/mL) in 96-well round bottom microtiter plates containing 200 μL culture medium. After 72 hours, cell-free supernatants were harvested. The interferon-γ (IFN-γ) and IL-4 release in the supernatants was measured by cytokine-specific sandwich enzyme-linked immunosorbent assay (ELISA) assays following the instructions of the manufacturer (CLB, Amsterdam, The Netherlands).
RESULTS
GM-CSF, IL-4, and TNF-α–treated AML cells efficiently present nominal and allo-antigens to established T-cell clones.
To assess the effect of cytokines on surface molecules involved in antigen presentation, AML cells were cultured with GM-CSF, IL-4, and TNF-α, and the expression levels of HLA and costimulatory molecules were measured by FACS. Cytokine treatment enhanced the expression of HLA molecules, but did not induce CD80 on AML cells, not even after 14 days of culture. Expression levels of CD86 and CD34 were not affected by cytokines (Fig 1A). Subsequently, untreated and cytokine-treated AML cells were used as APC for antigen-specific and alloreactive T-cell clones. As shown in Fig 1B, untreated, HLA-DR15, DRB1*1302 positive AML cells presented the mycobacterial recombinant 65-kD protein (hsp65) and its peptide to an HLA-DR15 restricted, hsp65 specific T-cell clone. Untreated AML cells also stimulated the DR13-specific alloreactive T-cell clone. Cytokine treatment significantly enhanced antigen presentation by AML cells. Both antigen-specific and alloreactive T-cell clones showed substantially higher levels of proliferative responses. When the cytokines were used separately, antigen presentation by AML cells cultured with IL-4 was also significantly increased (Fig 1B). A synergistic effect of TNF-α with IL-4 was observed, whereas the addition of GM-CSF did not augment antigen presentation by AML cells (Fig 1B). Similar phenotypical and functional results were obtained using three other AML cells derived from patients with AML-M1 or AML-M5 classifications (data not shown).
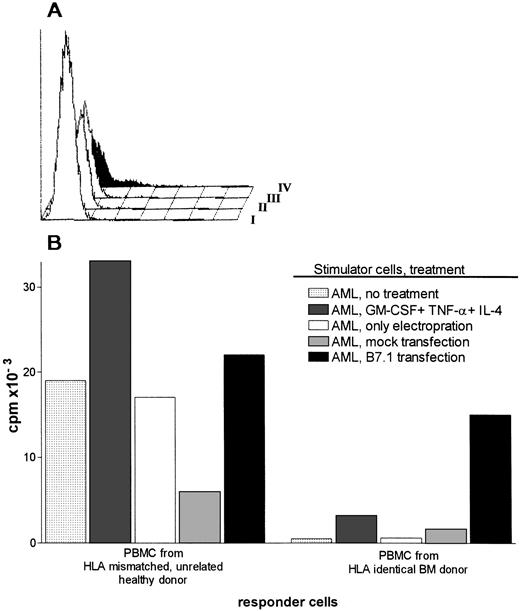
(A) Surface expression of HLA class I, HLA class II, and CD80, CD86, and CD34 molecules on untreated AML cells and AML cells cultured with GM-CSF, IL-4, and TNF-. I, No MoAb; II, anti-CD80; III, anti-CD86; IV, anti-DR; V, anticlass I; VI, CD34. Cells were labeled with fluorescein-conjugated MoAbs and analyzed on a FACS. (B) Proliferative responses of hsp65 (aa418-427)-specific T-cell clone R2F10 and HLA-DR13-specific alloreactive clone against untreated or cytokine treated (72 hours) AML cells. Antigens (hsp65, 5μg/mL; and hsp65 peptide 418-427, 1 μg/mL) were added in the assay. The results are expressed as stimulation index (cpm in the presence of stimulator [APC]-cpm in the absence of stimulator/cpm in the absence of the stimulator).
(A) Surface expression of HLA class I, HLA class II, and CD80, CD86, and CD34 molecules on untreated AML cells and AML cells cultured with GM-CSF, IL-4, and TNF-. I, No MoAb; II, anti-CD80; III, anti-CD86; IV, anti-DR; V, anticlass I; VI, CD34. Cells were labeled with fluorescein-conjugated MoAbs and analyzed on a FACS. (B) Proliferative responses of hsp65 (aa418-427)-specific T-cell clone R2F10 and HLA-DR13-specific alloreactive clone against untreated or cytokine treated (72 hours) AML cells. Antigens (hsp65, 5μg/mL; and hsp65 peptide 418-427, 1 μg/mL) were added in the assay. The results are expressed as stimulation index (cpm in the presence of stimulator [APC]-cpm in the absence of stimulator/cpm in the absence of the stimulator).
CD80 transfected AML cells, but not cytokine-treated AML cells, induce T-cell proliferation in HLA identical MLR.
Antigen presentation by AML cells was significantly enhanced by GM-CSF, IL-4, and TNF-α, but this treatment did not induce CD80 expression as discussed above. Because CD28/CD80 signaling pathway is considered essential for the induction of primary immune responses, we evaluated the role of CD80 in an HLA identical setting. Malignant cells from a patient with AML M-1 classification were transfected with a CD80 containing plasmid vector pCDNA-1. The transfections showed only in some attempts a transient expression of CD80 on a small fraction of AML cells (Fig 2A). CD80 transfected or cytokine-treated AML cells were used as stimulator cells in primary MLR using PBMC from HLA-identical sibling BM donor as responder cells. Control responder cells were HLA mismatched PBMC(HLA-mismatched MLR) (Fig 2B). Cytokine treatment potentiated the stimulatory capacity of AML cells significantly in an HLA-mismatched MLR (Fig 2B). Cytokine-treated AML cells were however not able to induce proliferation in HLA-identical MLR. In contrast, CD80-transfected AML cells stimulated both HLA mismatched and HLA identical PBMC (Fig 2B). Unmodified AML cells, AML cells transfected with the empty plasmid (mock transfection), and AML cells that underwent electroporation without DNA were able to trigger HLA mismatched PBMC, but did not induce T-cell proliferation from HLA identical PBMC (Fig 2B).
(A) Expression of CD80 on AML cells transfected with pCDNA-B7 plasmid vector. CD80 expression was determined by FACS 48 hours after transfection of the cells. I, pCDNA-B7- transfected cells + no MoAb; II, untransfected cells + anti-CD80; III, mock-transfected cells + anti-CD80; IV, pCDNA-B7– transfected cells + anti-CD80. (B) HLA mismatched and HLA identical MLR reactions induced by cytokine-treated or CD80-transfected AML cells. Control stimulator cells are AML cells transfected with empty plasmid (mock transfection), AML cells electroporated without DNA, and untreated AML cells. Results are expressed as the mean cpm of triplicate cultures. The SEM did not exceed 15%.
(A) Expression of CD80 on AML cells transfected with pCDNA-B7 plasmid vector. CD80 expression was determined by FACS 48 hours after transfection of the cells. I, pCDNA-B7- transfected cells + no MoAb; II, untransfected cells + anti-CD80; III, mock-transfected cells + anti-CD80; IV, pCDNA-B7– transfected cells + anti-CD80. (B) HLA mismatched and HLA identical MLR reactions induced by cytokine-treated or CD80-transfected AML cells. Control stimulator cells are AML cells transfected with empty plasmid (mock transfection), AML cells electroporated without DNA, and untreated AML cells. Results are expressed as the mean cpm of triplicate cultures. The SEM did not exceed 15%.
The specificity of allogeneic T-cell response induced by CD80-transfected AML cells.
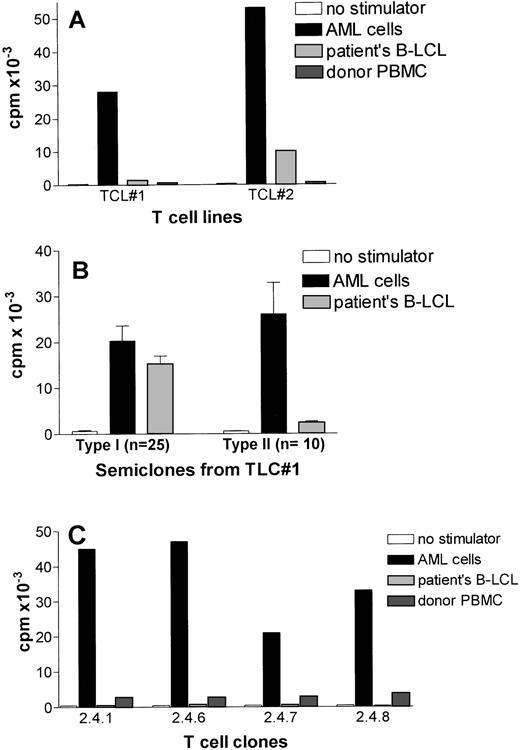
CD80-transfected AML cells were subsequently used as stimulator cells for unprimed T cells of the HLA identical BM donor to generate leukemia reactive T-cell lines. Two independent T-cell lines displayed strong proliferative activity against AML cells and did not recognize autologous PBMC (Fig 3A). The T-cell line (TCL#2) showed some proliferation against patients’ EBV-LCL suggesting the presence of patient specific, rather than leukemia-specific T cells.
Target cell specificity of leukemia reactive T cells generated by CD80-transfected AML cells. (A) Leukemic-cell reactivity of two T-cell lines generated against CD80-transfected AML cells. T-cell lines were tested against untransfected AML cells and patients’ EBV-LCL after second stimulation. (B) Proliferative activity of oligoclonal T-cell cultures (semiclones) generated from T-cell line #1. The results are expressed as the mean cpm of triplicate cultures. The SEM did not exceed 15%. (C) Preferential recognition of leukemic cells by four T-cell clones generated from a type II semiclone shown in Fig3B.
Target cell specificity of leukemia reactive T cells generated by CD80-transfected AML cells. (A) Leukemic-cell reactivity of two T-cell lines generated against CD80-transfected AML cells. T-cell lines were tested against untransfected AML cells and patients’ EBV-LCL after second stimulation. (B) Proliferative activity of oligoclonal T-cell cultures (semiclones) generated from T-cell line #1. The results are expressed as the mean cpm of triplicate cultures. The SEM did not exceed 15%. (C) Preferential recognition of leukemic cells by four T-cell clones generated from a type II semiclone shown in Fig3B.
Because the activity of the T-cell line, TCL#1, was preferentially directed against AML cells (Fig 3A), this T-cell line was analyzed in more detail. First, several semiclones were generated by dilution to a cell concentration of 5,000 cells per well. As shown in Fig 3B, two types of semiclones could be identified: the majority of the obtained semiclones (n = 25) showed type I reactivity, which did not discriminate between leukemic cells and EBV-LCL. Type II semiclones (n = 10) showed strong proliferation towards AML cells with little or no proliferation against patients’ EBV-LCL. One of these type II semiclones (designated as 2.4), was subjected to limiting dilution at 0.3 cell per well. The limiting dilution showed several T-cell clones (n = 25), which did not discriminate between EBV-LCL and leukemic cells (data not shown), four CD4+ T-cell clones (ie, 2.4.1, 2.4.6, 2.4.7, and 2.4.8 ) that strongly proliferated against AML cells and did not recognize patients’ EBV-LCL (Fig 3C). These clones showed a very low reactivity against autologous PBMC (1,000 to 1,700 cpm) (Fig 3C).
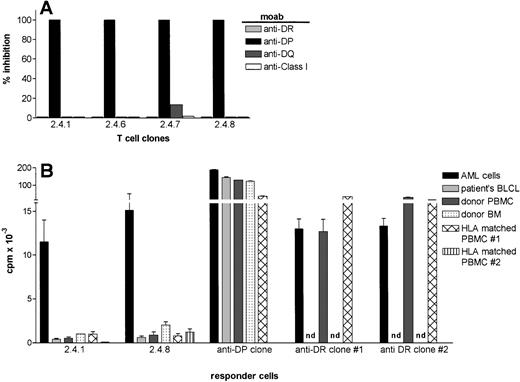
The recognition of AML cells by the four leukemia reactive T-cell clones was blocked by a MoAb against HLA-DP (B7/21), but not by MoAbs against HLA-DR (B8.11.2), -DQ (SPV.L3), or against HLA class-I (W6/32) indicating the HLA-DP restricted recognition of the target antigens (Fig 4A).
HLA-restriction and the target cell specificity of leukemia reactive T-cell clones 2.4.1 and 2.4.8. (A) MoAbs against HLA-DR (B.8.11.2), -DP( B7/21), -DQ(SPV.L3) and HLA class I (W6/32) were added in the proliferation assays at a final dilution of 1:200. The results are expressed as % inhibition of the proliferative response in the absence of antibodies. (B) The proliferative response of HLA-DP–restricted T-cell clones, 2.4.1 and 2.4.8, against leukemic cells and nonleukemic cells. The data represent the summary of eight independent experiments. HLA-DPB1*1301–specific and HLA-DRB1*1302–specific T-cell clones were used as control. Besides patients’ EBV-LCL, autologous BM, and PBMC, two HLA-DP and DR-matched PBMC from unrelated donors were tested as stimulator cells.
HLA-restriction and the target cell specificity of leukemia reactive T-cell clones 2.4.1 and 2.4.8. (A) MoAbs against HLA-DR (B.8.11.2), -DP( B7/21), -DQ(SPV.L3) and HLA class I (W6/32) were added in the proliferation assays at a final dilution of 1:200. The results are expressed as % inhibition of the proliferative response in the absence of antibodies. (B) The proliferative response of HLA-DP–restricted T-cell clones, 2.4.1 and 2.4.8, against leukemic cells and nonleukemic cells. The data represent the summary of eight independent experiments. HLA-DPB1*1301–specific and HLA-DRB1*1302–specific T-cell clones were used as control. Besides patients’ EBV-LCL, autologous BM, and PBMC, two HLA-DP and DR-matched PBMC from unrelated donors were tested as stimulator cells.
The fine specificity of the AML cell recognition was subsequently analyzed in more detail. Clones 2.4.1 and 2.4.8 were tested for proliferation against several stimulator cells including patients’ EBV-LCL, autologous PBMC, autologous BM cells, and PBMC derived from HLA-DP matched healthy individuals. One HLA-DP specific and two HLA-DR specific alloreactive clones were used as controls. In Fig 4B, the results of eight independent experiments are summarized. The T-cell clones, 2.4.1 and 2.4.8, showed strong proliferation against AML cells in all experiments, but never proliferated against patients’ EBV-LCL. The clones showed weak proliferative activity against autologous PBMC, autologous BM, and PBMC derived from HLA-DP matched individuals. The control class II specific alloreactive T cells recognized both leukemic and nonleukemic cells efficiently (Fig 4B). The low reactivity of clones 2.4.1 and 2.4.8 against nonleukemic cells could be inhibited by anti–HLA-DP antibodies and was never observed when HLA mismatched PBMC or BM cells were used as stimulator cells (data not shown), thereby ruling out a nonspecific proliferation. This low reactivity against nonleukemic cells could not be enhanced by culturing stimulator cells with cytokines like GM-CSF, IL-4, IFN-γ, or TNF-α (data not shown).
Leukemia reactive T-cell clones secrete high levels of IFN-γ, but little or no IL-4 in response to leukemic cells.
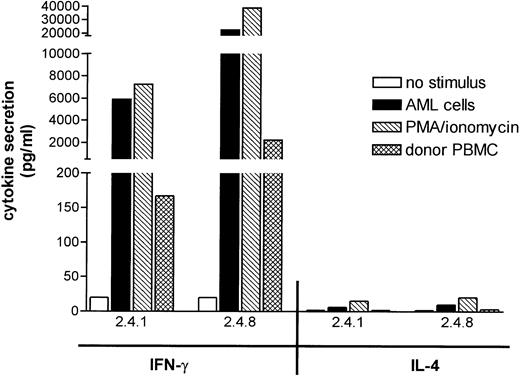
To gain insight into the cytokines secreted by leukemia reactive T-cell clones 2.4.1 and 2.4.8, production of IFN-γ and IL-4 was measured in response to patients’ leukemic cells, donor PBMC, and PMA/ionomycin (Fig 5). Both T-cell clones secreted high levels of IFN-γ, but hardly detectable levels of IL-4 in response to patients’ AML cells. A similar cytokine secretion profile was found after nonspecific stimulation with PMA/ionomycin, suggesting that both clones displayed a Th1 like cytokine secretion profile. Both T-cell clones showed significantly lower, but detectable, IFN-γ responses to donor PBMC, similar to the low reactivity observed in the T-cell proliferation studies, which suggested that the target antigens of the clones may be expressed at low levels on nonleukemic cells (Fig 5).
Cytokine secretion by leukemia reactive T-cell clones. T-cell clones, 2.4.1 and 2.4.8, were stimulated with the indicated irradiated stimulator cells or with PMA/ionomycin for 72 hours. The release of IFN-γ and IL-4 in the culture medium was assessed by cytokine-specific sandwich ELISA and expressed as the mean of the triplicate cultures.
Cytokine secretion by leukemia reactive T-cell clones. T-cell clones, 2.4.1 and 2.4.8, were stimulated with the indicated irradiated stimulator cells or with PMA/ionomycin for 72 hours. The release of IFN-γ and IL-4 in the culture medium was assessed by cytokine-specific sandwich ELISA and expressed as the mean of the triplicate cultures.
DISCUSSION
In this study, we have generated leukemia-cell reactive T-cell lines and clones from unprimed T cells in an HLA identical BM donor/patient combination using CD80-transfected AML cells. Our results indicate that the majority of allogeneic T-cell responses against AML cells is directed at patients’ specific minor histocompatibility antigens. A minority of the T cells can also be triggered by antigens that are preferentially expressed by leukemic cells.
Donor-derived T cells play a key role in the the graft-versus-leukemia (GvL) effect seen after BMT or after donor lymphocyte infusions. It is therefore important to characterize the leukemia-associated target antigens recognized by donor derived T cells. These studies have been hampered because leukemic cells are not potent APC and usually fail to induce T-cell responses in vitro. We therefore searched for in vitro conditions that would improve the antigen presenting function of AML cells and facilitate the generation of leukemia reactive T cells in HLA identical combinations. First, we explored the effect of GM-CSF, TNF-α, and IL-4 on the antigen presenting function of AML cells. These cytokines are known to generate dendritic, superior APC from monocytes, myeloid cell precursors, or malignant precursors of CML cells.15,28-30 Cytokine treatment significantly enhanced HLA expression levels of AML cells, but did not induce CD80 expression, neither increased the low expression levels of CD86 in four different AML cells classified as AML-M1 or AML M5. AML cells with other subclassifications were not tested in this study. Although the possibility remains that other AML types may respond to cytokines differently, our results indicate that unlike CD34+, CML precursors,15 CD34+ AML-M1 and AML-M5 cells seem not to change their phenotype into dendritic-like cells after culture with GM-CSF, TNF-α, and IL-4. After cytokine treatment, AML cells efficiently presented nominal antigens to T-cell clones, induced T-cell proliferation in HLA mismatched MLR, but failed to induce primary T-cell response in HLA identical MLR. Additional attempts to generate T-cell lines using cytokine-treated AML cells were also unsuccessful (data not shown). Thus, despite the expression of low levels of costimulatory CD86 molecule, cytokine-treated AML cells did not costimulate unprimed T cells sufficiently. Upon the introduction of CD80 gene, AML cells became adequate stimulator cells and triggered significant T-cell proliferation from HLA identical PBMC. This finding underscores the importance of CD80/CD28 interactions during the induction of primary antitumor T-cell responses and is consistent with some recent data where costimulation via CD80, but not via CD86, was associated with a clear antitumor effect.17,31 It is not clear yet why CD80 provides a superior costimulation than CD86. It has been suggested that CD80 and CD86 are involved in the differentiation of reciprocal T-cell subsets. CD80 costimulation may drive naive T cells to differentiate into Th1-like cells involved in inflammatory and antitumor responses.31 CD86-costimulated T cells may in contrast differentiate into Th2 type cells, which inhibit antiinflammatory responses (reviewed in Lu et al32). The cytokine secretion profile of the leukemia reactive T-cell clones generated in this study resembles a Th1-like pattern, which again supports the role of CD80 in the induction of these T cells. However, it should be noted that the CD80-transfected AML cells that we used coexpressed low levels of CD86. We, therefore, cannot exclude the possibility that the proper costimulatory signals are generated by the synergistic action of both CD80 and CD86, as also suggested by other investigators.19 31
AML cells transfected with the CD80 gene enabled us to investigate the target cell specificity of leukemia reactive T cells in an HLA identical patient/donor combination. The generated T-cell lines and clones contained mainly CD4+, proliferative T cells without detectable cytotoxic activity (data not shown). In our experiments, we were unable to generate CD8+ CTL using CD80-transfected AML cells. It is possible that CD4+ T cells display an in vitro growth advantage. Yet, these results imply that CD4+ T cells may significantly contribute to antileukemia reactivity after BMT. Our analysis indicates that leukemic cells induce different types of CD4+ T cells with distinct target cell specificities. The majority of T cells induced by leukemic cells do not discriminate leukemic from nonleukemic cells of the patient. Although we have not performed a thorough analysis of the reaction patterns, these “patient-specific” T cells are most probably directed to host specific mHag, which are present on both leukemic and nonleukemic cells. This also supports the general notion that mHag expressed by leukemic cells are important targets of post-BMT T-cell responses (reviewed in Goulmy9).
Interestingly, in our analysis, T-cell responses to AML cells were not confined to patients’ specific antigens. Some T-cell lines and clones reacted preferentially against AML cells. These cells strongly proliferated in response to leukemic cells, but did not recognize patients’ nonleukemic EBV-LCL suggesting that the ligands were absent on B cells. In a detailed analysis, two leukemia reactive, HLA-DP restricted T-cell clones showed strong proliferative reactivity and IFN-γ secretion in response to leukemic cells and never reacted to patients’ EBV-BLCL. The clones however showed a weak, but reproducible, reactivity against donor PBMC, donor BM, as well as against two unrelated HLA matched PBMC. Our results thus indicate that the target antigens of the latter clones are (1) strongly expressed by leukemic cells, (2) are absent on EBV-LCL, but (3) are expressed at low levels on nonleukemic myeloid cells. We, therefore, consider that the target antigens of these HLA-DP restricted clones are “leukemia-associated” rather than “leukemia-specific.” The preferential reactivity of T-cell clones exclude the possibility that they may react to antigens that are derived from extracellular sources such as FCS. It is possible that the target antigens are derived from membrane-associated or secreted proteins, as it is the case for the vast majority of HLA class II associated peptides.33
To our knowledge, a genuinely leukemia cell-specific T-cell clone has not yet been reported. Attempts to generate CTL responses against CML-specific Bcr/Abl fusion sequence have not been successful. Other T-cell clones that were initially considered as leukemia-specific appeared to recognize host specific mHag.34-37Lineage-specific differentiation antigens or developmentally regulated antigens can be recognized by T cells. Recently, Dolstra et al38 have described a leukemia-associated mHag antigen expressed only by EBV-LCL and by leukemic cells of B-cell origin. We have also identified an allo-HLA restricted T-cell clone that recognizes an antigen that is shared only by AML cells and CD34+ early precursor cells, indicating that its expression is developmentally regulated.25 Also, in other models, tumor reactive T cells are often directed to differentiation antigens, developmentally regulated embryonal antigens, or antigens that are overexpressed by tumor cells, rather than tumor-specific antigens.39 As in other tumors, also in leukemia tumor-cell selectivity rather than genuine tumor-specificity may result in leukemic load reduction. The leukemia reactive T-cell clones isolated in this study support these findings and will be used for the biochemical identification of such leukemia-associated antigens.
ACKNOWLEDGMENT
We thank Prof Dr F. Claas and Dr M. Oudshoorn for critically reading the manuscript.
Supported by grants from the Dutch Cancer Foundation and the J.A Cohen Institute for Radiopathology and Radiation Protection.
Address reprint requests to Tuna Mutis, MD, PhD, The Department of Immunohematology and Blood Bank, Leiden University Medical Center, Albinusdreef 2, 2333 ZA, Leiden, The Netherlands.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. (A) Surface expression of HLA class I, HLA class II, and CD80, CD86, and CD34 molecules on untreated AML cells and AML cells cultured with GM-CSF, IL-4, and TNF-. I, No MoAb; II, anti-CD80; III, anti-CD86; IV, anti-DR; V, anticlass I; VI, CD34. Cells were labeled with fluorescein-conjugated MoAbs and analyzed on a FACS. (B) Proliferative responses of hsp65 (aa418-427)-specific T-cell clone R2F10 and HLA-DR13-specific alloreactive clone against untreated or cytokine treated (72 hours) AML cells. Antigens (hsp65, 5μg/mL; and hsp65 peptide 418-427, 1 μg/mL) were added in the assay. The results are expressed as stimulation index (cpm in the presence of stimulator [APC]-cpm in the absence of stimulator/cpm in the absence of the stimulator).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/5/10.1182_blood.v92.5.1677/4/m_blod41714001x.jpeg?Expires=1769220757&Signature=bfpAM7ak3IL6fMq8~KGI8Hq7wjcSNq6btq1wL2ZXJY9DKyUNWQdMDbknBhOleq-9Ckb48q5VhPqciVOKvk90JuwuDVUsNN4D578KsDVAdpaToozMNEsRxCt6NzwweGn7Er~PBsNN7bBiCN~R5zLln~2jCLfVIiLdBAoSQvMZf2VkRvPs6N3FYHs~x7lP8v7spfF9SHdCrCkw9wP6IjtO1v5XNO2r5Joo~3A9JAGuyiN8VFv2idZ4s~t-Aa87~jyYCqFu0dYHCSnRrhLQLD~KKNcpiD46oED6TEhQhtAHnkzBbJBxbC3~AkDIkyJs9ORAo5ewL2eealpLfZaWL7xKng__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal