Abstract
Cyclin E and the cyclin-dependent kinase inhibitor p27 are two important regulators of the G1-S transition modulating the activity of cyclin-dependent kinases. Aberrations in the cell cycle control are often observed in tumors and might even be mandatory in tumor development. To investigate the importance of cell-cycle defects in malignant lymphomas we have characterized the expression of cyclin E and p27 in 105 newly diagnosed lymphomas using immunohistochemistry. A significant, inverse correlation between p27 and cyclin E expression was observed (rs = −.24, P = .02) and both proteins correlated with the S-phase fraction (rs = −.35, P < .001 andrs = .45, P < .001, respectively). The inverse relationship between p27 expression and proliferation was abrogated in some lymphomas, suggesting that p27 downregulation can represent a genuine aberration. Survival analysis was performed in 105 patients with a median observation time of 86 months. Low p27 and high cyclin E expression were significantly associated with a poor prognosis (P = .0001 and .03, respectively). In a multivariate Cox analysis, p27 expression, stage, serum lactate dehydrogenase level, grade, and age were independent prognostic factors, in contrast to S-phase fraction and cyclin E expression. This is the first report showing that p27 expression in malignant lymphomas has independent prognostic significance, which necessitates future studies regarding its more precise biological role in lymphoid tumorogenesis.
© 1998 by The American Society of Hematology.
THE G1-S TRANSITION is controlled by families of highly conserved proteins consisting of cyclin dependent kinases (CDKs) and sets of activating and inhibitory proteins.1,2 Cyclins D1 and E sequentially activate CDKs triggering phosphorylation of key substrates such as the retinoblastoma protein (pRB), thereby initiating DNA replication and passage through the restriction point.3 There are two families of CDK-inhibitors affecting the activity of the kinase complexes contributing to proper control of the G1-S transition. The INK family of proteins (p15, p16, p18, and p19) consists of specific CDK-inhibitors mainly affecting the cyclin D-CDK4/CDK6 complexes.4 The other class of inhibitors, the CIP/KIP family (including p21, p27, and p57), has a less selective inhibitory effect on many CDK-complexes with a main activity during G1.5 p27 is affected by intrinsic and extrinsic factors, such as transforming growth factor-β (TGF-β), cell-cell contact, and elevated cyclic adenosine monophosphate (cAMP) levels causing increased expression of the inhibitor with subsequent arrest in G1 or cell-cycle exit.6,7 p27 might also be involved in terminal differentiation as observed for the promyelocytic cell line HL-60.8 In lymphoid tissues p27 is expressed in nonproliferating lymphocytes whereas activated lymphocytes, eg, in the germinal centers, are negative, suggesting an inverse relation between proliferation and p27 expression in normal lymphocytes.9
The cell cycle is often deregulated in tumors and several reports have shown a high frequency of aberrant expression of cyclins, CDKs, CDK-inhibitors, or suppressor proteins like p53 and pRB.10,11 It has even been suggested that G1-S transition defects are mandatory in tumor development.12 Genes coding for the INK-family of CDK-inhibitors are often mutated in tumors in contrast to the CIP/KIP-family where mutations are rare.13Cyclin D1 is overexpressed in many tumors and can also transform cells in collaboration with other oncogenes, proving that cyclin D1 is a proto-oncogene.10,12 Despite the fact that cyclin E is highly expressed in many tumors, it is not clear if cyclin E is a proto-oncogene or not. Interestingly, high cyclin E expression in breast cancer is associated with an increased risk of death in the disease.14,15 Similar findings have been observed for low p27 expression in several solid tumors and leukemia, suggesting that specific cell-cycle defects are associated with aggressive tumor growth and poor prognosis.15-20
In the present study we have characterized the expression of cyclin E and p27 in malignant lymphomas and can establish a relationship between these proteins and parameters such as tumor cell proliferation, tumor grade, stage, and serum lactate dehydrogenase (LDH) level. Of special interest is the finding that p27 expression was a significant, independent prognostic factor for malignant lymphomas.
MATERIALS AND METHODS
Patient material.
All newly diagnosed malignant lymphomas between 1987 and 1993 at Umeå University hospital with available archive specimens and data regarding immunophenotype, S-phase fractions, and serum LDH levels were included in this study, giving a total of 105 patients. No patient had received any treatment before the biopsy. Lymphomas were initially classified according to the Kiel classification21 and reclassified according to the Revised European-American Lymphoma classification (REAL)22 based on morphological examination of imprints and paraffin sections and immunophenotyping with flow cytometry. Chronic lymphocytic leukemia (CLL, n = 19), follicular lymphoma (FL, n = 23), and other small cell lymphomas (n = 7) were included in the indolent lymphoma group and diffuse large B-cell (n = 39), mantle cell (MCL, n = 11), and large T-cell lymphomas (n = 3) in the aggressive group.23 Three cases of lymphoblastic lymphomas were included in the aggressive group. The treatments applied were, with few exceptions, chlorambucil with or without steroids for advanced indolent lymphomas and anthracyclin containing chemotherapy combinations for aggressive lymphomas. Radiotherapy was given to stage I patients and combined with three courses of chemotherapy for aggressive cases before radiotherapy. Asymptomatic patients with indolent lymphoma received deferred treatment. For control stainings benign lymphoid tissues, mainly tonsils, were used.
Antibodies and immunohistochemistry.
Five-micrometer paraffin sections were dehydrated and deparaffinized according to standard procedures. Optimal antigen retrieval for the cyclin E and p27 proteins was achieved by microwave treatment 3 × 5 minutes in citrate buffer, pH 8.0 for cyclin E and pH 6.0 for p27. Staining was performed in an automatic immunohistochemistry staining machine (Ventana 320-202; Ventana Inc, Tucson, AZ) using anti-cyclin E (HE12, 1:500; Santa Cruz Biotechnology, Inc, Santa Cruz, CA) and anti-p27 antibodies (K25020, 1:200; Transduction Lab, Inc, Lexington, KY) according to the Ventana program. The percentage of positively stained cells was evaluated using a 10 × 10 square grid fitted into the eyepiece of the microscope using an objective lens of × 40. At least 400 cells were counted from randomly chosen fields covering the specimen.
Cell kinetics and flow cytometry.
Cell suspensions were prepared by mechanical desintegration of freshly obtained lymph node tissues, and DNA-staining was performed according to Vindeløv et al.24 A FACScan flow cytometer was used (Becton Dickinson Immunocytometry Systems, Inc, San Jose, CA) and S-phase fractions were calculated by the Cellfit software using the RFIT evaluation model (Becton Dickinson).
Clinical evaluation and statistical analysis.
Results from clinical investigations, staging and outcome were studied retrospectively from the records. The Mann-Whitney rank sum test was used for comparing two different groups, Kruskal-Wallis test for variations between subgroups, and χ2-test for comparing proportions. Correlations between variables were tested according to Spearman's test. Kaplan-Meier and Cox multiple regression analysis were used for survival analysis. Statistical analysis was performed using SPSS software (SPSS, Inc, Chicago, IL).
RESULTS
Cyclin E.
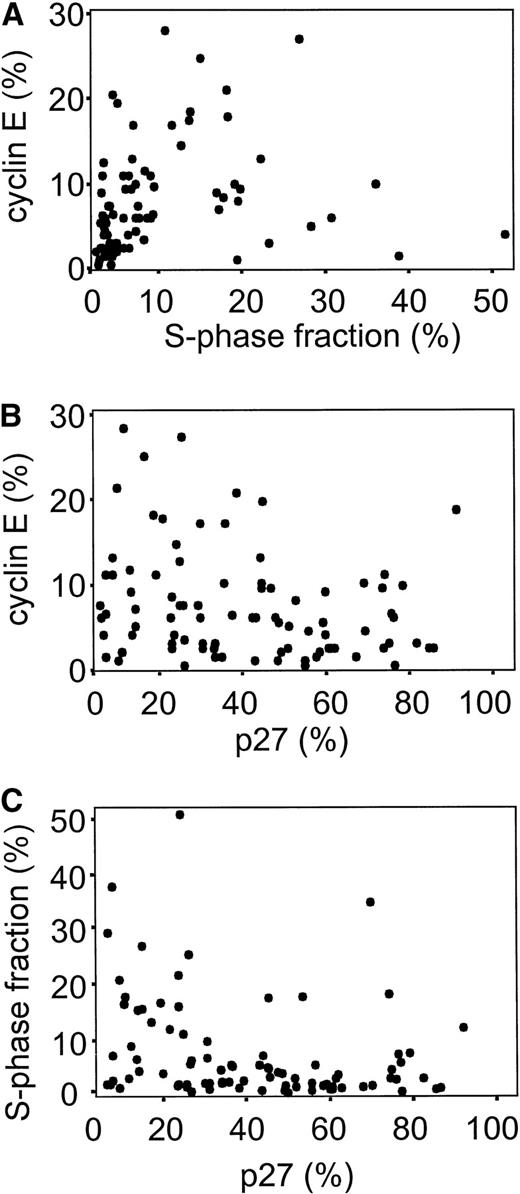
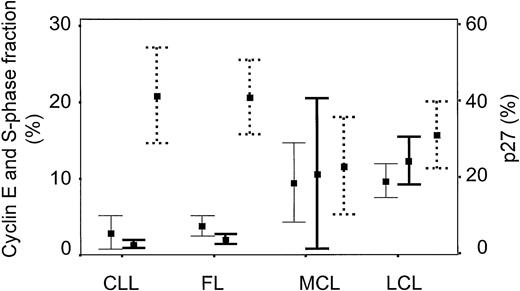
Immunohistochemical cyclin E expression could be evaluated in 98 of 105 cases, showing nuclear staining with various numbers of positive cells often in combination with a quite distinct cytoplasmic staining (Fig 1A). Only the nuclear staining was evaluated because of earlier reports suggesting that the HE12 antibody can react with unrelated cytoplasmic antigens22 and a median fraction of 5.3% cyclin E positive cells was found. A statistically significant correlation between the percentage of cyclin E+ cells and S-phase fraction was observed (rs = .45, P < .001) as shown in Fig 2A. Regarding subgroups of malignant lymphomas, low cyclin E positivity was observed in CLL and FL (median labeling index = 2%), with higher values in MCL and, as expected, the highest fraction of cyclin E+ positive cells in large cell lymphomas (Fig 3). A significant difference in cyclin E expression existed between the subgroups (P < .001) as well as between aggressive and indolent lymphomas (Table 1). Patient characteristics in relation to cyclin E expression are presented in Tables 1 and2.
Immunostainings of cyclin E and p27 in normal lymphoid tissues and malignant lymphomas. (A) Cyclin E staining in an indolent lymphoma showing both nuclear and cytoplasmic reactivity. (B) p27 staining of a reactive follicle in a tonsil. Notice the staining pattern in the dark and light zone. (C) p27 staining of a malignant follicular in an FL lymphoma. The staining pattern is representative for indolent lymphomas. (D) CLL with p27−pseudofollicles. (E) Large cell lymphoma with p27−blastic cells and occasional positive small lymphocytes (S-phase fraction = 11.5%). (F) Large cell lymphoma with p27+(S-phase fraction = 3.4%).
Immunostainings of cyclin E and p27 in normal lymphoid tissues and malignant lymphomas. (A) Cyclin E staining in an indolent lymphoma showing both nuclear and cytoplasmic reactivity. (B) p27 staining of a reactive follicle in a tonsil. Notice the staining pattern in the dark and light zone. (C) p27 staining of a malignant follicular in an FL lymphoma. The staining pattern is representative for indolent lymphomas. (D) CLL with p27−pseudofollicles. (E) Large cell lymphoma with p27−blastic cells and occasional positive small lymphocytes (S-phase fraction = 11.5%). (F) Large cell lymphoma with p27+(S-phase fraction = 3.4%).
Scatter diagram showing the distribution of cyclin E, p27, and S-phase fraction in lymphoma samples. (A)rs = .45, P < .001, n = 98; (B)rs = −.24, P = .02, n = 93; (C)rs = −.35, P < .001, n = 100.
Scatter diagram showing the distribution of cyclin E, p27, and S-phase fraction in lymphoma samples. (A)rs = .45, P < .001, n = 98; (B)rs = −.24, P = .02, n = 93; (C)rs = −.35, P < .001, n = 100.
Diagram showing cyclin E (—), S-phase fraction (), and p27 (· · ·) expression for different lymphoma entities. Error bars represent mean values and 95% confidence intervals. The histological subgroups are: CLL (n = 18), FL (n = 18), MCL (n = 11), diffuse large cell lymphoma (LCL) (n = 34). Data concerning 12 cases with different other morphological types are not shown in the figure.
Diagram showing cyclin E (—), S-phase fraction (), and p27 (· · ·) expression for different lymphoma entities. Error bars represent mean values and 95% confidence intervals. The histological subgroups are: CLL (n = 18), FL (n = 18), MCL (n = 11), diffuse large cell lymphoma (LCL) (n = 34). Data concerning 12 cases with different other morphological types are not shown in the figure.
Median Value for Cyclin E and p27
| . | Cyclin E % . | (n) . | P Value . | p27 % . | (n) . | P Value . |
|---|---|---|---|---|---|---|
| ≤65 yr | 5.0 | (52) | 32 | (53) | ||
| >65 yr | 5.5 | (46) | .88 | 25 | (47) | .05 |
| Indolent | 2.0 | (45) | 42 | (46) | ||
| Aggressive | 7.8 | (53) | <.001 | 20 | (54) | .004 |
| LDH ≤8 μkat/L | 4.5 | (61) | 37 | (63) | ||
| LDH >8 μkat/L | 6.0 | (37) | .18 | 24 | (37) | .03 |
| Stage I | 9.0 | (19) | 33 | (21) | ||
| II | 9.6 | (15) | 20 | (15) | ||
| III | 4.6 | (16) | 25 | (15) | ||
| IV | 5.4 | (48) | .006 | 39 | (49) | .21 |
| All lymphomas | 5.3 | (98) | 30 | (100) |
| . | Cyclin E % . | (n) . | P Value . | p27 % . | (n) . | P Value . |
|---|---|---|---|---|---|---|
| ≤65 yr | 5.0 | (52) | 32 | (53) | ||
| >65 yr | 5.5 | (46) | .88 | 25 | (47) | .05 |
| Indolent | 2.0 | (45) | 42 | (46) | ||
| Aggressive | 7.8 | (53) | <.001 | 20 | (54) | .004 |
| LDH ≤8 μkat/L | 4.5 | (61) | 37 | (63) | ||
| LDH >8 μkat/L | 6.0 | (37) | .18 | 24 | (37) | .03 |
| Stage I | 9.0 | (19) | 33 | (21) | ||
| II | 9.6 | (15) | 20 | (15) | ||
| III | 4.6 | (16) | 25 | (15) | ||
| IV | 5.4 | (48) | .006 | 39 | (49) | .21 |
| All lymphomas | 5.3 | (98) | 30 | (100) |
The number of patients are denoted in parentheses. Differences between subgroups were calculated with Kruskal-Wallis test.
Clinical Information in Relation to Cyclin E and p27
| (A) . | Cyclin E ≤2% . | Cyclin E >2% . | P Value . |
|---|---|---|---|
| Age (yr) | 61 | 64 | .29 |
| LDH (μkat/L) | 6.5 | 7.7 | .05 |
| S-phase fraction (%) | 1.7 | 5.4 | <.001 |
| p27 (%) | 44 | 31 | .05 |
| Indolent/aggressive | 24/5 | 21/48 | <.001 |
| Stage I/II/III/IV | 4/2/5/18 | 15/13/11/30 | .26 |
| General symptom A/B | 22/7 | 45/23 | .24 |
| Male/female | 24/5 | 38/31 | .008 |
| n | 29 | 69 | |
| (B) | p27 ≤15% | p27 >15% | P Value |
| Age (yr) | 72 | 61 | .008 |
| LDH (μkat/L) | 9.0 | 7.0 | .004 |
| S-phase fraction (%) | 12.7 | 2.8 | .001 |
| Cyclin E (%) | 6.8 | 5.0 | .09 |
| Indolent/aggressive | 6/17 | 40/37 | .02 |
| Stage I/II/III/IV | 3/3/5/12 | 21/12/10/37 | .58 |
| General symptom A/B | 13/9 | 55/22 | .20 |
| Male/female | 15/8 | 49/28 | .55 |
| n | 23 | 77 |
| (A) . | Cyclin E ≤2% . | Cyclin E >2% . | P Value . |
|---|---|---|---|
| Age (yr) | 61 | 64 | .29 |
| LDH (μkat/L) | 6.5 | 7.7 | .05 |
| S-phase fraction (%) | 1.7 | 5.4 | <.001 |
| p27 (%) | 44 | 31 | .05 |
| Indolent/aggressive | 24/5 | 21/48 | <.001 |
| Stage I/II/III/IV | 4/2/5/18 | 15/13/11/30 | .26 |
| General symptom A/B | 22/7 | 45/23 | .24 |
| Male/female | 24/5 | 38/31 | .008 |
| n | 29 | 69 | |
| (B) | p27 ≤15% | p27 >15% | P Value |
| Age (yr) | 72 | 61 | .008 |
| LDH (μkat/L) | 9.0 | 7.0 | .004 |
| S-phase fraction (%) | 12.7 | 2.8 | .001 |
| Cyclin E (%) | 6.8 | 5.0 | .09 |
| Indolent/aggressive | 6/17 | 40/37 | .02 |
| Stage I/II/III/IV | 3/3/5/12 | 21/12/10/37 | .58 |
| General symptom A/B | 13/9 | 55/22 | .20 |
| Male/female | 15/8 | 49/28 | .55 |
| n | 23 | 77 |
Median values and number of cases are noted. Differences are calculated with Mann-Whitney's test and χ2-test.
p27.
The expression of p27 was successfully analyzed in 100 of 105 cases. A distinct nuclear staining was observed with very low background staining making the p27 expression easy to evaluate, and a median value of 30% positive cells was determined in the lymphomas. Small lymphocytes were usually distinctly positive in contrast to larger often negative cells. In benign germinal centers the centroblasts were typically negative whereas centrocytes were weakly to moderately positive corresponding to the dark and light areas of each follicle. In the germinal center scattered cells were intensely p27+(Fig 1B). In FL these follicular subcompartments had disappeared, giving a positive appearance in the neoplastic follicles (Fig 1C) with a diffuse mixture of p27+ and p27− cells, but also in the lymphomas the smaller cells were mostly negative. In so-called pseudofollicles in CLL, containing proliferating cells, most cells were p27− (Fig 1D). Large cell lymphomas were, in general, p27− (Fig 1E) but cases were noted with distinct staining of large, blastic cells (Fig 1F).
The expression of p27 was inversely correlated with both cyclin E expression and S-phase fraction as illustrated in Fig 2B and C (rs = −.24, P = .02 andrs = −.35, P < .001, respectively). There was no significant difference regarding p27 expression in subgroups of lymphomas, but MCL cases showed the lowest p27 expression (Fig 3). The fraction of p27+ cells did not correlate to the fraction of T cells in the B-cell lymphomas (data not shown). Low p27 expression was associated with high serum LDH levels and age as summarized in Tables 1 and 2, and for aggressive lymphomas also with advanced stage (not shown). A weak significant positive correlation was observed between p27 expression and leukocyte count and a negative correlation to platelet count (not shown in figures). A strong correlation was found between low p27 expression and higher age (Table2).
Survival.
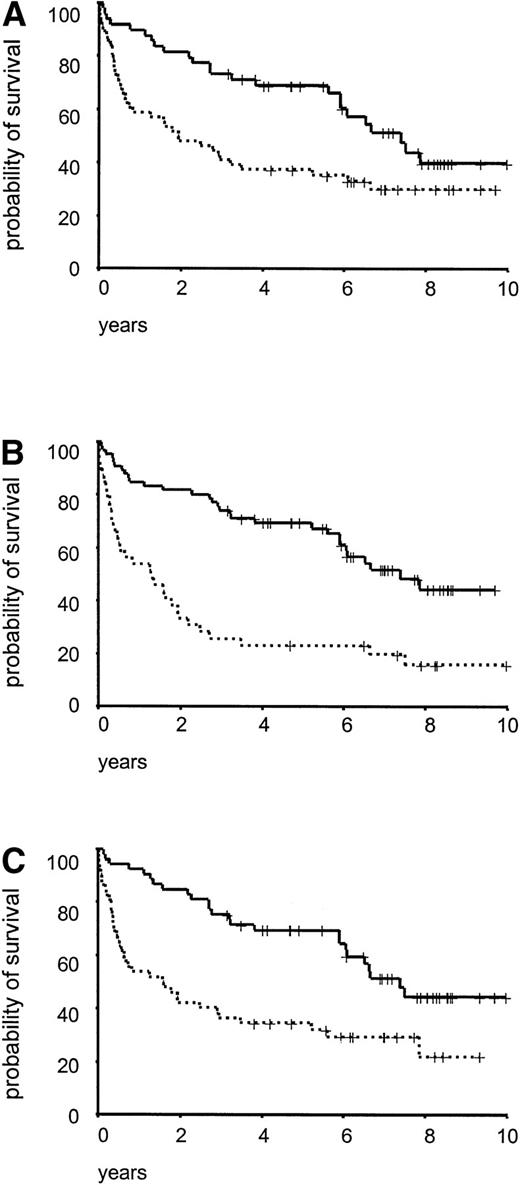
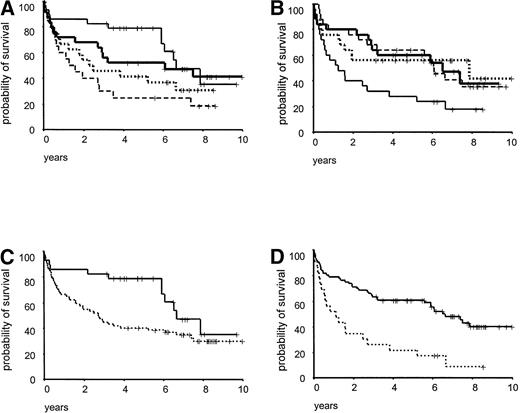
Survival analysis was performed in 105 malignant lymphoma patients, with a median observation time of 86 months. As illustrated in Fig 4 there was a significant difference in survival between cases with indolent/aggressive lymphomas (P= .007), elevated/normal serum LDH values (P < .001), and high/low S-phase fractions (P < .001). When the patients were subdivided according to cyclin E and p27 expression using median values as cut off, no significant impact on survival was seen. Due to uncertainty in the definition of the normal range of p27 and cyclin E expression in lymphoid tissues, division into quartiles was performed and for both p27 and cyclin E the lowest quartile versus the other quartiles divided the patient material in statistically different prognostic groups (Fig 5A and B). Patients with low p27 expression (≤15% positive cells) had a significantly reduced survival (P < .0001, Fig 5C) and a three times increased risk of death in the disease compared with other patients (data not shown). For cyclin E the results were more complex, with no linear trend for the quartiles, but when using 2% as cut off (the lowest quartile), patients with high cyclin E expression had a significantly reduced survival (P = .03, Fig 5D).
Crude overall survival for 105 lymphoma cases subdivided in: (A) indolent (———, n = 49) and aggressive (· · ·, n = 56), P = .007; (B) LDH ≤8 μkat/L (———, n = 66) and LDH >8 μkat/L (· · ·, n = 39), P < .001; (C) S-phase fraction ≤4% (———, n = 53) and S-phase fraction >4% (· · ·, n = 52), P < .001.
Crude overall survival for 105 lymphoma cases subdivided in: (A) indolent (———, n = 49) and aggressive (· · ·, n = 56), P = .007; (B) LDH ≤8 μkat/L (———, n = 66) and LDH >8 μkat/L (· · ·, n = 39), P < .001; (C) S-phase fraction ≤4% (———, n = 53) and S-phase fraction >4% (· · ·, n = 52), P < .001.
Overall survival in relation to cyclin E and p27 expression. (A) Cyclin E expression divided in quartiles, first (——, n = 29), second (– – –, n = 20), third (, n = 25), and fourth (· · ·, n = 24), P = .03; (B) p27 expression divided in quartiles, first (——, n = 25), second (· · ·, n = 25), third (– – –, n = 25), and fourth quartile (, n = 25), P < .001; (C) cyclin E expression divided with a cut-off between the first and second quartile, cyclin E ≤2% (——, n = 29), and cyclin E >2% (· · ·, n = 69), P = .03; (D) p27 expression divided with a cut-off between the first and second quartile, p27 ≤15% (· · ·, n = 23) and p27 >15% (——, n = 77), P < .001.
Overall survival in relation to cyclin E and p27 expression. (A) Cyclin E expression divided in quartiles, first (——, n = 29), second (– – –, n = 20), third (, n = 25), and fourth (· · ·, n = 24), P = .03; (B) p27 expression divided in quartiles, first (——, n = 25), second (· · ·, n = 25), third (– – –, n = 25), and fourth quartile (, n = 25), P < .001; (C) cyclin E expression divided with a cut-off between the first and second quartile, cyclin E ≤2% (——, n = 29), and cyclin E >2% (· · ·, n = 69), P = .03; (D) p27 expression divided with a cut-off between the first and second quartile, p27 ≤15% (· · ·, n = 23) and p27 >15% (——, n = 77), P < .001.
Multiple regression was performed by Cox analysis in 93 lymphoma patients including cyclin E, p27, LDH, age, stage, S-phase fraction, and morphology. Cyclin E and p27 were dichotomized with cut off at 2% and 15%, respectively, because no linear relation between predicted survival and the variables was found (Fig 5). As presented in Table 3, LDH, age, grade, stadium, and p27 expression were independent significant factors in contrast to S-phase fraction and cyclin E expression. When the patients were subdivided according to a combination of the independent parameters LDH and p27, groups with highly different outcome were obtained (Fig 6). Patients having lymphomas with a p27 index >15% and an LDH value ≤8 microkatal (μkat)/L had a comparably good prognosis with 50% survival rate after 8 years, in contrast to the poor outcome for the group of patients with a p27 index ≤15% and LDH > 8 μkat/L (Fig6).
Result of the Cox Analysis After Backward Stepwise Reduction in 93 Lymphomas With 57 Events
| Variable . | Exp (B) . | Lower . | Upper . | P Value . | R Value . |
|---|---|---|---|---|---|
| . | 95% CI . | . | . | ||
| Age | 1.04 | 1.01 | 1.06 | .001 | .14 |
| Indolent vaggressive | 2.59 | 1.37 | 4.90 | .03 | .12 |
| LDH | 1.11 | 1.06 | 1.15 | .0001 | .20 |
| Stage | 1.98 | 1.50 | 2.62 | <.0001 | .22 |
| p27 ≤15% v >15% | 0.54 | 0.29 | 0.98 | .04 | −.066 |
| Excluded Variables After Stepwise Reduction | |||||
| S-phase fraction | .09 | .039 | |||
| Cyclin E ≤2% v >2% | .20 | .042 | |||
| Variable . | Exp (B) . | Lower . | Upper . | P Value . | R Value . |
|---|---|---|---|---|---|
| . | 95% CI . | . | . | ||
| Age | 1.04 | 1.01 | 1.06 | .001 | .14 |
| Indolent vaggressive | 2.59 | 1.37 | 4.90 | .03 | .12 |
| LDH | 1.11 | 1.06 | 1.15 | .0001 | .20 |
| Stage | 1.98 | 1.50 | 2.62 | <.0001 | .22 |
| p27 ≤15% v >15% | 0.54 | 0.29 | 0.98 | .04 | −.066 |
| Excluded Variables After Stepwise Reduction | |||||
| S-phase fraction | .09 | .039 | |||
| Cyclin E ≤2% v >2% | .20 | .042 | |||
All variables except cyclin E, p27, and grade were entered as continuous variables. The confidence interval of the risk, Exp (B), for each independent factor and the regression coefficient are listed.
Overall survival for 100 lymphomas divided according to a combination of p27 expression and serum LDH levels. LDH ≤8 μkat/L and p27 >15% (——, n = 52); LDH >8 μkat/L and p27 >15% (– – –, n = 25); LDH ≤8 μkat/L and p27 ≤15% (, n = 11); LDH >8 μkat/L and p27 ≤15% (· · ·, n = 12), P < .001.
Overall survival for 100 lymphomas divided according to a combination of p27 expression and serum LDH levels. LDH ≤8 μkat/L and p27 >15% (——, n = 52); LDH >8 μkat/L and p27 >15% (– – –, n = 25); LDH ≤8 μkat/L and p27 ≤15% (, n = 11); LDH >8 μkat/L and p27 ≤15% (· · ·, n = 12), P < .001.
DISCUSSION
In the present study immunohistochemistry was used to characterize the expression of cyclin E and p27 in malignant lymphomas, and to optimize the staining conditions we used antigen retrieval methods and a semiautomatic instrument giving reproducible staining patterns. The p27 reactivity was easily evaluated as reported earlier,9,15and even if occasional cells showed cytoplasmic staining various fractions of clearly p27+ nuclei were detected in the lymphomas. Cyclin E antibodies produced a mixed staining pattern with an often notable cytoplasmic staining, making the evaluation process more laborious. A similar, presumably unspecific reactivity has been reported using the same anti-cyclin E antibody25 and more specific cyclin E antibodies applicable on formalin-fixed material are needed for routine purposes. Concerning the choice of immunohistochemistry as a method for analysis of p27 and cyclin E protein expression, we have observed a good agreement between immunohistochemical and immunoblotting analyses of cyclin E and cyclin D1 (unpublished data). Immunohistochemical protein detection also gives the opportunity to characterize specific tissue compartments, such as follicle centers, regarding protein expression.
The cyclin E expression was in the present lymphoma series linked with proliferation, but this relationship seems ambiguous for other tumor types. Some investigators claim that there is no relation between cyclin E levels and proliferation in tumors overexpressing the protein and that the cell-cycle phase-specific expression of cyclin E is disturbed in these tumor cells.26,27 Other reports have observed a connection between cyclin E expression and proliferation as well as a cell-cycle phase-specific expression of the protein determined with immunohistochemistry or flow cytometry.15Our data showed a rather strong association between cyclin E expression and proliferation determined by the S-phase fraction and all lymphomas, except aggressive lymphomas, had in principle higher cyclin E index than S-phase fractions. Despite no obvious cell-cycle independent overexpression of cyclin E in lymphomas, a potential deregulation of the protein could not be excluded for some cases. Higher levels of cyclin E have been reported in low-and intermediate-grade malignant lymphomas compared with chronic lymphocytic leukemia,28 and in lymphoblastic leukemias the cyclin E levels were higher in recurrent cases, suggesting an association between cyclin E expression and disease progress.29
MCL lymphomas have a characteristic translocation, t(11;14), leading to an overexpression of cyclin D1, but additional aberrations in the RB-pathway have also been reported.30 The MCL cases included in the present study, with a presumable cyclin D1 overexpression, showed high cyclin E expression, which is in contrast to the inverse relation between cyclin E and cyclin D1 expression observed in breast cancer.31 Further studies are needed to better define patterns of aberrations in the cyclin E-cyclin D1–RB-pathway in MCL.
Small lymphocytes were in general p27+ whereas activated cells, such as centroblasts and immunoblasts, displayed weak or no p27 staining, suggesting an inverse association between proliferation and p27 expression. This association, verified statistically, was expected from the theoretical function of p27 as a CDK-inhibitor and cell-cycle blocker. Normal lymphocytes also display an inverse correlation between p27 and proliferation,9 but studies on various tumors have reported conflicting results.15 17 In our material the link between p27 positivity and S-phase fraction was not observed in several lymphoma cases, suggesting that p27 has biological functions aside from cell-cycle regulation.
Expression of the p27 protein was associated with high lymphocyte and low platelet counts and male gender, which could be explained by a strong connection to CLL lymphomas. More difficult to explain is the relationship between high age and low p27 expression. A feasible hypothesis might be that downregulation of p27 protein expression is age related, possibly indicating stepwise-occurring alterations where p27 is affected in a late stage. Age has been reported as an important prognostic32 factor for malignant lymphomas, and one reason for this might be the p27 downregulation observed here.
Our patient series seemed to represent a nonbiased material of lymphomas based on general patient characteristics and survival rates when subdivided according to LDH-values, S-phase fraction, and morphological grade.32-35 From a clinical point of view, the impact of cell-cycle regulators in relation to outcome is interesting. Regarding cyclin E, a weak but significant association between high cyclin E expression and poor prognosis was observed, but the impact on prognosis was not significantly independent in the Cox analysis. p27 expression alone or in combination with one or two other factors was highly significant as a prognostic factor, but when additional factors were introduced the prognostic impact decreased. This implicates an association between p27 and other prognostic factors, but importantly p27 seemed to have an independent impact on prognosis in contrast to S-phase fraction or cyclin E expression. Low p27 expression has been found to be associated with a poor prognosis in tumors such as breast cancer, lung cancer, colorectal cancer, and gastric cancer, suggesting that downregulation of p27 is a general phenomenon in malignancies associated with aggressive tumor growth.15-19 36
The adverse effect of low p27 values in malignancies like lymphomas is not fully understood, but several contributing explanations are possible. Most obvious is an abrogated cell-cycle block due to downregulation of p27 leading to less strict G1/S checkpoint surveillance with facilitated transit into the S-phase. Furthermore, at low p27 levels G0 cells are recruited into the cell cycle and aberrant p27 expression might therefore stimulate the recruitment of tumor cells into the cell cycle with effects on the tumor growth fraction.6,12 Expression of p27 is influenced by cell-to-cell contact and downregulation of p27 can inhibit cell adhesion.37 Tumors with low p27 expression may consequently have an impaired cell adhesion, promoting tumor dissemination. A recent report also defines a role for p27 in the regulation of apoptosis, proposing that lymphomas with low p27 expression might have a growth advantage due to few apoptotic events.38 Interestingly, preliminary data indicate that in some large cell lymphomas, p27 expression seems to be present in cases with high apoptotic rate.
Our data suggest that p27 expression is an important parameter that could be included in a future panel of diagnostic markers for lymphomas and, when combined with serum LDH levels, distinct prognostic subgroups of patients can be defined. The results clearly demonstrate the importance of G1-S transition regulators in lymphomas illuminating an important research area in hematologic neoplasias.
ACKNOWLEDGMENT
The authors thank Britta Lindgren (Department of Pathology, Umeå University) for technical assistance and Björn Tavelin (Oncological Center, Umeå University Hospital) for statistical help.
Supported by grants from the Swedish Cancer Society, The Medical Faculty, Umeå University, and Lion's Cancer Research Foundation, Umeå University.
Address reprint requests to Göran Landberg, MD, Department of Pathology, Umeå University, S-901 87 Umeå, Sweden; e-mail: goran.landberg.us@vll.se.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal