Abstract
CXCR4 is the receptor for the α-chemokine stromal cell-derived factor 1 (SDF-1) and has been shown to be expressed on a diversity of leukocytes. In this report, the expression of the CXCR4 receptor in cells of megakaryocytic lineage and the role of SDF-1 in megakaryocytopoiesis were investigated. Using flow cytometry in combination with reverse transcriptase-polymerase chain reaction (RT-PCR), we observed that bone marrow CD34+, CD61+ cells, blood platelets, and megakaryocytic leukemia cell lines all expressed the CXCR4 receptor. To examine the expression of the CXCR4 receptor on megakaryocyte progenitors (colony-forming units-megakaryocyte [CFU-Meg]), CXCR4-positive and -negative CD34+ populations were separated from bone marrow and cultured in a plasma clot culture system. A subpopulation of the CFU-Meg was found in the CXCR4-positive fraction. The functional significance of CXCR4 expression on cells of the megakaryocytic lineage was examined by studying the effects of SDF-1α on migration and proliferation of megakaryocyte progenitor cells in vitro. We found that SDF-1α potently induced megakaryocyte progenitor migration and significantly enhanced adhesion of mature marrow megakaryocytes to endothelium. No marked effects of SDF-1α alone or in combination with thrombopoietin and stem cell factor/kit ligand on megakaryocyte production in vitro were noted. These results demonstrate for the first time that the CXCR4 α-chemokine receptor is expressed on cells of the megakaryocytic lineage from progenitors to platelets and that its ligand SDF-1α may modulate several aspects of megakaryocytopoiesis.
© 1998 by The American Society of Hematology.
CHEMOKINES ARE A family of relatively low molecular weight proteins that mediate cell migration. Their classification is based on the sequence of arranged cysteine groups, with four subsets of chemokines reported to date. The α subset is characterized by an intervening amino acid between two cysteines and is thus termed CXC chemokines.1 2
The receptors for chemokines are seven transmembrane structures that are linked to G-proteins and mediate calcium flux upon activation.3,4 The surface expression of different chemokine receptors and the biological functions of their cognate ligands have largely been studied on leukocytes.5-10 The α-chemokine receptor CXCR4 has been shown to be abundantly expressed in a diversity of white blood cells, including peripheral blood lymphocytes, monocytes,11-15 thymocytes,16pre-B cells,17 and dendritic cells.18,19Stromal cell-derived factor 1 (SDF-1), the natural ligand for CXCR4, was initially cloned from bone marrow stroma.20,21 It has been characterized as a pre-B–cell stimulating factor (PBSF) as well as a potent chemoattractant for T lymphocytes, monocytes,11-15 and pre-B cells.17 Targeted disruption of the SDF-1 gene in mice was shown to be lethal with severe abnormalities in B-cell lymphopoiesis and bone marrow myelopoiesis.22 Recently, CXCR4 was identified as a coreceptor for the entry of T-tropic human immunodeficiency virus (HIV), and SDF-1 has been shown to inhibit T-tropic HIV infection.14,15 SDF-1 was recently observed to potently mediate chemotaxis of CD34+ hematopoietic progenitor cells.23 This observation indicates that SDF-1/CXCR4 may play a role in other aspects of hematopoiesis.
The expression of the CXCR4 receptor on megakaryocytic cells and the potential functions of its ligand, SDF-1, in such cells have not been addressed. No data exist to our knowledge about megakaryocytopoiesis in SDF-1 knockout mice. To that end, we have examined bone marrow megakaryocyte progenitors (colony-forming units-megakaryocyte [CFU-Meg]), mature bone marrow megakaryocytes, and blood platelets for CXCR4 expression. We have studied the effects of SDF-1 on modulating in vitro megakaryocytopoiesis in response to known lineage growth factors, on effecting megakaryocyte progenitor migration, and on altering adhesive properties of marrow megakaryocytes to endothelium. Our results suggest that SDF-1 is a novel cytokine that may regulate megakaryocytopoiesis through multiple functional activities.
MATERIALS AND METHODS
Preparation of human bone marrow cells.
Light-density bone marrow mononuclear cells were obtained from normal consenting donors and depleted of adherent cells as previously described.24
Isolation of CD34+ bone marrow cells by immunoadsorption.
CD34+ cells were isolated by immunoadsorption using the CellPro (Bothell, WA) Ceprate LC system according to the manufacturer's instructions. After elution, cells were washed with Ca2+ Mg2+-free phosphate-buffered saline (PBS) with 1% bovine serum albumin (BSA) and resuspended in the appropriate medium for the migration assay or in the appropriate medium for megakaryocyte liquid culture (see below). The purity of the CD34+ cell-enriched fraction ranged between 90% and 95%.
Cell separation or isolation by immunomagnetic beads.
Nonadherent light-density bone marrow cells or cultured bone marrow cells were washed with and resuspended in ice-cold PBS with 10% fetal calf serum (FCS) and 0.5% BSA (isolation medium). For megakaryocyte isolation, an isolation medium (megakaryocyte medium25), which consisted of Ca2+ Mg2+-free PBS containing 13.6 mmol/L sodium citrate, 1 mmol/L theophylline, 1% BSA (fraction V), and 11 mmol/L glucose (Sigma Chemical Co, St Louis, MO), adjusted to pH 7.3 and an osmolarity of 290 mOsmol/L, was used. The cells were incubated for 20 minutes at 4°C and then incubated with monoclonal antibodies (MoAbs; anti-CD34 [Immunotech, Westbrook, ME], anti-CD61 [Dako, Carpinteria, CA], or anti-CXCR4 [12G5] [PharMingen, San Diego, CA]) at 2 μg/106 cells for 1 hour at 4°C. The cells were washed with PBS twice and incubated with magnetic beads coated with sheep antimouse IgG (Dynabeads M-450; Dynal Inc, Lake Success, NY) for 30 minutes at 4°C. Positive or negative cells were separated by washing four times with isolation medium. In some experiments, CD34+ cells were selected by the Dynal CD34 Progenitor Cell Selection system (Dynalbeads M-450 CD34 and DETACHaBEAD CD34; Dynal) to detach the beads binding on the cells and to remove antibody and beads from the cells. The purity of the CD34+ cells isolated by this method ranged between 90% and 95%.
Platelet preparation.
Human platelets were isolated from blood freshly drawn from healthy volunteers and anticoagulated with 0.1 vol of 3.8% sodium citrate, as described previously.26
Culture of megakaryocytic cell lines and human umbilical vein endothelial cells (HUVECs).
The permanent human megakaryocytic cell lines HEL and UT-7 were obtained from the American Type Tissue Culture Collection (Bethesda, MD). Other megakaryocyte cell lines studied were generous gifts to our laboratory. The CMK cell line was provided by Dr T. Sato (Chiba University, Chiba, Japan), the Dami cell line by Dr S. Greenberg (Brigham and Women's Hospital, Harvard Medical School, Boston, MA), and the Mo7E cell line by Dr J. Hoxie (University of Pennsylvania, Philadelphia, PA). The murine pre-B–cell line L1.2 transfected with CXCR4 vector was obtained from LeukoSite, Inc (Cambridge,MA). HEL, CMK, and Dami cell lines were cultured in RPMI-1640 medium with 10% FCS. The growth factor-dependent cell line, UT-7, was maintained with 10 ng/mL of interleukin-3 (IL-3) in RPMI-1640 medium with 10% FCS, and Mo7E was cultured with 10 ng/mL IL-3 in RPMI-1640 medium with 20% FCS. HUVECs were obtained from Clonetics Corp (San Diego, CA) and were maintained in endothelial cell growth medium (EGM), which contained 10 ng/mL epidermal growth factor, 1 μg/mL hydrocortisone, 12 μg/mL bovine brain extract, 1.6 U/mL heparin, and 2% fetal bovine serum (Clonetics). Third through fifth passages of HUVECs were used for all experiments.
Antibodies and flow cytometric analysis.
Cells collected from human bone marrow or liquid culture were washed with ice-cold megakaryocyte medium with 2.5% FCS and then resuspended at 106/100 μL. The cells were incubated with purified normal mouse IgG (Santa Cruz, Santa Cruz, CA), 2 μg/106 cells at 4°C for 20 minutes to block any possible nonspecific binding. Fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated MoAbs or control isotype antibodies were then incubated with the cells at 4°C for 40 minutes. After extensive washing, cells were resuspended in 1% paraformaldehyde in PBS at 4°C in the dark. Cell-associated immunofluorescence was analyzed by a FACscan flow cytometer (Becton Dickinson, San Jose, CA) using CellQuest software. Several MoAbs were used for flow cytometry: FITC-conjugated anti-CD34, FITC-conjugated anti-IIIa (CD61), PE-conjugated anti-CXCR4 (12G5); the corresponding control isotype antibodies were purchased from Coulter (Miami, FL), Dako, and PharMingen, respectively.
CFU-Meg culture.
To assay for human CFU-Meg, the plasma clot culture system was used. The culture medium consisted of 280 μg/mL CaCl2, 10 mmol/L α-TG (Sigma), and 20 μg/mL L-asparagine constituted in complete α-medium (α-medium supplemented with 1 mmol/L minimum essential medium [MEM] sodium pyruvate, 0.05 mmol/L MEM nonessential amino acids, 1× MEM amino acids, 5 × 10−5 mol/L of β-mercaptoethanol, and 50 μg/mL penicillin and 50 μg/mL streptomycin; GIBCO Laboratories, Grand Island, NY) and 15% platelet-poor plasma (PPP). Thrombopoietin (TPO) at 50 ng/mL (a generous gift from Genentech, South San Francisco, CA) and 100 ng/mL stem cell factor (SCF; Amgen, Thousand Oaks, CA) were used as megakaryocyte growth factors. Cultures were incubated at 37°C in 5% CO2 for 12 days. Quantitation of colonies was performed by immunofluorescent staining using anti-GpIIIa MoAb and each cluster of three or more megakaryocytes was scored as a colony as read under a fluorescent microscope.
Liquid culture for megakaryocytes.
Isolated bone marrow CD34+ cells were plated in 96 flat-bottomed multiwell plates in growth factor-free and serum-free medium optimized for megakaryocyte cell culture (MegaCult, Serum-free “Base” Medium; StemCell Technologies Inc, Vancouver, British Columbia, Canada). Cells then were cultured for 10 days with various growth factors and assessed for megakaryocyte production. The number of megakaryocytes produced was determined by immunofluorescence staining using anti-CD61 MoAb and cell counting.
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis for CXCR4 gene expression.
Total RNA was extracted from cells using the RNA isolation system ULTRASPEC-II (Biotecx Laboratories, Inc, Houston, TX). One microgram of total RNA was denatured by heating and reverse transcribed with 20 U Moloney murine leukemia virus (MMLV) RT into first-strand cDNA using 30 pmol of primers (dt). The reaction was performed for 1 hour at 37°C in a 20 μL final volume containing 5 mmol/L dithiothreitol (DTT), 40 U RNAsin, 5 mmol/L dNTP mixture, and 5× buffer (200 mmol/L Tris, pH 8.3, 40 μmol/L MgCl2). PCR was performed in a DNA thermal cycler (Perkin-Elmer Cetus Corp, Norwalk, CT) using 5 μL of the transcription mixture and 2.5 U of Taq polymerase. dNTPs (0.2 mmol/L), 10× reaction buffer (100 mmol/L Tris-HCl, pH 8.3, 50 mmol/L KCl, 15 mmol/L MgCl2, 0.01% gelatin) and 30 pmol of each primer were added in a 50 μL reaction volume for 30 cycles at 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds. The following specific oligomers were used for CXCR4 detection: 5′-AGC TGT TGG CTG AAA AGC TGG TCT ATG-3′ and 5′-GCG CTT CTG GTG GCC CTT GGA GTG TG-3′.
Chemotaxis assays.
Chemotaxis assays were performed in triplicate using 5-μm pore filters (Transwell, 24-well cell clusters; Costar, Boston, MA). The filters were rinsed with migration medium (complete α-medium with 0.5% BSA) and the supernatant was aspirated immediately before loading cells. CD34+ cells (1.5 × 105) suspended in 100 μL migration medium with or without various concentrations of SDF-1α (R & D Systems, Minneapolis, MN) were loaded into each Transwell filter (upper well). Filters were then carefully transferred to another well (lower well) containing 650 μL migration medium consisting of different concentrations of SDF-1α. The plates were incubated at 37°C in 5% CO2 for 3.5 hours. Next, the upper wells were carefully removed and the cells in the lower wells were collected. The cells were washed and resuspended in proper volume and quantitated for viable cells using trypan blue exclusion. The number of CFU-Meg in the migrated CD34+ cell population was assessed by culturing the migrated cells in the plasma clot culture system described above.
Adhesion assay.
CD34+ cell-derived megakaryocytes were used in an endothelial adhesion assay. CD34+ cells first were cultured in a liquid culture system using serum-free MegaCult Serum-free “Base” Medium (StemCells Technology, Inc) with 50 ng/mL TPO and 100 ng/mL SCF for 10 days. Megakaryocytes then were isolated by magnetic beads using anti-GpIIIa MoAb from this culture and the cells were washed three times in megakaryocytic medium. The cells were subsequently incubated in growth factor-free Meg medium at 5 × 105/mL with 10 μCi of 51Cr carrier-free (DuPont NEN, Boston, MA) at 37°C overnight. The cells were washed three times and resuspended in Meg medium at 105/mL before treatments with different concentrations of SDF-1α for 6 hours at 37°C. After treatments, the cells were washed and resuspended at 2.5 × 104/100 μL in modified HEPES-Tyrode's buffer supplemented with 1% BSA, 2 mmol/L CaCl2, and 1 mmol/L MgCl2, as reported previously.27 The cell suspension was applied onto a confluent HUVEC monolayer in 96-well plates and incubated for 1 hour at 37°C. Nonadherent cells were removed by washing four times. Quantitation of adherent cells per well was performed by liquid scintillation counting for 51Cr.
Statistical analysis.
The results are expressed as the mean ± SD of data obtained from three or more experiments performed in duplicate or triplicate. Statistical significance was determined using the Student'st-test.
RESULTS
CXCR4 gene expression in megakaryocytes and platelets.
To detect the expression of the CXCR4 receptor, RT-PCR was performed using specific primers as described in Materials and Methods. In these experiments, mRNA isolated from L1.2 cells transfected with a human CXCR4-expressing vector was used as a positive control; RT-PCR performed without RNA was used as a negative control.
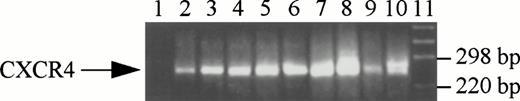
The CXCR4 transcripts were specifically amplified at their expected size (250 bp) in isolated bone marrow megakaryocytes and CD34+ progenitor cells. Platelets isolated from peripheral blood as well as the megakaryocytic leukemia cell lines HEL, CMK, Dami, Mo7E, and UT-7 were also found to express CXCR4 mRNA by RT-PCR (Fig 1).
CXCR4 gene expression in normal bone marrow CD34+ cells, megakaryocytes, platelets, and megakaryocytic cell lines. RT-PCR was performed as described in Materials and Methods. The PCR products were electrophoresed on a 3% agarose gel and visualized by ethidium bromide staining. RNA isolated from CXCR4-transfected L1.2 cells was used as a positive control and RT-PCR without RNA was used as a negative control. Lanes 1 through 11 represent negative control, CD34+ cells, CD61+ cells, platelets, HEL, CMK, Dami, Mo7E, UT-7, positive control, and DNA marker, respectively.
CXCR4 gene expression in normal bone marrow CD34+ cells, megakaryocytes, platelets, and megakaryocytic cell lines. RT-PCR was performed as described in Materials and Methods. The PCR products were electrophoresed on a 3% agarose gel and visualized by ethidium bromide staining. RNA isolated from CXCR4-transfected L1.2 cells was used as a positive control and RT-PCR without RNA was used as a negative control. Lanes 1 through 11 represent negative control, CD34+ cells, CD61+ cells, platelets, HEL, CMK, Dami, Mo7E, UT-7, positive control, and DNA marker, respectively.
Cell surface expression of the CXCR4 receptor on megakaryocytes and platelets.
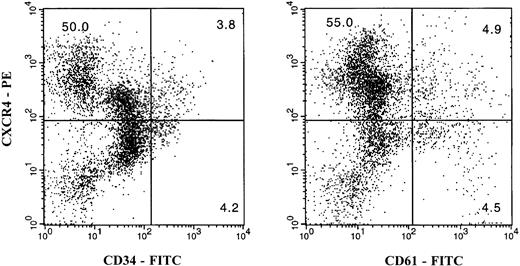
The cell surface expression of the CXCR4 receptor was analyzed by flow cytometry. About 46.9 ± 20.7% (n = 9) of nonadherent bone marrow mononuclear cells expressed this protein. To detect CXCR4 expression on CD34+ cells or megakaryocytes, two-color immunofluorescence staining was performed using PE-conjugated anti-CXCR4 MoAb (12G5) with FITC-conjugated anti-CD34 or anti-CD61 MoAb as described in Materials and Methods. Flow cytometry analysis showed that CXCR4 was coexpressed with CD34 as well as CD61. The percentage of expression of CXCR4 with CD34 or CD61 was found to be different from donor to donor. The coexpression of CXCR4 with CD34 ranged from 13% to 93%, with an average of 60.6% ± 25.8%. The coexpression of CXCR4 with CD61 ranged from 13% to 89%, with an average of 61.2% ± 26.7%. One representative experiment is shown in Fig2. These data indicate that the CXCR4 receptor was expressed on cells of the megakaryocytic lineage.
Two-color flow cytometry analysis of the cell surface expression of the CXCR4 receptor on bone marrow CD34+cells and megakaryocytes. Nonadherent light-density bone marrow cells were labeled simultaneously with a PE-conjugated anti-CXCR4 MoAb (12G5) and either an FITC-conjugated anti-CD34 (left panel) or anti-CD61 (right panel) MoAb. Double staining with PE- or FITC-labeled normal isotype mouse IgG was used as the negative control (data not shown). The figure in each quadrant represents the percentage of cells.
Two-color flow cytometry analysis of the cell surface expression of the CXCR4 receptor on bone marrow CD34+cells and megakaryocytes. Nonadherent light-density bone marrow cells were labeled simultaneously with a PE-conjugated anti-CXCR4 MoAb (12G5) and either an FITC-conjugated anti-CD34 (left panel) or anti-CD61 (right panel) MoAb. Double staining with PE- or FITC-labeled normal isotype mouse IgG was used as the negative control (data not shown). The figure in each quadrant represents the percentage of cells.
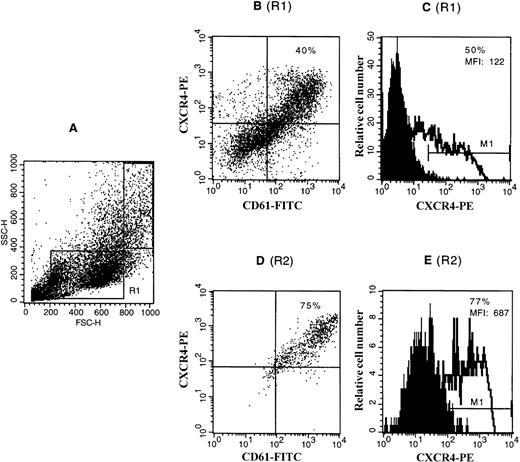
To confirm the expression of the CXCR4 receptor on megakaryocytes, CD34+ cells were cultured in liquid medium in the presence of TPO and SCF to obtain a population highly enriched in megakaryocytes (up to 50%). Cells were dually labeled with an FITC-conjugated anti-GpIIIa MoAb and a PE-conjugated anti-CXCR4 MoAb. Staining was analyzed by flow cytometry. Most of the GpIIIa-positive cells were stained by anti-CXCR4 antibody, whereas almost all (>90%) of the CXCR4-positive cells were shown to be GpIIIa-positive. Further analysis was performed by selectively gating the cells with different light scatter properties. As shown in Fig 3, megakaryocytes with large forward and side scatter properties (R2), which correspond to megakaryocytes of larger size and higher ploidy levels, as described previously,28 exhibited the stronger staining for CXCR4 than megakaryocytes with a relatively small size (R1).
Expression of the CXCR4 receptor on CD34+cell-derived megakaryocytes by flow cytometry. CD34+cells were cultured in a serum-free liquid culture system in the presence of TPO and SCF for 12 days. Cultured cells were stained with a PE-conjugated anti-CXCR4 MoAb (12G5) and either an FITC-conjugated anti-CD61 MoAb or an isotype control IgG and analyzed on FACscan. (A) The side scatter (SSC) versus forward scatter (FSC) of the analyzed cells. R1 represents the gating of the cells with relatively smaller forward and side scatter properties (which correspond to megakaryocytes with lower ploidy level and smaller size); R2 represents the gating of the cells with relatively larger forward and side scatter properties (which correspond to megakaryocytes with higher ploidy level and larger size). (B) and (D) show the coexpression of CXCR4 with CD61 shown in the dot plot as analyzed with gating in R1 and R2, respectively. The figures in these two quadrants represent the percentage of the double-positive cells. (C) and (E) show the expression of CXCR4 shown in the histogram plot as analyzed with gating in R1 and R2, respectively. The staining with control antibody is shown as the solid profile and the staining with the anti-CXCR4 antibody is shown as the thin line. The percentage of positive expression is provided in the inset. MFI, mean fluorescence intensity.
Expression of the CXCR4 receptor on CD34+cell-derived megakaryocytes by flow cytometry. CD34+cells were cultured in a serum-free liquid culture system in the presence of TPO and SCF for 12 days. Cultured cells were stained with a PE-conjugated anti-CXCR4 MoAb (12G5) and either an FITC-conjugated anti-CD61 MoAb or an isotype control IgG and analyzed on FACscan. (A) The side scatter (SSC) versus forward scatter (FSC) of the analyzed cells. R1 represents the gating of the cells with relatively smaller forward and side scatter properties (which correspond to megakaryocytes with lower ploidy level and smaller size); R2 represents the gating of the cells with relatively larger forward and side scatter properties (which correspond to megakaryocytes with higher ploidy level and larger size). (B) and (D) show the coexpression of CXCR4 with CD61 shown in the dot plot as analyzed with gating in R1 and R2, respectively. The figures in these two quadrants represent the percentage of the double-positive cells. (C) and (E) show the expression of CXCR4 shown in the histogram plot as analyzed with gating in R1 and R2, respectively. The staining with control antibody is shown as the solid profile and the staining with the anti-CXCR4 antibody is shown as the thin line. The percentage of positive expression is provided in the inset. MFI, mean fluorescence intensity.
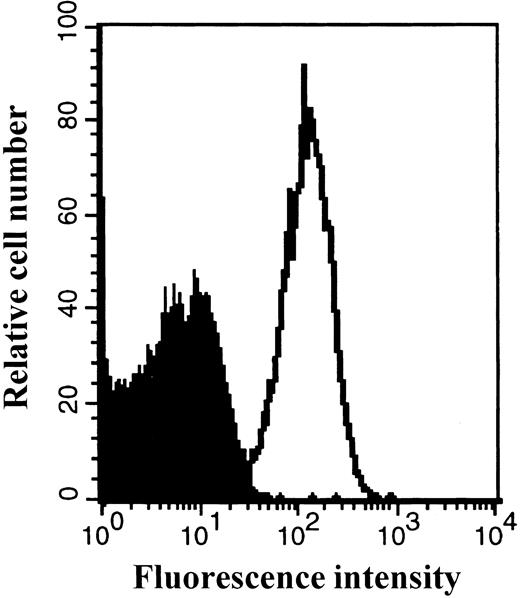
The expression of the CXCR4 receptor on platelets freshly isolated from peripheral blood was also detected by FACS analysis (Fig 4).
Expression of the CXCR4 receptor on peripheral blood platelets. Platelets were prepared as described in Materials and Methods. Platelets were stained with a PE-conjugated anti-CXCR4 MoAb or isotype control antibody and then analyzed by flow cytometry. The staining with control antibody is shown as the solid profile and the staining with the anti-CXCR4 antibody is shown as the thin line.
Expression of the CXCR4 receptor on peripheral blood platelets. Platelets were prepared as described in Materials and Methods. Platelets were stained with a PE-conjugated anti-CXCR4 MoAb or isotype control antibody and then analyzed by flow cytometry. The staining with control antibody is shown as the solid profile and the staining with the anti-CXCR4 antibody is shown as the thin line.
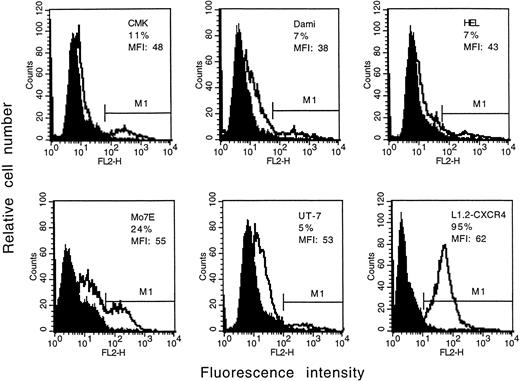
Expression of the CXCR4 receptor on megakaryocytic leukemia cell lines.
Cells from the megakaryocytic leukemia cell lines CMK, Dami, HEL, Mo7E, and UT-7 were stained with PE-conjugated anti-CXCR4 MoAb (12G5) and analyzed by flow cytometry. As shown in Fig5, a relatively lower surface expression of CXCR4 was found on megakaryocytic cell lines as compared with primary bone marrow megakaryocytes and platelets. Similar to the results observed with normal megakaryocytes, the expression of the CXCR4 receptor on megakaryocytic leukemia cells varied according to their maturation stage, with higher expression found in more mature megakaryocytic cells (data not shown).
Expression of the CXCR4 receptor on megakaryocytic leukemia cell lines. Cells were stained with a PE-conjugated anti-CXCR4 MoAb or isotype control antibody and then analyzed by flow cytometry. The staining with control antibody is shown as the solid profile and the staining with the anti-CXCR4 antibody is shown as the thin line. The percentage of positive expression is provided in the inset. MFI, mean fluorescence intensity.
Expression of the CXCR4 receptor on megakaryocytic leukemia cell lines. Cells were stained with a PE-conjugated anti-CXCR4 MoAb or isotype control antibody and then analyzed by flow cytometry. The staining with control antibody is shown as the solid profile and the staining with the anti-CXCR4 antibody is shown as the thin line. The percentage of positive expression is provided in the inset. MFI, mean fluorescence intensity.
Expression of the CXCR4 receptor on megakaryocytic progenitor cells.
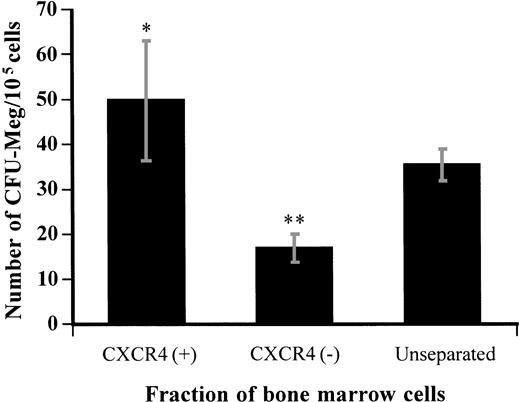
To address whether the CXCR4 receptor is expressed on megakaryocyte progenitors, CXCR4-positive and -negative populations were separated from bone marrow by magnetic beads using the specific anti-CXCR4 MoAb. About 85.6% ± 6.4% of the total cell population were recovered after selection. We obtained 35.3% ± 6.1% of the recovered cells in the CXCR4-positive fraction.
After selection, CXCR4-positive or -negative bone marrow cells were cultured in a plasma clot culture system for CFU-Meg in the presence of TPO and SCF. We observed a 1.4-fold enrichment of CFU-Meg in the cells selected for CXCR4 expression compared with that in unseparated cells. The frequency of CFU-Meg (the number of colonies formed per number of cells plated) in the CXCR4-positive fraction was about 3 times higher than in the CXCR4-negative fraction (Fig6). There were no significant differences in the size or morphology of the CFU-Meg colonies formed by CXCR4-positive or -negative bone marrow cells, as observed by microscopy. After selection by this method, approximately 72.4% ± 10.5% of the total CFU-Meg were recovered and 53.9% ± 10.6% of them were found in the CXCR4-positive fraction. These results indicate that the CXCR4 receptor is expressed on a subpopulation of megakaryocytic progenitor cells.
Megakaryocyte colony formation from the CXCR4-positive or -negative fraction of bone marrow cells separated by immunomagnetic bead isolation. Nonadherent light-density bone marrow cells were separated by immunomagnetic bead isolation as described in Materials and Methods and then cultured in the plasma clot system for CFU-Meg. The number of megakaryocyte colonies was determined after 12 days of culture. Results represent the mean ± SD of three separate experiments performed in triplicate. * and ** show statistical significance compared with unseparated control and are assessed asP < .05 and P < .01, respectively.
Megakaryocyte colony formation from the CXCR4-positive or -negative fraction of bone marrow cells separated by immunomagnetic bead isolation. Nonadherent light-density bone marrow cells were separated by immunomagnetic bead isolation as described in Materials and Methods and then cultured in the plasma clot system for CFU-Meg. The number of megakaryocyte colonies was determined after 12 days of culture. Results represent the mean ± SD of three separate experiments performed in triplicate. * and ** show statistical significance compared with unseparated control and are assessed asP < .05 and P < .01, respectively.
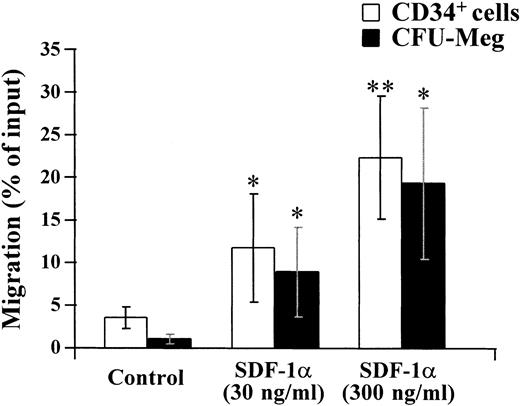
The effects of the SDF-1α ligand for the CXCR4 receptor on migration of megakaryocytic progenitor cells.
SDF-1 has been shown to be a chemoattractant for CD34+hematopoietic progenitor cells.23 The observed expression of the CXCR4 receptor on megakaryocytic progenitors led us to examine if SDF-1 had any effects on the migration of cells of this lineage. To address this question, CD34+ cells were isolated from bone marrow by an immunoaffinity column and studied in a chemotaxis assay. The migrated CD34+ cells were collected and cultured in the plasma clot system for CFU-Meg.
As shown in Fig 7, CFU-Meg as well as CD34+ cells migrated in response to SDF-1α in a dose-dependent manner.
Modulation of migration of CD34+ cells and megakaryocytic progenitor cells by SDF-1α. CD34+ cells were isolated from bone marrow by immunoadsorption using the CellPro Ceprate LC system. Migration of isolated CD34+ cells and CFU-Meg was measured as described in Materials and Methods. The migration of CD34+ cells or CFU-Meg was shown as the percentage of cell input. Results represent the mean ± SD of four separate experiments. * and ** show statistical significance compared with control and are assessed as P < .05 andP < .01, respectively.
Modulation of migration of CD34+ cells and megakaryocytic progenitor cells by SDF-1α. CD34+ cells were isolated from bone marrow by immunoadsorption using the CellPro Ceprate LC system. Migration of isolated CD34+ cells and CFU-Meg was measured as described in Materials and Methods. The migration of CD34+ cells or CFU-Meg was shown as the percentage of cell input. Results represent the mean ± SD of four separate experiments. * and ** show statistical significance compared with control and are assessed as P < .05 andP < .01, respectively.
Cell migration induced by chemokines or cytokines has been considered to be by two different mechanisms: chemotaxis and chemokinesis. Chemotaxis is the attraction of cells only in a positive concentration gradient, whereas chemokinesis is inducing cell migration in a random direction by activating cell motility.29 30 To confirm the chemotaxic effects of SDF-1α on CD34+ or CFU-Meg, migration assays were performed by adding different concentrations (0 to 300 ng/mL) of SDF-1α in the upper well, with 300 ng/mL of SDF-1α in the lower well. As shown in Fig 8, addition of SDF-1α in upper well significantly inhibited the migration of CD34+ or CFU-Meg response to SDF-1α in lower well.
Inhibitory effect of SDF-1α in the upper well on the migration of CD34+ cells, or CFU-Meg in response to SDF-1α in the lower well. CD34+ cells were loaded into the upper well with 0 to 300 ng/mL of SDF-1α and then applied to the lower well containing medium with or without 300 ng/mL of SDF-1α for migration assays. The migrated CD34+ cells were collected and counted. The number of CFU-Meg in the migrated CD34+cell population was assessed by culturing the migrated cells in the plasma clot culture system as described in Materials and Methods. The migration of the CD34+ cells or CFU-Meg was shown as the percentage of cell input. Results represent the mean ± SD of three separate experiments. * and ** show statistical significance compared with control (with 300 ng/mL of SDF-1α in the lower well and no SDF-1α in the upper well) and are assessed as P < .05 andP < .01, respectively.
Inhibitory effect of SDF-1α in the upper well on the migration of CD34+ cells, or CFU-Meg in response to SDF-1α in the lower well. CD34+ cells were loaded into the upper well with 0 to 300 ng/mL of SDF-1α and then applied to the lower well containing medium with or without 300 ng/mL of SDF-1α for migration assays. The migrated CD34+ cells were collected and counted. The number of CFU-Meg in the migrated CD34+cell population was assessed by culturing the migrated cells in the plasma clot culture system as described in Materials and Methods. The migration of the CD34+ cells or CFU-Meg was shown as the percentage of cell input. Results represent the mean ± SD of three separate experiments. * and ** show statistical significance compared with control (with 300 ng/mL of SDF-1α in the lower well and no SDF-1α in the upper well) and are assessed as P < .05 andP < .01, respectively.
These results indicated that SDF-1α is a chemoattractant factor for megakaryocytic progenitor cells.
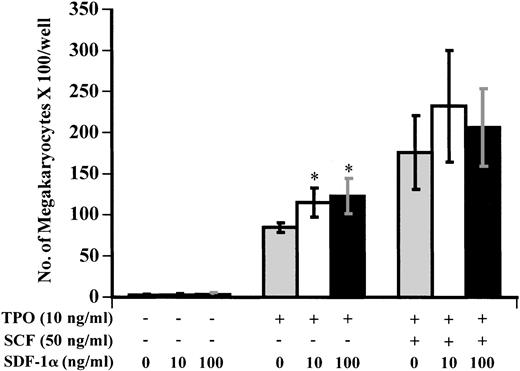
The effects of SDF-1α on the production of megakaryocytes from CD34+ cells in vitro.
SDF-1 has been shown to be involved in B-cell lymphopoiesis and myelopoiesis.20-22 We therefore asked whether SDF-1 modulated the production of mature GpIIb/IIIa+megakaryocytes from CD34+ progenitor cells.
As shown in Fig 9, SDF-1α alone had no significant effect on megakaryocyte production under serum-free liquid culture conditions. However, SDF-1α modestly promoted megakaryocyte production when combined with TPO. SDF-1α at 10 to 100 ng/mL increased megakaryocyte production by about 30% to 40% in combination with this growth factor. No significant increase in megakaryocyte production was observed when SDF-1α was combined with TPO + SCF under these culture conditions.
Effects of SDF-1α on the production of megakaryocytes from CD34+ cells. CD34+cells were isolated from bone marrow by immunoadsorption using the CellPro Ceprate LC system as described in Materials and Methods. The cells were cultured in a liquid culture using serum-free medium with growth factors as indicated. After 10 days of culture, megakaryocyte production was assessed by immunofluorescence staining using an anti-CD61 MoAb and cell counting. Results represent the mean ± SD of three separate experiments. * shows statistical significance compared with the control without SDF-1α and is assessed as P < .05.
Effects of SDF-1α on the production of megakaryocytes from CD34+ cells. CD34+cells were isolated from bone marrow by immunoadsorption using the CellPro Ceprate LC system as described in Materials and Methods. The cells were cultured in a liquid culture using serum-free medium with growth factors as indicated. After 10 days of culture, megakaryocyte production was assessed by immunofluorescence staining using an anti-CD61 MoAb and cell counting. Results represent the mean ± SD of three separate experiments. * shows statistical significance compared with the control without SDF-1α and is assessed as P < .05.
SDF-1α promotes the adhesion of megakaryocytes to endothelial cells.
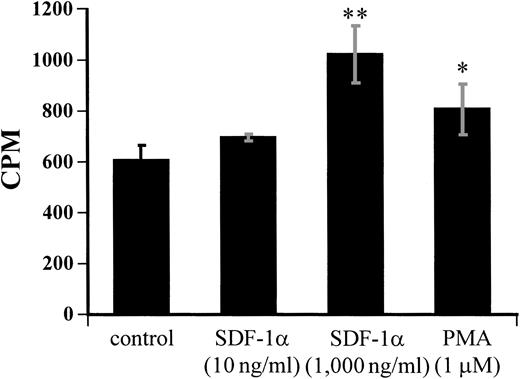
The endothelial cell is one of the major components of the hematopoietic microenvironment.31,32 HUVECs have been used as a cell model to study the interaction of megakaryocytes with endothelium.33 Previous studies have shown that SDF-1 induces hematopoietic progenitor cell migration on an endothelial monolayer.23 Because mature megakaryocytes express high levels of CXCR4 receptor, we asked if SDF-1 modulated the interaction of such megakaryocytes with endothelial cells. Adhesion assays were performed using CD34+ cell-derived megakaryocytes and confluent HUVECs as described in Materials and Methods. We observed that SDF-1α significantly increased the adhesion of megakaryocytes on HUVEC monolayer at a higher concentration (1,000 ng/mL; Fig 10).
Modulation of the adhesion of megakaryocytes to HUVEC by SDF-1α treatment. 51Cr-labeled megakaryocytes were treated with SDF-1α at the indicated concentrations for 6 hours before plating onto a HUVEC monolayer for the adhesion assay, as described in Materials and Methods. The adherent cells were determined by measuring the cpm value. The data are representative of three independent experiments performed in triplicate. * and ** show statistical significance compared with control and are assessed asP < .05 and P < .01, respectively.
Modulation of the adhesion of megakaryocytes to HUVEC by SDF-1α treatment. 51Cr-labeled megakaryocytes were treated with SDF-1α at the indicated concentrations for 6 hours before plating onto a HUVEC monolayer for the adhesion assay, as described in Materials and Methods. The adherent cells were determined by measuring the cpm value. The data are representative of three independent experiments performed in triplicate. * and ** show statistical significance compared with control and are assessed asP < .05 and P < .01, respectively.
DISCUSSION
In this study, we investigated the expression and functions of the CXCR4 receptor on cells of the megakaryocytic lineage.
Megakaryocytes freshly isolated from normal human bone marrow were shown to express CXCR4 as analyzed by both RT-PCR and flow cytometry. CD34+ cell-derived, highly enriched megakaryocyte populations allowed us to obtain enough cell numbers for more detailed analysis. The degree of expression of CXCR4 was found to increase in parallel with the stage of megakaryocyte maturation. In agreement with this observation, CXCR4 was detected at robust levels on platelets both by RT-PCR and flow cytometry.
The cell surface expression of the CXCR4 receptor on megakaryocytic leukemia cell lines appeared lower than on normal cells. Similar to the results with normal megakaryocytes, higher levels of expression were found on the cells of larger size and higher ploidy levels as analyzed by flow cytometry.
Our data indicate that CXCR4 is expressed on all cells of the megakaryocytic lineage, from progenitors (CFU-Meg) to peripheral blood platelets. The level of expression as detected by flow cytometry increased with increased maturational stage and was nearly uniform on terminally differentiated circulating platelets. This observation suggests that CXCR4 likely mediates different cell functions at the different maturational stages, given the marked differences in biological roles between the extremes of CFU-Meg and, at the other end, circulating platelets.
Indeed, we observed a marked potentiating effect of SDF-1α on CFU-Meg migration. This result is consistent with the previously reported chemotactic effect of SDF-1 on a heterogeneous CD34+population in vitro.23 These data indicate that SDF-1 may play a role in modulating megakaryocytopoiesis by triggering the trafficking and homing of megakaryocytic progenitors.
We then examined if SDF-1 can modulate the production of megakaryocytes from their progenitors. Unlike other CXC or CC chemokines that have been shown to inhibit the proliferation of megakaryocyte progenitor cells34 as well as hematopoietic stem cells,35-37 SDF-1α had no inhibitory effects on megakaryocyte production from early progenitors in vitro. Instead, we observed that SDF-1α modestly enhanced megakaryocyte production (∼30% to 40%) from CD34+ cells in the presence of TPO. SDF-1α alone had no discernible effects on megakaryocyte production (Fig 9).
To further address the effects of SDF-1 on megakaryocytopoiesis, we studied the adhesive interactions of primary megakaryocytes with endothelium. We used primary HUVECs in these experiments as a model system, because they have yielded useful insights in the past with regard to effects of a variety of cytokines on megakaryocyte attachment.33 We observed that SDF-1α significantly increased megakaryocyte adhesion to HUVECs. Because endothelium is believed to be an important source for hematopoietic growth factors in the marrow cavity, as well as to provide a supporting matrix for maturing cells,31 32 our in vitro observations provide further evidence for a potential role of SDF-1 in regulating human megakaryocytopoiesis.
The finding of robust expression of CXCR4 on peripheral blood platelets suggests that SDF-1 might alter platelet adhesion to endothelium or otherwise modulate platelet functions. These possibilities are currently being investigated.
In conclusion, our results demonstrate for the first time that the CXCR4 α-chemokine receptor is expressed on the megakaryocytic lineage from progenitor to platelets. Its ligand SDF-1 appears to modulate in vitro several aspects of megakaryocytopoiesis. Further studies of cells of the megakaryocytic lineage, particularly those derived from knock out mice, should provide novel insights into the physiological regulation of megakaryocytopoiesis by SDF-1.
ACKNOWLEDGMENT
The authors are grateful to Janet Delahanty for her editing and for the preparation of the figures for this manuscript and to Nancy DesRosiers for her assistance with the figures. Finally, we appreciate Youngsun Jung and Tee Trac for their typing assistance, Stephanie Brubaker and Delroy Heath for facilitating our receipt of the needed reagents for the experiments, and Dr In-Woo Park for his scientific suggestions.
Supported in part by National Institutes of Health Grants No. HL 53745-02, HL 43510-07, HL 55187-01, HL 51456-02, and HL 55445-01.
Address reprint requests to Jerome E. Groopman, MD, Division of Experimental Medicine, Beth Israel Deaconess Medical Center, Harvard Institutes of Medicine, 4 Blackfan Circle, Boston, MA 02115; e-mail:jgroopma@west.bidmc.harvard.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal