Abstract
Epstein-Barr virus (EBV) causes potentially lethal immunoblastic lymphoma in up to 25% of children receiving bone marrow transplants from unrelated or HLA-mismatched donors. Because this complication appears to stem from a deficiency of EBV-specific cytotoxic T cells, we assessed the safety and efficacy of donor-derived polyclonal (CD4+ and CD8+) T-cell lines as immunoprophylaxis and treatment for EBV-related lymphoma. Thirty-nine patients considered to be at high risk for EBV-induced lymphoma each received 2 to 4 intravenous infusions of donor-derived EBV-specific T lymphocytes, after they had received T-cell–depleted bone marrow from HLA-matched unrelated donors (n = 33) or mismatched family members (n = 6). The immunologic effects of this therapy were monitored during and after the infusions. Infused cells were identified by detection of the neo marker gene. EBV-specific T cells bearing theneo marker were identified in all but 1 of the patients. Serial analysis of DNA detected the marker gene for as long as 18 weeks in unmanipulated peripheral blood mononuclear cells and for as long as 38 months in regenerated lines of EBV-specific cytotoxic T cells. Six patients (15.5%) had greatly increased amounts of EBV-DNA on study entry (>2,000 genome copies/106 mononuclear cells), indicating uncontrolled EBV replication, a complication that has had a high correlation with subsequent development of overt lymphoma. All of these patients showed 2 to 4 log decreases in viral DNA levels within 2 to 3 weeks after infusion and none developed lymphoma, confirming the antiviral activity of the donor-derived cells. There were no toxic effects that could be attributed to prophylactic T-cell therapy. Two additional patients who did not receive prophylaxis and developed overt immunoblastic lymphoma responded fully to T-cell infusion. Polyclonal donor-derived T-cell lines specific for EBV proteins can thus be used safely to prevent EBV-related immunoblastic lymphoma after allogeneic marrow transplantation and may also be effective in the treatment of established disease.
© 1998 by The American Society of Hematology.
AN INCREASED understanding of the mechanisms by which T lymphocytes recognize virus- and tumor-specific antigens1,2 has stimulated much interest in the use of cytotoxic T cells as adoptive immunotherapy for viral and malignant diseases.3-8 An early candidate for such treatment was Epstein-Barr virus (EBV) infection. In immunocompetent hosts, EBV causes a mild, self-limiting illness followed by a life-long period of latency in which the activity of the virus in B cells is controlled by the host’s EBV-specific cytotoxic T cells.9 In otherwise healthy persons, EBV may be related to the development of malignancies such as Hodgkin’s disease and nasopharyngeal carcinomas.10-12 By contrast, in hosts with impaired T-cell immunity, EBV can cause unchecked lymphoproliferation evolving to immunoblastic lymphoma. This complication has developed in 1% to 25% of transplant recipients, with the highest frequencies in patients receiving bone marrow from mismatched family members or unrelated donors, particularly if the marrow was depleted of T cells to prevent graft-versus-host disease.13-17 In our own center, such patients developed lymphoma with an incidence in the first year after transplantation of 11.5% (7/61).18
Unselected populations of lymphocytes from the peripheral blood of the donor usually contain EBV-specific T cells and therefore can be used to control EBV lymphoproliferative disease.19,20 However, the utility of such therapy is limited by potentially fatal complications that arise from alloreactive T cells also present in the lymphocyte infusion.19,20 To overcome this obstacle, we generated EBV-specific T-cell lines from donor lymphocytes and used them as prophylaxis against EBV-induced lymphoma in transplant recipients considered at high risk for this disease. The infused T cells were genetically marked so that their persistence and localization within the body could be reliably monitored. The feasibility of this approach was established in preliminary studies in which the gene-modified T cells were shown to persist for extended periods in vivo and to retain anti-EBV activity.21 22 We report here the results of a trial of adoptive T-cell prophylactic therapy in 39 patients undergoing bone marrow transplantation at St Jude Children’s Research Hospital (Memphis, TN).
PATIENTS AND METHODS
Patients.
Patients receiving T-cell–depleted bone marrow transplants from a mismatched family member or closely matched unrelated donor between June 1, 1993 and October 20, 1996 were eligible for this study. Follow-up of enrolled patients ranged from 15 to 54 months posttransplantation. EBV-specific lines of cytotoxic T lymphocytes (CTLs) were successfully prepared for 69 of the 70 patients who received transplants during this time period. One donor was seronegative and a CTL line could not be established. The recipient of this marrow did not develop EBV-related problems.
Thirty-nine of these children subsequently became eligible for immunoprophylaxis with the CTL lines and were enrolled in the study (Table 1). Thirty-three had closely matched unrelated marrow donors and 6 had mismatched family member donors. The remaining patients were excluded because of active graft-versus-host disease (GVHD; n = 1), pneumonitis (n = 6), diseases recurrence (n = 6), death before day 45 (n = 7), graft failure (n = 2), veno-occlusive disease (n = 1), intercurrent infection (n = 4), or refusal to participate (n = 3). Donor marrow was depleted of T cells using anti–T-cell monoclonal antibodies CD6 and CD8 as previously described,23 and all patients received cyclosporin A to maintain targeted plasma concentrations.23 Sixty-one other recipients who were treated either before the availability of EBV-CTL or who did not receive prophylaxis because they were ineligible or declined consent, were controls. The clinical characteristics of these patients closely matched those of the study group. Blood from patients was monitored at regular intervals for EBV-DNA levels, marker gene levels, and immunologic assessment (see below). The clinical protocol24 was approved by the St Jude Institutional Review Board, the Recombinant DNA Advisory Committee of the National Institutes of Health, and the Food and Drug Administration.
Clinical Characteristics of 100 Patients Who Received Allogeneic Transplants With or Without Subsequent Infusions of Prophylactic Cytotoxic T Cells
| . | Study Group (n = 39) . | Control Group (n = 61) . | . |
|---|---|---|---|
| Age at treatment (mean and range) | 9.5 yr (9 mo to 20 yr) | 9.8 yr (8 mo to 23 yr) | |
| Male:female | 18:21 | 33:28 | |
| Diagnostic category | |||
| Standard risk* | 19 | 24 | |
| High risk-151 | 20 | 37 | |
| Type of transplant received | |||
| Mismatched family member | |||
| 5/6 match | 2 | 11 | |
| 4/6 match | 3 | 9 | |
| 3/6 match | 1 | 3 | |
| Unrelated donor | |||
| 6/6 match | 20 | 22 | |
| 5/6 match | 13 | 16 | |
| No. of developing EBV lymphoma | 0 | 7 (2 mismatched family member, 5 unrelated donor) | P = .03 |
| . | Study Group (n = 39) . | Control Group (n = 61) . | . |
|---|---|---|---|
| Age at treatment (mean and range) | 9.5 yr (9 mo to 20 yr) | 9.8 yr (8 mo to 23 yr) | |
| Male:female | 18:21 | 33:28 | |
| Diagnostic category | |||
| Standard risk* | 19 | 24 | |
| High risk-151 | 20 | 37 | |
| Type of transplant received | |||
| Mismatched family member | |||
| 5/6 match | 2 | 11 | |
| 4/6 match | 3 | 9 | |
| 3/6 match | 1 | 3 | |
| Unrelated donor | |||
| 6/6 match | 20 | 22 | |
| 5/6 match | 13 | 16 | |
| No. of developing EBV lymphoma | 0 | 7 (2 mismatched family member, 5 unrelated donor) | P = .03 |
*Chronic myeloid leukemia in chronic or accelerated phase; acute myeloid or lymphoblastic leukemia in first or second remission; and metabolic storage diseases.
Chronic myeloid leukemia in or after blast crisis; acute myeloid or lymphoblastic leukemia in relapse or greater than second remission; myelodysplasia; secondary acute myeloid leukemia; and familial erythrophagocytic lymphohistiocytosis.
Preparation and administration of gene-marked T-cell lines.
The preparation of EBV-specific CTLs has been described in detail previously.21,25 In summary, EBV-specific T-cell lines were prepared from fresh or frozen samples of peripheral blood (20 mL each) collected from donors on the day of marrow harvest. Donor-derived peripheral blood mononuclear cells were plated in growth medium at 2 × 106 cells per well and stimulated with 5 × 104 irradiated autologous lymphoblastoid cells. Live cells isolated 10 days later were subcultured at 5 × 105per well and restimulated with irradiated lymphoblastoid cells at 1.25 × 105 per well. Four days later, the cultures were treated with interleukin-2 (Proleukin; Chiron, Emoryville, CA) at 20 U/mL. They continued to be expanded with interleukin-2 (3 times a week, each time at 20 U/mL) and restimulated weekly with irradiated autologous lymphoblastoid cells at a T lymphocyte:B lymphoblastoid cell ratio of 4:1. When sufficient numbers of cytotoxic T lymphocytes were attained, the cells were transduced with the neo-containing G1Na retroviral vector (Genetic Therapy, Inc, Gaithersberg, MD), which confers resistance to neomycin and its G418 analogue. The efficiency of transduction ranged from 1% to 10%, as determined by semiquantitative polymerase chain reaction (PCR) analysis.25
Before cryopreservation, T cells were examined for EBV specificity, immunophenotype, HLA compatibility, sterility, and the presence of competent retrovirus, as previously described.25 Samples with satisfactory test results were thawed rapidly at 37°C and administered intravenously to patients at a median of 88 days posttransplantation. By protocol design, 6 patients received 4 doses of 1 × 107 cytotoxic T cells/m2 and 6 others received 2 doses of 1 × 107 cytotoxic T cells/m2 and 2 doses of 5 × 107cells/m2. Because both these dose levels appeared safe and efficacious, all remaining patients received 2 doses of 2 × 107 cells/m2. The phenotype of the infused lines is shown in Table 2.
Characteristics of EBV-CTL Lines Generated From Marrow
| . | Donors . | |||||
|---|---|---|---|---|---|---|
| Phenotype . | Percent Specific Lysis . | |||||
| CD4+ . | CD8+ . | γδ . | Auto* LCL . | Allo† LCL . | HSB-2‡ . | |
| Mean | 19 | 71 | 2 | 40 | 13 | 26 |
| Range | 2-98 | 3-99 | 0-34 | 12-88 | 0-67 | 21-86 |
| . | Donors . | |||||
|---|---|---|---|---|---|---|
| Phenotype . | Percent Specific Lysis . | |||||
| CD4+ . | CD8+ . | γδ . | Auto* LCL . | Allo† LCL . | HSB-2‡ . | |
| Mean | 19 | 71 | 2 | 40 | 13 | 26 |
| Range | 2-98 | 3-99 | 0-34 | 12-88 | 0-67 | 21-86 |
*EBV-lymphoblastoid cell lines from marrow donor.
EBV-lymphoblastoid cell lines from HLA-mismatched donor.
Target for activated killer cells.
Detection of EBV DNA.
Peripheral blood mononuclear cells (PBMCs) were isolated on lymphocyte separation medium (Nycomed, Oslo, Norway) from blood samples (10 to 40 mL each) that were collected weekly postinfusion for 6 weeks, monthly for 1 year, and then every 3 months for 2 years. DNA was then isolated on an anion exchange column (Qiagen) from 1 × 106 to 107 mononuclear cells, and samples of 0.01 to 103 ng were amplified with primer sequences that detect a single copy of an EBV-DNA BamHI-H segment, as previously described.21 Positivity was defined as a detectable EBV-specific signal on Southern blotting compared with 0.01 ng of DNA from BL2/B95-8 or IB4 cells (which both contain 2 integrated EBV genomes per cell) diluted in 1 μg of DNA from an EBV-genome–negative cell line, BL41 (2 copies).
Monitoring of infused T-cell lines.
To detect neo-positive cells in vivo, we analyzed DNA by the same PCR technique used to estimate the proportion of infused cells bearing the neo marker gene.26 Control DNA was prepared by diluting G1Na-transduced K-562 cells (1 integrant per cell) with nontransduced cells to give mixtures containing 0.01% to 10%neo-positive cells.
Spontaneous outgrowth of EBV+ B lymphocytes.
Where sufficient PBMCs were available, we used the spontaneous outgrowth of EBV-positive B cells ex vivo as an additional functional measure of the onset of uncontrolled EBV-driven lymphoproliferation. As previously reported, such outgrowth occurs within 3 weeks of culture initiation, when the development of overt immunoblastic lymphoma is imminent.25
In brief, PBMCs were cultured at doubling dilution from 6 × 105/mL well to 7.5 × 104/mL in complete medium in the absence of additional stimuli or cyclosporin. The cultures were examined at weekly intervals, and outgrowing cells were stained with CD19, LMP1, and EBNA 2-specific antibodies.
Limiting dilution analysis of EBV-specific precursor frequency.
PBMCs were seeded at between 5 × 105 and 500 cells/well in 96-well plates with a dilution factor of 2. Each well was stimulated with 104 irradiated donor lymphoblastoid cell line (LCL) (40 Gy); when PBMC dilutions reached 105 cells or lower, 5 × 104 irradiated (30 Gy) autologous PBMCs were added as feeder cells. After 3 to 6 weeks in culture, responder cells were present in sufficient numbers to allow the cultures to be assayed for cytotoxic activity against autologous LCL and HLA class I mismatched targets. Target cells were labeled with 51Cr, and lysis of 10% of the cells or greater was taken to indicate a positive well, because this value exceeded the spontaneous release of51Cr by 3 standard deviations. The frequency of CTL precursors was estimated from the slope of a regression plot of the log percentage of negative wells versus the number of responder lymphocytes.
Cytotoxic activity of donor-derived EBV-specific CTL lines.
The cytotoxicity of each CTL line was analyzed in a standard 4-hour chromium-5l release assay. Target cells included autologous and HLA-class I-mismatched LCL and the T-cell line HSB-2, which is sensitive to killing by lymphokine-activated killer cells. After labeling with chromium-51, the target cells were washed 5 times and plated at 5 × 103 cells per well with effector cells to give effector:target ratios of 40:1, 20:1, 10:1, and 5:1. After 4 hours, supernatants were harvested and chromium release was measured to determine specific cytotoxicity. To determine whether cytolysis was restricted by HLA class I, target cells were preincubated for 30 minutes with 16.5 ng/mL of W6/32 (Dako, Carpinteria, CA), a monoclonal antibody that recognizes a monomorphic HLA class I determinant. In some cases, the presence of HLA class II-restricted CTLs was measured using CR3/43 (Dako), which recognizes HLA-DR, DP, DQ, and DX as the blocking antibody. Where no antibody inhibition was obtained, the experiments were repeated after depletion of CD56+ and CD16+ cells to remove nonspecific natural killer/antibody-dependent cellular cytotoxicity (NK/ADCC) effectors.
In situ PCR analysis of tumor-infiltrating neo-positive cells.
To document the presence of infused EBV-specific T cells in tumor tissue, we examined biopsied tumor specimens in paraffin forneo-bearing cells using PCR in situ, as described in detail elsewhere.27 After 30 cycles of amplification on a Hybaid Omnislide thermocycler (National Labnet Co, Woodbridge, NJ), the preserved material was hybridized to an oligonucleotide probe internal to primer sequences26 that had been 3′ end-labeled with digoxigenin (Boehringer Mannheim, Indianapolis, IN) according to the manufacturer’s instructions. Bound probe was detected by antidigoxigenin antibody conjugated to alkaline phosphatase (Boehringer Mannheim). Paraffin-embedded BL2 and G1Na-transduced K562 served as negative and positive controls, respectively.
Toxicity monitoring.
All patients received their infusions of gene-marked T cells in the BMT unit or outpatient clinic of St Jude Children’s Research Hospital, where their vital signs were monitored before and immediately after each infusion. Complete blood counts were obtained, and liver function was evaluated 1 day after each infusion and then twice weekly until day 30. GVHD was graded by standard criteria.28
RESULTS
A total of 39 patients could be assessed for their responses to infusions of EBV-specific cytotoxic T-cell lines administered as immunoprophylaxis against EBV-related lymphoma (Table 1). We also report the responses of 2 patients who did not receive prophylactic CTL therapy (1 ineligible and 1 declined consent) and subsequently developed EBV lymphoma. CTL lines were successfully generated from 69 of 70 donors. A CTL line could not be generated from the single EBV-seronegative donor. Antibody inhibition studies showed that the CTL lines were class I HLA-restricted in 77% of cases. In cell lines with apparently unrestricted killing, removal of CD56+ and CD16+ invariably showed HLA class I-restricted killing. Some of the lines contained both HLA class I- and class II-restricted killing, but none of the lines was exclusively HLA class II-restricted.
Reconstitution of T-cell–mediated immunity to EBV.
We have reported that gene-marked, EBV-specific CTLs can be readily detected for prolonged periods after their infusion into patients.22 We sought to determine if such immune reconstitution is a consistent outcome of CTL therapy. In fact, gene-marked T cells were found in the peripheral blood of patients for a median of 11 weeks (range, 7 to 18 weeks) and in regenerated EBV-specific T-cell lines for up to 38 months. Measurement of the CTL precursor frequency in 12 patients (Table3) showed a median 32-fold increase (range, 2- to >500-fold) 1 month after completion of infusion, reaching levels equivalent those found in the normal range.29 The majority of patients had CTLp levels considerably below the normal range before CTL infusion. In the 3 patients with higher levels (including unique patient no. [UPN] 345 with both high CTLp and an elevated EBV DNA), the activity was likely to be nonspecific, because precursor frequency against HLA-mismatched targets was as high or higher than that against autologous targets. After treatment, the CTLp frequency for autologous LCL increased to a much greater extent than did the frequency of CTLp for allogeneic CTLs.
Increase in CTL Precursor Frequency After Adoptive Transfer of EBV-Specific CTL
| UPN . | Before CTL Infusion . | 1 Mo After Infusion . |
|---|---|---|
| 282 | 1/285,176 | 1/7,860 |
| 293 | 1/31,250 | 1/975 |
| 296 | <1/500,000 | 1/975 |
| 314 | <1/500,000 | 1/15,700 |
| 317 | 1/25,000 | 1/1,952 |
| 324 | <1/500,000 | 1/3,905 |
| 337 | 1/145,833 | 1/3,905 |
| 345 | 1/3,900 | 1/1,952 |
| 347 | 1/207,622 | 1/1,560 |
| 401 | 1/107,638 | 1/31,300 |
| 414 | 1/604,525 | 1/32,005 |
| 419 | 1/207,622 | 1/71,905 |
| UPN . | Before CTL Infusion . | 1 Mo After Infusion . |
|---|---|---|
| 282 | 1/285,176 | 1/7,860 |
| 293 | 1/31,250 | 1/975 |
| 296 | <1/500,000 | 1/975 |
| 314 | <1/500,000 | 1/15,700 |
| 317 | 1/25,000 | 1/1,952 |
| 324 | <1/500,000 | 1/3,905 |
| 337 | 1/145,833 | 1/3,905 |
| 345 | 1/3,900 | 1/1,952 |
| 347 | 1/207,622 | 1/1,560 |
| 401 | 1/107,638 | 1/31,300 |
| 414 | 1/604,525 | 1/32,005 |
| 419 | 1/207,622 | 1/71,905 |
CTL precursor frequency was calculated as described previously22 and in Patients and Methods.
Direct evidence of antiviral and antitumor activity.
Six of the patients (15.5%) had high EBV-DNA levels before study entry. High levels of EBV-DNA result from poorly controlled EBV reactivation and are highly predictive of the onset of overt immunoblastic lymphoma.30-32 In the control group, who did not receive EBV-CTLs, 11.5% (7/61) of the recipients of T-cell–depleted unrelated/mismatched donor marrow developed high levels of EBV-DNA (>2,000 genomes per 1 × 106mononuclear cells) after transplantation, and all rapidly progressed to overt lymphoma.30 Table 4 shows details of the 6 patients in the CTL group who developed increased levels of EBV-DNA (>2,000 genomes per 1 × 106mononuclear cells) early in the posttransplantation course, before administration of CTLs. Sequential molecular analysis of EBV-DNA in these patients’ peripheral blood showed direct evidence for the antiviral action of the infused donor-derived T-cell lines, because EBV-DNA levels decreased by 3 to 5 logs within 2 to 3 weeks of the first T-cell infusion. In addition, spontaneously growing lines of EBV-transformed B cells could no longer be derived from peripheral blood (Table 4). None of the treated patients developed EBV lymphoma, in contrast to the uniform occurrence of this complication in the patients who had high EBV-DNA levels and/or spontaneous transformation and did not receive CTL.30
Effect of CTL Therapy on EBV DNA Levels and Spontaneous Outgrowth of LCL Lines
| UPN . | Genomes per 106 Mononuclear Cells . | Spontaneous Outgrowth of B Cells . | ||
|---|---|---|---|---|
| Preinfusion . | Postinfusion . | Preinfusion . | Postinfusion . | |
| 219 | 40,000 | 400 | NT | NT |
| 227 | 40,000 | 400 | + | − |
| 282 | 4,000 | 100 | + | − |
| 292 | 20,000 | 100 | − | − |
| 327 | 4,000 | 40 | + | − |
| 345 | 20,000 | 400 | NT | NT |
| UPN . | Genomes per 106 Mononuclear Cells . | Spontaneous Outgrowth of B Cells . | ||
|---|---|---|---|---|
| Preinfusion . | Postinfusion . | Preinfusion . | Postinfusion . | |
| 219 | 40,000 | 400 | NT | NT |
| 227 | 40,000 | 400 | + | − |
| 282 | 4,000 | 100 | + | − |
| 292 | 20,000 | 100 | − | − |
| 327 | 4,000 | 40 | + | − |
| 345 | 20,000 | 400 | NT | NT |
Abbreviation: NT, not tested.
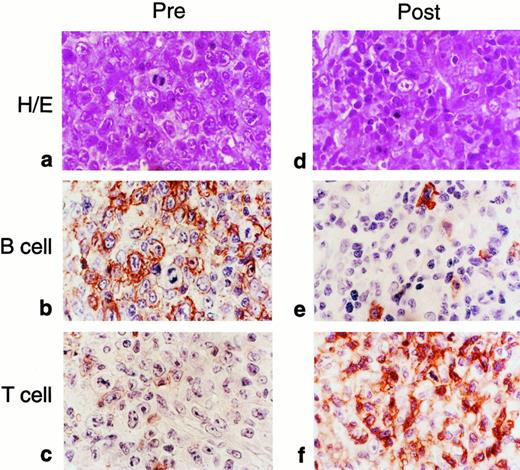
The most compelling illustration of the efficacy of our cytotoxic T-cell lines was provided by 2 patients who received their infusions after the onset of overt lymphoma. There was a complete and sustained response in both. The first patient has been previously reported.21 In the second patient we were able to quantify the marker gene in tumor biopsies to demonstrate unequivocally that the infused T-cell line was present at sites of tumor destruction. This 11-year-old boy declined the prophylaxis study and subsequently developed bulky cervical and nasopharyngeal lymphomatous disease at 210 days posttransplantation that was characterized by the presence of EBV-specific antigens and CD20+ B lymphoblasts (Fig 1). Treatment with infusions of stored, EBV-specific cytotoxic T lymphocytes was begun immediately with informed parental consent. A follow-up biopsy examination, performed 10 days after the patient had received 2 × 107 cytotoxic T cells/m2, showed an infiltrate of smaller lymphocytes, primarily CD3+ T cells (Fig 1). One percent of these cells carried the neo marker gene, a proportion identical to that found in the EBV-specific cell line that had been administered to the patient (Figs 2 and3). By contrast, fewer than 0.01% of the circulating peripheral blood mononuclear cells contained theneo marker (Fig 3). Thus, EBV-specific T lymphocytes from the infused cell line had infiltrated this lymphoma, either accumulating or selectively expanding at the tumor site. The inflammatory response associated with this immune infiltrate produced airways obstruction and mucosal sloughing, necessitating mechanical ventilation. However, the child made a full recovery and remains in remission at 24 months after the last T-cell infusion.
Comparison of tumor biopsy samples before and after adoptive T-cell therapy for EBV lymphoma in an 11-year-old boy who had undergone allogeneic bone marrow transplantation from a matched unrelated donor. The initial specimen had the histologic appearance of immunoblastic lymphoma (a; hematoxylin and eosin). Most of the cells reacted to staining for the CD20 B-cell marker (b), whereas a few were positive for the T-cell marker (c). After adoptive immunotherapy, the follow-up biopsy showed an infiltrate of smaller lymphocytes, the majority of which reacted with the T-cell marker (d through f).
Comparison of tumor biopsy samples before and after adoptive T-cell therapy for EBV lymphoma in an 11-year-old boy who had undergone allogeneic bone marrow transplantation from a matched unrelated donor. The initial specimen had the histologic appearance of immunoblastic lymphoma (a; hematoxylin and eosin). Most of the cells reacted to staining for the CD20 B-cell marker (b), whereas a few were positive for the T-cell marker (c). After adoptive immunotherapy, the follow-up biopsy showed an infiltrate of smaller lymphocytes, the majority of which reacted with the T-cell marker (d through f).
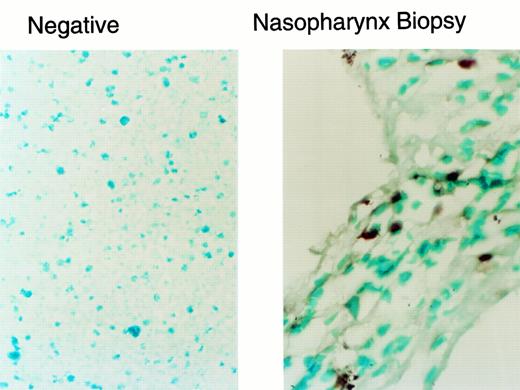
In situ PCR analysis of tumor-infiltrating lymphocytes illustrated in Fig 1d through f. The neo gene was detected in 1% of the infiltrating lymphocytes, a rate that corresponds to the gene-marking efficiency for the EBV-specific T-cell line administered to this patient.
In situ PCR analysis of tumor-infiltrating lymphocytes illustrated in Fig 1d through f. The neo gene was detected in 1% of the infiltrating lymphocytes, a rate that corresponds to the gene-marking efficiency for the EBV-specific T-cell line administered to this patient.
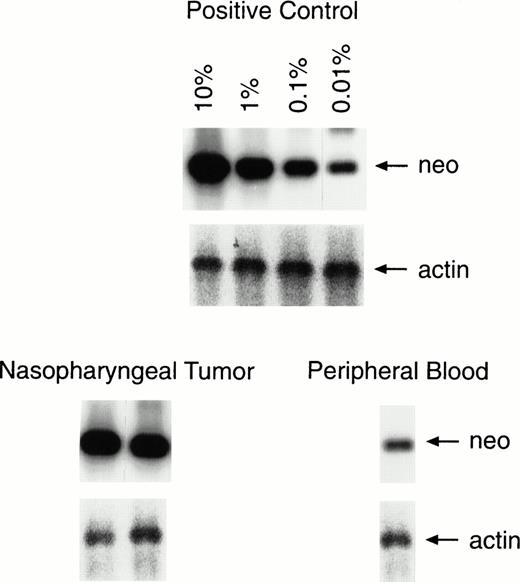
Semiquantitative PCR analysis of lymphocytes infiltrating the tumor and in peripheral blood. One percent of the lymphocytes infiltrating the second nasopharyngeal biopsy was positive for the marker gene, compared with a level of 0.01% to 0.001% in a peripheral blood sample taken on the same day. The results of PCR analysis for the actin gene are provided for comparison of equal amounts of DNA.
Semiquantitative PCR analysis of lymphocytes infiltrating the tumor and in peripheral blood. One percent of the lymphocytes infiltrating the second nasopharyngeal biopsy was positive for the marker gene, compared with a level of 0.01% to 0.001% in a peripheral blood sample taken on the same day. The results of PCR analysis for the actin gene are provided for comparison of equal amounts of DNA.
Safety of T-cell infusions.
None of the patients had appreciable changes in liver function, renal function, or chest x-ray appearances during the administration of cytotoxic T cells. Acute GVHD did not appear de novo in any patient after an infusion of T cells, although in 1 child there was exacerbation of pre-existing chronic GVHD. Twenty-seven of the patients in the prophylaxis study remain alive 15 to 54 months after transplantation (mean follow-up, 30 months). Twelve patients have died: 9 from relapse, 2 from infection, and 1 from pneumonitis.
DISCUSSION
These results support the belief that infusions of EBV-specific CD4+ CD8+ T cells are effective as prophylaxis or treatment for EBV-associated immunoblastic lymphoma. Six patients received infusions of EBV-CTLs in the presence of extremely high levels of circulating EBV-DNA (>2,000 genome copies/106mononuclear cells). None subsequently developed lymphoma, in contrast to the uniform association between this complication and similarly elevated EBV-DNA levels in previous studies.18,30 Indeed, in the group receiving cytotoxic T cells, the elevated levels of viral DNA returned to normal within 3 weeks after the first infusion. Overall, 0 of 39 patients receiving CTL prophylaxis developed EBV lymphoma, compared with an incidence of 11.5% (7/61) in the control group in our own center. It is noteworthy that infusion of CTLs was effective at reducing EBV-DNA levels even in a patient with apparently high levels of CTLp. However these CTLp were likely to be nonspecific. Such activity would not be expected to be as effective as specific CTLs in preventing EBV-driven lymphoproliferation and could therefore coexist with a high viral load. This patient, UPN 345, had a high EBV viral load, malaise, and fevers and all symptoms resolved after CTL administration. Although we cannot rule out the possibility that the decrease in viral load was a result of recovery of endogenous EBV-specific immunity, our own results and data from other groups15 have shown that endogenous recovery does not usually occur in the first 3 months after transplantation.
The most direct evidence that T cells restore antiviral and antitumor immunity comes from the eradication of frank EBV lymphoma in 2 children who did not receive prophylactic CTLs. In the second child, we were able to demonstrate the infiltration, accumulation, and expansion of gene-marked T cells within the tumor site, as well as the association between such infiltration and the destruction of EBV-positive B lymphoblasts. However, marked local inflammatory and necrotic reactions associated with the rapid expansion of infused T cells in the nasopharynx necessitated prolonged mechanical ventilation, suggesting that the use of CTLs to prevent lymphoma in transplant recipients is preferable and more cost effective than treatment of established disease.
We postulate that the high degree of in vivo expansion and long-term persistence of the infused T lymphocytes were a consequence of both the continued presence of viral antigen and the use of polyclonal lines containing both CD4+ and CD8+ cells, rather than CD8+ T-cell clones alone.22 It has been argued on theoretical grounds that single clones are preferable to multiple clones because of their lower probability of containing cells that cross-react with normal host antigens.33 However, in a number of different human and murine systems, CD4+ cells have been shown to play a crucial role in establishing or maintaining CD8+ T-lymphocyte–mediated antiviral or antitumor immunity.34 35 Hence, the lack of CD4+virus-specific helper T cells may limit the capacity of the CD8+ T-cell clones to survive for extended periods or to be recalled should viral reactivation occur late in the clinical course. We cannot rule out the possibility that our polyclonal lines contain T cells that cross-react with host alloantigens; however, none of the T-cell infusions was associated with GVHD induction.
What are the practicalities of preparing cytotoxic T lymphocytes for the prevention or treatment of EBV-related immunoblastic lymphoma in allogeneic transplant recipients? Because EBV is ubiquitous and persistent, a majority of donors carry a high frequency of EBV-specific cytotoxic T-cell precursors that can be readily reactivated and expanded in vitro for infusion into patients requiring bone marrow transplantation. Unique target antigens against which the T cells can be directed are provided by the 9 virus-encoded latency-associated proteins expressed by EBV-infected tumor cells.9 Hence, specific T-cell lines that meet the necessary criteria for infusion could be routinely generated; in our study, viable T-cell lines were generated from more than 98% of the donors. Moreover, because the infused cells can be expected to expand markedly in vivo and persist in the circulation for extended periods, the preparation and infusion of even limited numbers of T-lymphocyte precursors (2 × 107/m2) is adequate to bring the frequency of EBV-specific T cells into the normal range, thus preventing or aborting EBV reactivation.
One of the drawbacks of our approach is the time required to generate CTL lines. It may be possible to simplify and accelerate the production of cytotoxic T cells by substituting dendritic cells that express EBV antigens for EBV-positive lymphoblastoid cell lines, such as those used in the present study. Dendritic cells can be prepared in 7 to 10 days,36,37 rather than the 4 weeks normally required for lymphoblastoid cells, and could then be used to stimulate T-lymphocyte responses not only to the set of latency antigens expressed by LCL, but also to the more restricted set of EBV antigens expressed by cells common to Hodgkin’s disease and nasopharyngeal carcinoma.38 Dendritic cells can also induce primary immune responses that may allow generation of EBV-specific CTL lines from seronegative donors.37 However, because the virus in EBV-driven lymphoproliferations of allogeneic bone marrow transplant recipients usually derives from the donor marrow, recipients of seronegative marrow may be low risk for EBV.
We estimate a total cost of less than $4,000 to prepare and validate each separate T-cell line. This expense compares favorably with that of chemoprophylaxis for other viral diseases that commonly arise after bone marrow transplantation and is substantially less than the cost of treating established immunoblastic lymphoma. Thus, the strategy of antiviral prophylaxis we have outlined would be feasible for routine clinical application in patients at high risk for development of EBV-related cancers, and perhaps in other malignancies in which viral antigens are readily found. The overall effectiveness of this approach may now be assessed in a prospective randomized trial.
ACKNOWLEDGMENT
The authors thank John Gilbert for scientific editing and the staff of the BMT inpatient and outpatient units for clinical care of these patients.
Supported by Grants No. P 30CA 21765 and CA 61384 from the National Cancer Institute, by the Assisi Foundation of Memphis, and by the American Lebanese Syrian Associated Charities (ALSAC).
Address reprint requests to Cliona M. Rooney, PhD, Center for Cell and Gene Therapy, Baylor College of Medicine, 1102 Bates St, Suite 1100, Houston, TX 77030.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal