Abstract
Several hematopoietic cytokines have been investigated for their potential to provide protection from the lethal consequences of bone marrow aplasia after total body irradiation (TBI). Some can increase the dose of irradiation tolerated by the animals; none allow endogenous recovery after doses such as administered in clinical blood or marrow transplantation. We tested the radioprotective potential of FLT-3 ligand, an early acting hematopoietic cytokine, alone and in combination with a late acting cytokine, granulocyte-colony stimulating factor (G-CSF). Adult outbred New Zealand White rabbits were submitted to TBI of 1,200 or 1,400 cGy by a Co60 source. Recombinant human (rh) FLT-3 ligand at a dose of 500 μg/kg and/or rhG-CSF at a dose of 10 μg/kg were administered for 14 days subcutaneously daily, beginning either 2 days before or the day after TBI. All control animals given no growth factors died of aplasia at day 10 (range, 5 to 16). All 8 animals given G-CSF had severe aplasia and 7 died at day 8 (range, 5 to 10); 1 animal survived, with G-CSF being administered before TBI. In contrast, 11 of 12 animals given FLT-3 ligand, with or without G-CSF, survived. Radioprotection was best in the group given FLT-3 ligand together with G-CSF before TBI. In these animals median platelet counts were never <10 × 109/L and median white blood cell counts never <0.5 × 109/L. These data show that hematopoietic recovery can occur after 1,400 cGy TBI in rabbits, if protected by FLT-3 ligand, and suggest a radioprotective clinical potential of this cytokine.
© 1998 by The American Society of Hematology.
SEVERE BONE MARROW (BM) aplasia with bleeding and infection is the first dose-limiting toxicity of total body irradiation (TBI).1-3 In animal models, toxicity is expressed as LD50/30, the dose at which 50% of animals survive at day 30 postirradiation. LD50/30 is influenced by dose rate and number of radiation fractions. It depends on the number of stem cells surviving and varies from species to species with a higher dose tolerated by smaller animals.4,5 The LD50/30 ranges from 300 to 400 cGy in dogs to about 700 cGy in mice, and is estimated in humans at 300 to 600 cGy.2,5The maximal tolerated dose, ie, the dose without early mortality, is less well defined but considerably lower. With stem cell support it is increased to about 1,400 to 1,600 cGy, limited then by central nervous, pulmonary, and gastrointestinal toxicity.2,6 7
Hematopoietic growth factors and a variety of cytokines have become available over the last decade and have been tested for their potential to protect BM from the lethal consequences of TBI.8-11 Some have been shown to promote recovery when administered after sublethal TBI, others were only effective when given before TBI or combined with other cytokines. Improvements in all experiments were marginal. Importantly, with the exception of stem cell factor,12 none of the single cytokines or their combinations provided for recovery after doses such as were administered in the clinical setting for blood or marrow transplantation (BMT).13
FLT-3 ligand is a novel hematopoietic cytokine, involved in regulation of early hematopoiesis.14-16 It stimulates alone, or in combination with other growth factors, the proliferation of highly enriched human and murine hematopoietic stem cells in vitro and leads to expansion and mobilization of progenitor cells in animals and humans in vivo.17-22 Serum levels of FLT-3 ligand are highly upregulated in patients with acquired or induced BM aplasia, suggesting that ligand plays a role in a compensatory hematopoietic response to stimulate or protect the stem cell compartment.23,24 Based on preliminary data in mice,25 we were therefore interested in testing the potential of FLT-3 ligand to protect animals submitted to TBI at a dose equivalent or higher to the dose used for allogeneic BMT. The present data show that FLT-3 ligand effectively promotes recovery and protects animals from otherwise lethal TBI.
ANIMALS AND METHODS
Animals.
Adult outbred New Zealand White rabbits (Biological Research Laboratories Ltd, Füllinsdorf, Switzerland) of both sexes were used. Animals were housed in single cages with standard pellet diet and water ad libitum in the supervised Animal Care Facility Center of the Department of Research at the Kantonsspital Basel.
Experimental design.
Animals were treated with TBI in groups of two to four animals each, given either no growth factors (control animals) or growth factors for a period of 14 days, beginning 2 days before TBI (pre) or the day after TBI (post), as outlined in Table 1. The protocol was approved by the Ethical Committee for Animal Care of the Canton of Basel-Stadt.
Treatment and Outcome of Rabbits Subjected to TBI
| Treatment Group . | TBI (cGy) . | N . | Nadir (×109/L)-150 . | Survival Outcome . | Day of Death-150 . | ||
|---|---|---|---|---|---|---|---|
| Growth Factor . | Time of Injection . | Plt . | WBC . | ||||
| None | 1,200 | 4 | 8 (7-11)† | 0.13 (0.06-0.26)‡ | 0/4 | 12 (9-16) | |
| None | 1,400 | 4 | 2 (1-9)† | 0.19 (0.11-0.23)‡ | 0/4 | 8 (5-12) | |
| G-CSF | Post | 1,400 | 4 | 5 (2-222) | 0.07 (0.05-0.11) | 0/4 | 9 (5-10) |
| G-CSF | Pre | 1,400 | 4 | 7 (3-46) | 0.09 (0.07-0.14) | 1/4 | 8 (7-8) |
| FL | Pre | 1,400 | 4 | 7 (6-7) | 0.10 (0.05-0.12) | 4/4 | NA |
| FL + G-CSF | Post | 1,200 | 2 | 4.5 (4-5) | 0.28 (0.14-0.41) | 1/2 | 8 |
| FL + G-CSF | Pre | 1,200 | 2 | 37 (20-54)† | 0.92 (0.3-1.54)‡ | 2/2 | NA |
| FL + G-CSF | Pre | 1,400 | 4 | 11 (5-15)† | 0.55 (0.36-2.39)‡ | 4/4 | NA |
| Treatment Group . | TBI (cGy) . | N . | Nadir (×109/L)-150 . | Survival Outcome . | Day of Death-150 . | ||
|---|---|---|---|---|---|---|---|
| Growth Factor . | Time of Injection . | Plt . | WBC . | ||||
| None | 1,200 | 4 | 8 (7-11)† | 0.13 (0.06-0.26)‡ | 0/4 | 12 (9-16) | |
| None | 1,400 | 4 | 2 (1-9)† | 0.19 (0.11-0.23)‡ | 0/4 | 8 (5-12) | |
| G-CSF | Post | 1,400 | 4 | 5 (2-222) | 0.07 (0.05-0.11) | 0/4 | 9 (5-10) |
| G-CSF | Pre | 1,400 | 4 | 7 (3-46) | 0.09 (0.07-0.14) | 1/4 | 8 (7-8) |
| FL | Pre | 1,400 | 4 | 7 (6-7) | 0.10 (0.05-0.12) | 4/4 | NA |
| FL + G-CSF | Post | 1,200 | 2 | 4.5 (4-5) | 0.28 (0.14-0.41) | 1/2 | 8 |
| FL + G-CSF | Pre | 1,200 | 2 | 37 (20-54)† | 0.92 (0.3-1.54)‡ | 2/2 | NA |
| FL + G-CSF | Pre | 1,400 | 4 | 11 (5-15)† | 0.55 (0.36-2.39)‡ | 4/4 | NA |
Abbreviations: Plt, platelets; WBC, white blood cells; FL, Flt 3 ligand; NA, not applicable.
Median (range).
Difference in platelet counts (†) and WBC (‡) between control group animals and FI + G-CSF pretreated animals according to unpairedt-test, P = .05 and P = .02, respectively.
TBI.
TBI was administered from a Co60 source at a dose rate of 20 cGy/min in general anesthesia in two doses on 2 consecutive days, as previously described in our transplant protocol.26 27 Total doses of 1,200 and 1,400 cGy were examined. TBI was applied as a lateral beam from the top to animals lying on the floor. Animals were anesthetized by intramuscular (im) injection of Hypnorm (Janssen-Cilag Pharmaceutica, Baar, Switzerland) and were administered 0.2 mg of dexamethason to protect from immediate central nervous toxicity. Supportive care after TBI was comprised of Bactrim (Roche, Ltd, Basel, Switzerland) administered orally daily and an injection of normal saline subcutaneously (sc) if required. No red blood cell or platelet transfusions were given.
Growth factors.
Recombinant human granulocyte colony-stimulating factor (rhG-CsF) (Neupogen; kindly provided by Roche Pharma, Reinach, Switzerland) at a dose of 10 μg/kg and FLT-3 ligand (kindly provided by Immunex Corp, Seattle, WA) at a dose of 500 μg/kg were used. These doses of human growth factors had been previously tested and shown to stimulate hematopoiesis in vitro and to synergize in vivo in the mobilization of hematopoietic precursor cells in rabbits.19 Growth factors were injected daily sc in the shaved back of the animal. FLT-3 ligand and G-CSF were administered either singly or combined, as outlined in Table 1.
Laboratory examinations.
Animals were inspected daily and their weight was recorded. Whole blood cell counts, including hemoglobin, reticulocyte, platelet counts, and white blood cell differential counts, were determined three times weekly.28 A postmortem examination was performed in all the deceased animals.
Statistical analysis.
Mean, median, and range of numerical variables were calculated by the Excel spreadsheet program. Survival of the groups was compared by Fisher's exact t-test, the blood values at nadir by unpairedt-test.
RESULTS
Survival.
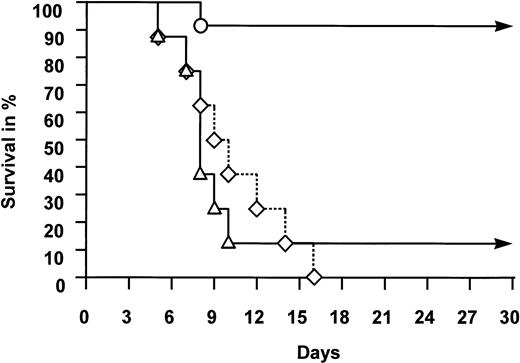
The outcome of animals following TBI is summarized in Table 1 and illustrated in Fig 1. All 8 control animals, subjected to 1,200 or 1,400 cGy but given no growth factors, died of aplasia between days 5 and 16 after TBI (median, day 10). No higher dose was considered because of excessive early toxicity of the central nervous system observed in previous studies.27Seven of 8 animals given 1,400 cGy TBI and treated with G-CSF alone died between days 5 and 10 (median, day 8). In this treatment group, only one animal administered G-CSF before TBI recovered from severe pancytopenia and survived. In contrast, 11 of 12 animals treated with FLT-3 ligand survived. The survivors included all 10 animals given FLT-3 ligand, with or without additional G-CSF, before TBI. Of the two animals subjected to 1,200 cGy and administered FLT-3 ligand and G-CSF after TBI, one animal failed to recover and died at day 8 in aplasia. The difference in survival between the control group or the G-CSF group and the FLT-3 ligand–treated group is highly significant (P < .01).
Survival of animals subjected to TBI. Rabbits were given no growth factors (◊), G-CSF (▵), or FLT-3 ligand with or without additional G-CSF (○). For experimental details, see Animals and Methods. Difference in survival between the three groups of animals was highly significant (P < .01, see text).
Survival of animals subjected to TBI. Rabbits were given no growth factors (◊), G-CSF (▵), or FLT-3 ligand with or without additional G-CSF (○). For experimental details, see Animals and Methods. Difference in survival between the three groups of animals was highly significant (P < .01, see text).
Extent of aplasia.
As a result of TBI, all control animals entered severe pancytopenia with platelet counts <10 × 109/L and white blood cell counts <0.2 × 109/L (Table 1). The extent of pancytopenia was no different in animals treated with G-CSF alone. Even the 1 surviving animal had a nadir of 8 × 109/L for platelets and 0.1 × 109/L for white blood cell counts. In contrast, none of the 4 animals given the combination of FLT-3 ligand and G-CSF before 1,400 cGy TBI had white blood cell counts <0.2 × 109/L at any time point and only 1 animal had a white blood cell count <0.5 × 109/L for 1 day. A platelet count was <10 × 109/L in only 1 animal on 1 day. Remarkably, 2 of 4 animals never had platelet counts <20 × 109/L nor white blood cell counts <1.0 × 109/L. The values of white blood cell counts and platelets at the time of nadir are significantly different between the control group and the FLT-3 ligand + G-CSF pretreated animals (P < .05).
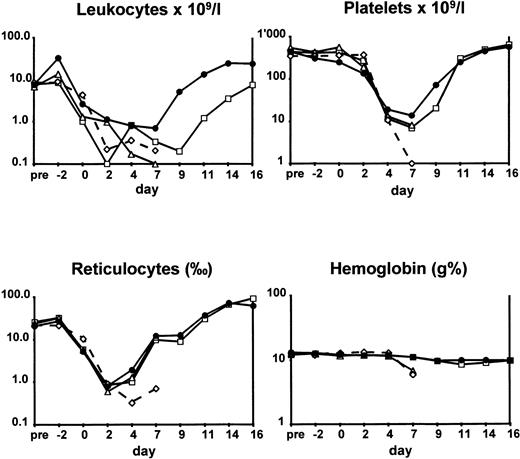
The kinetics of entering and recovering from aplasia in terms of median leukocyte, platelet, reticulocyte counts, and hemoglobin values are illustrated in Fig 2. Control animals and those administered G-CSF alone entered rapid aplasia and failed to recover. Among animals given FLT-3 ligand alone and those given the combination of FLT-3 ligand and G-CSF, there was a difference concerning white blood cell kinetics. In animals administered FLT-3 ligand alone, median white blood cell counts were already <1 × 109/L at day 2 and recovered to >1 × 109/L at day 11 only. In contrast, in the combined treatment group, white blood cell counts went to <1 × 109/L at day 4 and recovered to >1 × 109/L at day 8.
Hematological values of animals subjected to TBI. Rabbits were given no growth factors (◊), G-CSF (▵), FLT-3 ligand (□), and FLT-3 ligand with G-CSF (•). For experimental details, see Animals and Methods. Differences in hematological values at nadir between the control and the FLT-3 ligand with G-CSF–treated animals were significant (P ≤ .05, see text).
Hematological values of animals subjected to TBI. Rabbits were given no growth factors (◊), G-CSF (▵), FLT-3 ligand (□), and FLT-3 ligand with G-CSF (•). For experimental details, see Animals and Methods. Differences in hematological values at nadir between the control and the FLT-3 ligand with G-CSF–treated animals were significant (P ≤ .05, see text).
DISCUSSION
The data from this study show that FLT-3 ligand, alone or in combination with G-CSF, can consistently rescue animals from otherwise lethal aplasia when administered before TBI. G-CSF alone is insufficient. All animals pretreated with FLT-3 ligand survived high-dose irradiation. The best results in terms of survival and the speed of recovery from aplasia were achieved when the two cytokines were combined and given before, not after, irradiation. With the combination, the nadir of white blood cell and platelet count was reduced in both intensity and duration. These remarkable radioprotective effects of FLT-3 ligand are by far superior to the effects of other growth factors and cytokines tested previously for their potential to protect animals against the toxicity of TBI. Of the hematopoietic cytokines, G-CSF was shown to have some radioprotective effect in mice when administered before TBI. Sixty percent of mice given high-dose G-CSF (40 μg/kg) before 700 cGy TBI survived in contrast to control animals. No protection was observed at 800 cGy.29 Similarly, modest effects were observed with granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukins (IL)-3 and -6, in sublethally, but not in lethally, irradiated animals.8,9,30 Most data have been accumulated using IL-1 alone or in combination with G-CSF, GM-CSF, stem cell factor or tumor necrosis factor-α (TNF-α) or interferon-γ.8,22,31,32 Better results were obtained with the combination of IL-1 and stem cell factor administered before TBI.32 Even there, effects on protection were relatively marginal with an increase in LD50/30 by factors of 1.2 to 1.3 from 800 to 950 cGy. In only one of the models tested did total doses of tolerated TBI exceed 1,000 cGy when mice were given stem cell factor before and after 1,150 cGy TBI.13
The mechanism of radioprotection by growth factors is unknown and the results appear, in part, surprising. In clinical studies of leukemia treatment, cytokines are used to prime target cells for higher susceptibility to chemotheraphy.33 Therefore, an even more rapid decline in peripheral blood values could have been expected in cytokine-pretreated animals. To the contrary, in irradiated animals receiving FLT-3 ligand and G-CSF the decrease of peripheral blood values was not only less pronounced but also delayed. In addition, decline in whole blood leukocyte counts was significantly slower in animals given FLT-3 ligand and G-CSF than in animals given FLT-3 ligand alone. Dose of G-CSF and FLT-3 ligand and the timing of administration were selected on the basis of previous experiments in the same animal model.21 We found that FLT-3 ligand and G-CSF synergistically mobilize hematopoietic precursor cells in rabbits and have their most pronounced effect at 10 to 12 days' administration, ie, at the time when aplasia after radiation is most severe. Effects of both cytokines on the kinetics of entry into and recovery from hematopoietic aplasia cannot be explained solely by expansion and proliferation of stem and progenitor cells.
Several decades ago, the property of bacterial lipopolysacharides to protect animals from lethal irradiation was described and for a long time provided the basis for considerations in radioprotection.34 It is now known that lipopolysaccharides elicit an inflammatory reaction by releasing a variety of cytokines, including IL-1, IL-6, CSFs, TNF, IFNs, and transforming growth factor, all shown to have some radioprotective activity of their own. Based on these effects, Neta and Oppenheim8 postulated that an inflammatory response would assist in recovery from radiation by the removal of damaged tissue and by promoting restoration of normal function. Later, it was postulated that growth factors induce or enhance repair mechanisms and, thus, may protect stem cells from an irradiation insult.9,31 Radiation is a powerful stimulator of apoptosis.35,36 Aplasia after TBI might be the consequence of radiation-induced apoptosis in hematopoietic precursor cells. It has been shown that FLT-3 ligand promotes the maintenance of hematopoietic progenitors in vitro37 and has antiapoptotic effects.38 Protection of radiation-induced apoptosis by combined FLT-3 ligand, stem cell factor, and IL-3 has recently been shown for human and primate hematopoietic precursor cells in vitro.39 We postulate that similar mechanisms might take place in vivo in animals subjected to TBI.
Our data illustrate the profound radioprotective effects of FLT-3 ligand and extend information from previous studies concerning the potential of cytokine therapy in the treatment and prevention of radiation injury. The results of this study open the way for further analyses of radioprotective effects of FLT-3 ligand in growth factor combinations which should also include stem cell factor, thrombopoietin, and erythropoietin. The clinical goal is to design optimized radioprotective regimens to allow high-dose radiation for cancer patients or as intensive immunosuppression for solid-organ transplant recipients without the need for stem cell rescue.
Supported in part by the Swiss National Research Foundation Grant No. 3200-042431-94/1.
Address reprint requests to A. Gratwohl, MD, Division of Hematology, Department of Internal Medicine, Kantonsspital, CH-4031 Basel, Switzerland; e-mail: hematology@ubaclu.unibas.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal