Abstract
Eosinophils are potent inflammatory cells involved in allergic reactions. Inhibition of apoptosis of purified eosinophils by certain cytokines has been previously shown to be an important mechanism causing tissue eosinophilia. To elucidate the role of Bcl-2 family members in the inhibition of eosinophil apoptosis, we examined the expression of the known anti-apoptotic genes Bcl-2, Bcl-xL, and A1, as well as Bax and Bcl-xS, which promote apoptosis in other systems. We show herein that freshly isolated human eosinophils express significant amounts of Bcl-xL and Bax, but only little or no Bcl-2, Bcl-xS, or A1. As assessed by reverse transcription-polymerase chain reaction, immunoblotting, flow cytometry, and immunocytochemistry, we show that spontaneous eosinophil apoptosis is associated with a decrease in Bcl-xL mRNA and protein levels. In contrast, stimulation of the cells with granulocyte-macrophage colony-stimulating factor (GM-CSF) or interleukin-5 (IL-5) results in maintenance or upregulation of Bcl-xL mRNA and protein levels. Moreover, Bcl-2 protein is not induced by GM-CSF or IL-5 in purified eosinophils. Bcl-2 protein is also not expressed in tissue eosinophils as assessed by immunohistochemistry using two different eosinophilic tissue models. Furthermore, Bcl-xL antisense but not scrambled phosphorothioate oligodeoxynucleotides can partially block the cytokine-mediated rescue of apoptotic death in these cells. These data suggest that Bcl-xL acts as an anti-apoptotic molecule in eosinophils.
© 1998 by The American Society of Hematology.
EOSINOPHILIC INFILTRATION into tissues is usually followed by elimination of these cells by apoptosis. Cytokine-mediated inhibition of apoptosis contributes to the accumulation of eosinophils in tissues.1 Such eosinophilia is often observed in patients with chronic allergic diseases such as bronchial asthma.2 3 In the past few years there has been some progress in defining the tyrosine kinases that are activated by the interleukin-3 (IL-3)/IL-5/granulocyte-macrophage colony-stimulating factor (GM-CSF) receptor β-subunit. In contrast, no information is available regarding the genetic control of eosinophil apoptosis in allergic diseases.
Members of the Bcl-2 family of proteins are important regulators of apoptosis in many cellular systems.4 The first member of the family, Bcl-2, was originally cloned from the breakpoint of a t(14;18) translocation present in many human B-cell lymphomas. Increased production of Bcl-2 protein as a result of t(14;18) translocation contributes to neoplastic B-cell expansion by preventing B-cell death.4 Besides Bcl-2, there are other members of the family that inhibit apoptosis. For instance, the proteins encoded by Bcl-xL4,5 and A16 genes are also potent blockers of apoptosis. In contrast, other members of the Bcl-2 family promote cell death. For example, Bax7 and Bcl-xS5 are such pro-apoptotic proteins.
There have been some reports on the expression of Bcl-2 in eosinophils. Whereas two groups8,9 obtained evidence for Bcl-2 expression, a third group10 did not observe significant Bcl-2 levels. Therefore, the expression of Bcl-2 and other members of the Bcl-2 family in eosinophils does not appear to be clear at the moment. Moreover, all previously published work analyzed gene expression, but no functional data are currently available on Bcl-2 family members in eosinophils. To better understand the regulation of eosinophil apoptosis in chronic eosinophilic inflammation, we have investigated the expression of Bcl-2, Bcl-xL, A1, Bax, and Bcl-xS in freshly purified ex vivo eosinophils from control individuals and patients with atopic dermatitis as well as in tissue eosinophils that reflect the in vivo situation of chronic eosinophilic inflammation. Moreover, we measured the expression of these genes in eosinophils cultured in the presence or absence of GM-CSF and IL-5 in vitro. Furthermore, this study provides functional evidence for a role of Bcl-xL in the control of eosinophil apoptosis.
MATERIALS AND METHODS
Antibodies.
Anti–Bcl-2 monoclonal antibody (MoAb), control IgG1 MoAb, swine anti-rabbit fluorescein isothiocyanate (FITC)-conjugated secondary IgG antibody, and control rabbit IgG were from Dako (Zurich, Switzerland). FITC-conjugated anti–Bcl-2 MoAb was from Ancell Corp (Bayport, MN). Polyclonal rabbit antibodies against Bcl-x and Bax were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Polyclonal rabbit anti-Bcl-xS antibody was from Calbiochem-Novabiochem Corp (San Diego, CA). Goat anti-rabbit and anti-mouse horseradish peroxidase (HRP)-labeled secondary antibodies were obtained from Amersham International (Bucks, UK). FITC-conjugated control IgG1 MoAb was from Coulter (Hialeah, FL). Anti–IL-3 and anti–GM-CSF MoAbs were purchased from Genzyme (Cambridge, MA). Anti–IL-5 MoAb (5A5) was a kind gift from Dr J. Tavernier (University of Gent, Gent, Belgium). Anti-eosinophil cationic protein (ECP) MoAb (EG1) was from Pharmacia (Uppsala, Sweden). Anti-CD16 MoAb microbeads were from Miltenyi Biotec (Bergisch-Gladbach, Germany).
Eosinophil purification and cell cultures.
Eosinophils were purified from patients with atopic dermatitis and healthy normal individuals as previously described.11-14Eosinophils were cultured at 1 × 106/mL (expression experiments) or 0.5 × 106/mL (antisense experiments) for the indicated times using complete culture medium (RPMI 1640 supplemented with 10% fetal calf serum [FCS]). GM-CSF was a kind gift from Dr T. Hartung (University of Konstanz, Konstanz, Germany). IL-5 was obtained from Genzyme. The final cytokine concentrations were 25 ng/mL. Phosphorothioate oligodeoxynucleotide were synthesized by Genset S.A. (Paris, France). Sequences used were as follows: antisense Bcl-xL, 5′-TGT ATC CTT TCT GGG AAA GC-3′; and scrambled Bcl-xL, 5′-TAA GTT CCG ATG CGA CTT GT-3′. These antisense molecules were selected from a panel of different oligodeoxynucleotides as previously described.15The oligodeoxynucleotides were given to purified eosinophils that had been cultured for 20 hours in complete culture medium (at this time, the cells did not express detectable Bcl-xL protein levels), and were delivered in the form of complexes with the cationic lipid DOTAP (Boehringer Mannheim, Mannheim, Germany). Equal volumes of oligodeoxynuleotide (6 μmol/L) and DOTAP (0.2 mmol/L) were mixed and allowed to complex for 10 minutes at room temperature. The final oligodeoxynucleotide concentrations were 0.45 μmol/L. The initial phase (first 4 hours) of incubation with the oligodeoxynucleotides was performed in medium without serum to increase the uptake of these molecules by the eosinophils. Cells were then cultured again in complete culture medium in the presence or absence of GM-CSF or IL-5 for another 24 hours (apoptosis assay) or 36 hours (cell death assay). Therefore, cell viability was determined after the eosinophils had been in culture for a total of 60 hours.
Reverse transcription-polymerase chain reaction (RT-PCR).
mRNA expression of Bcl-2 family members was studied using Southern blot analysis linked to RT-PCR.1,11,12 Primers for Bcl-2 (5′-ACA ACA TCG CCC TGT GGA TGA C-3′ and 5′-ATA GCT GAT TCG ACG TTT TGC C-3′), Bcl-x (5′-GGA ATT CTT GGA CAA TGG ACT GGT TGA-3′ and 5′-CCC AAG CTT GTA GAG TGG ATG GTC AGT G-3′), Bax (5′-GGA ATT CTG ACG GCA ACT TCA ACT GGG-3′ and 5′-GGA ATT CTT CCA GAT GGT GAG CGA GG-3′), and A1 (5′-GAA GAT GAC AGA CTG TGA AT-3′ and 5′-TCA ACA GTA TTG CTT CAG GAG-3′) amplifications were synthesized (Bcl-2: HSC Biotechnology Service Centre, Toronto, Ontario, Canada; Bcl-x and Bax: R & D Systems, Abingdon, UK; A1: Microsynth, Balgach, Switzerland) according to previously published sequences.6 16 For positive controls, PCR amplifications were performed at the same time using cDNAs from HL-60 (Bcl-2, Bcl-x, Bax) and Jurkat (A1) cells. For negative controls, PCR reactions were performed without template DNA. Primers for β-actin control amplification were obtained from Clontech (Palo Alto, CA). The cDNAs used for the probes were cloned by PCR amplification of positive template DNA (cDNAs from HL-60, Jurkat cells, and neutrophils), and their specificities confirmed by sequencing.
Immunofluorescence.
Purified eosinophils (0.1 × 106) were stained with FITC-conjugated anti–Bcl-2 or control MoAb for 15 minutes at room temperature after a 45-minute permeabilization of the cell membrane with Permeafix (Ortho Diagnostic Systems, Raritan, NJ). To determine Bcl-x and Bax expression, cells were initially incubated with 10 μg/mL anti-Bcl-x, anti-Bcl-xS, anti-Bax, or control rabbit antibody for 15 minutes at room temperature, washed, and then incubated with FITC-conjugated purified swine anti-rabbit IgG antibody for 15 minutes at room temperature. Directly or indirectly stained cells were washed, and fixed in phosphate-buffered saline (PBS)-buffered 2% paraformaldehyde solution. Eosinophils were analyzed by flow cytometry in an EPICS XL (Coulter).
Immunocytochemistry.
Bcl-x, Bcl-xS, and Bax protein expression was also investigated by immunocytochemistry using a commercial kit (Histostain SP kit; Zymed Laboratories, San Francisco, CA) according to the manufacturer's instructions. Briefly, cytospins were prepared from 0.1 × 106 purified eosinophils. Slides were fixed in freshly made and filtered PBS-buffered 4% paraformaldehyde solution for 20 hours at room temperature in the dark. After washing with H2O and PBS, slides were incubated with Peroxo-Block (Zymed Laboratories) for 10 minutes to quench endogenous peroxidase activity. After blocking with nonimmune serum, 1 μg/mL primary antibody was added for 1 hour. This incubation was followed by addition of biotinylated secondary antibody and streptavidin-peroxidase conjugate. Bound peroxidase was detected by addition of substrate chromogen mixture, followed by hematoxylin counterstaining. Slides were mounted, and examined under a Zeiss Axioscope microscope (Oberkochen, Germany) at a magnification of ×1,000.
For Bcl-2 staining, the alkaline phosphatase-anti-alkaline phosphatase (APAAP) method was used.11 Staining was performed with a commercial kit (Dako) according to the manufacturer's instructions.
Immunoprecipitation, gel electrophoresis, and immunoblotting.
Immunoprecipitation, gel electrophoresis, and immunoblotting were performed as previously described,13 using anti–Bcl-x antibody.
Immunohistochemistry.
Immunohistochemistry was performed using nasal polyp and bladder cancer tissues as previously described.1 Anti-ECP, anti–Bcl-2, anti–IL-3, anti–IL-5, or anti–GM-CSF MoAb immunostainings were performed using the APAAP method with a commercial kit (Dako) according to the manufacturer's instructions. In additional experiments, sections were stained with anti–Bcl-x and anti-Bax antibody. These immunostainings were performed using a kit for rabbit primary antibody (Zymed Laboratories). Sections were examined under a Zeiss Axioscope microscope at a magnification of ×400 or ×1,000.
Determination of eosinophil death and apoptosis.
Cell death of eosinophils was assessed by uptake of 1 μmol/L ethidium bromide and flow cytometric analysis (EPICS XL) as previously described.12-14 To determine whether eosinophil death was apoptosis, eosinophils were morphologically examined.17Cytospin preparations were made, stained with Diff-Quik (Baxter, Düdingen, Switzerland), and analyzed as well as photographed under a Zeiss Axioscope microscope at a magnification of ×1,000.
RESULTS AND DISCUSSION
Bcl-xL, but not Bcl-xS, is significantly expressed by eosinophils.
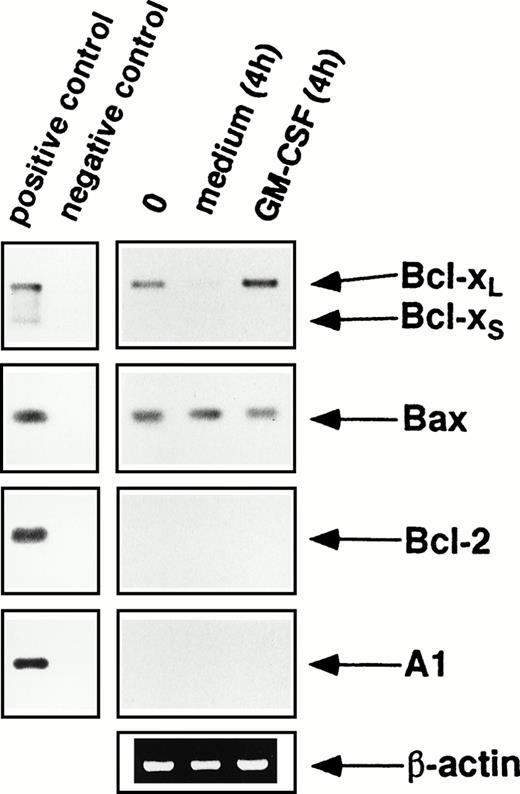
We first measured mRNA levels by RT-PCR in freshly isolated as well as cultured eosinophils in the presence or absence of the survival factors GM-CSF or IL-5. Because the PCR primers used bind to sequences shared by Bcl-xL and Bcl-xS, this technique allowed simultaneous identification of both Bcl-x mRNAs. As shown in Fig 1, freshly purified eosinophils expressed Bcl-xL but not Bcl-xS mRNA. The expression of Bcl-xL appeared to increase after GM-CSF stimulation of the eosinophils in vitro but rapidly decreased when eosinophils were cultured without cytokine support (Fig 1). To better quantify the expression of Bcl-xL mRNA in response to eosinophil hematopoietins, RT-PCR was performed using different numbers of cycles. These experiments clearly showed that Bcl-xLmRNA expression in eosinophils is downregulated in the absence and upregulated in the presence of eosinophil survival factors such as GM-CSF (Fig 2D) or IL-5 (not presented).
mRNA expression of Bcl-2 family members by purified blood eosinophils as assessed by RT-PCR using 20 cycles of amplification. Freshly isolated peripheral blood eosinophils (0) expressed significant levels of mRNA for Bcl-xL and Bax, but not for Bcl-2, Bcl-xS, and A1. When eosinophils were cultured in medium without cytokine support, the levels of Bcl-xL rapidly decreased, but were maintained or slightly increased after GM-CSF stimulation in vitro. In contrast, Bax mRNA levels remained unchanged when eosinophils were cultured in the presence or absence of GM-CSF. The same results were observed when eosinophils were cultured with IL-5 (not presented). Furthermore, Bcl-2 and A1 mRNA levels did not increase after incubation with eosinophil hematopoietins. As a control, human β-actin cDNA was amplified and PCR products were stained by ethidium bromide in an agarose gel. Positive control amplifications were performed using cDNAs from HL-60 (Bcl-x, Bax, Bcl-2) and Jurkat (A1) cells. All data are representative of eight independent experiments.
mRNA expression of Bcl-2 family members by purified blood eosinophils as assessed by RT-PCR using 20 cycles of amplification. Freshly isolated peripheral blood eosinophils (0) expressed significant levels of mRNA for Bcl-xL and Bax, but not for Bcl-2, Bcl-xS, and A1. When eosinophils were cultured in medium without cytokine support, the levels of Bcl-xL rapidly decreased, but were maintained or slightly increased after GM-CSF stimulation in vitro. In contrast, Bax mRNA levels remained unchanged when eosinophils were cultured in the presence or absence of GM-CSF. The same results were observed when eosinophils were cultured with IL-5 (not presented). Furthermore, Bcl-2 and A1 mRNA levels did not increase after incubation with eosinophil hematopoietins. As a control, human β-actin cDNA was amplified and PCR products were stained by ethidium bromide in an agarose gel. Positive control amplifications were performed using cDNAs from HL-60 (Bcl-x, Bax, Bcl-2) and Jurkat (A1) cells. All data are representative of eight independent experiments.
Bcl-xL, but not Bcl-xS, protein is significantly expressed by purified blood eosinophils. (A) Immunocytochemistry. Bcl-x (the antibody used reacts with both Bcl-xL and Bcl-xS proteins) proteins were expressed in freshly isolated peripheral blood eosinophils (0), and levels rapidly decreased under conditions of cytokine withdrawal in vitro, but were maintained in the presence GM-CSF. (B) Flow cytometry. The Bcl-x protein expression observed in freshly isolated peripheral blood eosinophils (0) was undetectable in the absence of eosinophil hematopoietins and appeared to be increased by cytokine exposure in cultured eosinophils after 20 hours. (C) Immunoprecipitation and immunoblot. Bcl-xL (arrow), but not Bcl-xS(arrowhead), protein expression was observed in freshly isolated peripheral blood eosinophils (0). Bcl-xL protein expression was increased after GM-CSF stimulation but decreased after cytokine withdrawal in vitro. (Right) The positions of molecular size standards. (D) Semiquantitative RT-PCR. Bcl-xL protein expression correlates with the expression of Bcl-xL mRNA. Cytokine withdrawal in vitro decreased Bcl-xL mRNA in eosinophils, whereas stimulation with GM-CSF or IL-5 (not presented) increased Bcl-xL mRNA expression. (Top) Numbers of PCR cycles. (E and F) Immunocytochemistry and flow cytometry. No detectable Bcl-xS (the antibody used reacts specifically with Bcl-xS) protein expression was observed in freshly isolated peripheral blood eosinophils (0). (A, B, E, and F) are representative of eight independent experiments, (C and D) of three independent experiments.
Bcl-xL, but not Bcl-xS, protein is significantly expressed by purified blood eosinophils. (A) Immunocytochemistry. Bcl-x (the antibody used reacts with both Bcl-xL and Bcl-xS proteins) proteins were expressed in freshly isolated peripheral blood eosinophils (0), and levels rapidly decreased under conditions of cytokine withdrawal in vitro, but were maintained in the presence GM-CSF. (B) Flow cytometry. The Bcl-x protein expression observed in freshly isolated peripheral blood eosinophils (0) was undetectable in the absence of eosinophil hematopoietins and appeared to be increased by cytokine exposure in cultured eosinophils after 20 hours. (C) Immunoprecipitation and immunoblot. Bcl-xL (arrow), but not Bcl-xS(arrowhead), protein expression was observed in freshly isolated peripheral blood eosinophils (0). Bcl-xL protein expression was increased after GM-CSF stimulation but decreased after cytokine withdrawal in vitro. (Right) The positions of molecular size standards. (D) Semiquantitative RT-PCR. Bcl-xL protein expression correlates with the expression of Bcl-xL mRNA. Cytokine withdrawal in vitro decreased Bcl-xL mRNA in eosinophils, whereas stimulation with GM-CSF or IL-5 (not presented) increased Bcl-xL mRNA expression. (Top) Numbers of PCR cycles. (E and F) Immunocytochemistry and flow cytometry. No detectable Bcl-xS (the antibody used reacts specifically with Bcl-xS) protein expression was observed in freshly isolated peripheral blood eosinophils (0). (A, B, E, and F) are representative of eight independent experiments, (C and D) of three independent experiments.
To determine whether the expression of Bcl-xL and Bcl-xS mRNAs correlates with the expression of their proteins, we performed immunocytochemistry, flow cytometry, and immunoblotting following immunoprecipitation using anti–Bcl-x (reacts with both Bcl-xL and Bcl-xS proteins) and specific anti–Bcl-xS antibodies. As shown in Fig 2A and B, and Fig 3A, freshly purified blood and tissue eosinophils expressed Bcl-x protein. Moreover, levels of Bcl-x protein were maintained or increased in GM-CSF–stimulated eosinophils. In contrast, levels of Bcl-x protein decreased under conditions of cytokine withdrawal in vitro (Fig 2A through C). Immunoblotting studies after anti–Bcl-x immunoprecipitation showed that the Bcl-x expression observed by immunocytochemistry and flow cytometry was totally caused by Bcl-xL expression, because no protein from the Bcl-xS splice form was detected (Fig 2C). In agreement with these data and recently published work,10Bcl-xS expression was also not seen using a specific anti–Bcl-xS antibody as assessed by immunocytochemistry (Fig 2E) and flow cytometry (Fig 2F). Together, these results suggest that expression of Bcl-xL transcripts correlates with the patterns of Bcl-xL protein expression in both freshly isolated or in vitro cultured eosinophils. Moreover, Bcl-x function does not appear to be regulated at the level of splicing in eosinophils.
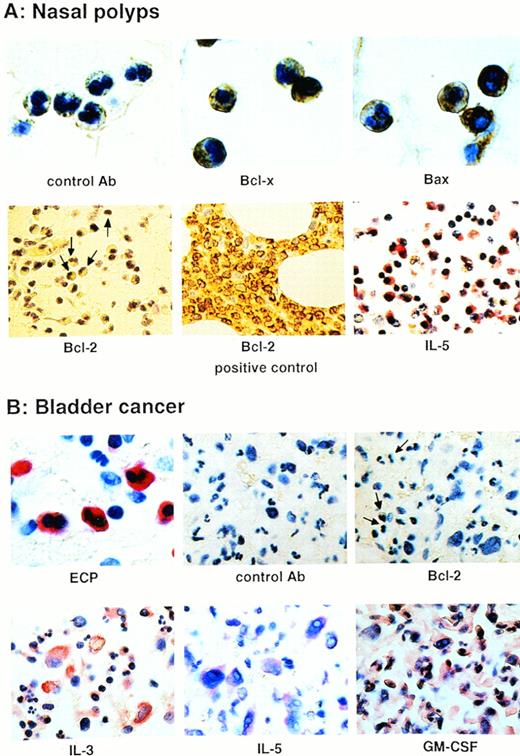
Bcl-x and Bax, but not Bcl-2, proteins are significantly expressed by eosinophils in eosinophilic tissues. (A) Immunohistochemical staining with the indicated antibody using nasal polyp tissues. Bcl-x and Bax, but not Bcl-2, proteins were detectable in tissue eosinophils. In the Bcl-2 panel, some representative eosinophils are marked with arrows. The Bcl-2+ control was bone marrow tissue from a patient with a B-cell lymphoma. Moreover, IL-5 protein was highly expressed in nasal polyp tissues. (B) Staining of tissue infiltrated by bladder cancer cells as well as eosinophils. Again, eosinophils expressed no detectable Bcl-2 protein (see cells marked with an arrow in the third panel), although high levels of the eosinophil hematopoietins were present in this tissue.
Bcl-x and Bax, but not Bcl-2, proteins are significantly expressed by eosinophils in eosinophilic tissues. (A) Immunohistochemical staining with the indicated antibody using nasal polyp tissues. Bcl-x and Bax, but not Bcl-2, proteins were detectable in tissue eosinophils. In the Bcl-2 panel, some representative eosinophils are marked with arrows. The Bcl-2+ control was bone marrow tissue from a patient with a B-cell lymphoma. Moreover, IL-5 protein was highly expressed in nasal polyp tissues. (B) Staining of tissue infiltrated by bladder cancer cells as well as eosinophils. Again, eosinophils expressed no detectable Bcl-2 protein (see cells marked with an arrow in the third panel), although high levels of the eosinophil hematopoietins were present in this tissue.
Bax is expressed at high levels by eosinophils.
As shown in Fig 1, Bax mRNA was highly expressed in freshly isolated eosinophils. In addition, Bax mRNA levels remained unchanged when eosinophils were cultured in the presence or absence of GM-CSF (Fig 1) or IL-5 (not presented).
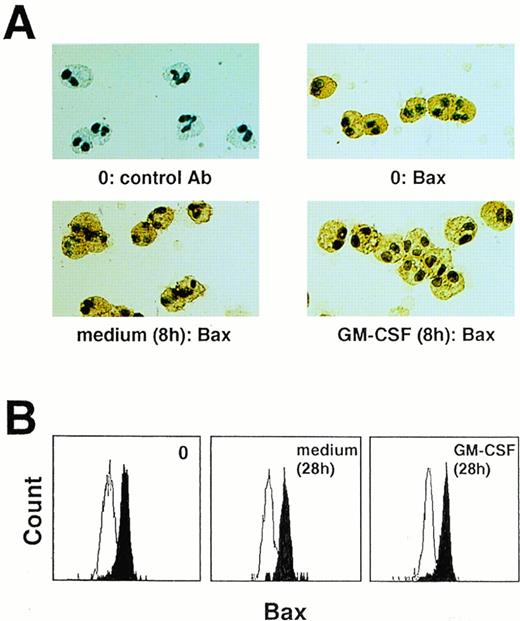
To determine Bax protein expression, an anti–Bax antibody was used. This antibody was able to stain tissue and purified blood eosinophils (Fig 3A, and Fig 4A and B). Moreover, we had no evidence for a change in Bax protein expression after in vitro cell cultures of eosinophils in the presence or absence of cytokines with anti-apoptotic properties (Fig 4A and B).
Bax protein is expressed by purified blood eosinophils. Bax protein levels remained unchanged when eosinophils were cultured in the presence or absence of eosinophil hematopoietins, as seen by immunocytochemistry (A) and flow cytometry (B). Each figure is representative of eight independent experiments.
Bax protein is expressed by purified blood eosinophils. Bax protein levels remained unchanged when eosinophils were cultured in the presence or absence of eosinophil hematopoietins, as seen by immunocytochemistry (A) and flow cytometry (B). Each figure is representative of eight independent experiments.
Bcl-2 and A1 are expressed at very low levels by eosinophils.
We again used RT-PCR to determine Bcl-2 and A1 mRNA levels. As shown in Fig 1, freshly isolated as well as cultured eosinophils in the presence or absence of GM-CSF from normal control individuals and allergic patients do not express Bcl-2 and A1 mRNA. Note that positive signals for Bcl-2 and A1 were only obtained when RT-PCR with a higher number of cycles (>25) was performed.
To determine whether eosinophils express Bcl-2 protein under conditions where they are exposed to eosinophil hematopoietins, we investigated tissues with significant eosinophilic infiltration using immunohistochemistry. We have previously shown that delayed eosinophil apoptosis occurs in nasal polyp tissues.1 Although high levels of IL-5 were present in these tissues, Bcl-2 expression was not observed in eosinophils (Fig 3A). Moreover, we had the chance to investigate eosinophil-infiltrated tissue from a patient with bladder cancer. Immunohistochemical examination of this tissue showed that the eosinophil hematopoietins IL-3, IL-5, and GM-CSF were highly expressed, especially by the cancer cells (Fig 3B). Again, the eosinophils under these pathologic conditions did not appear to express significant amounts of Bcl-2 protein (Fig. 3B). Furthermore, we used immunocytochemistry and flow cytometric analysis to measure Bcl-2 protein levels in purified blood eosinophils. We did not observe immunoreactive Bcl-2 in resting or GM-CSF–stimulated eosinophils (not presented). Together, these results show that Bcl-2 and A1 are either not present or are present at very low levels in human eosinophils.
Role for Bcl-xL in the regulation of eosinophil apoptosis by cytokines.
Bcl-xL is an anti-apoptotic protein that regulates apoptosis in many cellular systems,4,5 perhaps by regulating the electrical and osmotic homeostasis of mitochondria.17 Although the expression of Bcl-xL in mature eosinophils might be little compared with umbilical cord blood–derived eosinophils as assessed by immunoblotting,10 our in vivo and in vitro expression studies suggested that Bcl-xL might be a good candidate involved in the anti-apoptotic pathway mediated by cytokines in eosinophils. To test this hypothesis, we determined the effect of decreasing the levels of Bcl-xL expression. Eosinophils that had been cultured for 20 hours did not express detectable levels of Bcl-xL protein (Fig 2B). Exposure of these eosinophils to an optimal dose of phosphorothioate-derivatized Bcl-xLantisense oligodeoxynucleotides for another 28 hours clearly inhibited GM-CSF– or IL-5–mediated upregulation of Bcl-xL protein levels, whereas scrambled control oligodeoxynucleotides had no effect (Fig 5A).
Bcl-xL antisense but not scrambled oligodeoxynucleotides partially inhibit cytokine-mediated rescue of spontaneous eosinophil apoptosis. (A) Eosinophils lost the expression of Bcl-xL protein after in vitro culture for 24 hours (see Fig 2B). In these cells, IL-5–induced Bcl-xL expression within 24 hours (total cell culture time: 48 hours). Whereas scrambled Bcl-xL oligodeoxynucleotides (sc Bcl-xL) had no effect on IL-5–mediated upregulation of Bcl-xL protein levels, no significant induction of Bcl-xL protein expression was observed in eosinophils treated with Bcl-xLantisense molecules (as Bcl-xL). The same data were observed using GM-CSF to upregulate Bcl-xL protein expression (not presented). This figure is representative of five independent experiments. (B) Bcl-xL antisense but not scrambled oligodeoxynucleotides partially inhibited the effects of GM-CSF and IL-5 on eosinophil viability (*; P < .001). In addition, no significant effects of the oligodeoxynucleotides on spontaneous eosinophil death were observed (not presented). Means ± SEM of six independent experiments are presented. (C) Bcl-xL antisense and scrambled oligodeoxynucleotides-treated eosinophils were cultured in the presence of IL-5 as described in Materials and Methods for a total of 48 hours. Cells were stained with Giemsa-May-Grünwald (Diff-Quik). Apoptotic eosinophils are characterized by reduced cell volume as well as nuclear condensation. Some cells demonstrate secondary necrosis (lower panel, the two cells in the right margin; these cells show complete nuclear fragmentation). In these experiments, we did not observe any cell death that was the result of primary necrosis. The Bcl-xL antisense-treated cell populations demonstrated much more apoptosis. Data are representative of three independent experiments.
Bcl-xL antisense but not scrambled oligodeoxynucleotides partially inhibit cytokine-mediated rescue of spontaneous eosinophil apoptosis. (A) Eosinophils lost the expression of Bcl-xL protein after in vitro culture for 24 hours (see Fig 2B). In these cells, IL-5–induced Bcl-xL expression within 24 hours (total cell culture time: 48 hours). Whereas scrambled Bcl-xL oligodeoxynucleotides (sc Bcl-xL) had no effect on IL-5–mediated upregulation of Bcl-xL protein levels, no significant induction of Bcl-xL protein expression was observed in eosinophils treated with Bcl-xLantisense molecules (as Bcl-xL). The same data were observed using GM-CSF to upregulate Bcl-xL protein expression (not presented). This figure is representative of five independent experiments. (B) Bcl-xL antisense but not scrambled oligodeoxynucleotides partially inhibited the effects of GM-CSF and IL-5 on eosinophil viability (*; P < .001). In addition, no significant effects of the oligodeoxynucleotides on spontaneous eosinophil death were observed (not presented). Means ± SEM of six independent experiments are presented. (C) Bcl-xL antisense and scrambled oligodeoxynucleotides-treated eosinophils were cultured in the presence of IL-5 as described in Materials and Methods for a total of 48 hours. Cells were stained with Giemsa-May-Grünwald (Diff-Quik). Apoptotic eosinophils are characterized by reduced cell volume as well as nuclear condensation. Some cells demonstrate secondary necrosis (lower panel, the two cells in the right margin; these cells show complete nuclear fragmentation). In these experiments, we did not observe any cell death that was the result of primary necrosis. The Bcl-xL antisense-treated cell populations demonstrated much more apoptosis. Data are representative of three independent experiments.
The ability of antisense oligodeoxynucleotides to specifically inhibit the upregulation of Bcl-xL protein allowed exploration of its role in the prevention of apoptosis by eosinophil hematopoietins in this cellular system. Apoptosis assays were performed after a total cell culture time of 48 hours and cell death assays after 60 hours. No significant effects of the oligodeoxynucleotides on the viability of eosinophils in the absence of IL-5 or GM-CSF were observed (not presented). In contrast, antisense but not scrambled Bcl-xLoligodeoxynucleotides consistantly inhibited by approximately 34% the ability of IL-5 or GM-CSF to prevent eosinophil death (Fig 5B). The effect of the antisense molecules was highly significant (t-test, P < .001). We also investigated whether the observed cell death was apoptosis. Using analysis of the eosinophil morphology,18 we observed much more pycnosis of the nucleus and cell shrinkage in cells that had been treated with antisense Bcl-xL oligodeoxynucleotides (Fig 5C). Quantitative analysis (counting of 300 cells) showed similar results as observed in the cell death experiments: The antisense molecules reduced the anti-apoptotic effect of IL-5 in average by 33% (not presented). Furthermore, no differences were observed between eosinophils from normal control individuals and allergic patients.
These data suggest that Bcl-xL is functionally active within the intracellular anti-apoptotic pathway mediated by cytokines such as IL-5 or GM-CSF in eosinophils. However, the inhibitory effect of Bcl-xL antisense oligodeoxynucleotides on GM-CSF– or IL-5–mediated delay of eosinophil apoptosis was only partial. This could be due in part to the fact that, although the antisense molecules significantly blocked Bcl-xL protein synthesis, some Bcl-xL expression was still induced by IL-5. It is possible that these relatively little Bcl-xL levels were enough to promote GM-CSF or IL-5 responses in the majority of the eosinophils. Another explanation for the incomplete block of cytokine-mediated inhibition of eosinophil apoptosis by Bcl-xL antisense oligodeoxynucleotides would be the involvement of other, as yet unidentified, gene products. Therefore, further definition of the genetic control of eosinophil apoptosis is required.
ACKNOWLEDGMENT
We thank T. Hartung (University of Konstanz, Konstanz, Germany) for GM-CSF, J. Tavernier (University of Gent, Gent, Belgium) for anti–IL-5 MoAb, A. Schapowal (Clinic for Allergy and Dermatology Davos, Davos, Switzerland) for nasal polyp tissues, and St. Gratzl (Kantonsspital Chur, Chur, Switzerland) for the cancer tissue.
Supported by grants from the Swiss National Science Foundation (32-49210.96) and the OPO-Foundation, Zurich, Switzerland (both to H.-U.S.).
Address reprint requests to Hans-Uwe Simon, MD, Swiss Institute of Allergy and Asthma Research, University of Zurich, Obere Strasse 22, CH-7270 Davos, Switzerland; e-mail: hus@siaf.unizh.ch.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal