Abstract
Systemic administration of ISIS 2302, a 20-mer antisense phosphorothioate oligonucleotide targeting human intercellular adhesion molecule-1 mRNA, causes prolongation of plasma clotting times in both monkey and human studies. The anticoagulant effects of ISIS 2302 were investigated with both in vitro coagulation assays in human plasma and purified enzyme systems. At high oligonucleotide plasma concentrations (>100 μg/mL), prolongation of the prothrombin and thrombin times was observed. In a thrombin time assay using purified components, high concentrations of ISIS 2302 inhibited thrombin clotting activity both by stimulating inhibition by heparin cofactor II and directly competing with fibrinogen for binding to anion binding exosite I. In contrast, low concentrations of ISIS 2302 (<100 μg/mL) showed a selective, linear prolongation of the activated partial thromboplastin time (PTT). The rate limiting effect of 50 μg/mL ISIS 2302, which prolonged the PTT to 1.5 times control, was identified by sequential modification of the clotting assay. Delaying addition of oligonucleotide until after contact activation failed to correct prolongation of the PTT. The calcium-dependent steps of the intrinsic pathway were individually assessed by adding sufficient activated coagulation factor to correct the PTT in plasma deficient in that specific factor. Addition of factor XIa, IXa, VIIIa, or Va failed to correct the PTT in the presence of ISIS 2302. In contrast, 0.2 nmol/L factor Xa corrected prolongation of the PTT in factor X–deficient plasma with or without oligonucleotide present. ISIS 2302 (50 μg/mL) did not prolong a modified Russel viper venom time, suggesting no significant inhibition of prothrombinase. Thus, 50 μg/mL ISIS 2302 prolonged the PTT by selectively inhibiting intrinsic tenase activity. ISIS 2302 showed partial inhibition of intrinsic tenase activity (to approximately 35% of control) at clinically relevant oligonucleotide concentrations in a chromogenic assay. This activity was oligonucleotide sequence–independent but required the phosphorothioate backbone, suggesting that inhibition of intrinsic tenase is a general property of this class of oligonucleotides. These results are relevant to both the therapeutic use of phosphorothioate oligonucleotides and the potential design of inhibitors of the intrinsic tenase complex, a novel target for anticoagulation.
© 1998 by The American Society of Hematology.
ANTISENSE THERAPY targets specific mRNA sequences in an attempt to selectively suppress expression of the corresponding protein and inhibit disease processes. This approach requires knowledge of the disease process and the relevant target gene sequence but promises theoretical specificity in the targeting of pathophysiologic mechanisms.1 Multiple antisense compounds are currently in phase I or II trials for indications in infectious disease, vascular restenosis, cancer, and inflammation.2Intercellular adhesion molecule-1 (ICAM-1), a receptor for the β2-integrins LFA-1 (CD11a/CD18) and Mac-1 (CD11b/CD18), participates in leukocyte emigration in response to inflammatory stimuli. ICAM-1 expression is induced by inflammatory mediators (tumor necrosis factor, interleukin-1, interferon-γ) and upregulated in a number of inflammatory disease states.3,4 Antisense oligonucleotides that inhibit ICAM-1 expression are efficacious in murine models of carrageenan-induced inflammation, collagen-induced arthritis, dextran sulfate–induced inflammatory bowel disease, and cardiac allograft rejection.5 ISIS 2302, an antisense oligonucleotide that inhibits human ICAM-1 expression, is currently in phase II trials for Crohn’s disease, ulcerative colitis, rheumatoid arthritis, psoriasis, and renal transplant rejection.2 6
Most first-generation antisense compounds (including ISIS 2302) are phosphorothioate oligonucleotides in which a nonbridging oxygen in the phosphodiester backbone is replaced with sulfur. This modification results in increased resistance to exonucleases while preserving high-affinity binding to the complementary RNA sequence. The phosphorothioate backbone supports RNAase H–dependent cleavage of oligo-RNA duplexes (an important antisense mechanism) and heavily influences the pharmacological behavior of these oligonucleotides. This modification also increases binding to a variety of protein targets, which may result in hybridization-independent effects.1These hybridization-independent effects may result in important side effects of therapy. Second- and third-generation antisense compounds contain alternative backbone or 2′ sugar modifications that may show less protein binding (and fewer hybridization-independent effects) than the phosphorothioates. However, these oligonucleotide modifications do not appear to support RNAase H–dependent degradation of target mRNA.7-9 The in vivo efficacy of these alternative modifications and their antisense mechanisms awaits confirmation.
Prolongation of the activated partial thromboplastin time (PTT) is commonly observed following administration of ISIS 2302 in both cynomolgus monkeys and humans.6,10 In the monkeys, prolongation of the thrombin time and complement activation have also been observed with doses greater than 3 mg/kg administered by 2-hour intravenous infusion. Prolongation of the PTT correlates directly with plasma concentrations of phosphorothioate oligonucleotides in the cynomolgus monkeys.1,11,12 The basis for the anticoagulant effects of these oligonucleotides was previously unknown. Phosphorothioate oligonucleotides and the anticoagulant drug heparin are both linear polyanionic polymers, suggesting the potential for binding to similar sites on protein surfaces. Several examples of phosphorothioate oligonucleotides interacting with heparin-binding proteins already exist, including basic fibroblast growth factor and platelet-derived growth factor.1 13 Likewise, numerous coagulation proteases (thrombin, factors Xa, IXa, XIa), cofactors (factors VIII and V), and serpin inhibitors (antithrombin and heparin cofactor II) can bind to heparin or heparan sulfate. Thus, these proteins represent potential targets for the interaction of phosphorothioate oligonucleotides with the coagulation cascade.
This investigation examines the anticoagulant effects of the phosphorothioate oligonucleotide ISIS 2302. The effects of this oligonucleotide were analyzed in both plasma-based clotting assays and purified enzyme systems to define potential anticoagulant mechanisms. These investigations show that ISIS 2302 selectively inhibits the intrinsic tenase complex (factor IXaB, factor VIIIa, phospholipid, and calcium) at plasma oligonucleotide concentrations that prolong the PTT to an extent similar to that observed in vivo (1.5 times control).6 This activity is independent of oligonucleotide sequence but requires the phosphorothioate backbone, suggesting inhibition of intrinsic tenase is a general property of this class of oligonucleotides. Higher plasma concentrations of ISIS 2302 also inhibit thrombin by stimulating heparin cofactor II (HCII) activity or directly competing with fibrinogen for binding. These findings are relevant to both the therapeutic use of systemically administered phosphorothioate oligonucleotides and the design of potential inhibitors of the intrinsic tenase complex, a novel target for anticoagulant therapy.
MATERIALS AND METHODS
Reagents.
Lyophilized, pooled normal human plasma (Dade Ci-trol I), rabbit brain partial thromboplastin containing ellagic acid (Dade Actin), and rabbit brain thromboplastin (Dade Thromboplastin C Plus) were obtained from Baxter Diagnostics, Inc (Deerfield, IL). Rabbit brain cephalin, recombinant hirudin, and reptilase (Atroxin) were purchased from Sigma (St Louis, MO). Factor-deficient plasmas (V, VIII, IX, X, and XI) were purchased from George King Biomedical, Inc (Overland Park, KS). HCII-immunodepleted plasma was obtained from Affinity Biologicals Inc (Hamilton, Ontario, Canada). Human factors XIa, IXaB, X, Xa, prothrombin, fibrinogen, HCII, and purified Russel viper venom Xa activator (RVV-Xa) were purchased from Enzyme Research (South Bend, IN). Human thrombin was purified from prothrombin activated withOxyuranus scutellatus venom preabsorbed with Amberlite CG-50 resin (Sigma) as previously described.14 Human factor V was obtained from Hematologic Technologies, Inc (Essex Junction, VT). Albumin-free factor VIII (Alphanate) was generously provided by the Alpha Therapeutic Corporation (Los Angeles, CA). Human antithrombin (ATIII) was obtained from Doug Tollefsen (Washington University, St Louis, MO). The chromogenic substrates S-2765 and S-2238 were purchased from Kabi-Pharmacia (Franklin, OH). Unfractionated, clinical-grade porcine heparin was purchased from Elkins-Sinn (Cherry Hill, NJ). All other chemicals were at least reagent grade and purchased from major suppliers.
Oligonucleotides.
All oligonucleotides were provided by ISIS Pharmaceuticals (Carlsbad, CA). ISIS 2302 (antisense) is a 20-mer phosphorothioate oligodeoxyribonucleotide molecule with the base sequence (5′-GCCCAAGCTGGCATCCGTCA-3′). Additional oligonucleotides include the phosphorothioate sense (5′-TGACGGATGCCAGCTTGGGC-3′) and scrambled versions (5′-GACGCATCGCGCCTACATCG-3′) of ISIS 2302, and a phosphodiester analogue of ISIS 2302.
Molecular masses (kD) and extinction coefficients (ε0.1%).
These values are: human factor XIa, 160,000 and 1.34; factor IXaB, 46,000 and 1.43; factor X, 58,900 and 1.16; factor Xa, 46,000 and 1.40; factor V, 330,000 and 0.96; thrombin 36,700 and 1.83; ATIII, 58,000 and 0.62; HCII, 65,600 and 0.59; and Russel viper venom factor X activator, 79,000 and 1.34.
Clotting times in human plasma.
All clotting times were performed in polystyrene cuvettes with a BBL Fibro System fibrometer (Becton Dickinson, Lincoln Park, NJ) warmed to 37°C. Dilutions of ISIS 2302 were made in phosphate-buffered saline. Prothrombin times (PT) were performed by incubating ISIS 2302 (0.05 mL) with normal pooled human plasma (0.1 mL) for one minute at 37°C, adding rabbit brain thromboplastin (0.15 mL), and determining the clotting time. The rabbit brain thromboplastin was resuspended in 75% of the standard volume to compensate for the reduced volume used in the PT assay. Activated PTTs were performed by incubating ISIS 2302 (0.05 mL), human plasma (0.1 mL), and rabbit brain partial thromboplastin (0.1 mL) for 3 minutes at 37°C; adding 0.04 mol/L CaCl2 (0.05 mL); and determining the clotting time. To assess the effect of ISIS 2302 on contact activation, the PTT was performed as above with or without 50 μg/mL of plasma oligonucleotide present, extending the first stage incubation from 3 to 10 minutes. Alternatively, addition of ISIS 2302 was delayed until just before recalcification. To assess the effect of ISIS 2302 on the calcium-dependent steps, the PTT was performed in specific factor-deficient plasmas (factor XI, IX, VIII, or V) as above with or without 50 μg/mL of plasma ISIS 2302. The minimal amount of activated coagulation factor (factor XIa, IXa, VIIIa, or Va) to correct the PTT to control levels in the absence of ISIS 2302 was added during recalcification, and the clotting time was determined. A modified Russel viper venom time was performed by incubating ISIS 2302 (0.05 mL), pooled normal or factor V–deficient human plasma (0.1 mL), 10% (vol/vol) rabbit brain cephalin (0.05 mL), and 1.2 nmol/L RVV-Xa (0.05 mL) for 30 seconds at 37°C; adding 0.04 mol/L CaCl2(0.05 mL); and determining the clotting time. Thrombin or reptilase times in human plasma were performed by incubating ISIS 2302 (0.05 mL) with human plasma (0.2 mL) for 3 minutes at 37°C. Human thrombin (0.05 mL, final concentration approximately 15 nmol/L) or reptilase (0.05 mL, 10 μg/mL) was added and the clotting time determined.
Factor V and VIII activation for PTT assays.
Factor V was activated with 0.2 nmol/L thrombin for 10 minutes at room temperature in 0.15 mol/L NaCl, 20 mmol/L HEPES, pH 7.4, 5 mmol/L CaCl2, and 0.01% Tween 20. Hirudin (0.4 nmol/L) was added to neutralize thrombin, and the activation mix was added to plasma at the time of recalcification. Factor VIII activity was quantitated by constructing a standard curve for clotting activity with serial dilutions of normal plasma into factor VIII–deficient plasma.A clotting activity of 1 U/mL was assumed equivalent to 0.7 nmol/L plasma factor VIII concentration.15 Factor VIII was activated with 40 nmol/L thrombin for 30 seconds at room temperature in the same buffer as above. Hirudin (80 nmol/L) was added to neutralize thrombin, and the activation mix was added immediately to plasma with recalcification. Controls without hirudin gave equivalent clotting times.
Purified thrombin time.
ISIS 2302 was incubated in 180 μL of clotting buffer (150 mmol/L NaCl, 10 mmol/L CaCl2, 10 mmol/L imidazole-HCl, pH 7.4, 6.6% PEG-8000) for 3 minutes at 37°C, followed by addition of20 μL plasma-derived or recombinant thrombin (approximately 20 nmol/L final concentration). The assay was initiated by addition of human fibrinogen (50 μL of a 2 mg/mL solution) and the clotting time determined. To assess for potential cofactor activity, either 500 nmol/L ATIII or 250 nmol/L HCII was included in the buffer, fibrinogen was added as above, and the clotting time was initiated by adding plasma-derived thrombin. Controls without ATIII or HCII gave identical clotting times when either fibrinogen or thrombin was added last.
Expression and purification of recombinant thrombins.
The plasmid constructs pCMVPT (wild-type), pR70E, and pR89E, stable transfection into CV-1 (wild-type, R70E) or BHK (R89E) cell lines and purification from conditioned media, were described previously.14 16 Thrombin amino acid residues are numbered sequentially from the first residue of the B chain. This corresponds to the alternative chymotrypsin numbering system (in parentheses) as follows: R70 (75) and R89 (93).
Chromogenic assay for intrinsic tenase activity.
A chromogenic assay for intrinsic tenase complex activity was performed under conditions of limiting factor VIIIa concentration, as previously described, with minor modifications.17 Albumin-free human factor VIII (4.2 nmol/L) was activated with 40 nmol/L thrombin in 0.15 mol/L NaCl, 20 mmol/L HEPES, pH 7.4, 5 mmol/L CaCl2, and 0.01% Tween for 30 seconds at room temperature. Thrombin was neutralized with recombinant hirudin (60 nmol/L), and the activation mixture was diluted 50-fold into 0.15 mol/L NaCl, 20 mmol/L HEPES, pH 7.4, 2 mmol/L CaCl2, and 0.1% PEG-8000 buffer containing 84 pmol/L human factor VIIIa, 3 nmol/L human factor IXaB, and 2% (vol/vol) rabbit brain cephalin. Human factor X was immediately added to 300 nmol/L, and the reaction was sampled (50 μL) at 15, 30, 45, and 60 seconds into 10 μL of 0.25 mol/L EDTA, pH 7.4. The chromogenic substrate S-2765 (100 μL) was added to 300 μmol/L with polybrene (70 μg/mL) present, and the amount of factor Xa was determined by comparing the rate of substrate hydrolysis (change in absorbance at 405 nm over 2 minutes) in a kinetic microtiter plate reader (Vmax, Molecular Devices Corp, Menlo Park, CA) to a standard curve constructed with purified factor Xa. The initial rate of intrinsic tenase complex activity (factor Xa generation) was determined by plotting factor Xa concentration versus time under conditions where less than 10% total substrate cleavage occurred. The rate of factor Xa generation (nmol/L/min) was linear over the time course of the assay (0 to 60 seconds), and with respect to factor VIIIa concentration (0 to 140 pmol/L).
RESULTS
Effect of ISIS 2302 on global tests of coagulation.
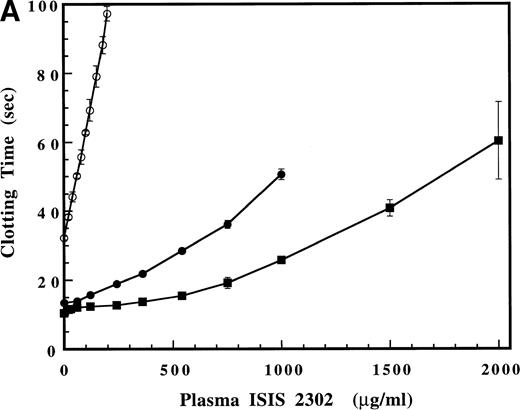
Significant prolongation of the PTT (to 1.5 times control) is observed during in vivo administration of ISIS 2302 (2 mg/kg over 2 hours) to humans subjects.6 This prolongation of the PTT correlates with the effects observed at similar plasma oligonucleotide concentrations in cynomolgus monkeys.11 Thus, the effect of increasing concentrations of ISIS 2302 on the PT, activated PTT, and thrombin time was evaluated in pooled normal human plasma (Fig 1A and B). ISIS 2302 was expressed as the equivalent plasma oligonucleotide concentration (not assay volume concentration). At low plasma concentrations of oligonucleotide (0 to 100 μg/mL), the predominant effect was a rapid, linear prolongation of the PTT (Fig 1B). Gradual prolongation of the PT (100 to 1,000 μg/mL) and thrombin time (>500 μg/mL) was observed at higher plasma concentrations of ISIS 2302 (Fig 1A). The selective prolongation of the PTT at low concentrations of ISIS 2302 (<100 μg/mL) suggests inhibition of factor(s) within the intrinsic coagulation pathway.
(A) Effect of ISIS 2302 on the prothrombin (•), activated partial thromboplastin (PTT) (○), and thrombin times (▪) in normal pooled human plasma. (B) Detail of the plasma oligonucleotide concentration range that shows selective prolongation of the PTT (see text). Clotting times are expressed as the mean of triplicate determinations with error bars representing ±2 SD.
(A) Effect of ISIS 2302 on the prothrombin (•), activated partial thromboplastin (PTT) (○), and thrombin times (▪) in normal pooled human plasma. (B) Detail of the plasma oligonucleotide concentration range that shows selective prolongation of the PTT (see text). Clotting times are expressed as the mean of triplicate determinations with error bars representing ±2 SD.
Effect of ISIS 2302 on thrombin, fibrinogen, and inhibition by ATIII and HCII.
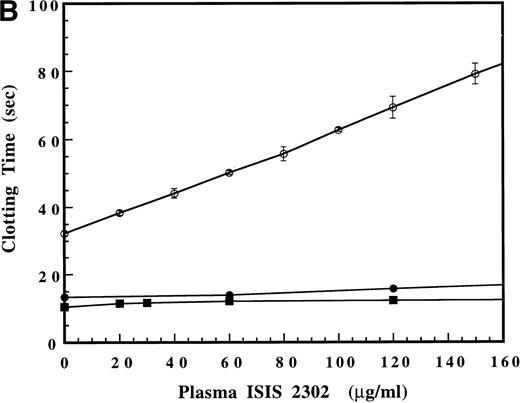
The effect of ISIS 2302 on fibrinogen cleavage was examined by comparing thrombin and reptilase clotting times in normal pooled human plasma. The amount of human thrombin added was reduced to match control values with the reptilase clotting time (18 to 20 seconds). Increasing amounts of oligonucleotide did not prolong the reptilase time, even at concentrations that significantly prolonged the thrombin time (Fig 2). Reptilase and thrombin cleave the identical peptide bond in fibrinogen (arg16-gly17) to release fibrinopeptide A.18 The failure of ISIS 2302 to prolong the reptilase time suggests that the oligonucleotide does not affect the ability of fibrinogen to be cleaved but interacts directly with thrombin to inhibit substrate recognition.
Comparison of the effect of ISIS 2302 on the reptilase (•) and dilute thrombin times (○). The thrombin concentration was reduced to give control values equivalent to the reptilase assay (18 to 19 seconds). Clotting times are expressed as the mean of triplicate determinations with error bars representing ±2 SD.
Comparison of the effect of ISIS 2302 on the reptilase (•) and dilute thrombin times (○). The thrombin concentration was reduced to give control values equivalent to the reptilase assay (18 to 19 seconds). Clotting times are expressed as the mean of triplicate determinations with error bars representing ±2 SD.
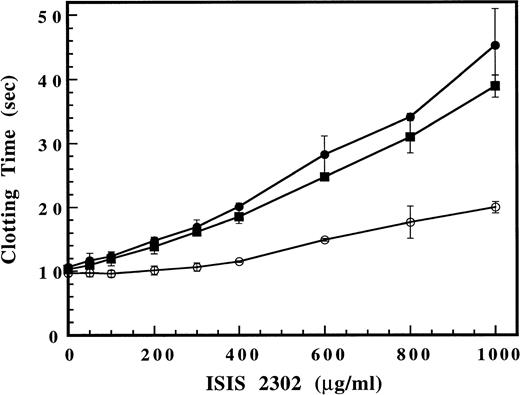
The mechanism for the inhibition of fibrinogen clotting by ISIS 2302 was examined in a purified thrombin time assay using selected recombinant thrombins with arginine to glutamate substitutions in anion binding exosite I (R70E) or exosite II (R89E). ISIS 2302 showed nearly identical dose-dependent prolongation of the thrombin time with both wild-type (Fig 3) and plasma-derived thrombin (Fig 4). This result indicates that ISIS 2302 can directly inhibit fibrinogen cleavage by thrombin and does not require a plasma cofactor. A similar prolongation of the thrombin time by ISIS 2302 was observed with thrombin R89E (exosite II) at slightly higher oligonucleotide concentrations. In contrast, the dose response to ISIS 2302 showed a marked shift to the right for thrombin R70E (exosite I), indicating resistance to oligonucleotide effects. Residue Arg 70 in anion binding exosite I of thrombin is not required for fibrinogen cleavage but is located in close proximity to the fibrinogen binding site.16 The resistance of thrombin R70E to inhibition by the oligonucleotide suggests that the mutation disrupts a binding site for ISIS 2302 in exosite I of thrombin. The comparative lack of effect of an identical mutation in exosite II (R89E in the heparin binding site) suggests this is not simply due to a change in total protein charge. Thus, ISIS 2302 can inhibit clotting by directly competing with fibrinogen for binding to exosite I of thrombin. This mechanism for thrombin inhibition is observed with other polyanions, including a 15-mer phosphodiester oligonucleotide aptamer that binds specifically to exosite I.19
Effect of ISIS 2302 on fibrinogen clotting in a purified thrombin time assay. Purified recombinant wild-type thrombin (•), thrombin R70E (exosite I mutant) (○), or thrombin R89E (exosite II mutant) (▪) was added to clotting buffer (see Materials and Methods) containing increasing amounts of ISIS 2302 (200 μL). The assay was initiated by addition of 50 μL of 2 mg/mL human fibrinogen and the time to clot formation determined. Clotting times are expressed as the mean of triplicate determinations with error bars representing ±2 SD.
Effect of ISIS 2302 on fibrinogen clotting in a purified thrombin time assay. Purified recombinant wild-type thrombin (•), thrombin R70E (exosite I mutant) (○), or thrombin R89E (exosite II mutant) (▪) was added to clotting buffer (see Materials and Methods) containing increasing amounts of ISIS 2302 (200 μL). The assay was initiated by addition of 50 μL of 2 mg/mL human fibrinogen and the time to clot formation determined. Clotting times are expressed as the mean of triplicate determinations with error bars representing ±2 SD.
Effect of ATIII and HCII on inhibition of clotting activity by ISIS 2302 in a purified thrombin time assay. The clotting time for plasma-derived thrombin was determined as described in the legend of Fig 3, except that thrombin was added last to initiate the clotting time. The ISIS 2302 dose response was compared in the absence of inhibitor (•), with 500 nmol/L ATIII (○), or 250 nmol/L HCII (▪). Clotting times are expressed as the mean of triplicate determinations with error bars representing ±2 SD.
Effect of ATIII and HCII on inhibition of clotting activity by ISIS 2302 in a purified thrombin time assay. The clotting time for plasma-derived thrombin was determined as described in the legend of Fig 3, except that thrombin was added last to initiate the clotting time. The ISIS 2302 dose response was compared in the absence of inhibitor (•), with 500 nmol/L ATIII (○), or 250 nmol/L HCII (▪). Clotting times are expressed as the mean of triplicate determinations with error bars representing ±2 SD.
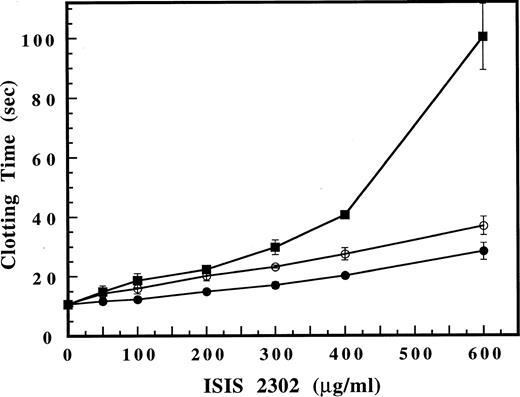
Phosphorothioate oligonucleotides are linear, polyanionic polymers, suggesting the possibility that their anticoagulant effects may involve mechanisms similar to glycosaminoglycans. The ability of ATIII and HCII to act as plasma cofactors for ISIS 2302 was addressed by addition of these serpins to the purified thrombin time. Addition of ATIII (500 nmol/L) slightly prolonged the baseline clotting time but had no significant effect on the dose response to ISIS 2302 (Fig 4). Likewise, ISIS 2302 failed to accelerate the inhibition of thrombin or factor Xa by ATIII with purified components under pseudo–first-order conditions (data not shown). These results suggest that ATIII is not a plasma cofactor for ISIS 2302. In contrast, HCII (250 nmol/L) did not affect the baseline clotting time but markedly enhanced the ISIS 2302 dose response (Fig 4). This shift in the oligonucleotide dose response suggests that inhibition of thrombin clotting activity by ISIS 2302 in plasma depends largely on the presence of HCII. Likewise, immunodepletion of HCII from plasma markedly reduced the ability of the oligonucleotide to prolong the thrombin time, confirming the role of HCII as a plasma cofactor for ISIS 2302 (Fig 5).
Effect of ISIS 2302 on the plasma thrombin time in parent (○) and HCII immunodepleted (•) plasma. Clotting times are expressed as the mean of triplicate determinations with error bars representing ±2 SD.
Effect of ISIS 2302 on the plasma thrombin time in parent (○) and HCII immunodepleted (•) plasma. Clotting times are expressed as the mean of triplicate determinations with error bars representing ±2 SD.
Effect of low doses of ISIS 2302 on the PTT.
Prolongation of the PTT is the predominant effect of ISIS 2302 at plasma concentrations below 100 μg/mL, suggesting selective inhibition of the intrinsic coagulation pathway (Fig 1B). To further evaluate effects on the intrinsic pathway, a plasma concentration of 50 μg/mL ISIS 2302 was selected. This concentration prolongs the PTT to a degree similar to that observed in phase II studies (1.5 times control).6 The rate-limiting step for clot formation in the presence of ISIS 2302 was identified by sequential modification of the PTT to bypass potential inhibitory effects of oligonucleotide. The effect of ISIS 2302 on contact activation was assessed by prolonging the incubation with contact activator (ellagic acid) or delaying addition of ISIS 2302 until after that incubation was complete (Table 1). Neither prolonged incubation with ellagic acid nor delayed addition of the oligonucleotide corrected prolongation of the PTT by ISIS 2302. Failure of these modifications to correct the PTT to control levels suggests that the rate-limiting defect induced by the oligonucleotide is distal to the contact activation system. This result also infers that ISIS 2302 inhibits a portion of the intrinsic pathway that is relevant to in vivo coagulation.
Effect of ISIS 2302 on the First Stage (Contact Activation) of the PTT
| First-Stage Incubation (min) . | Control (sec) . | 50 μg/mL ISIS 2302 (sec) . |
|---|---|---|
| 3 | 31.9 ± 0.5 | 49.2 ± 3.1 |
| 10 | 30.1 ± 0.3 | 48.9 ± 0.9 |
| ISIS 2302 added after 3 | 31.6 ± 0.3 | 48.7 ± 0.3 |
| First-Stage Incubation (min) . | Control (sec) . | 50 μg/mL ISIS 2302 (sec) . |
|---|---|---|
| 3 | 31.9 ± 0.5 | 49.2 ± 3.1 |
| 10 | 30.1 ± 0.3 | 48.9 ± 0.9 |
| ISIS 2302 added after 3 | 31.6 ± 0.3 | 48.7 ± 0.3 |
The PTT was performed as described with modification of the first stage as above. Results are expressed as the mean clotting time (seconds) ± SD.
The PTT was further modified to identify the rate-limiting step for oligonucleotide inhibition of the calcium-dependent portion of the intrinsic pathway. Briefly, sufficient activated coagulation factor (factor XIa, IXa, VIIIa, Xa, or Va) was added to plasma deficient in that factor (during recalcification) to correct the PTT to control levels in the absence of oligonucleotide. Correction of the PTT with activated factor in the presence of 50 μg/mL ISIS 2302 suggests that the oligonucleotide acts proximally to that factor activity. Likewise, failure to correct the PTT with activated factor in the presence of oligonucleotide suggests that the rate-limiting defect is distal to the activation of that factor. The addition of sufficient factor XIa, IXa, or VIIIa to correct the PTT to control levels in the absence of the oligonucleotide failed to correct the clotting time in the presence of 50 μg/mL ISIS 2302 (Table 2). The failure of these activated factors to correct the PTT suggests that the rate-limiting step in inhibition by the oligonucleotide is at or below the level of the intrinsic tenase complex (factors IXa, VIIIa, phospholipid, and calcium). Furthermore, addition of maximal amounts of plasma factor XIa (30 nmol/L) or IXa (90 nmol/L; equivalent to complete activation) cannot correct the PTT to control values, further indicating that the defect is distal to the activation of these factors (data not shown). In contrast, addition of 0.2 nmol/L factor Xa corrects prolongation of the PTT in both the absence and presence of ISIS 2302 (Table 2). The ability of subnanomolar amounts of factor Xa (<1% plasma factor X levels) to bypass the inhibitory effect of ISIS 2302 suggests that the rate-limiting step lies proximal to generation of factor Xa (ie, the intrinsic tenase complex). Thus, 50 μg/mL ISIS 2302 prolongs the PTT via inhibition of intrinsic tenase complex activity.
Effect of ISIS 2302 on the Correction of the PTT by Activated Coagulation Factor in Factor-Deficient Plasma
| Plasma . | Factor Added . | Control (sec) . | 50 μg/mL ISIS 2302 (sec) . |
|---|---|---|---|
| Normal | None | 31.9 ± 0.5 | 49.2 ± 3.1 |
| Factor XI deficient | None | 99.9 ± 4.1 | ND |
| 15 nmol/L fXIa | 31.8 ± 0.6 | 50.0 ± 1.2 | |
| Factor IX deficient | None | 102.6 ± 3.6 | ND |
| 20 nmol/L fIXa | 31.9 ± 1.0 | 52.9 ± 1.3 | |
| Factor VIII deficient | None | 82.7 ± 1.0 | ND |
| 0.01 U/mL fVIIIa | 31.1 ± 0.5 | 50.9 ± 0.5 | |
| Factor X deficient | None | 147.2 ± 11.9 | ND |
| 0.2 nmol/L fXa | 30.5 ± 0.1 | 32.1 ± 0.8 | |
| Factor V deficient | None | 183.6 ± 0.1 | ND |
| 0.015 U/mL fVa | 31.1 ± 0.3 | 48.4 ± 0.5 |
| Plasma . | Factor Added . | Control (sec) . | 50 μg/mL ISIS 2302 (sec) . |
|---|---|---|---|
| Normal | None | 31.9 ± 0.5 | 49.2 ± 3.1 |
| Factor XI deficient | None | 99.9 ± 4.1 | ND |
| 15 nmol/L fXIa | 31.8 ± 0.6 | 50.0 ± 1.2 | |
| Factor IX deficient | None | 102.6 ± 3.6 | ND |
| 20 nmol/L fIXa | 31.9 ± 1.0 | 52.9 ± 1.3 | |
| Factor VIII deficient | None | 82.7 ± 1.0 | ND |
| 0.01 U/mL fVIIIa | 31.1 ± 0.5 | 50.9 ± 0.5 | |
| Factor X deficient | None | 147.2 ± 11.9 | ND |
| 0.2 nmol/L fXa | 30.5 ± 0.1 | 32.1 ± 0.8 | |
| Factor V deficient | None | 183.6 ± 0.1 | ND |
| 0.015 U/mL fVa | 31.1 ± 0.3 | 48.4 ± 0.5 |
PTT was performed as described (see Materials and Methods) in normal or factor-deficient plasma in the presence or absence of oligonucleotide. Sufficient activated coagulation factor was added during recalcification to correct the clotting time to control values without oligonucleotide present. Clotting times are expressed as the mean (sec) ± SD.
Abbreviation: ND, not determined.
The determination that intrinsic tenase activity is rate limiting in the presence of ISIS 2302 does not rule out additional effects on downstream enzyme complexes (ie, the prothrombinase complex). Addition of sufficient factor Va to correct the PTT in factor V–deficient plasma to control levels failed to do so in the presence of the oligonucleotide (Table 2). In principle, this may result from insufficient generation of factor Xa by the intrinsic tenase complex or from additional inhibitory effects of the oligonucleotide on prothrombinase complex activity. To assess the effect of ISIS 2302 on plasma prothrombinase activity, a modified Russel viper venom time was performed. Purified factor X activator from Russel viper venom (RVV-Xa) and rabbit brain cephalin were added to pooled normal human plasma in the presence and absence of 50 μg/mL ISIS 2302, followed by recalcification (Table 3). No difference in the clotting times was observed, suggesting that 50 μg/mL ISIS 2302 does not significantly inhibit the prothrombinase complex. The clotting time was markedly prolonged when performed in factor V–deficient plasma, indicating that the assay is dependent on formation of the prothrombinase complex (factors Xa, Va, phospholipid, and calcium) rather than excess factor Xa activation by the venom. Thus, the failure of factor Va to correct the PTT to control levels in the presence of oligonucleotide is secondary to inadequate generation of factor Xa by the intrinsic tenase complex. The lack of effect of 50 μg/mL ISIS 2302 on plasma prothrombinase activity (Table 3), and minimal effect on plasma thrombin time (Fig 1B), confirms that prolongation of the PTT is primarily due to inhibition of intrinsic tenase complex activity.
Effect of ISIS 2302 on the Modified Russel Viper Venom Time
| Plasma . | RVV-Xa . | Control (sec) . | 50 μg/ml ISIS 2302 . |
|---|---|---|---|
| Normal | 0.2 nmol/L | 32.1 ± 0.8 | 32.2 ± 0.6 |
| FV deficient | 0.2 nmol/L | >200 | ND |
| Plasma . | RVV-Xa . | Control (sec) . | 50 μg/ml ISIS 2302 . |
|---|---|---|---|
| Normal | 0.2 nmol/L | 32.1 ± 0.8 | 32.2 ± 0.6 |
| FV deficient | 0.2 nmol/L | >200 | ND |
The factor X activator purified from Russel viper venom and 2% (vol/vol) rabbit brain cephalin were incubated with normal or factor V–deficient plasma for 30 seconds and recalcified as described. The clotting times are expressed as the mean (sec) ± SD.
Abbreviation: ND, not determined.
Effect of ISIS 2302 on intrinsic tenase activity.
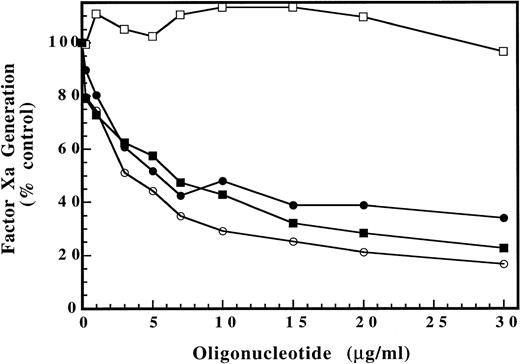
To further characterize the inhibitory effects of ISIS 2302, a chromogenic assay designed to measure the rate of factor Xa generation by the intrinsic tenase complex was used.17 A limiting amount of thrombin-activated human factor VIIIa was added to a reaction mix containing human factor IXaB, rabbit brain cephalin, and buffer containing 2 mmol/L CaCl2. Human factor X was immediately added and the reaction mixture sampled over time to determine the rate of factor Xa generation by chromogenic substrate cleavage (see Materials and Methods). ISIS 2302 markedly inhibited intrinsic tenase activity (factor Xa generation), reaching near maximal effect at 10 to 15 μg/mL oligonucleotide (Fig 6). Further increases in ISIS 2302 concentration showed approximately 35% residual activity, consistent with partial inhibition of the enzyme complex. This marked inhibition of intrinsic tenase activity at low oligonucleotide concentrations is consistent with results of the clotting assays, confirming that this enzyme complex is the major molecular target for the anticoagulant effects of ISIS 2302.
Effect of oligonucleotides on intrinsic tenase complex activity. Increasing concentrations of oligonucleotide were added to an intrinsic tenase reaction containing final concentrations of 84 pmol/L human factor VIIIa, 3 nmol/L human factor IXaB, 2% (vol/vol) rabbit brain cephalin, in 0.15 mol/L NaCl, 20 mmol/L HEPES, pH 7.4, 2 mmol/L CaCl2, and 0.1% PEG-8000 buffer. Human factor X was added to 300 nmol/L and the reaction sampled (50 μL) at 15, 30, 45, and 60 seconds into 10 μL of 0.25 mol/L EDTA, pH 8.0. The chromogenic substrate S-2765 was then added to 300 μmol/L with polybrene 70 μg/mL and the amount of factor Xa generated determined by comparison of the rate of cleavage with a standard curve (see Materials and Methods). The dose response for ISIS 2302 (antisense) (•), sense (○), scrambled (▪), and phosphodiester oligonucleotides (□) is shown. The rate of factor Xa generation (nmol/L/min) in the presence of oligonucleotide was expressed as a percentage of the control value without oligonucleotide present. Data points represent the average of two determinations.
Effect of oligonucleotides on intrinsic tenase complex activity. Increasing concentrations of oligonucleotide were added to an intrinsic tenase reaction containing final concentrations of 84 pmol/L human factor VIIIa, 3 nmol/L human factor IXaB, 2% (vol/vol) rabbit brain cephalin, in 0.15 mol/L NaCl, 20 mmol/L HEPES, pH 7.4, 2 mmol/L CaCl2, and 0.1% PEG-8000 buffer. Human factor X was added to 300 nmol/L and the reaction sampled (50 μL) at 15, 30, 45, and 60 seconds into 10 μL of 0.25 mol/L EDTA, pH 8.0. The chromogenic substrate S-2765 was then added to 300 μmol/L with polybrene 70 μg/mL and the amount of factor Xa generated determined by comparison of the rate of cleavage with a standard curve (see Materials and Methods). The dose response for ISIS 2302 (antisense) (•), sense (○), scrambled (▪), and phosphodiester oligonucleotides (□) is shown. The rate of factor Xa generation (nmol/L/min) in the presence of oligonucleotide was expressed as a percentage of the control value without oligonucleotide present. Data points represent the average of two determinations.
Effect of oligonucleotide structure on inhibition of intrinsic tenase complex activity.
The specific oligonucleotide structure required for inhibition of intrinsic tenase activity was also evaluated in the chromogenic assay. The role of oligonucleotide sequence specificity was addressed by comparison of the dose response for antisense, sense, and scrambled versions of ISIS 2302 (Fig 6). Each of these phosphorothioate oligonucleotides showed a similar dose response for inhibition of intrinsic tenase activity, indicating that a specific oligonucleotide base sequence was not required. The role of the oligonucleotide backbone was addressed by comparison of ISIS 2302 with the identical phosphodiester oligonucleotide (Fig 6). The phosphodiester analogue of ISIS 2302 did not show any significant inhibitory activity, suggesting that the phosphorothioate backbone was required for inhibition of intrinsic tenase. These results suggest that inhibition of the intrinsic tenase complex activity is a general property of phosphorothioate oligonucleotides. Consistent with these conclusions, prolongation of the PTT has been reported for a number of oligonucleotides of the phosphorothioate class.11
DISCUSSION
Phosphorothioate oligonucleotides exhibit a number of hybridization-independent effects modulated by protein-oligonucleotide interactions. Inhibition of coagulation is an important hybridization-independent effect of this class of oligonucleotides.1 ISIS 2302 showed two major anticoagulant effects, the predominant mechanism depending on the plasma oligonucleotide concentration. High concentrations of ISIS 2302 (>100 μg/mL) resulted in significant inhibition of fibrin clot formation by thrombin, shown by prolongation of the thrombin time (Fig 1). In part, the oligonucleotide inhibits clotting by direct interaction with exosite I of thrombin (the fibrinogen recognition site), as evidenced by a lack of effect on the reptilase time (Fig 2), dose-dependent prolongation of the purified thrombin time, and the relative resistance to inhibition of thrombin R70E (Fig 3). However, HCII appears to be a major plasma cofactor for ISIS 2302, based on the marked enhancement of the oligonucleotide dose response with addition of this serpin to the purified thrombin time (Fig 4) and the significantly reduced potency of ISIS 2302 in plasma immunodepleted of HCII (Fig 5). Glycosaminoglycans accelerate the inhibition of thrombin by HCII through an allosteric (or conformational) mechanism.20 Numerous polyanions, including polyphosphates and phosphodiester oligonucleotides, accelerate this inhibition in purified systems.21 Stimulation of HCII activity by phosphorothioate oligonucleotides in normal human plasma has not been previously reported. In contrast, ATIII is not a plasma cofactor for ISIS 2302, as it shows minimal effects on the dose response in the purified thrombin time. Unlike heparin, ISIS 2302 failed to accelerate protease inhibition by ATIII through either a conformational (factor Xa) or template mechanism (thrombin; data not shown).14,22 Thus, the predominant mechanism for prolongation of the thrombin time was stimulation of HCII activity by the oligonucleotide. However, this effect of the oligonucleotide requires plasma oligonucleotide concentrations that are at least several fold higher than commonly observed in either animal or human studies.6 11 Likewise, prolongation of the PT by ISIS 2302 suggests that additional inhibitory effects may occur at high plasma oligonucleotide concentrations (>100 μg/mL).
In contrast, low plasma concentrations of ISIS 2302 (<100 μg/mL) selectively prolonged the PTT, suggesting inhibition of the intrinsic coagulation pathway (Fig 1B). Systemic administration of ISIS 2302 (2 mg/kg over 2 hours) in humans results in transient prolongation of the PTT to approximately 1.5 times control, similar to the therapeutic target range for heparin.6 A plasma concentration of ISIS 2302 (50 μg/mL) that prolonged the PTT to 1.5 times control in vitro was analyzed in a series of modified clotting assays. The oligonucleotide did not prolong the PTT by inhibiting contact activation (Table 1), but it inhibited the calcium-dependent portion of the intrinsic pathway. Analysis of the calcium-dependent factors indicated that the rate-limiting effect of the oligonucleotide was at the level of the intrinsic tenase complex activity. This conclusion is supported by the ability to bypass the effect of ISIS 2302 with subnanomolar factor Xa and the failure of proximally acting factors (factors XIa, IXa, or VIIIa) to correct prolongation of the PTT (Table2). This concentration of factor Xa (0.2 nmol/L, <1% plasma factor X) is similar to that observed during the clotting of minimally altered whole blood, suggesting this mechanism may exist in vivo as well.23 The failure of factor VIIIa to correct the inhibition is consistent with a defect in intrinsic tenase complex activity or assembly but does not address additional potential defects in cofactor activation. Intrinsic tenase complex activity is selectively inhibited at this oligonucleotide concentration, as shown by the lack of effect on prothrombinase (Table 3) and thrombin clotting times (Fig 1B). Thus, prolongation of the PTT by 50 μg/mL ISIS 2302 is secondary to inhibition of the intrinsic coagulation pathway at the level of intrinsic tenase activity.
The effect of ISIS 2302 on intrinsic tenase complex activity in plasma was confirmed in a purified assay system under conditions of limiting factor VIIIa concentration. ISIS 2302 showed a marked partial inhibition of intrinsic tenase activity with near maximal inhibition at 10 to 15 μg/mL oligonucleotide and approximately 35% (of control) residual intrinsic tenase activity at higher concentrations (Fig 6). This pattern of inhibition is very similar to antithrombin-independent inhibition of intrinsic tenase activity by heparin.24Furthermore, inhibition of intrinsic tenase complex appears to be a general property of phosphorothioate oligonucleotides, based on the lack of sequence specificity and requirement for the phosphorothioate backbone (Fig 6). Prolongation of the PTT by unrelated phosphorothioate oligonucleotides has also been observed, suggesting that inhibition of the intrinsic tenase may be relevant to all first-generation antisense compounds.11 In principle, this inhibition may represent an effect on assembly, catalytic rate, or stability of the enzyme complex. Defining the specific mechanism of inhibition will require determining the effects of ISIS 2302 on factor VIII activation, binding interactions of the individual intrinsic tenase components, steady state kinetic constants for factor X activation, and stability of factor VIIIa.
Inhibition of the intrinsic tenase complex by phosphorothioate oligonucleotides has important implications for the therapeutic use of these first-generation antisense compounds. More than 10 antisense phosphorothioate oligonucleotides are currently in clinical trials in the United States and Europe.2 ISIS 2302 has recently advanced to a large phase IIb trial (300 patients) for steroid-dependent Crohn’s disease. In a smaller phase II trial (20 patients), intravenous administration of ISIS 2302 (2 mg/kg over 2 hours) was associated with transient prolongation of the PTT to approximately 1.5 times control.6 In the cynomolgus monkey studies, prolongation of the PTT is dose related, correlates with plasma oligonucleotide levels, and is observed with a number of phosphorothioate oligonucleotides.11 The results of the present investigation indicate that prolongation of the PTT by ISIS 2302 is secondary to inhibition of the intrinsic tenase complex and that this inhibition is a potential side effect of all systemically administered phosphorothioate oligonucleotides. The risk of bleeding complications due to these coagulation abnormalities is unknown. The plasma half-life of phosphorothioate oligonucleotides is relatively short (30 to 60 minutes), and adjustment of dosing regimens to reduce peak plasma oligonucleotide levels may further reduce the theoretical risk of hemorrhagic complications. This potential toxicity may be more problematic if concurrent surgical or invasive procedures are contemplated. However, because of uncertainties in extrapolating from in vitro to in vivo coagulation, the ultimate risk assessment must come from clinical trials. In this regard, it should be noted that serious hemorrhagic complications have not been recorded in any clinical trials performed by ISIS Pharmaceuticals to date, including studies in patients with Crohn’s disease and a spectrum of malignancies (personal communication, December 1997, J. Tami, ISIS Pharmaceuticals).
These findings may impact on the design of future antisense oligonucleotide modifications. The effect of alternative backbone or 2-O sugar modifications on intrinsic tenase activity will need to be addressed for second- and third-generation antisense oligonucleotides. Furthermore, RNA-DNA duplexes containing these alternative backbones or 2-O sugar modifications (in either strand) appear to be resistant to RNAase H cleavage.25 If efficient inhibition of a particular mRNA target requires an RNAase H–dependent mechanism, then hybrid oligonucleotides may be required in which a phosphorothioate cleavage site is flanked by alternative oligonucleotide modifications. The rate of RNAase H–dependent cleavage decreases with reduction in the deoxynucleotide portion of chimeric oligonucleotides, and gaps of ≤ 4 nucleotides do not support enzyme activity.25 The minimal phosphorothioate structure that inhibits intrinsic tenase activity has not been defined. Rational design of hybrid oligonucleotides to maximize RNAase H cleavage rates and minimize effects on coagulation will require determination of this minimal inhibitory oligonucleotide structure.
Inhibition of the intrinsic tenase complex may also have potential advantages for dissociating the hemorrhagic and antithrombotic effects of anticoagulant therapy. Unfractionated, low molecular weight, and low-affinity (for antithrombin) forms of heparin inhibit the intrinsic tenase complex by a similar antithrombin-independent, partial inhibition mechanism. This inhibition occurs at concentrations within the therapeutic range of both unfractionated and low molecular weight heparin, suggesting it may contribute to the antithrombotic efficacy of these drugs.24 Low-affinity heparin shows antithrombotic activity in a rabbit thrombosis model, further suggesting the importance of antithrombin-independent mechanisms.26Analysis of tissue factor (TF)-initiated clotting in minimally altered whole blood suggests that factor Xa is the limiting factor in thrombin generation, which does not reach maximal rates until well after initial clot formation occurs.23 In a purified system, TF–factor VIIa concentration affects the lag phase (initiation) for thrombin generation but does not affect the ultimate rate of thrombin generation in the propagation phase (amplification). Thus, the intrinsic tenase complex is primarily responsible for activating sufficient factor Xa to result in an explosive increase in thrombin generation during clot formation.27 Partial inhibition of intrinsic tenase would thus be expected to dampen thrombin generation during the propagation phase without inhibiting initiation of coagulation. Selective dampening of the propagation phase may disproportionately increase the antithrombotic effect relative to hemorrhagic risk, improving the risk to benefit ratio of anticoagulant therapy.24 Development of specific intrinsic tenase inhibitors will facilitate testing of this hypothesis. Inhibition of intrinsic tenase by phosphorothioate oligonucleotides shows that this anticoagulant effect can be achieved with diverse chemical structures. Thus, determining the minimal inhibitory oligonucleotide structure may facilitate rational design of synthetic intrinsic tenase inhibitors.
ACKNOWLEDGMENT
We thank Pete Lollar (Emory University, Atlanta, GA) for helpful discussions regarding the chromogenic assay for intrinsic tenase activity and Dr P. Bhattacharya (Alpha Therapeutic Corp, Los Angeles, CA) for providing human factor VIII.
Supported in part by National Institutes of Health Grant 1K08 HL 02923 and a grant from ISIS Pharmaceuticals (to J.P.S.).
Address reprint requests to John P. Sheehan, MD, Department of Medicine/Hematology, 7703 Floyd Curl Dr, San Antonio, TX 78284; e-mail:sheehan@uthscsa.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal