Abstract
Inorganic arsenic trioxide (As2O3) and the organic arsenical, melarsoprol, were recently shown to inhibit growth and induce apoptosis in NB4 acute promyelocytic leukemia (APL) and chronic B-cell leukemia cell lines, respectively. As2O3 has been proposed to principally target PML and PML-RAR proteins in APL cells. We investigated the activity of As2O3 and melarsoprol in a broader context encompassing various myeloid leukemia cell lines, including the APL cell line NB4-306 (a retinoic acid–resistant cell line derived from NB4 that no longer expresses the intact PML-RAR fusion protein), HL60, KG-1, and the myelomonocytic cell line U937. To examine the role of PML in mediating arsenical activity, we also tested these agents using murine embryonic fibroblasts (MEFs) and bone marrow (BM) progenitors in which the PML gene had been inactivated by homologous recombination. Unexpectedly, we found that both compounds inhibited cell growth, induced apoptosis, and downregulated bcl-2 protein in all cell lines tested. Melarsoprol was more potent than As2O3 at equimolar concentrations ranging from 10−7 to 10−5 mol/L. As2O3 relocalized PML and PML-RAR onto nuclear bodies, which was followed by PML degradation in NB4 as well as in HL60 and U937 cell lines. Although melarsoprol was more potent in inhibiting growth and inducing apoptosis, it did not affect PML and/or PML-RAR nuclear localization. Moreover, both As2O3 and melarsoprol comparably inhibited growth and induced apoptosis of PML+/+ and PML−/− MEFs, and inhibited colony-forming unit erythroid (CFU-E) and CFU granulocyte-monocyte formation in BM cultures of PML+/+ and PML−/− progenitors. Together, these results show that As2O3 and melarsoprol inhibit growth and induce apoptosis independent of both PML and PML-RAR expression in a variety of myeloid leukemia cell lines, and suggest that these agents may be more broadly used for treatment of leukemias other than APL.
© 1998 by The American Society of Hematology.
THE USE OF ORGANIC arsenicals was pioneered by Paul Ehrlich, who showed that Salvarsan (arsphenamine) was an extremely effective antispirochetal agent.1 Most organic arsenicals have been superseded by other drugs, but melarsoprol remains an important therapy for certain tropical diseases, such as African sleeping sickness.2 Moreover, potassium arsenite (administered orally as Fowler’s solution) was widely used in the West for controlling leukocytosis in patients with chronic myelocytic leukemia in the early 1900s.3 However, this therapy gradually waned with the advent of radiotherapy in the 1930s and the identification of potent antileukemic agents after the Second World War.
Recently, inorganic arsenic trioxide (As2O3) was reported to induce complete remission in a high proportion of patients with refractory acute promyelocytic leukemia (APL).4-6 Chen et al showed that As2O3 induced apoptosis in the APL cell line, NB4, which may be mediated through downregulation of bcl-2, and modulation of the PML-RARα fusion protein, the specific product of the t(15;17) of APL,7-10 and/or the PML protein.11 More recently, the antileukemic effects of As2O3 were proposed to be directly mediated by its ability to induce the relocalization and degradation of PML, as well as the degradation of PML-RARα in APL cells.12-14 We found that the organic arsenical, melarsoprol, exhibited effects similar to As2O3 on chronic B-cell leukemic cell lines,15 a finding which suggested that the cytotoxic action of this drug may not be dependent on PML or PML-RARα expression.
In the present study we have explored the effects of As2O3 and melarsoprol in a variety of myeloid leukemia cell lines in which the PML-RARα fusion protein is or is not expressed. Furthermore, to examine whether PML mediated the growth inhibitory and apoptotic activities of these compounds, we also evaluated both As2O3 and melarsoprol in cells from mice in which PML had been disrupted by homologous recombination, using both murine embryonic fibroblasts (MEFs) and bone marrow (BM) progenitors from PML−/− mice.16-18
We unexpectedly found that As2O3 and melarsoprol can inhibit growth and induce apoptosis in a PML and PML-RARα independent manner, thus suggesting that both compounds may be useful for treatment of leukemias other than APL.
MATERIALS AND METHODS
Reagents.
Arsenic trioxide (As2O3) was purchased from Sigma Chemicals, Inc (Milwaukee, WI). A stock solution was made with phosphate-buffered saline (PBS) at a concentration of 10−2 mol/L. Melarsoprol (Arsobal; purchased from Rhône Poulenc Rorer, Paris, France) was dissolved in propylene glycol at a stock concentration of 0.09 mol/L (36 mg/mL) and stored at room temperature in the dark. Both solutions were diluted to working concentrations before use.
Cell culture and cell viability assays.
Cell lines, including NB4 cells, the RA-resistant NB4 subclone 306, HL60, U937, and KG-1, were cultured in RPMI 1640 medium containing 10% fetal bovine serum (FBS), 2 mmol/L L-glutamine, penicillin G (100 U/mL), and streptomycin (100 μg/mL). MEFs were isolated from wild-type and PML−/− mouse embryos at 13.5 days of gestation and cultured in Dulbecco’s Modification of Eagle’s Medium (DMEM) supplemented with 20% FBS. Early passage MEFs were used. For cell growth and viability assays, NB4, NB4.306, HL60, U937, and KG-1 cells were seeded at an initial concentration of 1 × 105 cells/mL, and incubated in RPMI 1640 medium plus 10% FBS with or without As2O3 or melarsoprol. Cell growth and viability were assessed in triplicate by an automatic counter (Zb1; Coulter Electronic, Miami, FL) and trypan blue exclusion at indicated days. The percentage of viable cells in treated samples was divided by the average viability of the untreated samples (controls). Each growth curve was repeated at least three times.
In situ terminal deoxynucleotidyl transferase mediated dUTP-biotin nick-end labeling (TUNEL).
The procedure for identification of programmed cell death in situ was performed as described by Gavrieli et al,19 with minor modifications. In brief, NB4, HL60, and U937 cells were treated with reagents described above for 24 hours, and then pelleted onto slides by centrifugation. The slides were quickly air-dried and fixed in 4% paraformaldehyde for 30 minutes at room temperature and washed with PBS. To quench endogenous peroxidase, cells were treated with 0.1% H2O2 in PBS for 15 minutes at room temperature and washed with dH2O and TdT buffer. The labeling reaction was performed with TdT-biotin dUTP labeling mix for 1 hour in a humid chamber and stopped in 2× SSC (0.3 mol/L NaCl, 0.03 mol/L sodium citrate, pH 7.0) for 15 minutes. The slides were incubated with Vectastain ABC reagent (Vector Laboratories, Burlingame, CA) for 30 minutes after blocking with 2% bovine serum albumin (BSA) in PBS. TUNEL-positive cells were visualized by 3, 3′-diaminobenzidene (DAB) staining and counterstaining with hematoxylin. The same assay was also performed with MEFs. In this case, 1 × 104 cells were plated in each chamber of Permanox slides (Nalge Nunc International, Naperville, IL) in 1 mL of DMEM medium with or without the above-mentioned reagents. After a 24-hour incubation, cells were fixed and the same procedures described above were performed. At least 300 cells were counted for each condition.
Western blot analysis.
Cells were lysed in a lysis buffer containing 20 mmol/L Tris-HCl, 1 mmol/L EGTA, 50 mmol/L NaVO4, 50 mmol/L NaF, 0.01 U/mL aprotinin, 10 μg/mL leupeptin, 10 μg/mL pepstatin, 1 mmol/L elastinal, and 1 mmol/L phenylmethylsulfonyl fluoride (PMSF) (all from Sigma). The lysates were then sonicated using a ultrasonic homogenizer (4710 series; Cole Parmer Instruments, Chicago, IL), centrifuged at 7,500g (Sorvall Instruments, Newtown, CT), and the protein content of the lysates was determined (BioRad Protein Assay Kit I, Melville, NY) at 595 nm with a BSA standard. A sample buffer containing 10% glycerol, 0.4% sodium dodecyl sulfate (SDS), 0.3% bromphenol blue, 0.2% pyronin Y, in 1× stacking buffer (Tris base 0.5 mol/L, 0.8% SDS), 20% 2-mercaptoethanol, was added to the cell lysates which were subsequently heat-denatured at 95°C for 3 minutes. Ten micrograms per lane of protein was loaded on an SDS-polyacrylamide gel containing 12.5% polyacrylamide, and size-fractionated by electrophoresis. Proteins were electroblotted onto Immobilon-P PVDF transfer membrane (Millipore, Bedford, MA) and immunostained with a mouse anti-human monoclonal bcl-2 antibody (clone 124, M0887; dilution 1:5,000; Dako, Carpinteria, CA). Bound antibody was detected using the ECL chemiluminescence detection system (Amersham, Arlington Heights, IL). Protein bands were quantified by computer densitometry.
Immunofluorescence analysis.
Cells were cultured in medium with As2O3 or melarsoprol at concentrations of 10−6 and 10−5 mol/L for 4 hours or 24 hours, and then pelleted onto slides by cytospin and fixed in 4% paraformaldehyde. A 1:100 dilution of a mouse monoclonal antibody raised against the N-terminal region of PML (clone PGM3, SC-966; Santa Cruz Biotechnology, Santa Cruz, CA) was applied to the slides for 1 hour. Subsequently, a 1:200 dilution of a Texas Red–conjugated rabbit anti-mouse antibody (115-075-003; Jackson ImmunoResearch Laboratories Inc, West Grove, PA) was used as secondary antibody to stain the PML protein.
In vitro BM culture.
BM cells from wild-type and PML−/− mice were obtained by flushing the femur and tibia BM in Iscove’s MEM containing 15% FBS and plated at a concentration of 5 × 104 cells per dish in “Complete” methycellulose medium (StemCell Technologies, Vancouver, BC, Canada) with or without 10−6 mol/L of As2O3 or melarsoprol. Colony-forming unit erythroid (CFU-E) and CFU granulocyte-monocyte (GM) were counted at days 2 and 6 after plating, respectively. The inhibition of colony formation was calculated by comparison with control.
RESULTS
Growth inhibition by arsenicals does not depend on PML-RARα.
We tested the effects of As2O3 and melarsoprol on the growth of NB4, HL60, U937, and KG-1 cells. At concentrations of As2O3 or melarsoprol ranging from 10−7 to 10−5 mol/L, both compounds inhibited cell growth in all cell lines tested. The effective concentration of As2O3 for growth inhibition was 10−6 mol/L, and at equimolar concentrations, melarsoprol was more potent than As2O3 in all cell lines but KG-1 (Fig 1). Inhibition of growth and survival of cell lines was not dependent on the presence or absence of PML-RARα whose expression is restricted to NB4 cells.
Growth inhibition of NB4, HL60, U937, and KG-1 cell lines after As2O3 or melarsoprol treatment. Various concentrations of As2O3 and melarsoprol were added to the media (Materials and Methods): Controls (○), 10−5 mol/L (▵), 10−6 mol/L (□), and 10−7 mol/L (•). As2O3 at a concentration of 10−4 mol/L (⊞) was also used. Representative results from one of three independent experiments are shown as the mean ± SD of triplicates. Inhibition of cell growth and loss of viability are comparable in four different cell lines in which PML-RAR fusion protein is or is not present.
Growth inhibition of NB4, HL60, U937, and KG-1 cell lines after As2O3 or melarsoprol treatment. Various concentrations of As2O3 and melarsoprol were added to the media (Materials and Methods): Controls (○), 10−5 mol/L (▵), 10−6 mol/L (□), and 10−7 mol/L (•). As2O3 at a concentration of 10−4 mol/L (⊞) was also used. Representative results from one of three independent experiments are shown as the mean ± SD of triplicates. Inhibition of cell growth and loss of viability are comparable in four different cell lines in which PML-RAR fusion protein is or is not present.
Growth inhibition by arsenicals of the RA-resistant NB4 subclone 306.
Both drugs were then tested in the RA-resistant subclone 306 derived from NB4 cells in which the intact PML-RARα protein is no longer expressed.20 This line was sensitive to As2O3 (10−6mol/L) and melarsoprol (10−7 mol/L) (Fig 2A and B). Growth inhibition was also in this case both time and dose dependent. Surprisingly, NB4.306 seemed to respond better than the parental NB4 cell line to both compounds. These results are in keeping with the notion that inhibition of growth and survival of NB4 cell lines does not depend on the presence of PML-RARα or its degradation, and suggest that both arsenicals can be used in the treatment of both RA-sensitive and RA-resistant APL.
As2O3 and melarsoprol induce growth inhibition of the RA-resistant cell line NB4.306 cells. Concentrations of As2O3 (A, growth curve; C, viability) and melarsoprol (B, growth curve; D, viability) are the same as depicted in Fig 1. As2O3 and melarsoprol inhibit cell growth in a dose-dependent manner.
As2O3 and melarsoprol induce growth inhibition of the RA-resistant cell line NB4.306 cells. Concentrations of As2O3 (A, growth curve; C, viability) and melarsoprol (B, growth curve; D, viability) are the same as depicted in Fig 1. As2O3 and melarsoprol inhibit cell growth in a dose-dependent manner.
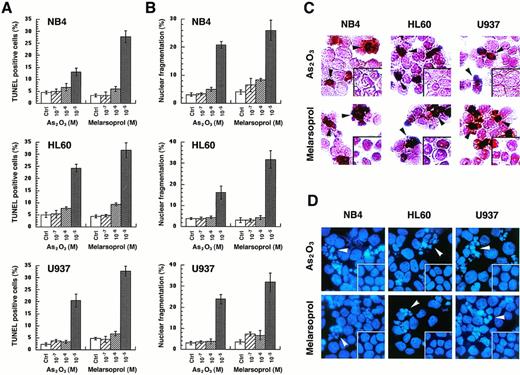
Induction of apoptosis by arsenicals in NB4, HL60, and U937 cell lines.
To show whether the growth inhibition by As2O3or melarsoprol is caused by induction of apoptosis, in situ TdT labeling or DAPI staining were performed 24 hours after cells were treated with different concentrations of As2O3or melarsoprol (see Materials and Methods). TUNEL-positive cells or cells with nuclear fragmentation which represent apoptotic cells were counted. Both As2O3 and melarsoprol induced apoptosis in all cell lines tested. Maximal apoptotic effects were observed at 10−5 mol/L with both drugs; however, melarsoprol was again somewhat more potent at this concentration (Fig 3A). At 10−6 mol/L concentration, apoptotic cells accounted for only 6% to 10% of the population examined. However, percent of mortality, in certain cases, exceeded these values, suggesting that arsenicals may also induce cell death through mechanisms other than apoptosis. The in situ TdT assay, in these experiments, seemed more sensitive than the morphological analysis (Fig 3). Apoptosis induced by these two compounds was comparable among the three different cell lines tested, irrespective of the expression of the PML-RARα fusion protein.
As2O3 and melarsoprol induce apoptosis in NB4, HL60, and U937 cell lines. Apoptotic cells were identified by the in situ TdT assay, as well as morphological changes of nuclei after DAPI staining. The percentages of TUNEL-positive cells (A) and cells with nuclear fragmentation (B) were expressed as the mean ± SD. Morphology of TUNEL-positive cells are indicated with black arrows and fragmented nuclei are indicated with white arrows, as shown in panels (C) and (D).
As2O3 and melarsoprol induce apoptosis in NB4, HL60, and U937 cell lines. Apoptotic cells were identified by the in situ TdT assay, as well as morphological changes of nuclei after DAPI staining. The percentages of TUNEL-positive cells (A) and cells with nuclear fragmentation (B) were expressed as the mean ± SD. Morphology of TUNEL-positive cells are indicated with black arrows and fragmented nuclei are indicated with white arrows, as shown in panels (C) and (D).
PML and PML-RARα are relocalized and degraded by As2O3 but not melarsoprol.
PML is typically concentrated within discrete speckled nuclear structures that have been variably named PML nuclear bodies (NBs), or Kremer bodies, ND10, or PODs (for PML oncogenic domains).21-25 The presence of PML-RARα leads to the delocalization of PML from the NBs in APL cells, where it acquires a microspeckled/granular nuclear localization. PML, RARα, RXRα, and the PML-RARα fusion protein colocalize in these aberrant structures. Upon RA treatment, these proteins reacquire their natural nuclear localization.23-25
A previous report suggested that As2O3-induced relocalization of PML or PML-RARα onto nuclear bodies and subsequent degradation of PML or PML-RARα was key in the induction of apoptosis in NB4 cells by this compound.12 To test this hypothesis, we cultured three different leukemic cell lines in the presence of As2O3 or melarsoprol at a concentration of 10−6 (Fig 4) or 10−5 mol/L (not shown) for 24 hours, time by which both compounds already induced cell death. Indirect immunofluorescence staining of PML with a specific monoclonal antibody was performed in control and treated NB4, HL60, and U937 cells after 4 and 24 hours. In NB4 cells, the microspeckled pattern of PML staining rapidly changed to a speckled pattern upon As2O3 treatment, followed by a reduction in the number of PML NBs (Fig 4). The normal nuclear distribution of PML was seen in untreated HL60 and U937 cells, as shown in Fig 4. However, after incubation with As2O3, the number of PML NBs was dramatically reduced also in these cells. Although melarsoprol was more potent in inducing apoptosis, treatment with this drug did not alter PML or PML-RARα nuclear localization patterns in NB4, HL60, and U937 cells either after 4 hours (not shown) or after 24 hours of incubation at both 10−6 (Fig 4) or 10−5 mol/L (not shown) concentrations, suggesting that these agents do not modulate PML or PML-RARα proteins in an identical manner.
PML relocalization and degradation in APL or non-APL cell lines after As2O3 and melarsoprol treatment. As2O3 induces PML relocalization onto nuclear bodies, followed by PML degradation not only in NB4 cells but also in HL60 and U937 cell lines. Melarsoprol does not affect PML nuclear localization.
PML relocalization and degradation in APL or non-APL cell lines after As2O3 and melarsoprol treatment. As2O3 induces PML relocalization onto nuclear bodies, followed by PML degradation not only in NB4 cells but also in HL60 and U937 cell lines. Melarsoprol does not affect PML nuclear localization.
As2O3 and melarsoprol downregulate bcl-2.
To identify what would be a possible mechanism underlying the ability of As2O3 and melarsoprol to induce apoptosis, we performed a Western blot analysis of bcl-2 expression on NB4, NB4-306, HL60, U937 cells treated with various concentrations of As2O3 and melarsoprol (Materials and Methods). Both compounds reduced the levels of bcl-2 protein, not only in NB4 cells but also in HL60, U937, and the RA-resistant NB4 subclone 306 upon 24-hour incubation with these drugs (Fig 5). These changes were dose dependent. Again, melarsoprol was more potent in this effect compared to As2O3. Thus, both As2O3and melarsoprol induce bcl-2 downregulation, and this process does not depend on the presence of PML-RARα.
Downregulation of bcl-2 by As2O3and melarsoprol in myeloid cell lines irrespective of PML-RAR expression. As2O3 and melarsoprol downregulate bcl-2 expression at concentrations of 10−6 and 10−5 mol/L in all cell lines tested.
Downregulation of bcl-2 by As2O3and melarsoprol in myeloid cell lines irrespective of PML-RAR expression. As2O3 and melarsoprol downregulate bcl-2 expression at concentrations of 10−6 and 10−5 mol/L in all cell lines tested.
Growth inhibition and induction of apoptosis by arsenicals in cells devoid of PML.
To study the role of PML in growth inhibition or apoptosis by arsenicals, we took advantage of mice in which the PML gene had been ablated by homologous recombination.16-18 We treated primary MEFs isolated from wild-type and PML−/− mice with different concentrations of As2O3 or melarsoprol for 24 hours. Both compounds triggered apoptosis of MEFs at concentrations of 10−6 and 10−5mol/L. No differences in apoptosis between wild-type and PML−/− MEFs were observed (Fig6A and B). Next, we performed in vitro cultures with BM cells from wild-type and PML−/− mice in the presence or absence of As2O3 or melarsoprol (Materials and Methods). Formation of CFU-E and CFU-GM from wild-type and PML−/− BM progenitors were comparably inhibited by 10−6 mol/L concentration of both compounds (Fig 6C and D). Thus, both As2O3 and melarsoprol can inhibit cell growth in a PML independent manner.
As2O3 and melarsoprol induce growth inhibition and apoptosis in a PML independent manner. Wild-type and PML−/− MEFs were incubated with the indicated concentrations of As2O3 (A) and melarsoprol (B) for 24 hours, and apoptotic cells were identified by in situ TdT. For each condition, at least 300 cells were counted. No differences in apoptosis and growth inhibition induced by As2O3 and melarsoprol between wild-type and PML−/− cells were found. For in vitro BM cultures (Materials and Methods), wild-type and PML−/− cells were incubated with As2O3 and melarsoprol at a concentration of 10−6 mol/L. At day 2 and day 6 CFU-E (C) and CFU-GM (D) were counted in triplicate. The data are expressed as a percentage of colony formation of arsenicals-treated versus untreated cells: untreated = 100%. The bars indicate the mean values ± SD from one representative experiment out of three.
As2O3 and melarsoprol induce growth inhibition and apoptosis in a PML independent manner. Wild-type and PML−/− MEFs were incubated with the indicated concentrations of As2O3 (A) and melarsoprol (B) for 24 hours, and apoptotic cells were identified by in situ TdT. For each condition, at least 300 cells were counted. No differences in apoptosis and growth inhibition induced by As2O3 and melarsoprol between wild-type and PML−/− cells were found. For in vitro BM cultures (Materials and Methods), wild-type and PML−/− cells were incubated with As2O3 and melarsoprol at a concentration of 10−6 mol/L. At day 2 and day 6 CFU-E (C) and CFU-GM (D) were counted in triplicate. The data are expressed as a percentage of colony formation of arsenicals-treated versus untreated cells: untreated = 100%. The bars indicate the mean values ± SD from one representative experiment out of three.
DISCUSSION
Clinical reports from two Chinese groups, confirmed by a preliminary study in the United States, indicate that As2O3is extremely effective for inducing complete remission in patients with APL who are resistant to both cytotoxic chemotherapy and all-trans retinoic acid.4-6 These studies have suggested that almost all patients who express PML-RARα fusion mRNA are clinically responsive. Recently, the cytotoxic effects of this agent were linked to its ability to relocalize the aberrant PML-RARα fusion protein onto a specific nuclear organelle, the PML nuclear body, which was closely followed by degradation of PML-RARα and the PML proteins.12,14 The direct implication of these results is that the antileukemic effects of As2O3 would thus be limited to APL patients who expressed the fusion protein. However, we have recently tested both As2O3 and melarsoprol (an organic arsenical) in various B-cell lymphoid cell lines and observed striking apoptotic effects,15 suggesting that in these cell lines, PML-RARα expression was not essential to the cytotoxic mechanism. Therefore, to further examine the dependency of arsenicals on PML or PML-RARα expression, we evaluated the effects of these agents in a number of myeloid cell lines that expressed wild-type PML, with or without the fusion PML-RARα, and also in cells in which wild-type PML had been knocked out by homologous recombination.
Results from our experiments show that the cytotoxic effect of both arsenicals in these cell lines is not mediated by mechanisms that are dependent on PML or PML-RARα expression. In most lines, melarsoprol was somewhat more potent compared with As2O3 in inhibiting growth and inducing apoptosis, and the effects of both drugs were dose dependent. As previously reported,12 we confirmed that As2O3 relocalized PML protein onto nuclear bodies and induced PML and PML-RARα degradation in NB4 cells while triggering apoptosis. However, similar effects were also observed in HL60 and U937 cells which do not harbor the PML-RARα fusion gene. Moreover, melarsoprol induced apoptosis in all the cell lines tested without altering PML and/or PML-RARα.
Similarly, the differentiating action of As2O3and melarsoprol, which in our hands was negligible, did not appear to depend on the expression and/or modulation of PML and/or PML-RARα either. In fact, the small effect we observed in long-term cultures (up to 2 weeks), was comparable in all the cell lines tested with both compounds (data not shown).
We also found that bcl-2 downregulation, which has been previously linked to the antileukemic effects of As2O3 in APL, was also not dependent on expression of PML-RARα protein, because it occurred in the NB4 subclone 306 in which the intact protein is not detectable. Finally, to test whether PML expression was essential to the antileukemic effects of arsenicals, we tested both agents in mouse embryonic fibroblasts and BM cells from animals wherein wild-type PML had been eliminated by homologous recombination. In these cells wholly lacking PML expression,18 both As2O3 and melarsoprol were equally effective in inhibiting growth and inducing apoptosis, and both had similar effects on normal CFU-E and CFU-GM colony formation. Moreover, no differences between wild-type and PML−/− cells were observed. Together, these data strongly support the notion that the antileukemic effects of these arsenicals occurs independently of both PML and PML-RARα expression. These results are in keeping with the medicinal history of arsenicals for diseases that are not characterized by alterations in PML protein such as, for instance, chronic myelocytic leukemia.3
Our results indicate that both As2O3 and melarsoprol are broadly active as antileukemic agents in both myeloid and lymphoid diseases. However, recent studies in solid tumor cell lines show that this activity is not indiscriminate, and does not occur merely as a nonselective cellular poison.26-28 Recent attempts to extend the clinical utility of melarsoprol have been constrained by previously unappreciated neurotoxicity.29Although severe neurotoxicity has also been reported with doses of As2O3 approximately fivefold in excess of that recently reported to be effective in APL,30 this effect has not been observed with the lower doses used clinically in recent series.4-6
In conclusion, our data indicate that cytotoxic activity is not mediated by the PML protein and therefore is not limited to diseases that are associated with alterations in PML expression. Thus, assuming a reasonable therapeutic index can be established clinically, arsenicals may have a potentially broader therapeutic role that is not confined to APL.
Supported in part by the National Cancer Institute, National Institutes of Health (CA-77136 to R.P.W. and CA-71692 to P.P.P.); the US Food and Drug Administration (FD-R-001364 to R.P.W.); and by a grant from the Lymphoma Foundation. P.P.P. is a Scholar of the Leukemia Society of America.
Address reprint requests to Pier Paolo Pandolfi, MD, PhD, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10021; e-mail: p-pandolfi@ski.mskcc.org
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal