Abstract
Several prospective randomized studies have shown that the treatment of chronic myeloid leukemia with interferon- (IFN-) prolongs the survival by comparison with conventional chemotherapy. However, although IFN- can induce cytogenetic responses, true complete remissions are rarely achieved and information on the long-term effects of IFN- treatment is limited. For that purpose, we updated and analyzed a prospective comparative trial of IFN- and conventional chemotherapy that was initiated in 1986. The first analysis of the trial was already published, and showed a survival advantage for IFN- (N Engl J Med 12:820, 1994). The observation period of living patients now ranges between 95 and 129 months and we examined the long-term effects of IFN- treatment, always by comparison with conventional chemotherapy and according to the intention-to-treat principle. The patients who were submitted to allogeneic bone marrow transplantation (BMT) in chronic phase (38 of 322 or 12%) were censored at the date of BMT. Seventy-three of the original 284 nontransplanted patients were alive, 56 (30%) in the IFN- arm and 17 (18%) in the chemotherapy arm. Forty-one patients overall (14%) were still receiving IFN-. In the IFN- arm 9 patients were in continuous complete cytogenetic remission and 11 were in major or minor cytogenetic remission. Median and 10-year survival of low-risk patients were 104 months (95% CI, 85 to 127 months) and 47% (95% CI, 36% to 59%) in IFN- arm versus 64 months (95% CI, 49 to 98 months) and 30% (95% CI, 16% to 44%) in chemotherapy arm (P = .03). Median and ten-year survival of non–low-risk patients were 69 months (95% CI, 56 to 76 months) and 16% (95% CI, 8% to 24%) in IFN- arm versus 46 months (95% CI, 39 to 61 months) and 5% (95% CI, 0% to 11%) in chemotherapy arm (P = .006). A low Sokal’s risk, hematologic response, and cytogenetic response were associated with a longer survival. No major or unusual side effects were recorded after the 5th year of IFN- treatment. Fourteen patients died in chronic phase, 9 (4%) in IFN- arm and 5 (5%) in chemotherapy arm, mainly of cardiovascular accidents (6 cases) and of other cancers (5 cases). We conclude that a policy of chronic treatment with IFN- maintained a significant survival advantage over conventional chemotherapy on a long-term basis and irrespective of the risk. However, the great majority of the long-term survivors were in the low-risk group. The question of treatment discontinuation was not addressed in this study.
© 1998 by The American Society of Hematology.
INTERFERON-α (IFN-α) was reported to be active in the treatment of chronic myeloid leukemia (CML) in 19831 and to induce cytogenetic remissions in 1986.2 In 1986 we enrolled 322 consecutive CML patients in a prospective randomized study of IFN-α versus conventional chemotherapy. Our reports on cytogenetic response and on survival appeared in 19923 and in 19944 and provided confirmation to the findings5-7 that IFN-α was able to induce cytogenetic remissions and to the suggestion5 that IFN-α treatment prolonged survival as compared with conventional chemotherapy. Subsequently, the therapeutic role of IFN-α in CML was confirmed again in three prospective multicenter trials.8-11 On the other hand the treatment is chronic, may be required for a very long period of time, and can interfere with other treatment procedures. The effect of IFN-α on long-term survival, the duration and the quality of the response, the late treatment complications, and the compliance with chronic treatment are all important issues that were described so far only in the patients who were originally recruited at a single center at M.D. Anderson Hospital (Houston, TX) because that center was the first to apply IFN-α to the treatment of CML on a large basis.5 12 We report on these issues, based on an unselected multicenter series of patients who were prospectively assigned to treatment with IFN-α or with conventional chemotherapy.
MATERIALS AND METHODS
Patients.
The recruitment for this study was opened July 1986 and was terminated July 1988. Approval was obtained from the Institutional Review Boards. Informed consent was provided according to the Declaration of Helsinki. During that 2-year period, 322 patients were assigned at random, with a ratio of 2:1, to receive either IFN-α (218 cases) or conventional chemotherapy (104 controls). In the IFN-α arm, the drug was human recombinant IFN-α2a (Roferon-A; HoffmannLa Roche), 9 million IU (mIU) daily for a minimum of 14 months and indefinitely in case of any cytogenetic response, until tolerated or until progression to accelerated or blastic phase. Chemotherapy could be used at any time in case of poor hematologic control, and after 14 months in case of no cytogenetic response. In the chemotherapy arm, first-line treatment was hydroxyurea in 94 patients and busulfan in 10 patients.
Definitions.
Accelerated or blastic phase was defined by at least two of the following predetermined criteria: (1) more than 10% blast cells or more than 30% blast cells and promyelocytes in the peripheral blood; (2) more than 15% blast cells or more than 50% blast cells and promyelocytes in the marrow aspirate; (3) splenomegaly (>10 cm below the costal margin) with a white blood cell (WBC) count of less than 25 × 109/L; (4) involvement of the central nervous system, bone, lymph nodes, or other extrahematologic sites; and (5) trisomy Ph, trisomy 8, or isochromosome 17. The cytogenetic response was evaluated after 8 and 14 months and yearly thereafter, based on a minimum of 10 evaluable metaphases, and was defined according to the percentage of Ph negative metaphases as none, minimal (1% to 32% Ph neg), minor (33% to 65% Ph neg), major (66% to 99% Ph neg), and complete (100% Ph neg). The hematologic response was defined as complete if blood counts were normal (hemoglobin > 110 g/L, platelet < 500 × 109/L, WBC < 10 × 109/L), if the differential leukocyte count did not contain immature cells, and if the spleen was not palpable. The formulation of Sokal et al13 was used to categorize the patients by risk.
Statistical analysis.
Survival was calculated according to the method of Kaplan and Meier14 from the date of randomization to the date of death or last contact. Follow-up information was available on all patients. At last contact (June 1997), the follow-up of living patients ranged between 95 and 129 months (median, 112 months). During the chronic phase, 38 patients underwent allogeneic bone marrow transplantation (BMT) (12%) and 6 patients were autografted (2%). Allogeneic BMT was allowed by the protocol, whereas autografts were protocol violations. For that reason, all survival calculations and comparisons were made either including, excluding, or censoring the patients who were submitted to transplant. The results were always identical, and the survival data that are reported hereforth include all randomized patients, with censoring for allogeneic transplant. The time to the progression to accelerated or blastic phase was calculated from randomization, with censoring for allogeneic BMT and for death in chronic phase. Analyses and comparisons were made by the chi-square test and Student’s t-test, by the log-rank method and by logistic regression. The Cox proportional hazards model for covariate analysis of censored data on survival15 was used whenever appropriate. P values were two sided. All the calculations were based on the intention-to-treat principle and always included all the randomized patients. To avoid any bias from using time-dependent variables like hematologic or cytogenetic response, landmark analysis at specific time points was performed whenever appropriate.
RESULTS
Patients’ status.
The status of the patients who were not transplanted is shown in Table1. In the IFN-α arm 56 of 188 patients (30%) were alive and 16 of these patients (8%) maintained a complete or a major cytogenetic remission. In the chemotherapy arm 17 of 96 patients (18%) were alive, with a minor cytogenetic response in one case.
Present Status and Current Treatment of the Patients Who Were Recruited and Randomized for the Study
| . | IFN-α . | Chemotherapy . |
|---|---|---|
| Total | 218 | 104 |
| Allogeneic BMT in chronic phase | 30 | 8 |
| Remaining | 188 | 96 |
| Alive, chronic phase | 52/188 (28%) | 14/96 (14%) |
| Alive, accelerated or blastic phase | 4/188 (2%) | 3/96 (3%) |
| Alive, total | 56/188 (30%) | 17/96 (18%) |
| Cytogenetic status (response) | ||
| Complete (Ph neg 100%) | 9/56 (16%) | 0/17 |
| Major (Ph neg 66% to 99%) | 7/56 (12%) | 0/17 |
| Minor (Ph neg 33% to 65%) | 4/56 (7%) | 1/17 (6%) |
| Minimal or none (Ph neg <33%) | 31/56 (55%) | 10/17 (59%) |
| Nonevaluable or unknown | 5/56 (9%) | 6/17 (35%) |
| Current treatment | ||
| IFN-α alone | 28/56 (50%) | 2/17 (12%) |
| IFN-α and chemotherapy | 10/56 (18%) | 1/17 (6%) |
| Chemotherapy | 16/56 (28%) | 14/17 (82%) |
| Unknown | 2/56 (4%) | 0/17 |
| . | IFN-α . | Chemotherapy . |
|---|---|---|
| Total | 218 | 104 |
| Allogeneic BMT in chronic phase | 30 | 8 |
| Remaining | 188 | 96 |
| Alive, chronic phase | 52/188 (28%) | 14/96 (14%) |
| Alive, accelerated or blastic phase | 4/188 (2%) | 3/96 (3%) |
| Alive, total | 56/188 (30%) | 17/96 (18%) |
| Cytogenetic status (response) | ||
| Complete (Ph neg 100%) | 9/56 (16%) | 0/17 |
| Major (Ph neg 66% to 99%) | 7/56 (12%) | 0/17 |
| Minor (Ph neg 33% to 65%) | 4/56 (7%) | 1/17 (6%) |
| Minimal or none (Ph neg <33%) | 31/56 (55%) | 10/17 (59%) |
| Nonevaluable or unknown | 5/56 (9%) | 6/17 (35%) |
| Current treatment | ||
| IFN-α alone | 28/56 (50%) | 2/17 (12%) |
| IFN-α and chemotherapy | 10/56 (18%) | 1/17 (6%) |
| Chemotherapy | 16/56 (28%) | 14/17 (82%) |
| Unknown | 2/56 (4%) | 0/17 |
Treatment discontinuation and adjustment.
The number of the patients who discontinued the assigned treatment and went off protocol before progression was 114 of 218 (52%) in the IFN-α arm and 37 of 104 (35%) in the chemotherapy arm. The difference was due to the fact that 39 patients (18%) discontinued IFN-α because of the side effects that are listed in Table2. The classic flu-like syndrome including fatigue, fever, musculoskeletal pain, and headache covered 37% of discontinuations, either early or late. Anorexia, nausea, and diarrhea came second, but only during the first 3 years of treatment. Neurologic side effects like depression, amnesia, lethargia, psychosis, reversible coma in one case, and polyneuropathy, mainly sensorial, developed during the first 5 years of treatment and were the third cause of treatment discontinuation.
IFN- Arm. Distribution by the Years of Treatment of the Side Effects That Caused Treatment Discontinuation
| Side Effects . | Years of Treatment . | ||||
|---|---|---|---|---|---|
| 1 . | 2-3 . | 4-5 . | 6 or More . | Total . | |
| Fatigue, fever, pain, headache | 10 | 6 | 5 | 4 | 25 (37%) |
| Anorexia, nausea, diarrhea | 8 | 6 | 0 | 0 | 14 (21%) |
| Neurologic, “central” * | 1 | 2 | 3 | 0 | 6 (9%) |
| Neurologic, “peripheral” † | 2 | 2 | 1 | 0 | 5 (7%) |
| Hematologic | 2 | 2 | 2 | 0 | 6 (9%) |
| Skin, itching, alopecia | 3 | 2 | 2 | 0 | 7 (10%) |
| Liver | 0 | 1 | 1 | 0 | 2 (3%) |
| Allergic reaction | 2 | 0 | 0 | 0 | 2 (3%) |
| Total | 28 | 21 | 14 | 4 | 67 (100%) |
| Side Effects . | Years of Treatment . | ||||
|---|---|---|---|---|---|
| 1 . | 2-3 . | 4-5 . | 6 or More . | Total . | |
| Fatigue, fever, pain, headache | 10 | 6 | 5 | 4 | 25 (37%) |
| Anorexia, nausea, diarrhea | 8 | 6 | 0 | 0 | 14 (21%) |
| Neurologic, “central” * | 1 | 2 | 3 | 0 | 6 (9%) |
| Neurologic, “peripheral” † | 2 | 2 | 1 | 0 | 5 (7%) |
| Hematologic | 2 | 2 | 2 | 0 | 6 (9%) |
| Skin, itching, alopecia | 3 | 2 | 2 | 0 | 7 (10%) |
| Liver | 0 | 1 | 1 | 0 | 2 (3%) |
| Allergic reaction | 2 | 0 | 0 | 0 | 2 (3%) |
| Total | 28 | 21 | 14 | 4 | 67 (100%) |
Treatment was discontinued in 39 patients overall, but usually more than one side effect contributed to the decision of discontinuing the treatment.
Depression, amnesy, lethargia, psychosis, reversible coma in one case.
Polyneuropathy, mainly sensorial.
In the IFN-α arm 84 patients overall (38%) also received chemotherapy before progression to accelerated or blastic phase, either because IFN-α was discontinued for side effects (39 cases) or because it was believed that IFN-α was unable to control the disease (45 cases). In the chemotherapy arm, no option was provided for crossing the treatment to IFN-α, but IFN-α was actually administered to 8 patients, 2 to 70 months after randomization. The overall survival of these 8 patients was long, ranging from 96 to 129 months (median, 106 months), with 2 of 8 alive at last contact.
The dose of IFN-α that was originally scheduled for this study was 9 mIU daily (corresponding to 63 mIU weekly) for 8 months, to be maintained thereafter at maximum tolerated dose if any cytogenetic response was obtained. During the first year, the mean IFN-α weekly dose was significantly higher in the patients who achieved any degree of cytogenetic response than in the others (Table3). Thereafter, in the majority of the nonresponders the dose was decreased to 9 mIU weekly, as it was prescribed by the protocol. In the responders the dose was also adapted, according to tolerance, and from the 5th year on was about 20 mIU weekly (Table 3).
Dose of IFN- in Cytogenetic Responders and Nonresponders
| Treatment Period (yr) . | IFN-α Weekly Dose (mIU, Total) . | |
|---|---|---|
| Cytogenetic Responders at 14 Months (n = 74) . | Cytogenetic Nonresponders at 14 Months (n = 84) . | |
| 1 | 50.4 ± 15.4 | 36.4 ± 17.5 |
| 2 | 43.4 ± 28.0 | 14.7 ± 11.2 |
| 3 | 30.8 ± 23.1 | 11.2 ± 18.2 |
| 4 | 25.2 ± 21.7 | 5.6 ± 13.3 |
| 5 | 21.0 ± 16.1 | 10.5 ± 19.6 |
| 6 | 19.6 ± 16.1 | — |
| 7 | 19.6 ± 18.2 | — |
| 8 | 22.4 ± 16.8 | — |
| 9 | 20.4 ± 15.4 | — |
| Treatment Period (yr) . | IFN-α Weekly Dose (mIU, Total) . | |
|---|---|---|
| Cytogenetic Responders at 14 Months (n = 74) . | Cytogenetic Nonresponders at 14 Months (n = 84) . | |
| 1 | 50.4 ± 15.4 | 36.4 ± 17.5 |
| 2 | 43.4 ± 28.0 | 14.7 ± 11.2 |
| 3 | 30.8 ± 23.1 | 11.2 ± 18.2 |
| 4 | 25.2 ± 21.7 | 5.6 ± 13.3 |
| 5 | 21.0 ± 16.1 | 10.5 ± 19.6 |
| 6 | 19.6 ± 16.1 | — |
| 7 | 19.6 ± 18.2 | — |
| 8 | 22.4 ± 16.8 | — |
| 9 | 20.4 ± 15.4 | — |
The dose of IFN that was received by the patients who achieved any degree of cytogenetic response was higher (P < .001, Student’s t-test) than the dose of IFN-α that was received by the patients who did not achieve any cytogenetic response during the first 14 months. Subsequently, the dose decreased progressively to about 20 mIU weekly in the responders and to about 10 mIU weekly in the nonresponders. After the 5th year only 5 nonresponder patients were still receiving IFN-α.
BMT.
The option for allogeneic BMT from an HLA-compatible family donor was always open and was actually used during the chronic phase in 33 patients, 2 to 65 months after registration (median, 15 months). In another 5 patients an allogeneic BMT was performed later (42 to 107 months after registration) from a matched unrelated or a partially matched related donor. In summary, 38 of 322 patients of any age (12%) and of 172 patients less than 51 years old (22%) received an allogeneic BMT during the chronic phase. Sixteen of the 38 transplanted patients (42%) died of transplant or transplant-related complications, two are alive with leukemia, and the remaining 20 (53%) are alive and in hematologic remission. Two additional patients, both in the IFN-α arm, were transplanted in blastic phase and died of transplant. Six patients (4 in the IFN-α arm and 2 in the chemotherapy arm) were autografted in chronic phase. Three of them died of leukemia and three are alive with leukemia. Ten patients (6 in the IFN-α arm and 4 in the chemotherapy arm) were autografted in accelerated or blastic phase. Seven died of transplant or leukemia, and three are alive with leukemia.
Survival and progression.
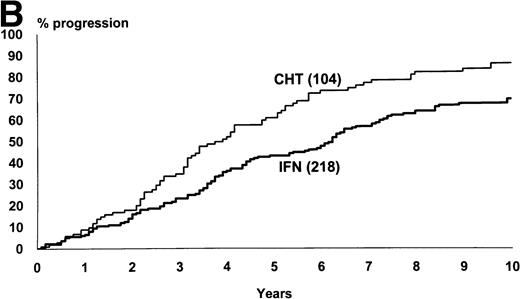
The median survival of the 218 patients who were assigned to IFN-α and of the 104 patients who were assigned to chemotherapy was 76 months and 52 months, respectively (95% CI, 69 to 86 months and 43 to 66 months, log-rank test, P = .002) (Fig1). The proportion of the patients who were projected to be alive after 10 years was 29% (95% CI, 23% to 36%) in IFN-α arm and 17% (95% CI, 9% to 25%) in chemotherapy arm. Figure 1 also shows the rate of the progression from chronic to accelerated or blastic phase. The median time from diagnosis to progression was 74 months with IFN-α and 46 months for chemotherapy (log-rank test, P = .0005).
(A) Kaplan and Meier’s plot of overall survival of all randomized patients, irrespective of any protocol violation and of the cause of death. The cases who were submitted to allogeneic BMT in chronic phase were censored at the date of transplant. Log-rank test,P = .002. The number of cases at risk at each year is shown below the graph. (B) Time from randomization to progression to accelerated or blastic phase. The cases who were submitted to allogeneic BMT in chronic phase and the cases who died in chronic phase were censored. Log-rank test, P = .0005.
(A) Kaplan and Meier’s plot of overall survival of all randomized patients, irrespective of any protocol violation and of the cause of death. The cases who were submitted to allogeneic BMT in chronic phase were censored at the date of transplant. Log-rank test,P = .002. The number of cases at risk at each year is shown below the graph. (B) Time from randomization to progression to accelerated or blastic phase. The cases who were submitted to allogeneic BMT in chronic phase and the cases who died in chronic phase were censored. Log-rank test, P = .0005.
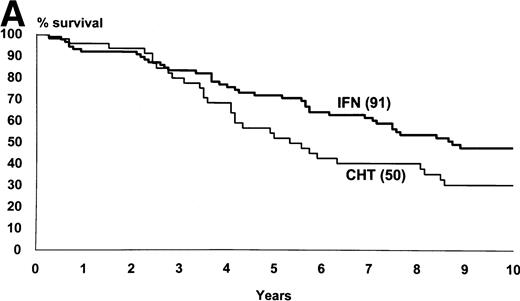
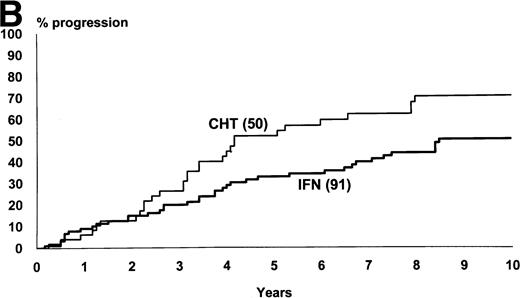
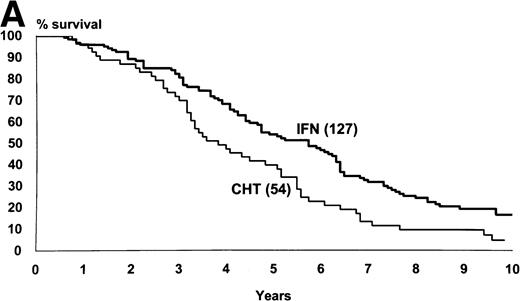
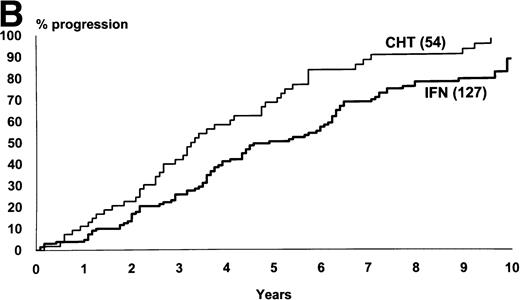
Figure 2 shows the survival and the progression from chronic to accelerated or blastic phase of Sokal’s low-risk patients as a function of treatment. Figure3 shows the same for Sokal’s intermediate- and high-risk patients. The difference was significant both in low-risk patients (P = .03 for survival and .02 for progression) and in intermediate- and high-risk patients (P = .006 for survival and .002 for progression).
Low-risk cases (Sokal’s relative risk < 0.8). Overall survival (A) and time to progression to accelerated or blastic phase (B). P values are .03 and .02. Notice that 10-year survival is 47% in IFN- arm and 30% in chemotherapy arm.
Low-risk cases (Sokal’s relative risk < 0.8). Overall survival (A) and time to progression to accelerated or blastic phase (B). P values are .03 and .02. Notice that 10-year survival is 47% in IFN- arm and 30% in chemotherapy arm.
Intermediate- and high-risk cases (Sokal’s relative risk ≥ 0.8). Overall survival (A) and time to progression to accelerated or blastic phase (B). P values are .006 and .002. Notice that although IFN- patients fared significantly better than chemotherapy patients, more than 80% of IFN- patients progressed and died within 10 years as well.
Intermediate- and high-risk cases (Sokal’s relative risk ≥ 0.8). Overall survival (A) and time to progression to accelerated or blastic phase (B). P values are .006 and .002. Notice that although IFN- patients fared significantly better than chemotherapy patients, more than 80% of IFN- patients progressed and died within 10 years as well.
Deaths in chronic phase.
Fourteen patients died in chronic phase; nine of them had been assigned to IFN-α (4%) and five had been assigned to chemotherapy (5%) (Table 4). The deaths occurred either early (3 to 14 months after diagnosis), and were due to infection, cardiac disease, or gastric bleeding; or late (32 to 99 months after diagnosis), and were due to cardiac or vascular disease, or to other cancers. Fatal cardiovascular events were slightly more frequent in IFN-α patients (5 of 218 or 2.3%) than in chemotherapy patients (1 of 104 or 1%). Cancers were slightly more frequent in chemotherapy patients (4 of 104 or 4%) than in IFN-α patients (2 of 218 or 1%). Among the nine IFN-α patients who died in chronic phase, four were in stable complete or major cytogenetic remission.
Deaths in Chronic Phase
| Treatment Arm . | IFN-α Dose (mIU/m2/d) . | Duration of IFN-α Treatment (mo) . | Time from D/C IFN-α to Death (mo) . | Overall Survival (mo) . | Age at Death (yr) . | Causes of Death . |
|---|---|---|---|---|---|---|
| IFN-α | 4.0 | 2 | 1 | 3 | 45 | Infection, acute, nonidentified |
| IFN-α | 1.5 | 2 | 6 | 8 | 63 | Cardiac, congestive heart failure |
| IFN-α | 1.2 | 1 | 9 | 10 | 69 | Cardiac, congestive heart failure |
| IFN-α | 4.2 | 10 | 0 | 10 | 66 | Gastric bleeding, liver cyrrhosis at necropsy |
| IFN-α | 4.0 | 99 | 0 | 99 | 47 | Cardiac, myocardial infarctus |
| IFN-α | 1.3 | 65 | 3 | 68 | 68 | Vascular, cerebral, thrombotic |
| IFN-α | 4.3 | 89 | 2 | 91 | 71 | Vascular, cerebral, hemorrhagic |
| IFN-α | 4.2 | 73 | 4 | 77 | 71 | Cancer, bronchogenic |
| IFN-α | 4.1 | 72 | 19 | 91 | 73 | Cancer, liver |
| CHT | — | — | — | 14 | 58 | Infection, pneumonitis |
| CHT | — | — | — | 64 | 63 | Vascular, cerebral, undefined |
| CHT | — | — | — | 32 | 65 | Cancer, pharingeal |
| CHT | — | — | — | 38 | 59 | Cancer, pancreatic |
| CHT | — | — | — | 67 | 61 | Cancer, bronchogenic |
| Treatment Arm . | IFN-α Dose (mIU/m2/d) . | Duration of IFN-α Treatment (mo) . | Time from D/C IFN-α to Death (mo) . | Overall Survival (mo) . | Age at Death (yr) . | Causes of Death . |
|---|---|---|---|---|---|---|
| IFN-α | 4.0 | 2 | 1 | 3 | 45 | Infection, acute, nonidentified |
| IFN-α | 1.5 | 2 | 6 | 8 | 63 | Cardiac, congestive heart failure |
| IFN-α | 1.2 | 1 | 9 | 10 | 69 | Cardiac, congestive heart failure |
| IFN-α | 4.2 | 10 | 0 | 10 | 66 | Gastric bleeding, liver cyrrhosis at necropsy |
| IFN-α | 4.0 | 99 | 0 | 99 | 47 | Cardiac, myocardial infarctus |
| IFN-α | 1.3 | 65 | 3 | 68 | 68 | Vascular, cerebral, thrombotic |
| IFN-α | 4.3 | 89 | 2 | 91 | 71 | Vascular, cerebral, hemorrhagic |
| IFN-α | 4.2 | 73 | 4 | 77 | 71 | Cancer, bronchogenic |
| IFN-α | 4.1 | 72 | 19 | 91 | 73 | Cancer, liver |
| CHT | — | — | — | 14 | 58 | Infection, pneumonitis |
| CHT | — | — | — | 64 | 63 | Vascular, cerebral, undefined |
| CHT | — | — | — | 32 | 65 | Cancer, pharingeal |
| CHT | — | — | — | 38 | 59 | Cancer, pancreatic |
| CHT | — | — | — | 67 | 61 | Cancer, bronchogenic |
Fourteen patients died in chronic phase, nine in the IFN-α arm and five in the chemotherapy arm.
Abbreviations: CHT, chemotherapy; D/C, discontinuation of.
Prognostic factors.
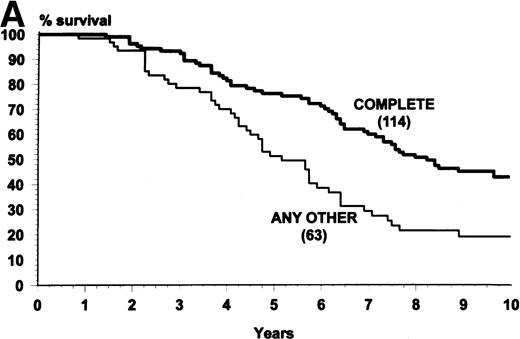
Prognostic factors for survival were examined in all the patients and in those who had been randomized to receive IFN-α (Table5). In all the patients, many factors were associated with survival, but by multivariate analysis only treatment arm remained statistically very significant, together with age, spleen, and Sokal’s score. In the patients who had been assigned to IFN-α the survival was significantly influenced by Sokal’s score, hematologic response, and cytogenetic response. Figure4 shows a landmark survival analysis of the patients who were assigned to IFN-α according to hematologic response and to cytogenetic response. Both responses were associated with a substantial and very significant prolongation of survival.
Results of Cox Proportional Hazards Model for Covariate Analysis of Censored Data on Survival (P values)
| . | All Patients (n = 306) . | IFN-α Patients (n = 162) . | ||
|---|---|---|---|---|
| Univariate . | Multivariate . | Univariate . | Multivariate . | |
| Sex | .332 | .558 | .214 | .255 |
| Age | .002 | .003 | .025 | .098 |
| Spleen | .002 | .006 | .002 | .344 |
| Hemoglobin | .009 | .435 | .052 | .857 |
| Platelet | .02 | .153 | .055 | .568 |
| WBC | .005 | .624 | .051 | .406 |
| Peripheral blood blast cells (%) | .003 | .348 | .024 | .034 |
| Sokal’s score | .0001 | .001 | .0001 | .0001 |
| Treatment arm | .001 | .001 | — | — |
| Hematologic response4-150 | — | — | .0001 | .002 |
| Cytogenetic response4-151 | — | — | .001 | .001 |
| . | All Patients (n = 306) . | IFN-α Patients (n = 162) . | ||
|---|---|---|---|---|
| Univariate . | Multivariate . | Univariate . | Multivariate . | |
| Sex | .332 | .558 | .214 | .255 |
| Age | .002 | .003 | .025 | .098 |
| Spleen | .002 | .006 | .002 | .344 |
| Hemoglobin | .009 | .435 | .052 | .857 |
| Platelet | .02 | .153 | .055 | .568 |
| WBC | .005 | .624 | .051 | .406 |
| Peripheral blood blast cells (%) | .003 | .348 | .024 | .034 |
| Sokal’s score | .0001 | .001 | .0001 | .0001 |
| Treatment arm | .001 | .001 | — | — |
| Hematologic response4-150 | — | — | .0001 | .002 |
| Cytogenetic response4-151 | — | — | .001 | .001 |
The model was applied to all cases, with the exclusion of 16 patients who were transplanted or were shifted to other treatments within the first 8 months. For IFN-α arm, the model was applied to the 162 patients (74% of total) who were alive and in chronic phase after 24 months, to avoid the bias related with time-dependent variables like hematologic and cytogenetic response (landmark analysis at 24 months).
Hematologic response was evaluated after 8 months.
Cytogenetic response was defined as a percentage of Ph neg metaphases ≥33% and was evaluated within 24 months, with examinations at 8, 14, and 24 months.
(A) Overall survival of the patients who were assigned to receive IFN- and were alive and in chronic phase after 8 months, when the hematologic response was assessed (landmark analysis). The patients who achieved a complete hematologic response (n = 114) survived more. Log-rank test, P = .0001. (B) Overall survival of the patients who were assigned to receive IFN- and were alive and in chronic phase after 24 months when the cytogenetic response was assessed (landmark analysis). The patients who achieved a complete or major cytogenetic response during the first 24 months (n = 34) survived longer. Log-rank test, P = .001.
(A) Overall survival of the patients who were assigned to receive IFN- and were alive and in chronic phase after 8 months, when the hematologic response was assessed (landmark analysis). The patients who achieved a complete hematologic response (n = 114) survived more. Log-rank test, P = .0001. (B) Overall survival of the patients who were assigned to receive IFN- and were alive and in chronic phase after 24 months when the cytogenetic response was assessed (landmark analysis). The patients who achieved a complete or major cytogenetic response during the first 24 months (n = 34) survived longer. Log-rank test, P = .001.
Cytogenetic response in IFN-α arm.
A cytogenetic response (Ph neg metaphases, 33% to 100%) was obtained in 70 of 218 cases (32%). The best cytogenetic response was complete (Ph neg, 100%) in 23 cases (10%), was major (Ph neg, 66% to 99%) in 23 cases (10%), and was minor (Ph neg, 33% to 65%) in the remaining 24 cases (11%). The time to achieve the first response ranged from less than 1 year to 7 years (median, 1 year), but the time to achieve the best response was even longer, with a median of 2 years. That was mainly due to the fact that the majority of the complete cytogenetic responses were detected rather late (4 after 1 year, 6 after 2 years, 5 after 3 years, and 8 after 5 years or more). Though many cytogenetic responses were first recorded after more than 1 year, many responders (48 of 70, 68%) received their first response within the first year of treatment, and other 14 patients (20%) were found to have some Ph neg metaphases within the same period. Only 8 of 70 cases (11%) did not show any Ph neg metaphases after the first year of treatment. One of these patients achieved a complete and stable response later, whereas the other 7 achieved only a major or a minor response, and that response was unstable and transient. Table6 shows the course and the fate of 70 patients who achieved a cytogenetic response. Seven of them (10%) were submitted to allogeneic BMT. Of the remaining 63 patients, 4 died in chronic phase (6%), 34 (54%) are alive and in chronic phase, and the remaining 25 (40%) progressed to accelerated or blastic phase and died of leukemia or related complications. The proportion of the cases with progression was negatively related with the quality of the cytogenetic response (13% for complete response, 39% for major response, and 68% for minor response, P = .005) (Table 6). In the 10 patients who progressed to accelerated or blastic phase after a complete or a major cytogenetic response, two modalities of progression were observed: in 5 cases the loss of the cytogenetic response was rapidly followed by an acute blast crisis, with a lymphoid phenotype in 4 of 5 cases; in the other 5 cases, the time from cytogenetic response loss to progression was much longer, ranging between 17 and 73 months, progression to blastic phase was slow, and the terminal phenotype was myeloid.
IFN- Arm. Disease Course and Evolution of the Patients Who Achieved a Cytogenetic Response (Ph neg metaphases ≥33%)
| Best Cytogenetic Response (Ph neg metaphases) . | Complete (100%) . | Major (66%-99%) . | Minor (33%-65%) . | Total (33%-100%) . |
|---|---|---|---|---|
| No. of cases | 23 | 23 | 24 | 70 |
| Allogeneic BMT | 0 | 5 | 2 | 7 |
| Remaining | 23 | 18 | 22 | 63 |
| Dead, accelerated or blastic phase | 3 (13%) | 7 (39%) | 15 (68%) | 25 (40%) |
| Dead, chronic phase | 3 (13%) | 1 (5%) | 0 | 4 (6%) |
| Alive, chronic phase | 17 (74%) | 10 (55%) | 7 (32%) | 34 (54%) |
| Discontinued IFN-α before progression | 0/23 | 3/18 (17%) | 9/22 (41%) | 12/63 (19%) |
| Best Cytogenetic Response (Ph neg metaphases) . | Complete (100%) . | Major (66%-99%) . | Minor (33%-65%) . | Total (33%-100%) . |
|---|---|---|---|---|
| No. of cases | 23 | 23 | 24 | 70 |
| Allogeneic BMT | 0 | 5 | 2 | 7 |
| Remaining | 23 | 18 | 22 | 63 |
| Dead, accelerated or blastic phase | 3 (13%) | 7 (39%) | 15 (68%) | 25 (40%) |
| Dead, chronic phase | 3 (13%) | 1 (5%) | 0 | 4 (6%) |
| Alive, chronic phase | 17 (74%) | 10 (55%) | 7 (32%) | 34 (54%) |
| Discontinued IFN-α before progression | 0/23 | 3/18 (17%) | 9/22 (41%) | 12/63 (19%) |
The proportion of the patients who progressed and died in accelerated or blastic phase was significantly related with the cytogenetic response (P = .005, chi-square test).
DISCUSSION
The main purpose of this updated report of the Italian study is to provide information on the long-term effects of a policy of chronic IFN-α treatment. The first point was to evaluate the effects of IFN-α on long-term survival, and more clearly to answer the question if the survival benefit over conventional chemotherapy would continue in the long term. This point was not settled in prior reports because the difference between conventional chemotherapy and IFN-α does not concern the cure rate, but the cytogenetic response rate. In this study complete and major cytogenetic responses occurred in 46 of 218 patients (21%) and only 9 patients (4%) were still in complete cytogenetic remission after more than 8 years. Although some of these patients were found to be sometimes also in molecular remission, as defined by nonquantitative reverse transcriptase polymerase chain reaction, and although some cases of molecular remission were described by others,16 in the great majority of the patients leukemia cannot be cleared off by current IFN-α treatment.17 18Therefore, this study cannot provide evidence that IFN-α can cure CML, but shows that the survival benefit of IFN-α over conventional chemotherapy did not die out and was maintained in the long term either in the low-risk patients or in the patients with a higher risk. However, whereas the relative benefit of IFN-α over chemotherapy was independent of the risk, the absolute benefit was not, because nearly all the long survivors were in the low-risk group (Figs 3 and 4). The second point was to evaluate the long-term compliance and the long-term side effects of chronic IFN-α treatment. Late treatment-related toxicity was neither more frequent nor different from early toxicity (Table 2). In particular, no more cases of neurologic toxicity were observed. However, it should be noticed that the number of cases “at risk” after 6 years was only 54 and that the mean dose that was administered after that time was 3 mIU daily. Eight patients (4%) died in chronic phase. In one case death was attributed to treatment-related pancytopenia. In the other 7 cases, a direct responsibility of treatment could not be established, but based on the causes of death (Table 4), special attention should be paid to the chronic administration of IFN-α to patients with established risk factors for cardiac or vascular disease. The development of other cancers was of concern, but the proportion was not different in the two treatment arms (1% with IFN-α and 4% with conventional chemotherapy).
This study was planned in 1985 and was started in 1986 with the aim of evaluating if IFN-α would prolong the survival over conventional chemotherapy. The design was not conceived with the purpose of answering other questions like the prediction, the kinetic, and the duration of the response. However, because these questions are important, the data were examined retrospectively and the results suggested that the cytogenetic response to IFN-α was predicted by Sokal’s risk and by hematologic response, and that the great majority of the cytogenetic responses were achieved within 1 year of treatment, though in many cases the responses improved significantly with time. The results also suggested that when the best cytogenetic response was only minor (Ph neg, 33% to 65%), the response was unstable and of a short duration, whereas the median duration of complete and major responses (Ph neg, ≥66% to 100%) was of about 60 months. These data fit with the observations from the M.D. Anderson Hospital trial.5,12 19
The treatment protocol prescribed a daily IFN-α dose of 9 mIU, corresponding to 63 mIU weekly to be administered for at least 14 months and indefinitely in the case of any cytogenetic response. The doses that were actually administered ranged widely, with a mean value decreasing from 50 mIU weekly during the first year to about 20 mIU weekly from the 5th year and continuing for the remainder of the study. These data are the result of a treatment policy that was targeted to the maximum tolerated dose and do not answer the questions regarding whether lower doses of IFN-α can be more cost-effective8,9,11,19,20 and if the treatment should be continued forever or could be discontinued once a durable complete or major cytogenetic response has been achieved, as suggested by the observations from M.D. Anderson Hospital.12,19 21
Evaluating and discussing the long-term effect of IFN-α treatment raises two main problems. The first problem is how to improve the cytogenetic response rate and how to prolong survival even more. The addition of low-dose arabinosyl cytosine was shown to be effective in one study22 and is currently being tested by our group. The second problem concerns the relationship with BMT. Autologous BMT can help to improve survival but there is no evidence of a cure,23,24 and although it is conceivable that the longer the time from diagnosis, the worse the results,25,26pretreating with IFN-α does not prevent from successful autografting.24-27 Pretreating with IFN-α does not prevent from allogeneic BMT either, although delaying the transplant would probably increase transplant-related mortality.28 One study raised the suspicion that IFN-α treatment may specifically adversely affect transplant outcome,29 but more studies showed that this was not the case.30-33 Allogeneic BMT from an HLA-identical sibling is currently considered as the first therapeutic option in the management of CML for patients under the age of 60, and CML is the first in the list of the indications for an allogeneic transplant from a matched unrelated donor.34,35This is conceivable because allogeneic BMT is the only procedure that can cure CML.19,28 34-36 However, CML is no longer a rapidly fatal disease and about 50% of the cases present with low-risk features. In this study, the 10-year survival of the low-risk patients who were assigned to IFN-α is 47% (95% CI, 36% to 59%) for all of the 91 patients and 64% (95% CI, 47% to 80%) for the 36 patients who achieved a cytogenetic response. It should not be overlooked that these are not the best results that can be obtained at a single specialized center, but rather, are the results of a nationwide multicenter study that pioneered the introduction of IFN-α in Italy.
APPENDIX
The active members of the group for this study were: N. Testoni, M.D. Zamagni, G. Martinelli: University of Bologna; D. Damiani, M. Michieli: University of Udine; A. Zaccaria, Ospedale Ravenna; G. Specchia, V. Liso: University of Bari; M. Lazzarino, C. Bernasconi: University of Pavia; E. Montefusco, G. Alimena, F. Mandelli: University La Sapienza, Roma; P. Leoni, S. Rupoli, M. Candela: University of Ancona; A. Nosari, L. Gargantini: Ospedale Niguarda Cà-Granda, Milano; I. Majolino, S. Tringali: Ospedale Cervello, Palermo; A.M. Liberati, F. Grignani, A. Tabilio, M. Martelli: University of Perugia; F. Paolino, M. Bertini: Ospedale Molinette, Torino; A. Di Tucci, G. Broccia: Ospedale Oncologico Businco, Cagliari; F. Leoni, S. Ciolli: University of Firenze; L. Luciano, B. Rotoli: University of Napoli; R. Perricone, A. Cajozzo: University of Palermo; A. Montuoro, A. De Laurenzi: Ospedale San Camillo, Roma; F. Palmieri, E. Volpe: Ospedale Avellino; A. D’Emilio, E. di Bona: Ospedale Vicenza; A. Capucci, T. Izzi: Ospedale Brescia; G.L. Scapoli, G.L. Castoldi: University of Ferrara; M. Lombardo, L. Ruberto: University of Chieti; S. Sica, G. Leone: Università Cattolica, Roma; C. Delfini, G. Nicolini: Ospedale Pesaro; F. Papineschi, E. Benedetti: University of Pisa; F. Gualandi, A.M. Marmont: Ospedale San Martino, Genova; D. Dini, G. Torelli: University of Modena; L. Mangoni, V. Rizzoli: University of Parma; M. Girino, E. Ascari: University of Pavia; C.A. Bodenizza, M. Carotenuto: Casa Sollievo della Sofferenza, San Giovanni Rotondo; A. Di Francesco, D. Quaglino: University of L’Aquila; S. Nardelli, F. Ciccone: Ospedale Latina; E. Miraglia, R. De Biasi: Ospedale Nuovo Pellegrini, Napoli; D. Ferrero, A. Pileri: University of Torino; A. Rambaldi, T. Barbui: Ospedale Bergamo; S. Morandi, C. Bergonzi: Ospedale Cremona; C. De Rosa, R. Cimino: Ospedale Cardarelli, Napoli; F. Ronca, F. Nobile: Ospedale Reggio Calabria; M. Cantonetti, S. Amadori: UniversitàTor Vergata, Roma; A. Gallamini, E. Gallo: Ospedale Cuneo; C. Musolino, G. Squadrito: University of Messina; A. Capaldi, M. Aglietta: Ospedale Mauriziano Umberto I, Torino; G. Pinotti, A. Venco: Ospedale Varese; V. Zagonel, A. Pinto: Centro Regionale di Riferimento Oncologico, Aviano; I. Gentilini, P. Coser: Ospedale Bolzano; P. Guglielmo, R. Giustolisi: University of Catania; M. Pizzuti, F. Ricciuti: Ospedale Potenza.
Writing Committee: Sante Tura, Gianantonio Rosti, Antonio de Vivo, Francesca Bonifazi, Mauro Fiacchini: the Institute of Hematology and Clinical Oncology “L. and A. Seràgnoli,” Bologna University; Michele Baccarani, Domenico Russo, Renato Fanin: the Division of Hematology and the Department of Bone Marrow Transplantation, Udine University; Eliana Zuffa: the Division of Hematology, Ravenna Hospital; Enrico Montefusco: the Institute of Hematology, Roma “La Sapienza” University.
Submitted September 29, 1997; accepted May 1, 1998.
Supported by the National Research Council, Italy, Progetto Finalizzato Applicazioni Cliniche della Ricerca Oncologica; and by AIRC, Milano, Italy.
Address reprint requests to Michele Baccarani, MD, Division of Hematology, University Hospital, 33100 Udine, Italy; e-mail:ematologia@Drmm.Uniud.it.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.