Abstract
Mechanisms of platelet production and release by mammalian megakaryocytes are poorly understood. We used thrombocytopenic knockout mice to better understand these processes. Proplatelets are filamentous extensions of terminally differentiated megakaryocytes that are thought to represent one mechanism of platelet release; however, these structures have largely been recognized in cultured cells and there has been no correlation between thrombocytopoiesis in vivo and proplatelet formation. Mice lacking transcription factor NF-E2 have a late arrest in megakaryocyte maturation, resulting in profound thrombocytopenia. In contrast to normal megakaryocytes, which generate abundant proplatelets, cells from these mice never produce proplatelets, even after prolonged stimulation with c-Mpl ligand. Similarly, megakaryocytes from thrombocytopenic mice with lineage-selective loss of transcription factor GATA-1 produce proplatelets very rarely. These findings establish a significant correlation between thrombocytopoiesis and proplatelet formation and suggest that the latter represents a physiologic mechanism of platelet release. We further show that proplatelet formation by normal megakaryocytes and its absence in cells lacking NF-E2 are independent of interactions with adherent (stromal) cells. Similarly, thrombocytopenia in NF-E2−/− mice reflects intrinsic defects in the megakaryocyte lineage. These observations improve our understanding of platelet production and validate the study of proplatelets in probing the underlying mechanisms.
© 1998 by The American Society of Hematology.
CIRCULATING BLOOD platelets develop within the cytoplasm of megakaryocytes, the rarest of mammalian hematopoietic cells. In the course of their differentiation, megakaryocytes acquire several unique attributes, including polyploid DNA content, platelet-specific granules, and an elaborate system of demarcation membranes. Mature megakaryocytes finally release thousands of platelets by mechanisms that are poorly understood and controversial. Whereas aspects of lineage specification, endomitosis, and cytoplasmic maturation have been studied through a combination of cell culture, hematopoietic colony assays, and electron microscopy, these approaches have led only to inferences about the dynamic process by which megakaryocytes ultimately release platelets. The extreme rarity of megakaryocytes in sites of hematopoiesis limits identification of cells in the act of releasing platelets.
Two nonmutually exclusive mechanisms of platelet release have been proposed. According to one model, terminally mature megakaryocytes leave their site of origin intact or nearly intact and are fragmented within the first capillary bed they encounter in the systemic circulation, usually the lungs.1 Observations of a difference between the number of megakaryocyte fragments found in the venous and arterial circulation2,3 and, rarely, of entire megakaryocytes in blood vessels4,5 are consistent with this model. The alternative model proposes that the bulk of thrombocytopoiesis occurs at the site of megakaryocyte maturation. To provide a mechanism for platelet release in situ, investigators have pointed out that mature megakaryocytes can extend long cytoplasmic processes, designated as proplatelets6,7 or compound platelets,8 that are comprised of nascent blood platelets in a tandem array. However, criticisms of the phenomenon of proplatelet generation include the fact that that it has largely been recognized in vitro and frequently only after nonphysiologic manipulations.9-12 More importantly, this model lacks in vivo correlation with platelet production per se.
Studies in thrombocytopoiesis have been facilitated greatly by the identification of the major cytokine that regulates megakaryocyte growth and differentiation, the ligand for the c-Mpl receptor.13-15 The c-Mpl ligand is an erythropoietin-related glycoprotein that greatly increases platelet counts as a single agent in mice and humans; a wealth of data now points to the c-Mpl ligand as a regulator of all aspects of megakaryocyte differentiation, including endomitosis and cytoplasmic maturation (reviewed in Kaushansky16). Although mature human megakaryocytes cultured in the presence of the c-Mpl ligand clearly generate proplatelets,17 18 a genuine correlation between proplatelet formation in vitro and thrombocytopoiesis in vivo has remained elusive.
We recently reported the phenotype of knockout mice lacking the hematopoietic-specific basic-leucine zipper (bZip) transcription factor p45 NF-E2.19 The most dramatic aspect of these animals is a profound thrombocytopenia, resulting from an arrest in late megakaryocyte cytoplasmic maturation. The megakaryocytes of p45 NF-E2−/− mice show a large cytoplasm with abundant demarcation membranes but few platelet-specific granules. Subsequently, we generated mice with megakaryocyte-selective loss of expression of the zinc-finger transcription factor GATA-120; these mice also are severely thrombocytopenic as a result of arrested megakaryocyte differentiation. Despite extensive characterization of megakaryocyte morphology in these mutant mice, the cellular and molecular basis of thrombocytopenia are as obscure as the mechanisms of platelet formation and release by normal megakaryocytes.
We report here on our studies on three aspects of thrombocytopoiesis by normal and mutant megakaryocytes. First, megakaryocytes cultured from murine fetal livers in the presence of the c-Mpl ligand develop large numbers of proplatelets. In contrast, megakaryocytes lacking NF-E2 never generate proplatelets and GATA-1–deficient megakaryocytes do so very rarely. These findings provide a strong correlation between proplatelet formation in vitro and thrombocytopoiesis in vivo and lend support to the view that proplatelet formation represents a physiologic mechanism of platelet release. Second, we provide more detailed characterization of normal murine proplatelets. Finally, we demonstrate that proplatelet formation by normal megakaryocytes in vitro and thrombocytopenia resulting from absence of NF-E2 in vivo occur independently of interactions with other cell types. The sum of these observations furthers our understanding of thrombocytopoiesis and enhances the utility of genetic models of thrombocytopenia to analyze the mechanism of platelet production.
MATERIALS AND METHODS
Megakaryocyte culture.
p45 NF-E2 and GATA-1 heterozygous mice were maintained on an inbred 129/Sv genetic background. Livers were recovered from mouse fetuses between embryonic day (E) 13 and 15. Single-cell suspensions, prepared by successive passage through 22- and 25-gauge needles, were cultured in Dulbecco’s modified Eagle’s medium (GIBCO BRL, Bethesda, MD) supplemented with 10% fetal bovine serum, 2 mmol/L L-glutamine, 50 U/mL penicillin, 50 μg/mL streptomycin, and 0.1 μg/mL polyethylene glycol-conjugated recombinant human c-Mpl ligand (Amgen, Thousand Oaks, CA). Between the days 4 and 6, these fetal liver cultures contained 50% to 60% acetylcholinesterase-positive cells. c-Mpl ligand was added only at the initiation of liquid cultures.
Conditioned medium was obtained from megakaryocyte liquid cultures on day 5 and contaminating cells were removed by passage through 0.45-μm filters. Undiluted conditioned medium, without determination of cytokine content, was used to replace the original medium in experiments testing its role in influencing proplatelet formation.
To establish stromal cell layers, fetal liver or bone marrow cells were cultured under identical conditions as described above and all nonadherent cells were removed by multiple washes with phosphate-buffered saline on day 5 of culture. Fresh fetal liver suspensions, depleted of greater than 95% adherent cells by three successive incubations on tissue culture-treated plastic plates over 8 to 10 hours, were then cultured on the stromal cell layers, as described above.
To generate megakaryocyte colony-forming units (CFU-Mk) in semisolid medium, 1 to 5 × 105 fetal liver cells were cultured in 1.2 mL of 50% methylcellulose in Iscove’s modified Dulbecco medium, supplemented with 30% fetal bovine serum (Stem Cell Technologies, Vancouver, British Columbia, Canada) and 0.1 μg/mL c-Mpl ligand, as described above. Proplatelet formation was studied after 7 days in culture at 37°C.
Detection and characterization of proplatelets.
Proplatelets were detected and scored by phase contrast microscopy of cells growing in suspension in liquid culture. A total of 103 to 105 cells were cytocentrifuged onto coated glass slides and acetylcholinesterase activity was detected as previously reported.21 For indirect immunofluorescence, cytocentrifuged cell preparations were fixed in methanol for 1 minute, washed, blocked with 2% goat serum in tris-buffered saline, and successively incubated with 1:100 dilutions of rabbit antimouse platelet antiserum (gift of C.W. Jackson, St Jude Children’s Research Hospital, Memphis, TN) and fluorescein isothiocyanate (FITC)-conjugated goat antirabbit IgG (Pharmingen, Los Angeles, CA).
Electron microscopy.
To preserve proplatelet integrity, cells were concentrated by gentle centrifugation (200g for 4 minutes) and then adhered by gravity to poly-L-lysine (Sigma, St Louis, MO) -coated glass cover slips resting in a Petri dish. After 15 minutes, fixative (1.5% glutaraldehyde in 0.1 mol/L cacodylate buffer, pH 7.4) was added slowly into the Petri dish and the cells were fixed for 4 hours. After embedding in Epoxy resin in an inverted Beem capsule, the single layer of megakaryocytes was detached from the cover slips by immersion in liquid nitrogen, and ultrathin sections were cut with a Dupont MT6000 microtome (Dupont, Newton, CT), stained with uranyl acetate and lead citrate, and examined with a JEOL 100CX-II transmission electron microscope (JEOL, Peabody, MA) at an accelerating voltage of 60 kV.
For scanning electron microscopy (SEM), cover slips with cells were taken through a series of alcohols and dried using a Ladd Model 28000 Critical Point Dryer (Ladd Research Industries, Inc, Hatfield, PA). The cover slips were then mounted onto SEM specimen tubs, sputter-coated with Polaron SEM coating system (Polaron Instruments, Inc, Burlington, VT), and examined with a JEOL JSM-35CF Scanning Electron Microscope at an accelerating voltage of 20 kV.
Fetal liver transplantation.
Livers were recovered from the fetuses of p45 NF-E2 heterozygote intercrosses on postcoital day 14 and single-cell suspensions were prepared by passage through a syringe and 22-gauge needle. Pending determination of the p45 NF-E2 genotype of each fetus, these cells were cultured overnight in Dulbecco’s modified Eagle’s medium supplemented with 20% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% spleen cell conditioned medium. The next day, 14 adult 129/SvJ adult females were treated with whole-body irradiation at two doses of 500 cGy each 3 hours apart. Six mice were then injected intravenously with 6 to 8 × 106 fetal liver cells derived from p45 NF-E2+/+ or p45 NF-E2+/−donors and 6 mice with the same number of cells cultured from p45 NF-E2−/− donor fetuses. One recipient from the test group and 2 mice from the control group showed either endogenous or chimeric reconstitution at 3 to 5 weeks, indicating sublethal irradiation; the analysis presented here is limited to the majority of mice with complete hematopoietic reconstitution by the donor cells. Histologic analysis of reconstituted spleen and bone marrow and Southern analysis, using a flanking genomic DNA probe, were performed as described previously.19 At various times, 50 to 100 μL of blood was removed from the retro-orbital sinus and diluted in Unopette buffer (Becton Dickinson, Franklin, NJ), and leukocyte and platelet counts were determined by manual counting under light microscopy.
RESULTS
Abundant proplatelet formation by cultured murine megakaryocytes.
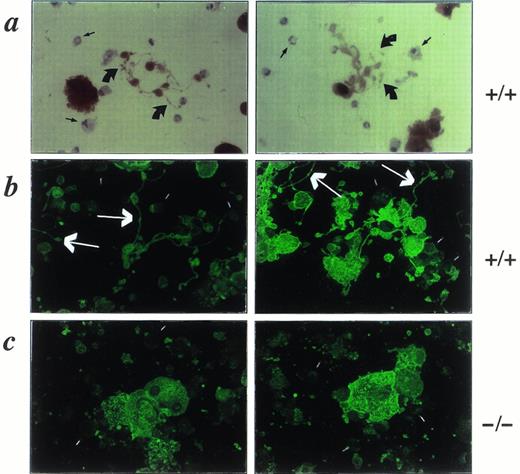
We cultured murine fetal liver cells in liquid or semisolid medium in the presence of fetal bovine serum and the c-Mpl ligand and observed the cultures over several days. Under these conditions, mature megakaryocytes develop in large numbers and produce an impressive array of cell projections (Fig 1a) that appear identical to structures previously described in human,17,22rat,10 guinea pig,11 and bovine12megakaryocytes cultured in vitro. These proplatelets, as they have been termed,6 are seen very rarely when c-Mpl ligand is excluded from the cultures. The formation of proplatelets correlates well with the degree of megakaryocyte maturity, being undetectable over the first 2 days and then increasing in frequency as the culture becomes dominated by large, polyploid, acetylcholinesterase-positive cells; in wild-type or p45 NF-E2+/− megakaryocyte cultures, 40% to 50% of the acetylcholinesterase-positive cells display proplatelets between culture days 4 and 6. In contrast, megakaryocytes lacking NF-E2 grow appreciably larger than control cells and acquire morphologic and molecular markers of maturity but are never observed to generate proplatelets (Fig 1b), even after prolonged culture. This finding provides the first correlation between proplatelet formation in vitro and thrombocytopoiesis in vivo.
Phase-contrast micrographs of control (a; +/+) and NF-E2-deficient (b; −/−) megakaryocytes on day 5 of culture of fetal liver cells in the c-Mpl ligand. The hematopoietic cells growing in suspension, and easily distinguished from the adherent stromal cells (straight arrow in [a], right panel), are mostly large and small megakaryocytes but include few myeloid and erythroid cells. Wild-type cultures (a) show abundant proplatelets characterized by platelet-size structures (curved arrows in [a]) on an extensive system of filamentous processes. Mutant cultures (b), here shown at higher cell density to include more cells in each microscopic field, never produce proplatelets. Original magnification × 200.
Phase-contrast micrographs of control (a; +/+) and NF-E2-deficient (b; −/−) megakaryocytes on day 5 of culture of fetal liver cells in the c-Mpl ligand. The hematopoietic cells growing in suspension, and easily distinguished from the adherent stromal cells (straight arrow in [a], right panel), are mostly large and small megakaryocytes but include few myeloid and erythroid cells. Wild-type cultures (a) show abundant proplatelets characterized by platelet-size structures (curved arrows in [a]) on an extensive system of filamentous processes. Mutant cultures (b), here shown at higher cell density to include more cells in each microscopic field, never produce proplatelets. Original magnification × 200.
Proplatelet formation has not previously been reported in cells isolated from the laboratory mouse, a species in which genetic models of thrombocytopenia are available. In large part, this reflects the relatively recent isolation of the c-Mpl ligand, which is required to propagate and drive complete maturation of sufficient numbers of megakaryocytes. A tendency to study cells derived from adult mice may also have been a factor; in our hands, culture of bone marrow cells under the same conditions as fetal liver cells yields significantly fewer megakaryocytes. However, mature megakaryocytes cultured from wild-type or p45 NF-E2+/− adult bone marrows also produce large numbers of proplatelets over the same culture period as do fetal liver cells, whereas cells derived from p45 NF-E2−/− adults fail to do so (data not shown).
Biochemical characterization of proplatelets.
Although proplatelets are believed to form by eversion of the megakaryocyte cytoplasm through the system of demarcation membranes, their specific composition is not fully characterized. Wild-type murine proplatelets show strong acetylcholinesterase activity (Fig 2a), a specific marker of the rodent megakaryocyte and platelet cytoplasm,21 throughout their length. This finding indicates the incorporation of cytoplasmic contents into these structures. Furthermore, a rabbit antimouse platelet antiserum23 readily stains the surfaces of the largest cells (megakaryocytes) in both control and p45 NF-E2−/− cultures, including the full length of proplatelets in the former (Fig 2b and c). Taken together, these data suggest that proplatelet formation represents cytoplasmic and membrane reorganization of mature megakaryocytes. This dramatic aspect of terminal megakaryocyte differentiation is notably missing in cells cultured from the profoundly thrombocytopenic mice lacking p45 NF-E2.
Staining of cytocentrifuge preparations of wild-type (a and b; +/+) and NF-E2-null (c; −/−) cultured megakaryocytes to show cytoplasmic acetylcholinesterase activity (a) and immunoreactivity with rabbit antimouse platelet antiserum (b and c). Proplatelets, indicated by the large arrows in (a) and (b), are hence shown to represent extensions of the megakaryocyte cytoplasm and membranes in wild-type cells and are not detected in the mutant megakaryocytes. Background staining of nonmegakaryocytic cells (short open bars) in (b) and (c) and absence of cholinesterase staining in granulocytes and monocytes (short straight arrows) in (a) provide internal controls for the antiserum and enzyme staining, respectively. Original magnification × 200.
Staining of cytocentrifuge preparations of wild-type (a and b; +/+) and NF-E2-null (c; −/−) cultured megakaryocytes to show cytoplasmic acetylcholinesterase activity (a) and immunoreactivity with rabbit antimouse platelet antiserum (b and c). Proplatelets, indicated by the large arrows in (a) and (b), are hence shown to represent extensions of the megakaryocyte cytoplasm and membranes in wild-type cells and are not detected in the mutant megakaryocytes. Background staining of nonmegakaryocytic cells (short open bars) in (b) and (c) and absence of cholinesterase staining in granulocytes and monocytes (short straight arrows) in (a) provide internal controls for the antiserum and enzyme staining, respectively. Original magnification × 200.
Electron microscopy of proplatelets.
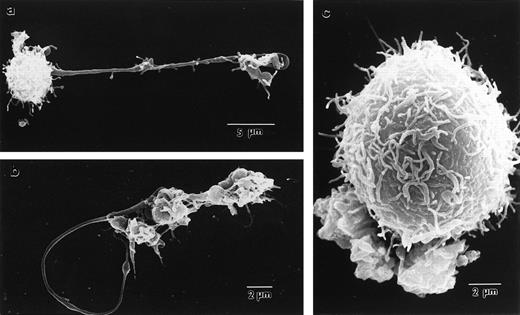
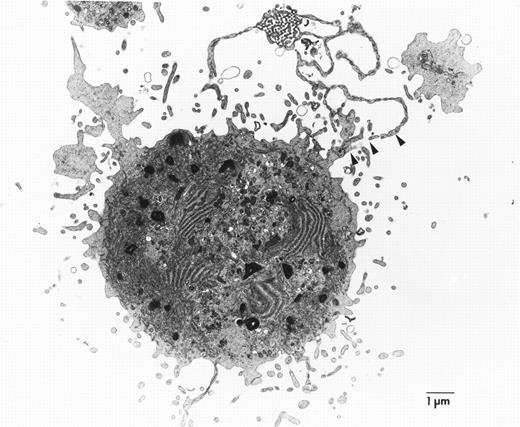
To better appreciate the structural basis of proplatelet formation in wild-type megakaryocytes and its absence in cells lacking NF-E2, we performed electron microscopy. Scanning electron micrographs of megakaryocytes cultured from normal fetal livers provide high-resolution images of the proplatelet surface and show these to be structures that emanate from the cell (Fig3a and b). The long filamentous connections between individual platelet-size structures are also readily shown by this technique. In contrast, the much larger megakaryocytes from NF-E2–deficient mice (Fig 3c) show numerous small, villous projections but do not display the proplatelets characteristic of normal mature megakaryocytes. Transmission electron microscopy confirms that the proplatelet cytoplasm is contiguous with that of the cell (Fig 4). Although the static images obtained by this technique are insufficient to draw conclusions about the dynamic process by which proplatelets are generated by mature megakaryocytes, these data are consistent with internal fragmentation of the megakaryocyte cytoplasm, as suggested by earlier studies.11,18 24 Indeed, ultrastructural features of cytoplasmic reorganization that appear to precede proplatelet formation, including dilatation of demarcation membranes, are also notably absent from megakaryocytes lacking NF-E2 (data not shown). Nevertheless, cultured p45 NF-E2−/−megakaryocytes have many of the same properties seen in vivo, including dearth of platelet-specific granules and abundance of demarcation membranes, indicating that maturation of the cells in culture parallels megakaryocyte differentiation in vivo.
Scanning electron micrographs of p45 NF-E2+/+ (a), p45 NF-E2+/− (b), and p45 NF-E2−/− (c) cultured megakaryocytes, showing extension of proplatelets from within normal cells and the surface appearance of the mutant cells, which are appreciably larger in size but do not develop proplatelets. Note the long filamentous processes separating individual platelet-size particles.
Scanning electron micrographs of p45 NF-E2+/+ (a), p45 NF-E2+/− (b), and p45 NF-E2−/− (c) cultured megakaryocytes, showing extension of proplatelets from within normal cells and the surface appearance of the mutant cells, which are appreciably larger in size but do not develop proplatelets. Note the long filamentous processes separating individual platelet-size particles.
Representative transmission electron micrograph of cultured p45 NF-E2+/− megakaryocytes, showing the proplatelet (arrowheads) as an extension of the cytoplasm. Ultrastructural analysis of numerous p45 NF-E2−/−megakaryocytes failed to show these structures.
Representative transmission electron micrograph of cultured p45 NF-E2+/− megakaryocytes, showing the proplatelet (arrowheads) as an extension of the cytoplasm. Ultrastructural analysis of numerous p45 NF-E2−/−megakaryocytes failed to show these structures.
Impaired proplatelet formation by GATA-1–deficient megakaryocytes.
We have recently established a distinct murine model of thrombocytopenia. Mice with a targeted deletion within the 5′-flanking region of the GATA-1 gene display megakaryocyte-selective loss of GATA-1 expression, dysregulated growth of megakaryocyte progenitors, and platelet counts that are reduced to approximately 15% of normal.20 The ultrastructure of GATA-1–deficient megakaryocytes shows a striking and early block in cytoplasmic maturation, with features distinct from those observed in the absence of p45 NF-E2. If proplatelet formation is a physiologically important aspect of terminal megakaryocyte differentiation, then GATA-1–deficient megakaryocytes also may be expected to be impaired in their ability to generate these structures. Indeed, proplatelet formation by GATA-1–deficient megakaryocytes is observed only rarely in liquid (Fig 5a, see page 1611) or semisolid (data not shown) cultures of the mutant cells or upon staining of the mutant cells with acetylcholinesterase or antiplatelet antiserum (Fig 5b and c, see page 1611). Scanning and transmission electron microscopy (data not shown) confirm the virtual absence of proplatelets and show cellular features similar to those observed in megakaryocytes found in the spleen or bone marrow of mutant animals,20 again providing correlation between our in vitro and in vivo findings. Thus, the absence or rarity of proplatelet formation by megakaryocytes cultured in the presence of the c-Mpl ligand correlates with profound or moderately severe thrombocytopenia in vivo in two distinct knockout mouse models of impaired megakaryocyte differentiation.
GATA-1–deficient megakaryocytes. Phase-contrast micrograph (a), acetylcholinesterase staining (b), and indirect immunofluorescence with a rabbit antimouse platelet antiserum (c) each show the rarity of proplatelets among megakaryocytes cultured from GATA-1–deficient mice. Control megakaryocytes (not shown here; see Figs 1 and 2) displayed abundant proplatelets by each of these techniques. For (a), (b), and (c), original magnifications × 200.
GATA-1–deficient megakaryocytes. Phase-contrast micrograph (a), acetylcholinesterase staining (b), and indirect immunofluorescence with a rabbit antimouse platelet antiserum (c) each show the rarity of proplatelets among megakaryocytes cultured from GATA-1–deficient mice. Control megakaryocytes (not shown here; see Figs 1 and 2) displayed abundant proplatelets by each of these techniques. For (a), (b), and (c), original magnifications × 200.
Cell-autonomous lack of proplatelet formation.
Expression of p45 NF-E2 is restricted to hematopoietic tissues and cultured cells of the erythroid, megakaryocyte, and mast cell lineages.25 Although this expression pattern suggests that the thrombocytopenia seen in the absence of p45 NF-E2 results from a requirement for NF-E2 function within the megakaryocyte itself, a role for other cell types, particularly stromal cells, remains possible. Indeed, previous studies have led to the hypothesis that various aspects of megakaryocyte differentiation, including platelet release, depend on interaction with the bone marrow stroma.26-28Lack of proplatelet formation by p45 NF-E2−/−megakaryocytes and the profound thrombocytopenia in NF-E2-null mice provide opportunities to address this question. We refer here to megakaryocytic processes that occur independently of other cells as being cell-autonomous and any necessity for NF-E2 function within megakaryocytes as reflecting a cell-autonomous requirement.
Supernatants from cultures of wild-type megakaryocytes fail to stimulate proplatelet formation in p45 NF-E2−/−cells; correspondingly, supernatants from the mutant cultures do not inhibit proplatelet formation by wild-type cells (Table 1). Previous studies have indicated that mature human megakaryocytes can generate proplatelets even when cultured as isolated cells in the absence of serum17,18; however, these experiments were performed on late megakaryocyte progenitors (human CD34+ bone marrow cells further purified by differential sedimentation or by flow cytometry for the CD38 marker) and thus leave open the possibility that signals delivered at an earlier stage in megakaryocyte differentiation are required for optimal proplatelet production. We therefore depleted the adherent cell fraction from a population of total wild-type fetal liver cells over the first few hours after tissue harvest. Over the following week, the remaining progenitors give rise to megakaryocytes that develop proplatelets in similar numbers and at the same rate as cells from nondepleted cultures (data not shown), indicating that contact with stromal cells is not a requirement for proplatelet formation. Moreover, when p45 NF-E2−/− fetal liver cells are cocultured from the outset with adherent cells derived from normal fetal livers or bone marrows, they remain unable to produce proplatelets; similarly, coculture of wild-type fetal liver cells with adherent cells of p45 NF-E2−/− origin does not inhibit proplatelet formation (Table 1). Hence, the lack of proplatelet formation by the defective megakaryocytes is very likely a cell-autonomous process reflecting a critical requirement for NF-E2 within the megakaryocyte lineage.
Cell-Autonomous Lack of Proplatelet Formation
| Source of Cultured Megakaryocytes . | Source of Conditioned Medium . | ||
|---|---|---|---|
| +/+ . | +/− . | −/− . | |
| +/+ | +++ | +++ | +++ |
| +/− | ND | ND | +++ |
| −/− | − | − | − |
| Source of Adherent (stromal) Cells | |||
| +/+ | +/− | −/− | |
| +/+ | +++ | ND | +++ |
| +/− | +++ | ND | +++ |
| −/− | − | ND | − |
| Source of Cultured Megakaryocytes . | Source of Conditioned Medium . | ||
|---|---|---|---|
| +/+ . | +/− . | −/− . | |
| +/+ | +++ | +++ | +++ |
| +/− | ND | ND | +++ |
| −/− | − | − | − |
| Source of Adherent (stromal) Cells | |||
| +/+ | +/− | −/− | |
| +/+ | +++ | ND | +++ |
| +/− | +++ | ND | +++ |
| −/− | − | ND | − |
Proplatelet formation by cultured murine megakaryocytes of different p45 NF-E2 genotypes (+/+, +/−, or −/−) when culture medium was replaced by conditioned medium from the indicated cultures (top panel) or nonadherent cells (including megakaryocytes) were transferred to an adherent cell monolayer established from fetal livers of the indicated genotype (bottom panel). Neither quantitative nor qualitative differences in proplatelet formation (represented here as abundant [+++] or absent [−]) were observed over 1 week of observation between these and unmanipulated cultures in two separate experiments for each type.
Abbreviation: ND, not done.
Cell-autonomous thrombocytopenia in the absence of NF-E2.
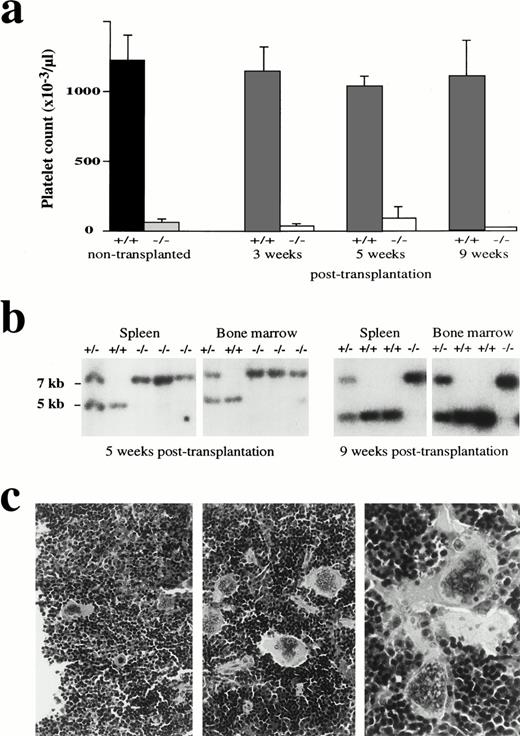
The mechanism underlying the inability of NF-E2-null megakaryocytes to produce platelets is unclear. To determine whether this mechanism is also cell-autonomous, we delivered lethal doses of γ-irradiation to wild-type adult mice and then attempted to rescue hematopoiesis in these animals by introducing fetal liver cells derived from p45 NF-E2−/− or control fetuses. All animals reconstituted by wild-type donor cells recover normal hematologic profiles, including platelet counts, within 3 weeks; in contrast, mice reconstituted by NF-E2−/− fetal liver cells rapidly recover normal numbers of leukocytes (data not shown) but remain profoundly thrombocytopenic ≥9 weeks after transplantation (Fig 6a); complete reconstitution by cells of donor origin is indicated by Southern analysis of the hematopoietic tissues (Fig 6b). In recipients of p45 NF-E2-null fetal liver cells, megakaryocytes harbor the same morphologic abnormalities seen in p45 NF-E2−/− cells (Fig 6C). These findings establish that the thrombocytopenia resulting from absence of NF-E2 function reflects deficiencies within the daughters of a radiation-sensitive cell population, most likely the megakaryocyte progenitors.
Cell-autonomous thrombocytopenia in mice lacking NF-E2 function. (a) Platelet counts from adult mice of the indicated p45 NF-E2 genotype (left 2 bars, dark shading) are reproduced after fetal liver cell transplantation. Lethally irradiated wild-type recipients were transplanted with fetal liver cells from p45 NF-E2+/+ or NF-E2−/− mice and hematologic profiles followed for 3 to 9 weeks. Severe thrombocytopenia is evident in recipients of p45 NF-E2−/− transplants. (b) Southern blot analysis of hematopoietic tissues from representative fetal liver transplant recipients verifies reconstitution in these animals by cells of the transplanted genotype. The wild-type allele is represented by a 5-kb band, and the knockout allele is represented by a 7-kb band. (c) Hematoxylin and eosin-stained microscopic sections of the spleen from representative recipient mice reconstituted with fetal liver cells from wild-type (left panel) or p45 NF-E2−/−(right 2 panels) donors. The numbers and morphology of the megakaryocytes are similar to those seen in adults of the donor genotypes. Original magnifications: for left two panels, ×40; for right panel, ×100.
Cell-autonomous thrombocytopenia in mice lacking NF-E2 function. (a) Platelet counts from adult mice of the indicated p45 NF-E2 genotype (left 2 bars, dark shading) are reproduced after fetal liver cell transplantation. Lethally irradiated wild-type recipients were transplanted with fetal liver cells from p45 NF-E2+/+ or NF-E2−/− mice and hematologic profiles followed for 3 to 9 weeks. Severe thrombocytopenia is evident in recipients of p45 NF-E2−/− transplants. (b) Southern blot analysis of hematopoietic tissues from representative fetal liver transplant recipients verifies reconstitution in these animals by cells of the transplanted genotype. The wild-type allele is represented by a 5-kb band, and the knockout allele is represented by a 7-kb band. (c) Hematoxylin and eosin-stained microscopic sections of the spleen from representative recipient mice reconstituted with fetal liver cells from wild-type (left panel) or p45 NF-E2−/−(right 2 panels) donors. The numbers and morphology of the megakaryocytes are similar to those seen in adults of the donor genotypes. Original magnifications: for left two panels, ×40; for right panel, ×100.
DISCUSSION
The cellular mechanisms by which megakaryocytes produce and release millions of platelets daily are uncertain and difficult to study. In large part, this is because megakaryocytes are among the rarest of hematopoietic cells and because platelet release is a dynamic process that occurs over an unspecified time period in an unknown location. Nevertheless, ultrastructural analysis of megakaryocytes in vivo has provided valuable insights into potential mechanisms of platelet release; images of parasinusoidal megakaryocytes extending processes that include nascent platelets (proplatelets) into the vascular space7,26,29 led to the hypothesis that formation, and subsequent fragmentation, of these structures contributes in some manner to the pool of circulating platelets. Before the identification of the c-Mpl ligand as the major regulator of megakaryocyte growth and differentiation (reviewed in Kaushansky16), proplatelet formation could consistently be demonstrated only in cells derived from a limited number of species and under specific culture conditions (reviewed in Choi30). Although wider use of the c-Mpl ligand to culture megakaryocytes in vitro has led to increasing recognition of the proplatelet as a genuine subcellular entity,17 18 it has remained difficult to establish a link between proplatelet formation in vitro and thrombocytopoiesis in vivo.
We show here that wild-type murine megakaryocytes develop a dramatic array of proplatelets during culture in the c-Mpl ligand, thus providing evidence that proplatelet formation is an integral aspect of late megakaryocyte maturation in all species studied to date. Furthermore, the kinetics of proplatelet formation closely parallels megakaryocyte differentiation, ie, both the frequency of proplatelet-bearing megakaryocytes and the average number of proplatelets per cell (data not shown) increase between 2 and 7 days of culture, before cell viability decreases. These proplatelets express both surface and cytoplasmic markers of the megakaryocyte/platelet lineage (Fig 2) and are shed into the culture medium in large numbers, where they either appear as platelet-size particles or remain in the beads on a string form that emanates from the mature megakaryocytes (Figs 1 and 3).
Proplatelets are observed infrequently when either human17 18 or murine (our observations) megakaryocytes are cultured in the absence of the c-Mpl ligand. This may explain why these structures proved difficult to demonstrate in early studies of thrombocytopoiesis in mice and other species. Our demonstration of abundant proplatelets in normal murine megakaryocytes may also be traced in part to the choice of cultured tissue. Culture of adult bone marrow or spleen cells under identical conditions yields many fewer megakaryocytes than that of mouse fetal liver cells (data not shown), perhaps reflecting the more limited proliferation potential of adult progenitors. However, we do not observe substantial differences in the acetylcholinesterase staining or ultrastructure of proplatelets obtained from fetal and adult sources. Moreover, known features of murine fetal liver-derived cells, such as the ability to produce circulating platelets in the fetus and to reconstitute hematopoiesis in lethally irradiated recipients, indicate that the fetal liver is a physiologically relevant source of megakaryocytes for study.
Our most important observation is that proplatelets are absent from cultures of p45 NF-E2−/− megakaryocytes and greatly reduced in cultures of GATA-1–deficient cells; the respective knockout mice display either profound or moderately severe thrombocytopenia. In each case, the development of megakaryocytes in culture shows other features that are also seen in vivo, such as unusually large megakaryocytes in the case of NF-E2 loss and smaller megakaryocytes admixed with an excess of immature cells in the case of GATA-1 deficiency (Vyas et al, manuscript in preparation). The striking differences between the wild-type and mutant cells with respect to generation of proplatelets thus establish a strong correlation between proplatelet formation in vitro and platelet production in vivo and support the view that proplatelets contribute to the mechanism by which terminally differentiated megakaryocytes release platelets.
Besides addressing the mechanism of platelet production in a species that is amenable to genetic manipulation, our studies underscore the value of examining this mechanism in culture, thus circumventing the low likelihood of identifying platelet-producing megakaryocytes in vivo. At the same time, it is worth emphasizing that the absence or decrease of proplatelet formation by megakaryocytes lacking NF-E2 or GATA-1, respectively, does not by itself point to the complete mechanism of thrombocytopenia in these animal models. Although our findings establish a critical link between proplatelet formation in vitro and thrombocytopoiesis in vivo, the arrest in megakaryocyte cytoplasmic maturation that characterizes these knockout mice19 20 might well precede the maturational stage at which generation of proplatelets is feasible. Although a requirement for NF-E2 or GATA-1 in aspects of proplatelet production cannot be ruled out, it is also possible that other cytoplasmic abnormalities of the defective megakaryocytes, such as reduced numbers of granules and disorganized demarcation membranes, simply preclude progression of the cell through terminal differentiation, including proplatelet formation.
Early observations of proplatelet formation in vivo by megakaryocytes in close contact with marrow stromal cells generated the notion that platelet production might depend on such cell-cell interactions. We have extended here previous studies17,18 to demonstrate that proplatelet formation can occur independently of interactions between megakaryocytes and stromal cells. The results summarized in Table 1 further indicate that the lack of proplatelet formation in the absence of NF-E2 function is not rescued by culture supernatant or adherent (stromal) cells from wild-type cultures, suggesting that this defect is intrinsic to the megakaryocyte. Additional proof for a cell-autonomous defect in NF-E2-null megakaryocytes is provided by our observation that the phenotype of NF-E2 knockout mice is reproduced in its entirety in lethally irradiated wild-type mice that receive a transplant of fetal liver cells derived from the knockout mice. Thus, it is unnecessary to invoke the existence of a secreted platelet-shedding factor solely on the basis of the phenotype of mice lacking NF-E2. Although it is likely that megakaryocytes receive, and depend on, signals delivered by other cells early in their differentiation, our results with the normal and mutant cells argue that terminal aspects of megakaryocyte differentiation, especially proplatelet formation, are largely independent of such signals. One might therefore speculate that platelet production and release are programmed aspects of the maturing megakaryocyte once the cell has committed to polyploidization and a megakaryocyte-specific pattern of gene expression. Of course, this does not exclude the possibility that extraneous signals serve to modify the number or qualitative characteristics of the proplatelets produced by mature megakaryocytes, as suggested previously.9,31 32
In conclusion, we report that proplatelet formation is absent or severely reduced in two distinct knockout mouse models of thrombocytopenia that result from primary disturbances in platelet production. These findings strongly suggest a physiologic role for proplatelet formation in the normal mechanisms of platelet production and release in vivo.
ACKNOWLEDGMENT
The authors are grateful to Amgen, Inc for providing recombinant c-Mpl ligand, to Carl Jackson for his generous gift of rabbit antimouse platelet antiserum, and to Stuart Orkin for comments on the manuscript.
P.L. and J.-L.V. contributed equally to this study.
Supported by grants from the National Institutes of Health, the Harcourt General Charitable Foundation, and the Dolphin Trust. P.L. was supported by a fellowship from the Association pour la Recherche Contre le Cancer (ARC, France), J.-L.V. by INSERM and the Fondation pour la Recherche Medicale (France), and P.V. by a fellowship from the Wellcome Foundation (UK).
Address reprint requests to Ramesh A. Shivdasani, MD, PhD, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail:ramesh_shivdasani@dfci.harvard.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. Phase-contrast micrographs of control (a; +/+) and NF-E2-deficient (b; −/−) megakaryocytes on day 5 of culture of fetal liver cells in the c-Mpl ligand. The hematopoietic cells growing in suspension, and easily distinguished from the adherent stromal cells (straight arrow in [a], right panel), are mostly large and small megakaryocytes but include few myeloid and erythroid cells. Wild-type cultures (a) show abundant proplatelets characterized by platelet-size structures (curved arrows in [a]) on an extensive system of filamentous processes. Mutant cultures (b), here shown at higher cell density to include more cells in each microscopic field, never produce proplatelets. Original magnification × 200.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/5/10.1182_blood.v92.5.1608/4/m_blod41727001w.jpeg?Expires=1769359503&Signature=ACn7hH~v8v4mai8I8VgLlTPvZSLPgNFnFbqPCGwWbFUFULwKGzmik-BGzFSsmYV3kc9ZPQ1nR0mcjV10hLiQMCyRfu3RQdCyrciKyEoVcvgOeu3c5JvwHXmJy6K0VTjozF0lCid-y8gPY6mMn8wOjsbm~bFCWcL1T7C4vlXJuQK2jxvkASMo9K090UJ-N9fDqqKPEhfOoogSKi~fJNNGD8~5q7TLPgjgWOP8Lspxg1LN7kMwy6aColKxCAyWtBasJBnxGWu5Qmn22h720uQWQQMz03M7cQeM4sH2R743VVM3Br~YCoCO6DCFROiNYvflTjLoN1JKBjRw5csKZTe1cg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal