Abstract
The binding of neutrophil β2 integrin to intercellular adhesion molecule-1 (ICAM-1) expressed on the inflamed endothelium is critical for neutrophil arrest at sites of tissue inflammation. To quantify the strength and kinetics of this interaction, we measured the adhesion between chemotactically stimulated neutrophils and ICAM-1–transfected mouse cells (E3-ICAM) in suspension in a cone-plate viscometer at shear rates typical of venular blood flow (100 s−1 to 500 s−1). The kinetics of aggregation were fit with a mathematical model based on two-body collision theory. This enabled estimation of adhesion efficiency, defined as the probability with which collisions between cells resulted in firm adhesion. The efficiency of β2-integrin–dependent adhesion was highest (∼0.2) at 100 s−1 and it decreased to approximately zero at 400 s−1. Both LFA-1 and Mac-1 contributed equally to adhesion efficiency over the initial 30 seconds of stimulation, but adhesion was entirely Mac-1–dependent by 120 seconds. Two hydrodynamic parameters were observed to influence integrin-dependent adhesion efficiency: the level of shear stress and the intercellular contact duration. Below a critical shear stress (<2 dyn/cm2), contact duration predominantly limited adhesion efficiency. The estimated minimum contact duration for β2-integrin binding was approximately 6.5 ms. Above the critical shear stress (>2 dyn/cm2), the efficiency of neutrophil adhesion to E3-ICAM was limited by both the contact duration and the tensile stress. We conclude that at low shear, neutrophil adhesion is modulated independently through either LFA-1 or Mac-1, which initially contribute with equal efficiency, but differ over the duration of chemotactic stimulation.
© 1998 by The American Society of Hematology.
THE ATTACHMENT of neutrophils to the vascular endothelium and their passage to sites of inflammation involves a sequence of events mediated by at least three classes of adhesion molecules: selectins, integrins, and members of the Ig gene superfamily.1 Attachment has been modeled as a multistep process involving neutrophil tethering, rolling, and firm adhesion.2,3 While the tethering and rolling of neutrophils on the endothelium is mediated by members of the selectin family,4-6 cell arrest is dependent on two members of the β2-integrin family, LFA-1 (αLβ2, CD11a/CD18) and Mac-1 (αMβ2, CD11b/CD18).7,8Intercellular adhesion molecule-1 (ICAM-1, CD54), a member of the Ig gene superfamily expressed on the vascular endothelium, has been identified as a major ligand for both of the CD18 integrins.9,10 The interaction between the β2integrins and ICAM-1 accounts for 50% to 70% of the neutrophil adhesion to endothelial cells.10,11 The remaining adhesion has been attributed to Mac-1 binding unknown ligands and to non-CD18 integrin-dependent adhesion mechanisms.7,11 12
Currently, little is known about the dynamics of β2-integrin–dependent neutrophil adhesion under defined shear flow. For example, what are the critical hydrodynamic parameters (shear rate, intercellular contact duration, and tensile stress) that modulate the rate of neutrophil recruitment onto the endothelium? Also, what are the relative contributions of LFA-1 and Mac-1 to the strength and binding kinetics of neutrophil adhesion with time after stimulation? To investigate these issues, we developed an assay in which cell suspensions are sheared in a cone-plate viscometer and aggregation rates are quantitated by fluorescence flow cytometry. This technique offers two major advantages over the conventional parallel-plate flow chamber assay used to quantitate leukocyte recruitment on substrates of endothelial cell monolayers or purified ligands.8,13 (1) Cone and plate viscometry of cell suspensions enables estimation of the number of cell-cell collisions based on two-body collision theory. This enables computation of the efficiency with which cell-cell collisions result in aggregate formation.14,15 In contrast, in the parallel-plate flow chamber, it is difficult to determine the flux of cells interacting with the planar substrate, since it depends not only on the bulk cell concentration, but also on the distance from the chamber entry point and the shear rate applied.16 (2) The amount of time that a leukocyte is activated before entry into the parallel plate flow chamber is difficult to determine due to its dependence on the residence time of the cells in the tubing before entry into the flow chamber. In the suspension assay, the entire cell population is activated at the same time, allowing the relationship between cell adhesivity and time to be determined with a resolution of approximately 1 second.
The objective of this report is to examine the kinetics and strength of formyl peptide (formyl-methionyl-leucyl-phenylalanine [FMLP])-stimulated neutrophil adhesion to ICAM-1–transfected mouse cells (E3-ICAM) under conditions where the shear rate, hydrodynamic forces, and encounter frequencies were precisely controlled. The experimental methodology combined with two-body collision theory allowed us to estimate adhesion efficiency under conditions in which the functions of Mac-1 and LFA-1 were assessed. Although several recent reports have investigated the biophysics of transient adhesion through the selectins,17-19 this is the first study that examines the kinetics and strength of adhesion through β2-integrin receptor bond formation independent of tethering through selectins. We conclude here that at low shear, LFA-1 and Mac-1 contribute equally to neutrophil adhesion initially, but their relative contributions differ markedly over the time course of chemotactic stimulation.
MATERIALS AND METHODS
Materials.
Paraformaldehyde (FMLP) and Ficoll were purchased from Sigma Chemical (St Louis, MO). Glutaraldehyde was obtained from Polysciences (Warrington, PA). Fluorescently labeled antibody to CD45 (CD45-fluorescein isothiocyanate [FITC]) was obtained from Becton Dickinson Immunocytometry Systems (San Jose, CA), and nuclear acid stain LDS-751 was purchased from Molecular Probes (Eugene, OR). Anti-CD11a monoclonal antibody (MoAb) R3.1 (IgG1), anti–ICAM-1 domain 2 MoAb R6.5 (IgG2a), and anti–ICAM-1 domain 1 MoAb RR1/1 (IgG1) were generous gifts from Dr Robert Rothlein (Boehringer-Ingelheim Pharmaceuticals, Ridgefield, CT), and anti–ICAM-1 domain 3 MoAb CBRIC1/7 (IgG1) was kindly provided by Dr Charles A. Parkos (Emory University, Atlanta, GA). A humanized anti-CD11b MoAb 60.1 (denoted h60.1, IgG1) was provided by Lora Whitehorse (Repligen, Cambridge, MA) and L-selectin–blocking MoAb LAM1-3 was kindly supplied by Cell Genesys (Foster City, CA). Fab fragments of MoAb R6.5, R3.1, and LAM1-3 were produced by digestion with papain and purified by passage over a protein-A–Sepharose column using an ImmunoPure Fab preparation kit from Pierce (Rockford, IL). In all of the adhesion experiments, unless otherwise mentioned, the blocking MoAbs were added at saturating concentrations: h60.1, RR1/1, and CBRIC1/7 were used at 20 μg/mL, MoAb R6.5 Fab was used at 25 μg/mL, and R3.1 Fab and LAM1-3 Fab were added at 30 μg/mL.
Cell preparation.
Fresh human blood was collected by venipuncture into a sterile syringe containing 10 U/mL of heparin. Neutrophils were isolated using a Ficoll-Hypaque density gradient (Mono-Poly resolving medium; Flow Laboratories, McLean, VA) as previously described20 and kept at 4°C in Ca2+-free HEPES buffer for up to 3 hours before the experiment. The purity of isolated neutrophils was more than 90% and the viability measured by trypan blue exclusion was more than 99%.
The parent mouse melanoma cell line B78H1 and the human ICAM-1 transfected cells (abbreviated by E3-ICAM) were generously provided by Dr Lloyd H. Graf (University of Illinois, Chicago).21 These cells were maintained in Dulbecco’s modified Eagle media (D-MEM; GIBCO, Grand Island, NY) with 10% fetal calf serum (FCS; Hyclone, Logan, UT), 1% penicillin-streptomycin (GIBCO), and 10 mmol/L HEPES (GIBCO). Transfected cells were selected in D-MEM media with 300 μg/mL Geneticin (GIBCO). Before each experiment, the B78H1 cells were detached from the tissue culture substrate using Hanks’ balanced salt solution (HBSS; Sigma) containing 5 mmol/L EDTA (Sigma). Cells were then pelleted by centrifugation (10 minutes at 250 g), resuspended in HBSS buffer, and kept at 4°C. Viability for the mouse cell line was determined to be approximately 95% by trypan blue exclusion. Furthermore, fluorescent microscopy demonstrated that E3-ICAM cells express a uniform distribution of ICAM-1 on their surface (data not shown).
Fluorescent conjugation of MoAb and determination of ICAM-1–binding sites.
The R6.5 Fab fragments were conjugated with a fluorescent cyanine dye, Cy3, using the fluorolink-antibody Cy3 labeling kit from Amersham Life Sciences (Pittsburgh, PA). To determine the equilibrium binding constant, Kd, of R6.5 Fab, the labeled antibody at various concentrations was added to the E3-ICAM cells at a concentration of 6 × 106 cells/mL. The mixture was incubated at room temperature for 20 minutes before being washed once and read on a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA). The MoAb equilibrium-binding constant,Kd, was determined to be 0.94 μg/mL as described earlier.22 R6.5 Fab conjugated with Cy3 was incubated with the parent B78H1 cells in control experiments.
Detection of the total number of ICAM-1 sites on the E3-ICAM cells was performed using whole R6.5 MoAb labeled with FITC using a QuickTag FITC Conjugation Kit (Boehringer-Mannheim, Indianapolis, IN). The number of cell-surface–binding sites was determined using Quantum Simply Cellular microbead standards purchased from Flow Cytometer Standards (Research Triangle Park, NC). These uniform microbeads have a calibrated number of goat-antimouse IgG sites on their surface. Both the calibrated microbeads and the E3-ICAM cells were labeled with R6.5-FITC at saturating concentrations. The number of binding sites per cell was then determined by quantifying the fluorescence intensity of the labeled cells and translating this value to the number of bound antibodies using the microbead standards.
Cone-plate viscometry.
Both the homotypic and heterotypic aggregation assays were performed in a cone-plate viscometer (Ferranti Electric, Commack, NY). The device consists of a stationary plate placed beneath a rotating cone maintained at 37°C.14 15 A cone angle of 1° was used in the current study, and the gap between the cone and plate ranged from less than 10 μm at the center to 610 μm at the outside edge. Neutrophil and E3-ICAM cell suspensions were placed in this gap before the experiment, cells were stimulated with FMLP, and shear was immediately applied. The geometry of the viscometer enables application of a uniform and linear shear rate to the entire cell suspension.
The neutrophil E3-ICAM cell suspension behaves like a newtonian fluid and its shear stress varies linearly with shear rate as follows: shear stress = viscosity · shear rate. The viscosity of the HEPES buffer was measured at 0.7 cp (or 0.007 poise) at 37°C in a Brookfield (Stoughton, MA) cone-plate viscometer. A shear rate of 100 s−1 in this system would thus correspond to a shear stress of 0.7 dyn/cm2 (0.007 × 100). In some experiments, the buffer viscosity was increased by addition of Ficoll (400,000 molecular weight; Sigma), a neutral hydrophilic polymer of sucrose commonly used in density gradients for cell separation. Addition of 6% (wt/vol) Ficoll to the HEPES buffer increased media viscosity to 1.7 cp at 37°C. A shear rate of 100 s−1 in these experiments corresponds to a shear stress of 1.7 dyn/cm2.
Homotypic aggregation assay.
Homotypic neutrophil aggregation experiments were performed as previously described.15 23 Briefly, neutrophil suspensions were stimulated with 1 μmol/L FMLP and sheared in a cone-plate viscometer. Aliquots of 30 μL were taken at each sampling time point for up to 3 minutes after stimulation and immediately fixed in 200 μL of 2% glutaraldehyde. A FACScan flow cytometer was used to analyze the particle distributions of fixed cell suspensions. Singlet neutrophils and aggregates were resolved using autofluorescence due to glutaraldehyde fixation, and aggregates were quantitated as integral multiples of the singlet fluorescence channel. The particle distribution of neutrophil aggregates were determined using the histograms of fluorescence intensity. The extent of homotypic aggregation was expressed as the fraction of singlets recruited into larger aggregates:
where the concentration of neutrophil singlets or aggregates with i cells is denoted by [Ni] (cell mL−1). [N6+] is the concentration of sextuplets and larger aggregates that were grouped since they could not be resolved by flow cytometric analysis. In the results presented here, aggregates with six or more neutrophils accounted for less than 5% of the total particles.
Heterotypic aggregation assay.
In the heterotypic aggregation assay, neutrophils and E3-ICAM (or B78H1) cells were labeled with spectrally distinct fluorescent stains. Neutrophils were labeled with 5 μg/mL anti–CD45-FITC for detection in the green (FL1) fluorescence channel, and E3-ICAM (or B78H1) cells were stained for 15 minutes at 25°C with the vital nucleic acid dye LDS-751 (0.5 μg/mL) for detection on the red (FL3) fluorescence channel. Independent experiments showed that neither of these fluorescent reagents either altered (1) the expression level of β2 integrin on resting neutrophils, (2) the rate of change of β2-integrin expression following stimulation, or (3) the adhesivity of β2 integrin (data not shown). After labeling, excess LDS-751 label was removed by a brief (5 to 6 seconds at 3,000g) centrifugation of E3-ICAM (or B78H1) cells. The two cell populations (typically between 3 × 106 to 6 × 106 cells/mL) were then mixed and incubated for 2 minutes in buffer containing 1.5 mmol/L Ca2+. The combined sample was stimulated with 1 μmol/L FMLP and sheared in a cone-plate viscometer as described earlier.14 15 Cell-suspension aliquots of 40 μL were taken at desired time points and fixed in 100 μL of 0.5% cold paraformaldehyde to avoid autofluorescence interfering with dual-color fluorescence discrimination.
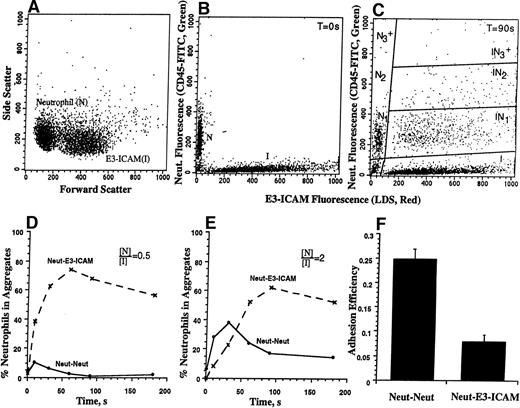
Labeling the cell populations with spectrally fluorescent dyes allowed the quantitation of homotypic neutrophil aggregates, as well as heterotypic neutrophil-E3-ICAM (or B78H1) aggregates by flow cytometric analysis (Fig 1). The neutrophil and E3-ICAM (or B78H1) population was isolated by gating on their characteristic forward versus side scatter. Aggregation between neutrophils and E3-ICAM (or B78H1) cells was quantitated by analyzing the dot plot between the green (due to CD45-FITC) and red (due to LDS-751) fluorescence channels (Fig 1C). Neutrophils are denoted byN, while the E3-ICAM (or B78H1) cells are denoted by I.This technique allowed us to resolve the homotypic neutrophil aggregate population of doublets (denoted by [N2]), and aggregates of three or more cells (denoted by [N3+]). Both flow cytometric detection and light microscopy observations showed that neither the B78H1 cells nor the transfected E3-ICAM cells aggregate homotypically following application of shear. However, these cells formed heterotypic aggregates with neutrophils. The population of E3-ICAM (or B78H1) cells was resolved into singlets and into aggregates composed of a single melanoma cell bound to either one, two, or more than two neutrophils. The concentrations of these particles is represented by [I], [IN1], [IN2], and [IN3+], respectively. Typically, less than 2% of the ICAM-1 cells appear off scale in the dot plot. Most of the experiments in this study (except Fig 1E) were performed in the presence of onefold excess E3-ICAM cells, ie, 3 × 106neutrophils/mL were stimulated and sheared with 6 × 106E3-ICAM cells/mL. Because of this, a majority of neutrophils were recruited into [IN1] (Fig 1C) and the number of aggregates in [IN2] and [IN3+] were relatively low. [IN3+] typically accounted for less than 5% of the heterotypic aggregates formed. The percentage of neutrophil recruitment into heterotypic aggregates was computed as follows:
Flow cytometric detection of heterotypic aggregation kinetics. neutrophils (3 × 106 cells/mL) were labeled green (CD45-FITC) and E3-ICAM cells (6 × 106cells/mL) were stained red (LDS-751) for 15 minutes at room temperature. Excess LDS-751 label was removed by centrifugation, cell populations were equilibrated in 37°C buffer containing 1.5 mmol/L Ca2+ for 2 minutes, stimulated with 1 μmol/L FMLP, and sheared in a cone-plate viscometer at a shear rate of 200 s−1. Samples were withdrawn at indicated time points, fixed with 0.5% cold paraformaldehyde, and analyzed on a flow cytometer. (A) Neutrophil and E3-ICAM gated on their characteristic forward versus side scatter. (B) Initial particle distribution at time zero. (C) Heterotypic aggregate distribution 90 seconds after stimulation and application of shear. (D) Kinetics of neutrophil–E3-ICAM aggregation for the experiment depicted in A through C. Dotted and solid lines denote the percentage of neutrophils in heterotypic and homotypic aggregates, respectively. (E) Kinetics of aggregation for a representative experiment where 3 × 106neutrophils/mL were stimulated and sheared with 1.5 × 106E3-ICAM cells/mL. (F) Adhesion efficiency (±SEM) for neutrophil-neutrophil and neutrophil–E3-ICAM collisions calculated from three independent experiments described in D and E.
Flow cytometric detection of heterotypic aggregation kinetics. neutrophils (3 × 106 cells/mL) were labeled green (CD45-FITC) and E3-ICAM cells (6 × 106cells/mL) were stained red (LDS-751) for 15 minutes at room temperature. Excess LDS-751 label was removed by centrifugation, cell populations were equilibrated in 37°C buffer containing 1.5 mmol/L Ca2+ for 2 minutes, stimulated with 1 μmol/L FMLP, and sheared in a cone-plate viscometer at a shear rate of 200 s−1. Samples were withdrawn at indicated time points, fixed with 0.5% cold paraformaldehyde, and analyzed on a flow cytometer. (A) Neutrophil and E3-ICAM gated on their characteristic forward versus side scatter. (B) Initial particle distribution at time zero. (C) Heterotypic aggregate distribution 90 seconds after stimulation and application of shear. (D) Kinetics of neutrophil–E3-ICAM aggregation for the experiment depicted in A through C. Dotted and solid lines denote the percentage of neutrophils in heterotypic and homotypic aggregates, respectively. (E) Kinetics of aggregation for a representative experiment where 3 × 106neutrophils/mL were stimulated and sheared with 1.5 × 106E3-ICAM cells/mL. (F) Adhesion efficiency (±SEM) for neutrophil-neutrophil and neutrophil–E3-ICAM collisions calculated from three independent experiments described in D and E.
Adhesion efficiency.
The rate at which neutrophils are incorporated into aggregates (as determined in equations 1 and 2) is not only dependent on the biologic properties of the cell that modulate its adhesivity, but also on the physical parameters of the system, which include the concentration and radius of the cells, and the applied shear rate. To quantify neutrophil adhesion under various experimental protocols, independent of the physical parameters, we estimated an index termed as adhesion efficiency (equation 3).15
Briefly, adhesion efficiency is defined as the fraction of intercellular collisions that result in firm adhesion and is always ≤1. It was estimated by fitting the data from homotypic and heterotypic aggregation experiments over the first 30 seconds after the application of shear with a mathematical model based on two-body collision theory. The total number of collisions (denominator) in equation 3 is dependent on the cell concentration, applied shear rate, and cell radius. For example, in the case two of unequal sized cells of radius ri and rj, at concentrations Ci and Cj, respectively, the collision frequency, fij (no. of collisions per second), at a shear rate G (1/s) is given byfij = 2/3(ri + rj)3CiCjG. Therefore, for the case of neutrophils (ri = 3.75 μm) and E3-ICAM cells (rj = 6 μm) being sheared at a relative cell concentration ratio, (Neutrophil)/(E3-ICAM) = 0.5, the number of heterotypic collisions is expected to exceed the homotypic collisions by approximately 4.4 times. The number of effective collisions (numerator) is measured based on the experimental aggregation kinetics. Adhesion efficiency estimated by this methodology is solely a function of the intrinsic biologic properties of the cell that determine its adhesivity. Important among these properties are the number, affinity, and distribution of adhesive receptors expressed on the cell surface, their response to applied shear, and the time after stimulation. This technique has previously been applied to estimate the efficiency of homotypic neutrophil aggregation.14 15 In the current report, we have extended it to estimate heterotypic adhesion efficiency of neutrophil–E3-ICAM collisions.
Transmission electron microscopy.
Neutrophil and E3-ICAM cell samples were prepared as described previously.24 Briefly, cell suspensions were fixed in 2% glutaraldehyde at room temperature for 30 minutes and postfixed for 1 hour in phosphate-buffered saline (PBS) containing 1% osmium tetroxide. Cells were then dehydrated in a graded series of ethanol and embedded in LX-112 (Ladd Research Industries, Burlington, VT). After polymerization, ultrathin sections were obtained on an RMC 7000 ultramicrotome (RMC, Tucson, AZ) equipped with a diamond knife. Sections were stained with uranyl acetate and lead citrate before being viewed on a JEOL 200CX electron microscope. The perimeter of the cells and the length of the intercellular contacts were measured using a SummaSketch III tablet (Seymour, CT) and Bioquant software (R&M Biometrics, Nashville, TN). The contact length index was defined as follows: (intercellular contact length)/(mean perimeter of the adherent cells). This index for homotypic aggregates was measured from 33 randomly chosen aggregates from three donors, while the contact length index for neutrophil–E3-ICAM aggregates was obtained from 12 images from a single donor.
Statistics.
Data were analyzed using analysis of variance (ANOVA). Posttests were performed using the Student-Newman-Keuls test and P values less than .05 were considered significant.
RESULTS
Kinetics of heterotypic aggregation of neutrophils and ICAM-1–transfected cells.
In the absence of any stimulus, shearing cells in the cone-plate viscometer did not lead to aggregation. Less than 5% of the neutrophils were observed to form homotypic aggregates and less than 2% of the E3-ICAM cells were incorporated into aggregates with neutrophils (data not shown). However, when stimulated by 1 μmol/L FMLP and sheared in a cone-plate viscometer, neutrophils were rapidly incorporated both into homotypic aggregates and into heterotypic aggregates with E3-ICAM transfectants (Fig 1). The aggregate size and composition was measured by flow cytometry over the time course of stimulation. Neutrophil and E3-ICAM transfectants were gated on their characteristic forward versus side scatter (Fig 1A). At time zero, all of the neutrophils were observed to elicit only green fluorescence (CD45-FITC), while the E3-ICAM transfectants elicited red fluorescence (LDS-751) (Fig 1B). Following stimulation and application of shear at 200 s−1 for 90 seconds, homotypic and heterotypic aggregates were observed in the red versus green fluorescence dot plot (Fig 1C).
The rate and extent of aggregation is a function of the initial cell concentration. Under conditions where stimulated neutrophils were sheared with onefold excess of E3-ICAM cells ([N]/[I] = 0.5), the frequency of heterotypic collisions is estimated to be approximately 4.4 times that of homotypic neutrophil collisions. Neutrophils were more rapidly recruited by E3-ICAM cells than by other neutrophils. Following 60 seconds of stimulation, approximately 75% of the neutrophils were incorporated into heterotypic aggregates (Fig 1B and D). Most aggregates were composed of single neutrophils with E3-ICAM cells (∼70%), while the rest of the E3-ICAM aggregates were attached to two or more neutrophils. E3-ICAM cells did not form aggregates with each other. The aggregation kinetics varied with neutrophil and E3-ICAM cell concentrations. For example, when neutrophils were stimulated and sheared with half as many E3-ICAM cells ([N]/[I] = 2, Fig 1E), approximately 40% of the neutrophils formed homotypic aggregates, while only approximately 20% were in heterotypic aggregates at the 30-second time point. Neutrophil homotypic aggregation peaked at 30 seconds, beyond which point they appeared to be recruited into heterotypic aggregates by E3-ICAM cells.
To quantify the adhesion between neutrophils and E3-ICAM cells independent of experimental parameters including cell concentration, aggregate size, and shear rate applied, we estimated adhesion efficiency. The kinetic data presented in Fig 1D and E were modeled over the first 30 seconds of stimulation, and adhesion efficiency was computed. Efficiency estimated in this fashion was equivalent for both the [N]/[I] cell ratios (Fig 1F). This further confirmed that adhesion efficiency as computed was invariant to changes in cell concentrations. At a shear rate of 200 s−1, homotypic collisions between neutrophils were approximately three times more effective compared to collisions between neutrophils and E3-ICAM cells. The efficiency of neutrophil-neutrophil collision was approximately 0.25, while that of neutrophil-E3-ICAM collisions was approximately 0.07. We have previously shown that neutrophil-neutrophil adhesion is dependent on L-selectin and β2 integrin binding their counterligand on opposing neutrophils.14 Blocking L-selectin function with MoAb, at low shear rates, results in purely β2-integrin–dependent homotypic aggregation. As will be shown later (Fig 4), the difference in adhesion efficiency between neutrophil-neutrophil and neutrophil-E3-ICAM can be abolished by blocking L-selectin.
The fraction of E3-ICAM cells incorporated into heterotypic aggregates did not change after the first 90 seconds of stimulation (Fig 1D and E) and the majority of the aggregates remained stable for at least 3 minutes. We tested the strength and stability of these heterotypic aggregates by allowing aggregate formation at 200 s−1 for 30 seconds before diluting the cell suspension in 20-fold excess buffer containing 1 μmol/L FMLP and 1.5 mmol/L Ca2+ (data not shown). This abrupt reduction in cell concentration caused the collision frequency to decrease by approximately 400-fold,15 without affecting the disaggregation kinetics. We observed less than a 10% change in the aggregate size distribution between 30 seconds and 180 seconds. This implies that heterotypic aggregates formed in the first 30 seconds remained stable and resistant to shear up to 180 seconds.
Molecular requirements for neutrophil–E3-ICAM aggregation.
Cells were preincubated with MoAb that have been shown to block adhesion function to determine the relative contributions of Mac-1 and LFA-1 on neutrophils, and ICAM-1 on transfectants to heterotypic aggregate formation (Fig 2). In these experiments, the percentages of neutrophils recruited into heterotypic aggregates 10 seconds after the application of shear were compared. The 10-second time point was chosen, because this is the earliest sampling point, and at this time less than 20% of the E3-ICAM cells have neutrophils adherent to them. Heterotypic aggregate formation before 10 seconds is thus the result of single neutrophils binding directly to the E3-ICAM cell surface. This is opposed to a situation in which neutrophils bind E3-ICAM when they collide with other neutrophils that are already adherent on the E3-ICAM cell. The latter phenomenon may play a significant role at times more than 30 seconds.
Adhesion of neutrophils to E3-ICAM transfectants. Neutrophils (3 × 106 cells/mL) were mixed with either E3-ICAM–transfected cells or B78H1 parent cells (6 × 106 cells/mL), stimulated with 1 μmol/L FMLP, and sheared at a shear rate of 200 s−1. The percentage of neutrophils in heterotypic aggregates 10 seconds after the application of shear is compared on addition of a panel of blocking antibodies at saturation concentration: R3.1 Fab (to LFA-1), h60.1 (to Mac-1), and R6.5 Fab (to ICAM-1). *The fifth bar represents experiments where R6.5 Fab was added to bind 50% of the ICAM-1 receptors on E3-ICAM cells.#Not statistically different. Error bars represent SEM from at least three independent experiments.
Adhesion of neutrophils to E3-ICAM transfectants. Neutrophils (3 × 106 cells/mL) were mixed with either E3-ICAM–transfected cells or B78H1 parent cells (6 × 106 cells/mL), stimulated with 1 μmol/L FMLP, and sheared at a shear rate of 200 s−1. The percentage of neutrophils in heterotypic aggregates 10 seconds after the application of shear is compared on addition of a panel of blocking antibodies at saturation concentration: R3.1 Fab (to LFA-1), h60.1 (to Mac-1), and R6.5 Fab (to ICAM-1). *The fifth bar represents experiments where R6.5 Fab was added to bind 50% of the ICAM-1 receptors on E3-ICAM cells.#Not statistically different. Error bars represent SEM from at least three independent experiments.
We have previously reported that resting neutrophils express approximately equal numbers of LFA-1 and Mac-1 receptors (∼50,000) on their cell surface.25 In comparison, the transfected E3-ICAM cells were found to homogeneously express at least 370,000 ± 50,000 (SD) ICAM-1 sites per cell. Heterotypic aggregation at a shear rate of 200 s−1 was found to be supported equally by LFA-1 and Mac-1, since addition of MoAbs R3.1 Fab (to LFA-1) or h60.1 (to Mac-1) blocked aggregation by approximately 55%. Simultaneous addition of both antibodies completely blocked aggregation, confirming that neutrophil-E3-ICAM adhesion was purely β2-integrin–mediated.
Aggregation studies were performed in the presence of a blocking antibody to ICAM-1 (R6.5 Fab). This MoAb binds domain 2 of ICAM-1 and has been previously shown to block both LFA-1-ICAM-1 and Mac-1–ICAM-1 interactions.10 Blocking 50% of the ICAM-1 sites by addition of this antibody at the measured Kd (0.94 μg/mL) decreased the level of aggregation by only 15%. At saturating concentrations of R6.5 Fab, heterotypic aggregation was inhibited by approximately 60%. Simultaneously, blocking both LFA-1 and ICAM-1 did not have any additive effect, while blocking Mac-1 and ICAM-1 inhibited aggregation to baseline levels.
Mac-1 has previously been shown to bind the third Ig-like domain of ICAM-1.26 We performed heterotypic adhesion experiments to determine if the inability of anti–ICAM-1 MoAb R6.5 to completely block adhesion was due to its inability to block domain 3 of ICAM-1, or due the presence of ICAM-1–independent ligands for Mac-1 on the mouse cell line. For these experiments, a blocking MoAb CBRIC1/7 against domain-3 of ICAM-1 was added. This MoAb has been previously shown to completely inhibit ICAM-1 binding to Mac-1 without significantly affecting binding via LFA-1.27 We observed that addition of CBRIC1/7 alone did not significantly reduce heterotypic aggregation from the levels seen in the absence of any MoAb. Furthermore, addition of CBRIC1/7 along with R6.5 only partially inhibited (∼60%) the level of neutrophil-E3-ICAM binding (data not shown). E3-ICAM cells apparently expressed a ligand for Mac-1 other than ICAM-1. This unidentified ligand for Mac-1 was also constitutively expressed on the parent B78H1 cells, since the extent of heterotypic aggregation of the parent cell with neutrophils was similar to that of neutrophil–E3-ICAM adhesion in the presence of saturating concentrations of R6.5 Fab. Binding of neutrophils to B78H1 parent cells could be abolished by addition of anti–Mac-1 MoAb (Fig 2). In our experimental system, LFA-1 on neutrophils apparently binds ICAM-1 on the transfectants, and Mac-1 binding to ICAM-1 could not be resolved. This may be due to Mac-1 binding to ICAM-1 with a lower affinity as compared with LFA-1, as previously reported.10,11 28 Alternatively, the Mac-1 ligand on the melanoma cells may be expressed in mass excess or bind with a higher affinity than ICAM-1.
Transient changes in the adhesivity of β2-integrin receptor– ligand interactions.
Within seconds of chemotactic stimulation of neutrophils, a rapid increase in β2-integrin–dependent adhesion has been observed.14,29 Mac-1 is also upregulated by 100% within 2 minutes after stimulation with 1 μmol/L FMLP, while the expression level of LFA-1 does not change.25 29 To examine how these changes in adhesivity may be reflected in changes in adhesion efficiency over time, we stimulated the neutrophil E3-ICAM cell suspension for a defined time period before applying shear in the cone-plate viscometer. Under these conditions, a lag period was introduced when cells were stimulated and receptor adhesivity changed, before shear was applied to initiate cell-cell collision.
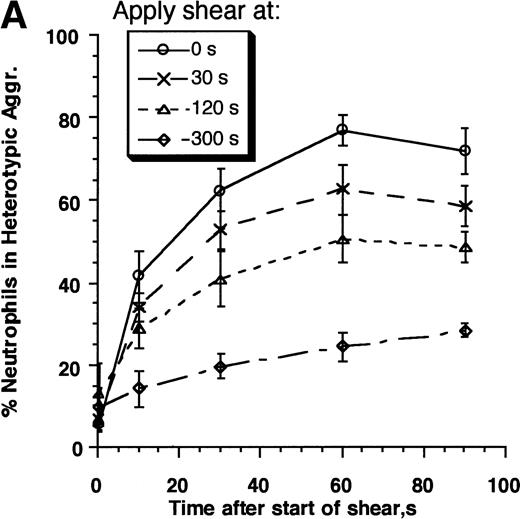
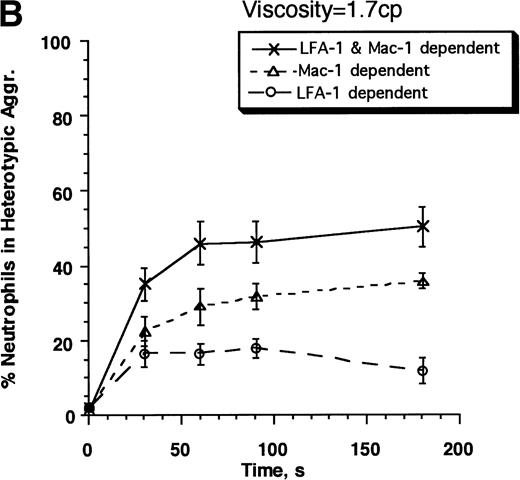
The kinetics of aggregate formation of E3-ICAM cells with neutrophils activated for 0, 30, 120, and 300 seconds before being sheared at a rate of 200 s−1 is shown in Fig3A. Both the rate and maximum extent of aggregation decreased with time following activation. We assessed the contributions of Mac-1 and LFA-1 to the time-dependent decrease in neutrophil adhesivity following FMLP stimulation by preblocking adhesion receptors with MoAbs (Fig 3B). LFA-1–dependent adhesion was studied by addition of an anti–Mac-1 blocking MoAb h60.1, while Mac-1–dependent adhesion was investigated in the presence of anti–ICAM-1 domain 1 antibody RR1/1. RR1/1 has been shown to behave identically to anti–LFA-1 MoAb R3.1 Fab, since they both specifically block the binding of LFA-1 to ICAM-1.10 We further verified that incubation of E3-ICAM cells with RR1/1 and R3.1 Fab simultaneously provided no more inhibition than either MoAb alone. Incubation with h60.1 and RR1/1 completely blocked aggregation (data not shown). The data in Fig 3b are presented using RR1/1 rather than R3.1, in part, due to reagent limitations in our laboratory. Also, treatment of E3-ICAM cells with RR1/1 allows complete inhibition of LFA-1/ICAM-1–mediated adhesion without intervening with neutrophil function. We observed that 120 seconds after stimulation, neutrophil adhesion was almost completely Mac-1–dependent, since blocking with anti–Mac-1 MoAb alone was sufficient to completely inhibit neutrophil–E3-ICAM adhesion (Fig3B).
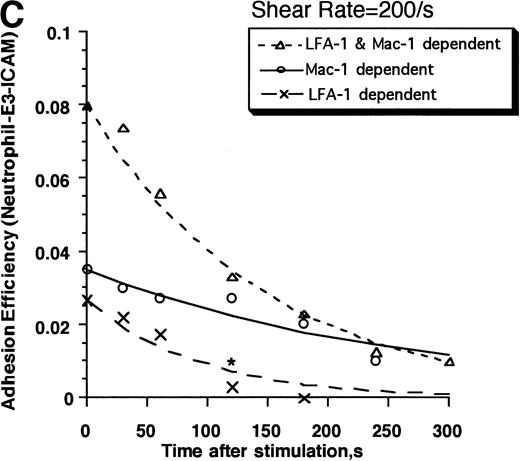
Contributions of LFA-1 and Mac-1 to time-dependent changes in cell adhesivity. Neutrophils (3 × 106cells/mL) were mixed with E3-ICAM transfectants (6 × 106 cells/mL) and stimulated with 1 μmol/L FMLP for fixed time periods (0, 30, 60, 120, 180, 240, or 300 seconds) before application of shear at 200 s−1. Cell aggregation kinetics was measured. (A) Percent neutrophils in heterotypic aggregates for experiments where shear was applied either 0, 30, 120, or 300 seconds after stimulation. (B) Percent neutrophils recruited in heterotypic aggregation when shear was applied 120 seconds after FMLP stimulation either in the absence of MoAb (LFA-1– and Mac-1–dependent adhesion) or on addition of anti–ICAM-1 domain 1 MoAb RR1/1 (Mac-1–dependent adhesion) or anti–Mac-1 MoAb h60.1 (LFA-1–dependent adhesion). Error bars in (A) and (B) represent SEM from three independent experiments. (C) Adhesion efficiency of neutrophil–E3-ICAM interactions with time at a shear rate of 200 s−1 either in the absence of MoAb, or upon addition of MoAbs to domain-1 of ICAM-1 (RR1/1) or Mac-1 (h60.1). Smooth lines represent curve fit to experimental data with a first-order exponential decay function as described in Results. Data are means from at least three independent experiments. *P < .05 with respect to adhesion efficiency estimated in the absence of MoAb and on addition of RR1/1.
Contributions of LFA-1 and Mac-1 to time-dependent changes in cell adhesivity. Neutrophils (3 × 106cells/mL) were mixed with E3-ICAM transfectants (6 × 106 cells/mL) and stimulated with 1 μmol/L FMLP for fixed time periods (0, 30, 60, 120, 180, 240, or 300 seconds) before application of shear at 200 s−1. Cell aggregation kinetics was measured. (A) Percent neutrophils in heterotypic aggregates for experiments where shear was applied either 0, 30, 120, or 300 seconds after stimulation. (B) Percent neutrophils recruited in heterotypic aggregation when shear was applied 120 seconds after FMLP stimulation either in the absence of MoAb (LFA-1– and Mac-1–dependent adhesion) or on addition of anti–ICAM-1 domain 1 MoAb RR1/1 (Mac-1–dependent adhesion) or anti–Mac-1 MoAb h60.1 (LFA-1–dependent adhesion). Error bars in (A) and (B) represent SEM from three independent experiments. (C) Adhesion efficiency of neutrophil–E3-ICAM interactions with time at a shear rate of 200 s−1 either in the absence of MoAb, or upon addition of MoAbs to domain-1 of ICAM-1 (RR1/1) or Mac-1 (h60.1). Smooth lines represent curve fit to experimental data with a first-order exponential decay function as described in Results. Data are means from at least three independent experiments. *P < .05 with respect to adhesion efficiency estimated in the absence of MoAb and on addition of RR1/1.
The data were further analyzed over the entire time course of chemotactic stimulation in terms of neutrophil–E3-ICAM adhesion efficiency (Fig 3C). Adhesion efficiency was highest immediately after stimulation and it decreased with time thereafter. The decrease in adhesion efficiency over time was fit by a first-order exponential function as previously described,15E =E0e−αt (Table1). In this equation,E0 is the adhesion efficiency immediately after stimulation (t = 0) and α is the decay constant that describes the time-dependent decrease in efficiency. Both LFA-1 and Mac-1 appear to contribute equally (∼30% to 40%) to neutrophil–E3-ICAM adhesion efficiency immediately following stimulation. However, adhesion mediated by LFA-1 decreased faster than that due to Mac-1. LFA-1–ICAM-1 binding did not contribute to adhesion by 120 seconds. The decrease in efficiency over time is quantitatively described by the decay constant for LFA-1, which is approximately fourfold greater than Mac-1–mediated adhesion (Table 1). Mac-1 accounted for most of the adhesion by 120 seconds. After this time point, Mac-1–dependent adhesion was not significantly different from that of the untreated control (with no MoAb addition). This is the first demonstration that adhesion mediated through LFA-1 binding to ICAM-1 is downmodulated within minutes of FMLP stimulation.
Time-Dependent Changes in β2-Integrin Avidity Following FMLP Stimulation
| Adhesion . | Adhesion Efficiency, E0 . | Decay Constant, α* . |
|---|---|---|
| LFA-1– & Mac-1–dependent | 0.080 | 0.007 |
| LFA-1–dependent | 0.027 | 0.011 |
| Mac-1–dependent | 0.035 | 0.003 |
| Adhesion . | Adhesion Efficiency, E0 . | Decay Constant, α* . |
|---|---|---|
| LFA-1– & Mac-1–dependent | 0.080 | 0.007 |
| LFA-1–dependent | 0.027 | 0.011 |
| Mac-1–dependent | 0.035 | 0.003 |
*Regression coefficient for curve fit, r > .97.
Kinetics and strength of the β2-integrin receptor–ligand interaction.
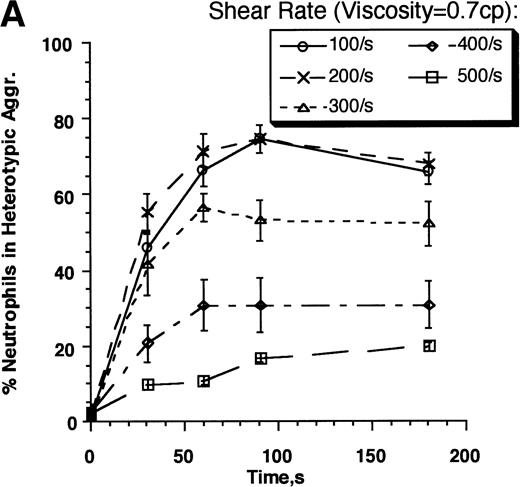
As shear rate increases, the contact duration of cells interacting in a linear shear field is predicted to decrease, and there is a concomitant increase in the amount of cell deformation and tensile forces acting to break intercellular bonds. A decrease in adhesion efficiency is thus expected to result. We examined the kinetics and strength of β2-integrin receptor–mediated adhesion between neutrophils and E3-ICAM cells over a range of shear rates from 100 s−1 to 500 s−1 (Fig4). The rate of neutrophil recruitment by E3-ICAM cells was greatest at the lower shear rates (100 to 200 s−1), and it decreased at higher shear rates (Fig 4A). The adhesion efficiency fit to these kinetics decreased approximately linearly with increasing shear rate between 100 s−1 and 300 s−1, reaching approximately zero at 400 s−1 (Fig 4B). The results were compared with homotypic neutrophil aggregation experiments performed in the presence of saturating concentrations of anti–L-selectin–blocking MoAb LAM1-3. We have previously established that on addition of LAM1-3, neutrophil adhesion was supported entirely by β2-integrin activation and binding.14 At a shear rate of 200 s−1, homotypic neutrophil adhesion efficiency was approximately 0.25 (Fig1F). However, on addition of MoAb LAM1-3, this efficiency decreased to approximately 0.11 (Fig 4B). At this shear rate, the efficiency of neutrophil–E3-ICAM adhesion closely matched the efficiency of homotypic neutrophil adhesion in the presence of L-selectin–blocking antibody. In fact, efficiency for both the cases was closely matched over the entire range of shear rates tested. In both cases, efficiency decreased from approximately 0.17 at 100 s−1 to zero at 400 s−1.
Kinetics and strength of β2-integrin receptor–ligand interactions. Neutrophils (3 × 106cells/mL) and E3-ICAM (5 × 106 cells/mL) cells were stimulated with 1 μmol/L FMLP and sheared in a cone-plate viscometer over a range of shear rates from 100 to 500 s−1 in normal HEPES buffer (media viscosity, =0.7 cp, A and B) or on addition of 6% Ficoll to the HEPES buffer (media viscosity, =1.7 cp, C and D). (A) Percent neutrophils in heterotypic aggregates in normal buffer. (B) Adhesion efficiencies for neutrophil–E3-ICAM collisions were computed from data presented in A, and compared with the adhesion efficiency for neutrophil-neutrophil collisions in independent homotypic aggregation experiments in the presence of 30 μg/mL anti–L-selectin MoAb, LAM1-3 Fab. (C) Percent neutrophils in heterotypic aggregates in HEPES buffer with 6% Ficoll. (D) Adhesion efficiencies for neutrophil–E3-ICAM were computed for the data in C and compared with neutrophil-neutrophil aggregation in the presence of LAM1-3 in media with viscosity 1.7 cp. Error bars represents SEM from three to eight independent experiments. *P < .05 with respect to neutrophil–E3-ICAM adhesion in the absence of Ficoll.
Kinetics and strength of β2-integrin receptor–ligand interactions. Neutrophils (3 × 106cells/mL) and E3-ICAM (5 × 106 cells/mL) cells were stimulated with 1 μmol/L FMLP and sheared in a cone-plate viscometer over a range of shear rates from 100 to 500 s−1 in normal HEPES buffer (media viscosity, =0.7 cp, A and B) or on addition of 6% Ficoll to the HEPES buffer (media viscosity, =1.7 cp, C and D). (A) Percent neutrophils in heterotypic aggregates in normal buffer. (B) Adhesion efficiencies for neutrophil–E3-ICAM collisions were computed from data presented in A, and compared with the adhesion efficiency for neutrophil-neutrophil collisions in independent homotypic aggregation experiments in the presence of 30 μg/mL anti–L-selectin MoAb, LAM1-3 Fab. (C) Percent neutrophils in heterotypic aggregates in HEPES buffer with 6% Ficoll. (D) Adhesion efficiencies for neutrophil–E3-ICAM were computed for the data in C and compared with neutrophil-neutrophil aggregation in the presence of LAM1-3 in media with viscosity 1.7 cp. Error bars represents SEM from three to eight independent experiments. *P < .05 with respect to neutrophil–E3-ICAM adhesion in the absence of Ficoll.
We next examined whether the decrease in efficiency with shear rate was primarily due to a decrease in the intercellular contact duration during collision, which in turn limits the number of adhesion bonds formed. Another mechanism that could account for the decrease in adhesion efficiency with increased shear is the concomitant increase in stresses acting to disrupt intercellular bonds.30 To differentiate between these two effects, experiments were performed in which buffer viscosity was increased by the addition of 6% Ficoll. At 37°C, normal HEPES buffer had a viscosity of 0.7 cp, while the addition of 6% Ficoll increased the buffer viscosity to 1.7 cp. Increasing the viscosity at the same shear rate caused a approximately 2.5-fold (=1.7/.7) increase in shear stress exerted on aggregates, without affecting the intercellular contact duration, which varies inversely with shear rate. We have previously shown that addition of Ficoll to the buffer did not itself activate neutrophils.14Cells remained in an unactivated spherical state. Moreover, Ficoll did not inhibit the amount of Mac-1 upregulation and L-selectin shedding characteristic of neutrophil stimulation with FMLP. At a low shear rate of 100 s−1, increasing viscosity did not alter the kinetics of neutrophil–E3-ICAM adhesion (Fig 4A and C). However, at shear rates ≥200 s−1, the increase in shear stress decreased the rate and extent of neutrophil–E3-ICAM adhesion. This effect is clearly evident in comparing the efficiency of neutrophil–E3-ICAM adhesion versus homotypic neutrophil adhesion in the presence of anti–L-selectin MoAb (Fig 4D). While increasing shear stress did not affect the linear decrease of efficiency with shear rate for homotypic neutrophil adhesion, it decreased the efficiency of neutrophil–E3-ICAM adhesion by 70% at a shear stress of approximately 2 dyn/cm2 (ie, on addition of 6% Ficoll at 200 s−1). Taken together, the data indicate that the predominant factor limiting homotypic aggregation over a range of shear between 100 s−1 and 400 s−1 was the decrease in the intercellular contact duration, rather than the concomitant increase in shear stress up to approximately 5 dyn/cm2. In contrast, neutrophil adhesion to E3-ICAM cells was markedly less efficient at shear stresses more than 2 dyn/cm2.
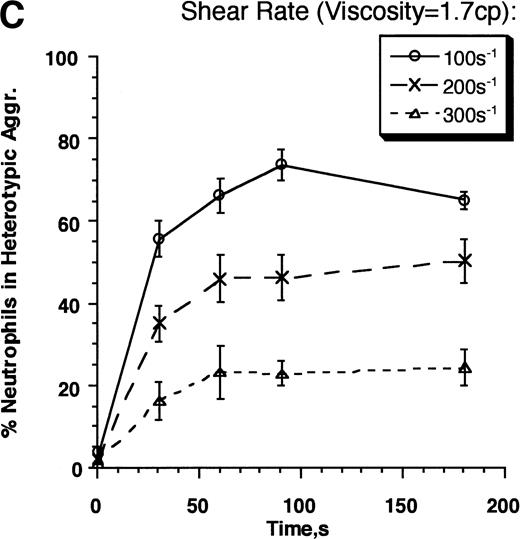
We next compared the relative contributions of LFA-1 and Mac-1 to the efficiency and strength of adhesion with increased shear stress. Experiments were performed in the presence of anti–LFA-1 MoAb (Mac-1–dependent adhesion) and anti–Mac-1 MoAb (LFA-1–dependent adhesion) at a shear rate of 200 s−1, both in normal buffer and in high viscosity buffer with 6% Ficoll (Fig 5A and B). Blocking either LFA-1 or Mac-1 caused an approximately 50% decrease in heterotypic aggregation in the first 30 seconds after neutrophil stimulation, consistent with the MoAb blocking data of Fig 2. However, over the time course of stimulation, blocking Mac-1 was significantly more effective in inhibiting aggregation. This implies that Mac-1, rather than LFA-1, is more effective in sustaining neutrophil adhesion. Blocking both the integrin subunits simultaneously abrogated heterotypic aggregation over the entire time course of the experiment. Increasing shear stress with Ficoll decreased the rate and extent of heterotypic aggregation for both integrin subunits (Fig 5B). Adhesion efficiency supported by either integrin subunits was inhibited by approximately 50% on increasing the shear stress (Fig 5C). The results suggest that LFA-1 and Mac-1 bonds formed in the first 30 seconds of stimulation mediate adhesion with comparable kinetics and strength, but their contributions differ markedly by 120 seconds at which time Mac-1 supports all the adhesion.
Strength of LFA-1– and Mac-1–mediated adhesion. Neutrophils (3 × 106 cells/mL) and E3-ICAM cells (5 × 106 cells/mL) were stimulated with 1 μmol/L FMLP and sheared in a cone-plate viscometer at 200 s−1 either in the absence of any MoAb (LFA-1– and Mac-1–dependent adhesion) or in the presence of anti–LFA-1 MoAb R3.1 Fab (Mac-1–dependent adhesion), anti–Mac-1 MoAb h60.1 (LFA-1–dependent adhesion), or on addition of both R3.1 and h60.1 (LFA-1– and Mac-1–independent adhesion): (A) in normal HEPES buffer (media viscosity, 0.7 cp), or (B) in buffer containing 6% Ficoll (media viscosity, 1.7 cp). The percent neutrophils in heterotypic aggregates is reported in the 2 panels. (C) Adhesion efficiencies for the experiment described in (A) and (B). Error bars represent SEM from at least three independent experiments. *P < .05 with respect to the same treatment in the absence of Ficoll.
Strength of LFA-1– and Mac-1–mediated adhesion. Neutrophils (3 × 106 cells/mL) and E3-ICAM cells (5 × 106 cells/mL) were stimulated with 1 μmol/L FMLP and sheared in a cone-plate viscometer at 200 s−1 either in the absence of any MoAb (LFA-1– and Mac-1–dependent adhesion) or in the presence of anti–LFA-1 MoAb R3.1 Fab (Mac-1–dependent adhesion), anti–Mac-1 MoAb h60.1 (LFA-1–dependent adhesion), or on addition of both R3.1 and h60.1 (LFA-1– and Mac-1–independent adhesion): (A) in normal HEPES buffer (media viscosity, 0.7 cp), or (B) in buffer containing 6% Ficoll (media viscosity, 1.7 cp). The percent neutrophils in heterotypic aggregates is reported in the 2 panels. (C) Adhesion efficiencies for the experiment described in (A) and (B). Error bars represent SEM from at least three independent experiments. *P < .05 with respect to the same treatment in the absence of Ficoll.
Intercellular contact areas of neutrophil–E3-ICAM aggregates.
The strength of adhesion between apposing cells has been shown to be dependent on the area and topography over which molecular bonds are distributed.31 32 The adhesion efficiency computed for neutrophil homotypic and neutrophil-E3-ICAM adhesion as demonstrated in Fig 4 was remarkably comparable over the range of shear rates in the low viscosity buffer. However, on increasing media viscosity, the adhesion efficiency of neutrophil–E3-ICAM adhesion was markedly lower than that due to neutrophil homotypic aggregation. We examined if these differences were due to differences in the relative contact areas of neutrophils engaged in heterotypic and homotypic aggregation (Fig6). At the time point of maximum aggregation, at a shear rate of 200 s−1, samples were fixed and prepared for transmission electron microscopy. In the absence of stimulation, neutrophils appeared as singlets with a round morphology (data not shown). E3-ICAM cells were also spherical with an average radius twice that of neutrophils. After stimulation and application of shear for 90 seconds, homotypic neutrophil aggregates were adherent either at multiple contact sites on their planar membrane or along continuous segments of the neutrophil surface (Fig 6A). The nature of the contacts in terms of the level of membrane interdigitation for homotypic and neutrophil–E3-ICAM aggregates were qualitatively similar (Fig 6B). We quantitatively compared the contact regions for the two types of aggregates by measuring the lengths of intercellular contacts in the plane of the electron micrograph sections as described in Materials and Methods. The contact length index for neutrophil-neutrophil aggregates (0.16 ± 0.01, n = 33) was not significantly different from that of neutrophil–E3-ICAM aggregates (0.14 ± 0.019, n = 12). This implied that the surface area over which adhesive bonds were formed in the two cases was equivalent.
Neutrophils and E3-ICAM cells observed by transmission electron microscopy. (A) Homotypic neutrophil aggregate formed 90 seconds after 1 μmol/L FMLP stimulation. (B) Neutrophil–E3-ICAM aggregate 60 seconds after FMLP stimulation. Arrow heads indicate regions of intercellular contact.
Neutrophils and E3-ICAM cells observed by transmission electron microscopy. (A) Homotypic neutrophil aggregate formed 90 seconds after 1 μmol/L FMLP stimulation. (B) Neutrophil–E3-ICAM aggregate 60 seconds after FMLP stimulation. Arrow heads indicate regions of intercellular contact.
DISCUSSION
The dynamics of β2-integrin–dependent adhesion between chemotactically stimulated neutrophils and an ICAM-1–transfected murine melanoma cell line were examined. Cell adhesion was quantified under defined hydrodynamic shear conditions where the shear rate, hydrodynamic forces, and intercellular encounter frequencies were precisely controlled.
The neutrophil–E3-ICAM system.
The efficiency of homotypic neutrophil aggregation mediated by L-selectin and β2 integrin was observed to be approximately 0.25 at a shear rate of 200 s−1. This is approximately threefold higher than that of neutrophil–E3-ICAM aggregation under the same shear conditions. In the presence of a MoAb that blocks L-selectin function, the efficiency of β2-integrin–mediated homotypic adhesion decreased to approximately 0.07. In the absence of L-selectin tethering, the kinetics and efficiency of adhesion were identical for neutrophils adhering to each other or to E3-ICAM transfectants. LFA-1 and Mac-1 contributed equally to adhesion efficiency and adhesion strength over the first minute of stimulation for both neutrophil-neutrophil and neutrophil–E3-ICAM adhesion. It appears that the dynamics of adhesion in this system is controlled primarily by the activation and binding of β2 integrin, rather than the availability of counterstructures that differ in homotypic and heterotypic aggregation.
The adhesion of neutrophils to E3-ICAM cells was found to depend entirely on LFA-1 and Mac-1. ICAM-1 accounted for all the adhesion via LFA-1 at 200 s−1, while Mac-1 binding to ICAM-1 was not a requirement under these conditions of shear. Mac-1–dependent adhesion of neutrophils to E3-ICAM cells was not significantly inhibited by treatment with MoAbs that block β2-integrin binding to domains 2 and 3 of ICAM-1. This may be attributed to Mac-1 binding ICAM-1 with a low affinity as previously reported in adhesion experiments under no shear conditions.10,11,28 These studies have also demonstrated other differences between the nature of Mac-1 and LFA-1 binding to their distinct domains on ICAM-1. Mac-1 recognition of ICAM-1 was more temperature sensitive than LFA-1–ICAM-1 interactions, and the former was influenced by the extent of N-linked glycosylation of ICAM-1.10,26 An alternate explanation for our experimental observations is that the endogenous Mac-1 ligand(s) expressed on both the parent B7H81 and E3-ICAM murine cells may bind Mac-1 with a higher affinity than ICAM-1. Regardless of the nature of the Mac-1 counterstructure in neutrophil adhesion to E3-ICAM-1, the current model provides a quantitative comparison of the strength and kinetics of LFA-1–ICAM-1 and Mac-1 binding under defined hydrodynamic shear. The presence of ligands for Mac-1 other than ICAM-1 is not surprising, and has been observed in previous studies of neutrophil adhesion to human endothelial cell monolayers10,11 and in vivo in animal models.12 Mac-1 is a promiscuous integrin with respect to its repertoire of ligands, which includes C3b, ICAM-1, factor X, heparan sulfate, fibrinogen, elastase, and denatured serum proteins, including albumin.33-35
β2-integrin–mediated adhesion is limited by intercellular contact duration.
Neutrophil adhesion mediated by β2 integrin was shown to decrease in a linear manner as shear was increased from 100 to 400 s−1. Over this range of shear rates, adhesion efficiency of neutrophil–E3-ICAM was virtually identical to that of neutrophil-neutrophil adhesion, provided L-selectin was blocked by MoAb. Below a critical threshold shear stress (∼2 dyn/cm2), the primary factor affecting the kinetics of neutrophil adhesion to E3-ICAM was the intercellular contact duration. A mathematical analysis of uniform spheres tumbling in a linear shear field has estimated that the average intercellular contact duration during cell-cell collisions varies inversely with shear rate as approximately 2.62/shear rate.14 36 Based on this relation and the current observation that adhesion was abrogated at shear rates more than 400 s−1, it follows that a minimum contact duration of 6.5 ms (∼2.62/400 seconds) was required for β2 integrin to bind in sufficient numbers to support neutrophil adhesion. Maximum adhesion was measured at a shear rate of 100 s−1, corresponding to a contact durations of approximately 25 ms.
This estimate of the duration of intercellular contact required for β2-integrin–mediated neutrophil adhesion is consistent with in vitro and in vivo observations. Parallel-plate flow chamber geometries are widely used to quantitate the recruitment of leukocytes from the flow stream to substrates expressing physiologic ligands. The contact duration between a cell and the substrate in this chamber is estimated to vary with shear rate as approximately 0.1/shear rate.37 Based on our estimate of the minimum contact duration for adhesion in sheared cell suspensions, we estimate that β2-integrin–mediated adhesion in a flow chamber can occur only at shear rates less than 15 s−1 (∼0.1/6.5 ms). This prediction is consistent with measurements of phorbol myristate acetate (PMA)-stimulated neutrophils becoming arrested on a planar bilayer expressing ICAM-1.8In these studies, adhesion was observed only at shear stresses less than 0.2 dyn/cm2 and corresponding shear rates of less than approximately 28 s−1.
The current data provides a quantitative framework to interpret the molecular dynamics that regulate the transition from selectin-dependent rolling to integrin-mediated leukocyte arrest on activated endothelium. Selectin-mediated rolling velocities of neutrophils typically range from 2 to 15 μm/s in vitro5,38 and 40 to 70 μm/s in vivo.39,40 In the multistep paradigm of leukocyte recruitment to the vessel wall, cell rolling is thought to increase the duration and extent of membrane contact on the endothelium, thereby facilitating the binding of inflammatory mediators such as platelet-activating factor (PAF) and interleukin-8 (IL-8). This in turn may enable activation and upregulation of β2-integrin affinity, a requirement of cell arrest and transmigration.41,42 In recent in vivo studies, it was estimated that during rolling, approximately 10% of the neutrophil surface is in contact with the endothelium.43 Assuming a maximum rolling velocity for neutrophils to be 70 μm/s, we estimate that the average contact duration between a rolling neutrophil and the endothelium is approximately 40 ms (=cell circumference · fraction of surface in contact/rolling velocity). This interval exceeds our estimate for the minimum time required for β2-integrin–mediated firm adhesion (∼6.5 ms) by approximately sixfold, and it corresponds to efficiencies that we would observe at shear rates of approximately 50 s−1 (>0.2). Hence, we hypothesize that if endothelial ligand density is not limiting, the distance that a neutrophil rolls before firm arrest in the vasculature is primarily determined by the extent of inflammatory mediator binding and the time required for signal transduction and activation of β2 integrin, rather than the rolling velocity of the cell.
High shear stresses decrease neutrophil-E3-ICAM adhesion.
While the adhesion efficiency for neutrophil-neutrophil and neutrophil–E3-ICAM aggregates was similar at low shear stress, the two interactions were different at a shear stress more than 2 dyn/cm2. Above this critical shear stress, the efficiency of neutrophil–E3-ICAM and not neutrophil-neutrophil adhesion decreased on increasing viscosity. Apparently, neutrophil–E3-ICAM adhesion was more susceptible to dissociation by tensile loading than homotypic neutrophil adhesion. This behavior was not unique to either α-subunit, since the efficiency of both LFA-1– and Mac-1–mediated adhesion decreased equally on increasing shear stress. There are two possible explanations for this phenomenon. (1) The number of bonds formed between neutrophils and E3-ICAM was lower, or the tensile strength of these bonds was weaker than that between two neutrophils. (2) The distribution of adhesion sites over the membrane contact area, or the stress acting on these bonds was different in the two adhesion systems. Several lines of evidence demonstrated that adhesion was not limited by the number of ICAM-1 receptors. If adhesion was limited by the availability of ICAM-1, we would expect more than the 15% decrease in adhesion observed when 50% of the ICAM-1 sites were blocked. Furthermore, analysis of the intercellular contact area by transmission electron microscopy (TEM) demonstrated that there was no apparent difference between the length and interdigitation of the contact regions between the neutrophil-neutrophil and neutrophil–E3-ICAM aggregates. The data suggest that the overall tensile strength of intercellular β2-integrin bonds, including the effects of bond number, distribution, and bond strength, is greater in the case of neutrophil-neutrophil adhesion as compared with neutrophil–E3-ICAM adhesion. Furthermore, it also demonstrates that changing the level of hydrodynamic shear may alter the critical parameter (intercellular contact duration or tensile stress) that controls neutrophil adhesion kinetics.
The lifetime for adhesion via LFA-1 and Mac-1.
Neutrophil adhesion is typically reversible over the time course of chemotactic stimulation under conditions of shear. This is a requirement for the transition from cell arrest to diapedesis and chemotaxis at sites of tissue inflammation. The current data indicate that the transience in neutrophil adhesivity was not due to a downregulation in the number of integrin receptors on the cell surface, since the expression levels of LFA-1 remained unchanged following stimulation. In contrast, Mac-1 was upregulated by at least 100% within 2 minutes of FMLP stimulation. Other possible explanations for the decreased adhesivity include (1) downregulation in the affinity of β2 integrin; (2) an increase in the tensile forces acting on each receptor over the time course of stimulation, which could be achieved by diffusion of β2-integrin sites to a smaller contact area on the neutrophil,44 and additionally by the generation of active forces exerted through the cytoskeleton45; and (3) activated Mac-1 sites may be prevented from engaging ligand on the E3-ICAM cells by competitive binding of endogenous ligands such as elastase released after neutrophil stimulation.35
The decrease in neutrophil adhesivity following stimulation was observed for both of the β2-integrin subunits. Adhesion mediated by LFA-1–ICAM-1 bonds decreased to approximately zero by 120 seconds of chemotactic stimulation. In contrast, adhesion mediated via Mac-1 decreased approximately four times slower. One possible explanation for the differential behavior of the β2-integrin subunits may be due to the adhesive contribution of the upregulated Mac-1 receptors, which are expressed on the neutrophil surface following stimulation.46Furthermore, receptor redistribution and/or clustering of LFA-1 and Mac-1 may also contribute to their differential contribution to cell adhesion. It has been recently reported that β2integrins (LFA-1 and Mac-1) are expressed predominantly on the cell body and not on the microvilli of resting polymorphonuclear neutrophils.47,48 Upon activation, Mac-1 expression is rapidly upregulated and it appears on the microvilli, cell surface ruffles, and on the cell body. The effect of surface distribution and the dynamics of redistribution to neutrophil adhesion is currently an issue being addressed in our laboratory. We have measured an identical decrease in LFA-1 adhesivity in a previous study of homotypic neutrophil aggregation where LFA-1 bound ICAM-3 on an apposing neutrophil (Taylor et al, in preparation). In this study, the exponential decay constant, α measured for LFA-1–dependent and Mac-1–dependent homotypic adhesion, was within 10% of the values reported in Table 1. This comparison suggests that the dynamics of LFA-1 adhesion to either ICAM-1 during heterotypic aggregation, or ICAM-3 during homotypic aggregation is dependent on the activation state and binding affinity of LFA-1, rather than the nature of the counterstructure. Although previous studies with lymphocytes have shown that LFA-1 alters its activation state following stimulation with either phorbol esters or antibody,49 50 this is the first study to demonstrate that neutrophil LFA-1–ICAM-1 adhesivity decreases following chemotactic stimulation.
In this study, we have introduced a new technique that combines cone-plate viscometry and flow cytometry to reveal the distinct nature of adhesion via LFA-1 and Mac-1. Consistent with published reports of β2-integrin–dependent neutrophil arrest on ICAM-1 bearing human umbilical vein endothelial cells, both activated LFA-1 and Mac-1 contributed equally to adhesion within the first minute of chemotactic stimulation.11 Furthermore, we show here that with increased time, Mac-1 becomes the predominant β2 integrin mediating neutrophil adhesion. Regardless of whether LFA-1 is binding to ICAM-1 in heterotypic interactions or ICAM-3 in homotypic aggregation, its contribution to the efficiency of capture and strength of adhesion rapidly decreases by 2 minutes of chemotactic stimulation. One implication is that the distinct functions attributed to LFA-1 and Mac-1 in mediating extravasation (LFA-1–predominant) and secretory and phagocytic processes (Mac-1–predominant)51 may be mediated by their differential responses over time of stimulation and level of hydrodynamic shear.
ACKNOWLEDGMENT
We acknowledge Dr J. David Hellums for the use of his laboratory facilities, Lisa Thurmon for assisting with the cell culture, and Evelyn Brown for preparing the TEM samples.
Supported by National Institutes of Health Grants No. AI23521, 5P50NS23327, AI31652, and HL42550. S.I.S. is an Established Investigator of the American Heart Association and a Fellow of the Whitaker Biomedical Foundation. A.R.B. is the recipient of a grant from the Methodist Hospital Foundation.
Address reprint requests to Scott I. Simon, PhD, Section of Leukocyte Biology, 1100 Bates, Room 6014, Houston, TX 77030-2600.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.








This feature is available to Subscribers Only
Sign In or Create an Account Close Modal