Abstract
The CD14-dependent and -independent dendritic cell (DC) pathways are instituted simultaneously when CD34+ progenitor cells are treated with granulocyte-macrophage colony-stimulating factor (GM-CSF)/tumor necrosis factor (TNF) ± stem cell factor (SCF) (GTS). If TNF activity is neutralized within 48 hours of cytokine exposure, DC development is halted and myelogranulocytic hematopoiesis takes place. In this study, we show that disruption of TNF activity at a later time point produced a distinct alteration within the DC system. Instead of downregulating DC development, treatment of GTS cultures with antibodies to TNF (anti-TNF) on day 3 provoked the selective expansion of the CD14-dependent (monocyte) DC pathway from progenitor cell populations lacking CD14 and CD1a. After an initial decrease in proliferation, anti-TNF produced a rebound in cell growth that yielded intermediate myeloid progenitors exhibiting CD14-dependent DC differentiation potential and CD14+CD1a+ DC precursors. Cultures enriched in CD14-dependent DCs were more potent stimulators of a mixed leukocyte reaction, compared with control GTS cultures containing both types of DCs. The intermediate progenitors expanded in the presence of anti-TNF were CD115+CD33+DR+, long-lived, and displayed clonogenic potential in methylcellulose. When exposed to the appropriate cytokine combinations, these cells yielded granulocytes, monocytes, and CD14-dependent DCs. Antigen-presenting function was acquired only when DC maturation was induced from these myelodendritic progenitors with GM-CSF + interleukin-4 or GTS. These studies show a novel mechanism by which TNF regulates the DC system, as well as providing a strategy for the amplification of the CD14-dependent DC pathway from immature progenitors. Although TNF is required to ensure the institution of DC hematopoiesis from CD34+ progenitor cells, its activity on a later progenitor appears to limit the development of CD14-dependent DCs.
© 1998 by The American Society of Hematology.
DENDRITIC CELLS (DCs) are antigen-presenting cells (APC) that are functionally characterized by their exceptional capacity to stimulate naive T-cell responses. They initiate both TH1- and TH2-driven immune responses, as well as CD8+ T-cell responses.1 Recent advances have shown that DCs do not represent a single cell type and that distinct hematopoietic lineages may give rise to DC subtypes.1-6 In humans, cells with DC phenotype and function may develop from lymphoid progenitors that also have the potential to develop into T and natural killer (NK) cells.1,6-9 These lymphoid progenitors may home to the thymus and lack myeloid cell markers.6,8 Their development into mature, lymphoid-derived DCs appears to be interleukin-3 (IL-3) dependent and granulocyte-macrophage colony-stimulating factor (GM-CSF) independent.6 9
Studies with multipotent CD34+ progenitor cells and peripheral blood mononuclear cells describe two other DC subtypes that are associated with the myeloid lineage.1-6,10 One DC subtype is represented by a CD14+CD1a+intermediate, while the other stems from a CD14− precursor.2-6,10 Both CD14-dependent and -independent pathways produce mature DCs exhibiting the capacity to stimulate naive T cells in the mixed leukocyte reaction (MLR) and expressing high levels of class II major histocompatability complex (MHC) antigens and costimulatory molecules required to deliver secondary signals for T-cell activation. Immature cells of the CD14-dependent DC pathway exhibit monocyte (mono)-associated markers such as nonspecific esterase (NSE) activity, complement receptors (CD11b), M-CSF receptors (CD115), and phagocytic potential, whereas cells of the CD14-independent DC pathway exhibit low or absent levels of these markers.4,10 It has been suggested that CD14-dependent DCs correspond to interstitial and/or circulating blood DCs and that CD1a+-derived DCs are related to epidermal DCs (Langerhans cells).4 5
Both CD14-dependent and -independent DC pathways develop from CD34+ hematopoietic progenitors when these cells are treated with GM-CSF/tumor necrosis factor (TNF) ± stem cell factor (SCF).4,5 10 Thus far, the earliest identifiable precursors for CD14-dependent DCs are nondividing CD14+CD1a+cells. For the CD1a+ DC pathway, the earliest precursors are CD14−CD1a+, which are also nonproliferating.
Members of the TNF superfamily are critical in controlling DC differentiation and survival. The presence of TNF during the earliest phases of DC maturation is an absolute requirement for the in vitro generation of DC hematopoiesis from multipotent CD34+progenitors.11,12 The addition of TNF to CD34+progenitors must occur within the first 48 hours of culture in order for DC development to proceed.11,12 If anti-TNF antibodies are added to GM-CSF/TNF/SCF (GTS)-treated CD34+ progenitor cell cultures during this period, DC hematopoiesis is blocked and the granulocyte pathway is favored.11 The mechanism of action involving TNF is emerging as a complex balance between its positive and negative regulatory functions. TNF induces the upregulation of GM-CSF receptors on DC intermediates, inhibits the granulocytic pathway, and induces specific apoptotic events.11-13 More recently, it has been shown that CD40 ligand, a member of the TNF receptor family, also drives a subset of CD34+ progenitors to mature into CD1a− DCs.14 Because both TNF and CD40 ligand result in NF-κB activation, there may be common mediators involving TNF/CD40L signaling of DC differentiation. RANK/TRANCE-R are novel members of the TNF receptor family that mediate the survival of mature DCs by preventing apoptosis.15,16 RelB, a member of the NF-κB/Rel family of transcription factors, has also been shown to be present selectively on mature DCs.17 This factor is increased upon DC maturation in the CD14+ pathway, and activates genes required for APC function when translocated to the nucleus.17
In this study we show that, despite the absolute requirement for TNF during the earliest phases of DC hematopoiesis from CD34+progenitors, neutralization of TNF activity at a later time point produces a distinct deviation within the myeloid-associated DC system. Instead of downregulating the DC pathway and favoring granulocyte development, anti-TNF treatment provoked the selective expansion of the CD14-dependent DC pathway from CD14−CD1a− progenitor cells present in GTS-treated cultures. After an initial decrease in cell proliferation, a rebound in cell growth yielded the expansion of CD14+CD1a+ DC precursors and the persistence of myelodendritic progenitors expressing CD115, CD33, DR (class II MHC proteins), and colony-forming unit (CFU) potential. The myelodendritic progenitors exhibited trilineage potential and differentiated along the granulocytic, monocytic, and mono-DC lineages when cultured with the appropriate cytokine combinations. However, potent MLR stimulatory activity was achieved only with cytokines known to produce mature DC progeny.
MATERIALS AND METHODS
Enrichment of neonatal cord blood–derived progenitor cells.
Cord blood was collected from healthy full-term infants into sterile heparinized containers during repeat caesarean sections, according to institutional guidelines. Mononuclear cells (MNC) were prepared by density centrifugation on Lymphoprep gradients (endotoxin poor; Nycomed, Oslo, Norway) and placed on nylon wool columns for the isolation of nonadherent cells, as previously described by us.11,18,19 Separation of CD34+ progenitor cells from the nonadherent population employed positive immunoselection using immunomagnetic beads (Dynabeads; Dynal Inc, Oslo, Norway) and yielded cell populations that were greater than 85% CD34+, as described elsewhere.11,18 19
Liquid cultures.
CD34+ cells were adjusted to 0.4 × 105cells/mL in RPMI 1640 medium (GIBCO-BRL, Grand Island, NY) containing 2 mmol/L L-glutamine, 10 mmol/L HEPES, 50 IU/mL penicillin, 50 μg/mL streptomycin, 5% pooled normal human serum (NHS/RPMI), and incubated in Teflon vials (Scientific Specialties Service, Randalstown, MD) at 37°C in a 5% CO2 humidified incubator. For induction of DC hematopoiesis, cultures were supplemented with GTS at the onset of the culture period.19 DC growth peaks around day 10-11 under these conditions, as previously described.11,13,18 19
Recombinant human (rh) SCF (Genzyme, Boston, MA) was used at 50 ng/mL, human rTNF-α (Knoll Pharmaceuticals, Whippany, NJ) at 500 U/mL, human rGM-CSF (Genzyme) at 100 U/mL, human rIL-4 (Genzyme) at 200 U/mL, human rM-CSF (Genzyme) at 50 ng/mL, and human rG-CSF (GIBCO-BRL) at 500 U/mL. The optimal concentration for each factor was calculated after dose analysis in prior studies.11,13,18,19The cytokine combinations used do not support the development of lymphocytes or erythrocytes.12,18,20 For in situ analyses and examination under phase microscopy, cultures were also established in either 24-well plates or Lab-Tek chamber slides (Nunc, Naperville, IL). Because of the profound effects of endotoxin on cell development and function,2,21-23 cultures were maintained under strict endotoxin-poor conditions, as previously described by us.18
On day 3, GTS cultures were split and a portion of the cells were treated with 15 μg/mL of rabbit polyclonal anti-TNF (Genzyme) or nonimmune rabbit Ig (Vector). Thereafter, the cultures were supplemented with fresh NHS/RPMI media without exogenous cytokines, on a weekly basis. As indicated, untreated and anti-TNF–treated GTS cultures received M-CSF, GM-CSF + G-CSF, GTS, or GM-CSF + IL-4.
Proliferation.
Proliferative events were measured by the uptake of [3H] thymidine and manual hemacytometer-assisted cell counts (Improved Neubauer, Fisher Scientific, Pittsburgh, PA). For thymidine uptake, 0.5 μCi of [3H] thymidine (specific activity, 25 Ci/mmol; Amersham, Arlington Heights, IL) was added to 100-μL aliquots taken from Teflon cultures and placed in 96-well microtiter plates. After 5 hours of incubation, cells were obtained using an automated sample harvester and counted in a liquid scintillation counter. Results are expressed as the mean of triplicate samples, and the SE was ≤20% in all experiments.
Immunofluorescence analysis.
Antibodies to DR, CD11b, CD13, CD33, CD34, CD38, and CD80 were obtained from Becton Dickinson (San Jose, CA). Anti-CD1a was obtained from Biosource (Camarillo, CA), anti-CD4 from the American Type Culture Collection (Rockville, MD), anti-CD14 from Sigma (St Louis, MO), anti-CD83 from Immunotech (Marseille, France), anti-CD86 from Pharmingen (San Diego, CA), and anti-CD115 from Serotec (Oxford, UK). In indirect assays, reactivity was detected with either fluorescein (FITC)- or phycoerythrin (PE)-linked anti-mouse Ig or anti-rabbit Ig (Fab′)2 fragments (Boehringer Manneheim Corp, Indianapolis, IN; Cappel, Westchester, PA; Jackson Immunoresearch, West Grove, PA). Matched nonimmune mouse or rabbit Ig isotypes were used as negative controls (Becton Dickinson; Coulter Immunology, Hialeah, FL). Cells were analyzed on a FACScan flow cytometer (Becton Dickinson) calibrated with Calbrite beads (Becton Dickinson) for FITC and PE. The distribution of debris, dead cells, and any contaminating red blood cells was assessed on the basis of forward and right-angle scatter before proceeding with the analysis. A total of 10,000 events were examined using a 488-nm wavelength excitation. Acquired events were analyzed using Cell Quest Software (Becton Dickinson). Results are expressed as percent positive cells after subtracting negative control values.
Allogeneic mixed leukocyte reaction.
Cells grown under various conditions were removed from Teflon cultures, centrifuged twice in RPMI, adjusted to equal concentrations in 5% NHS/RPMI, and irradiated with a 60Co source (for a total of 2,000 rads). Varying numbers of these stimulator cells were then added to 96-well microtiter plates containing 5 × 104responder cells/well (nylon wool enriched T-cell populations obtained from normal peripheral blood). A proliferative response was measured after 7 days by adding 0.5 μCi of [3H] thymidine to each well and harvesting the cells as described above. Thymidine uptake in control cultures containing irradiated stimulator cells or responder cells cultured alone was less than 200 cpm.
Colony-forming assays.
Cells from untreated GTS cultures and GTS cultures receiving anti-TNF on day 3 were removed from culture on the day indicated and seeded in duplicate in 24-well plates at 2 × 104 cells/mL. Colony growth was assessed in semisolid cultures containing: RPMI 1640 medium (GIBCO), 2 mmol/L L-glutamine, 10 mmol/L HEPES, 50 IU/mL penicillin, 50 μg/mL streptomycin, 5% pooled NHS, 15% fetal bovine serum, 1% StemPro methylcellulose (GIBCO), and cytokine combinations, as specifically indicated.
Cytochemistry.
Cells cultured in suspension (Teflon) were prepared for Wright stain (Hemacolor; EM Diagnostic Systems, Gibbstown, NJ) and NSE analysis by depositing them onto slides by means of cytocentrifugation (Shandon, Pittsburgh, PA). No fewer than 500 cells were analyzed.
Statistics.
Where indicated, Student's t-test was used to analyze data using Sigma Stat (Jandel Scientific, San Rafael, CA).
RESULTS
Phenotypic characteristics of GTS cultures on day 3.
In a previous study, we showed that by day 3, progenitor cell cultures treated with GTS contain CD33+CD13+ cells that undergo subsequent expansion into the DC pathway.13 In Fig 1 we provide an extended cell-surface profile of the day 3 cultures. Cells were positive for CD33, CD13, and DR, and negative or mostly negative for molecules associated with later stages of DC development, including CD86, CD80, CD14, CD1a, CD83, CD4, and CD11b. The anti-TNF–treated GTS cells on day 3 exhibited decreased levels of CD34 (≤10%), did not exhibit NSE activity, and were nonphagocytic (data not shown).
Flow-assisted characterization of GTS cultures on day 3. Each histogram depicts a representative experiment (N = 5 to 8 for each marker). The numbers inside the panels depict positive reactivity for the experiment shown, after assessing negative controls.
Flow-assisted characterization of GTS cultures on day 3. Each histogram depicts a representative experiment (N = 5 to 8 for each marker). The numbers inside the panels depict positive reactivity for the experiment shown, after assessing negative controls.
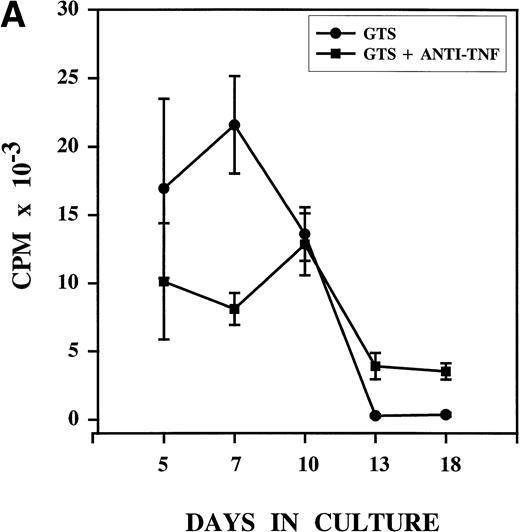
Anti-TNF treatment on day 3 alters the proliferation pattern associated with DC development in GTS cultures. Thymidine uptake confirmed that, as previously described, proliferation in GTS cultures is marked by day 5, peaks on day 7, and declines sharply after day 10 (Fig 2A).19 Anti-TNF treatment of GTS cultures on day 3 produced decreases in proliferation that were evident on day 5. The decreases between the two cultures were significant (P = .0012) by day 7, with anti-TNF treatment reducing proliferative potential by about 60%. Notwithstanding, after day 7, the proliferative capacity of the anti-TNF–treated GTS cultures remained stable whereas that of the GTS cultures decreased sharply. By day 10, proliferation in the two groups was virtually identical (P = .760).
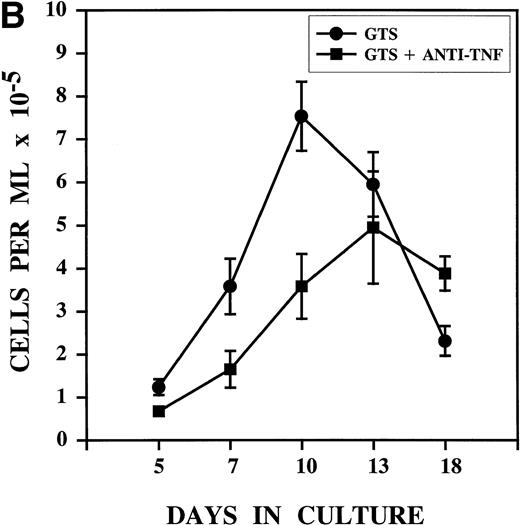
Anti-TNF treatment on day 3 alters the growth pattern associated with DC development in GTS cultures. GTS and anti-TNF–treated GTS cultures were compared in parallel. Results represent the mean of multiple experiments. (A) Proliferation was measured by thymidine uptake, N = 3 to 8 for all days. (B) Cell content was assessed by hemacytometer-assisted cell counts, N = 7 to 16 for all days. The addition of nonimmune rabbit IgG to GTS cultures on day 3 did not alter the growth pattern.
Anti-TNF treatment on day 3 alters the growth pattern associated with DC development in GTS cultures. GTS and anti-TNF–treated GTS cultures were compared in parallel. Results represent the mean of multiple experiments. (A) Proliferation was measured by thymidine uptake, N = 3 to 8 for all days. (B) Cell content was assessed by hemacytometer-assisted cell counts, N = 7 to 16 for all days. The addition of nonimmune rabbit IgG to GTS cultures on day 3 did not alter the growth pattern.
A kinetic analysis of cell content (Fig 2B) showed significant decreases in the absolute number of cells present in the anti-TNF–treated GTS cultures on days 5, 7, and 10, compared with the untreated GTS cultures (P < .006 for all days). Despite this effect, cell content steadily increased in the anti-TNF–treated GTS cultures to the point that on day 13 differences between the control GTS cultures and the anti-TNF treated GTS cultures were no longer significant (P = .4). On day 18, in fact, the anti-TNF–treated GTS cultures contained a significantly higher (P = .005) number of cells than the GTS cultures. Thymidine uptake showed the persistence of proliferative events (>3,500 cpm) in the anti-TNF GTS cultures on days 13 and 18 and almost no proliferation in the untreated GTS cultures (<300 cpm, P ≤ .03, Fig 2A). These results imply that progenitor/precursor cells with proliferative capacity endure in the anti-TNF–treated GTS cultures.
Anti-TNF treatment alters the phenotypic characteristics of progeny on day 10.
We previously noted that the period between days 3 and 7 in GTS cultures marks a critical time for DC development, as judged by the tremendous expansion (>80-fold) of DR+CD13+CD33+ DC intermediates and the distribution of CD14 and CD1a antigens.13 Later assessment on day 10 (peak DC development) showed that further maturation of DCs occurred, as revealed by reactivity with antibodies to DR, CD86, CD1a, CD80, and DR. In agreement with prior reports, we observed the presence of both CD14+CD1a+ and CD1a+ DC intermediates before peak DC development on day 10.4,5,10 The percentage of CD14+CD1a+ cells did not exceed 10%, supporting the implication that the CD14+-dependent DC pathway exists as a secondary DC pathway when hematopoiesis is instituted from CD34+ progenitors with GTS.10
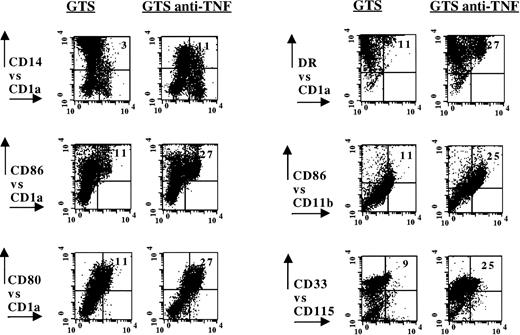
The data depicted in Fig 3 show that the cellular rebound observed in anti-TNF–treated GTS cultures produced a distinctive cell-surface profile that is associated with the development of the CD14+-dependent DC pathway.4,5,10 On day 10, the percentage of cells coexpressing CD14 and CD1a was increased sevenfold versus GTS cultures (P = .0007, N = 9). Anti-TNF also decreased the total percentage of CD14+ cells and the fluorescence intensity of CD14, while increasing the percentage of CD1a+ cells (approximately threefold, P = .0001, N = 9). These changes are similar to those previously reported to accompany the development of mono-derived DCs from CD14+ precursors isolated from either peripheral blood or cord blood GTS cultures.1-3 However, because CD14+ cells were less than 0.5% at the time we added anti-TNF (day 3, Fig 1), our results signify that the amplification of the CD14-dependent DC pathway must stem from an earlier, CD14− myeloid progenitor. Additional evidence in favor of the expansion of the CD14-dependent DC pathway in the anti-TNF–treated GTS cultures includes increases in cells which coexpress CD11b and CD86 (approximately fourfold, P= .02, N = 3) and NSE (Table1).4,5 10 Interestingly, despite the increases in markers denoting the CD14-dependent DC pathway, anti-TNF treatment did not significantly alter the distribution of CD13+CD33+ or CD83+ cells.
Flow-assisted characterization of anti-TNF–treated GTS cultures on day 10 revealed the expansion of the CD14-dependent DC pathway. A representative experiment for each group is shown. For CD14 versus CD1a, N = 9, for all other combinations, N ≥ 3. The numbers in the upper right quadrants depict percent positive cells for the experiment shown, after subtracting negative controls. With the exception of the CD80 versus CD1a combination, all comparisons yielded statistically significant differences (P ≤ .03). The addition of nonimmune rabbit IgG to control GTS cultures did not alter the distribution of any of the markers.
Flow-assisted characterization of anti-TNF–treated GTS cultures on day 10 revealed the expansion of the CD14-dependent DC pathway. A representative experiment for each group is shown. For CD14 versus CD1a, N = 9, for all other combinations, N ≥ 3. The numbers in the upper right quadrants depict percent positive cells for the experiment shown, after subtracting negative controls. With the exception of the CD80 versus CD1a combination, all comparisons yielded statistically significant differences (P ≤ .03). The addition of nonimmune rabbit IgG to control GTS cultures did not alter the distribution of any of the markers.
Kinetics of Nonspecific Esterase Activity in Untreated GTS and Anti-TNF–Treated GTS Cultures
| Condition: . | Day 5 . | Day 7 . | Day 10 . | Day 18 . |
|---|---|---|---|---|
| GTS | 1.23% | 6.6% | 8.0% | 40% |
| GTS anti-TNF | 0.8% | 5.7% | 24% | 68% |
| P value | .03 | .82 | .036 | .01 |
| Condition: . | Day 5 . | Day 7 . | Day 10 . | Day 18 . |
|---|---|---|---|---|
| GTS | 1.23% | 6.6% | 8.0% | 40% |
| GTS anti-TNF | 0.8% | 5.7% | 24% | 68% |
| P value | .03 | .82 | .036 | .01 |
For days 7, 10, and 18 N = 3; for day 5 N = 4.
Anti-TNF treatment of GTS cultures on day 3 produces distinct progeny in methylcellulose and liquid cultures.
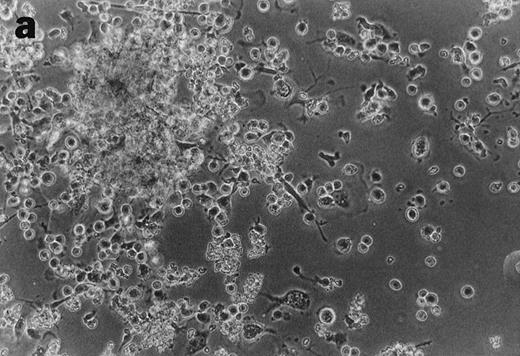
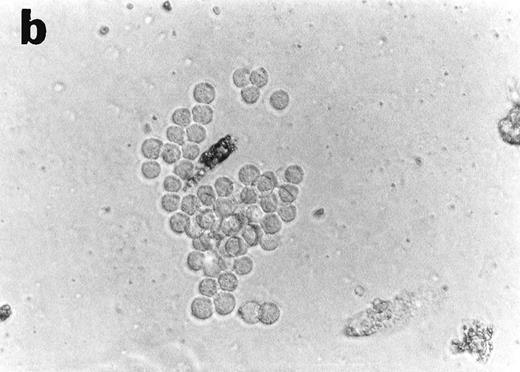
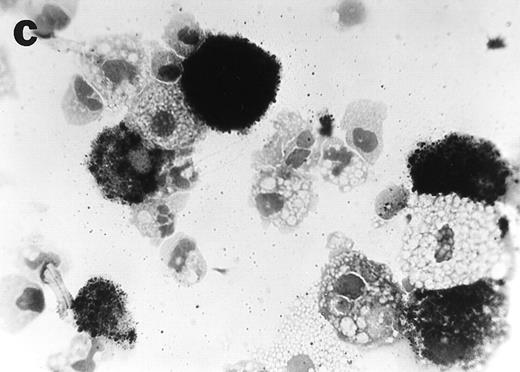
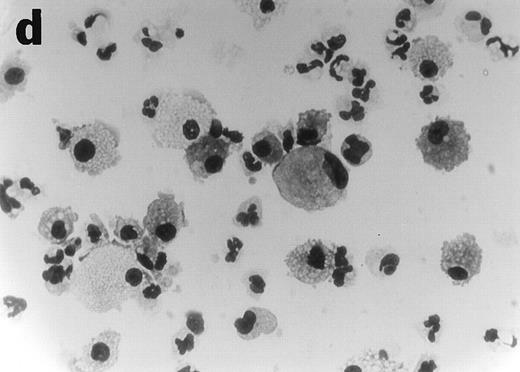
CD34+cells treated with GTS yielded numerous typical DC colonies in methylcellulose after ≈12 days, as expected (Fig 4a).19 GTS cultures treated with anti-TNF also yielded numerous CFUs. However, most of the colonies consisted of small round cells (Fig 4b) and few, if any, typical DC colonies were observed. In comparison to liquid GTS cultures which contained many adherent DC clusters (Fig 4c), the liquid anti-TNF–treated GTS cultures contained predominantly nonadherent cells around day 10 (Fig 4d). Examination of these nonadherent cells under higher magnification showed that they were either small round cells or veiled cells.
Anti-TNF–treated GTS cultures produce distinct progeny in methylcellulose and liquid cultures, compared with GTS cultures. GTS cultures yielded typical DC colonies around day 12 in methylcellulose (a) and adherent DC clusters in liquid cultures (c). In methylcellulose, anti-TNF–treated GTS cultures produced large CFUs containing small round cells and lacking typical DCs (b). The liquid anti-TNF–treated cultures (d) contained predominantly nonadherent cells. Original magnification × 20.
Anti-TNF–treated GTS cultures produce distinct progeny in methylcellulose and liquid cultures, compared with GTS cultures. GTS cultures yielded typical DC colonies around day 12 in methylcellulose (a) and adherent DC clusters in liquid cultures (c). In methylcellulose, anti-TNF–treated GTS cultures produced large CFUs containing small round cells and lacking typical DCs (b). The liquid anti-TNF–treated cultures (d) contained predominantly nonadherent cells. Original magnification × 20.
The increased APC functions of anti-TNF–treated GTS cultures correlate with increases in DR and costimulatory molecules.
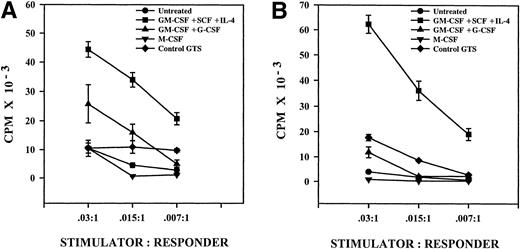
The coexpression of class II MHC antigens (DR) and costimulatory molecules (CD80/CD86) with CD1a was greater in the anti-TNF–treated GTS cultures, compared with control GTS cultures by at least twofold on day 10. In Fig 5, we investigated the functional outcome of these differences using the allogeneic MLR. T-cell activation was increased when stimulators were derived from the anti-TNF–treated GTS cultures. In wells containing only one stimulator cell per 200 responder cells (.015:1), anti-TNF–treated GTS cultures produced T-cell responses that were at least three times greater than those produced by control GTS cultures. Thus, there was a positive correlation between the increased expression of class II MHC molecules/costimulatory molecules with CD1a and the increased MLR stimulatory capacity of the anti-TNF–treated GTS cultures.
Anti-TNF–treated GTS cultures exhibit increased MLR capacity on day 10, when compared with untreated GTS cultures. Results represent the mean ± the SE of triplicate samples. One of three representative experiments is shown.
Anti-TNF–treated GTS cultures exhibit increased MLR capacity on day 10, when compared with untreated GTS cultures. Results represent the mean ± the SE of triplicate samples. One of three representative experiments is shown.
Anti-TNF–treated GTS cultures, but not control GTS cultures, contain myeloid progenitors with CFU potential on day 10.
The results shown in Fig 3 demonstrate that in addition to promoting increases in precursors associated with the CD14-dependent DC pathway, anti-TNF treatment of GTS cultures produced approximately a threefold increase in CD33+CD115+ cells on day 10 (P = .008, N = 4). CD115, the receptor for M-CSF, is distributed throughout the myelomonocytic lineage on cells both lacking and exhibiting proliferative and CFU potential.24-27 CD115 may also exist on nonproliferating mono-DC precursors, but is promptly lost during the DC maturation process.27-29
Because of the persistence of late proliferative events and the pattern of CD115 reactivity, we speculated that myeloid progenitors with CFU potential were sustained in the anti-TNF–treated GTS cultures on day 10. Figure 6 depicts the CFU capacity of cells that were removed from the anti-TNF–treated GTS cultures on day 10 and recultured with cytokines promoting myeloid colony formation (GM-CSF + SCF) in methlycellulose. Two principal types of CFUs developed under these conditions. One resembled a mono-CFU (Fig 6a), the other a granulocytic colony (Fig 6b). NSE and Wright stain analysis of cells obtained from these methylcellulose cultures confirmed the presence of both monos and granulocytes (Fig 6c and d). Cells obtained from control GTS cultures on day 10 had lost their capacity to yield CFUs in methylcellulose, which is consistent with their low proliferative potential in liquid cultures (Fig 2A).
Anti-TNF–treated GTS cultures contain myeloid progenitors with CFU potential on day 10. Cells removed from anti-TNF–treated GTS cultures on day 10 and placed in methylcellulose with GM-CSF+SCF yielded colonies resembling mono CFU (a) and granulocyte CFU (b). Nonspecific esterase (c) and Wright stain (d) analysis confirmed the presence of both mono-macrophages and granulocytes. Original magnification for (a), (b), and (d) = 40×, for (c) = 60×. Cells removed from untreated GTS cultures on day 10 and cultured in parallel failed to yield CFU.
Anti-TNF–treated GTS cultures contain myeloid progenitors with CFU potential on day 10. Cells removed from anti-TNF–treated GTS cultures on day 10 and placed in methylcellulose with GM-CSF+SCF yielded colonies resembling mono CFU (a) and granulocyte CFU (b). Nonspecific esterase (c) and Wright stain (d) analysis confirmed the presence of both mono-macrophages and granulocytes. Original magnification for (a), (b), and (d) = 40×, for (c) = 60×. Cells removed from untreated GTS cultures on day 10 and cultured in parallel failed to yield CFU.
To further characterize the progenitors present on day 10, developmental potential was also tested in liquid cultures in the presence of lineage-restricted myeloid cytokines. M-CSF treatment of cells removed from anti-TNF–treated GTS cultures produced greater than 90% mono-macrophages, whereas GM-CSF + G-CSF treatment yielded predominantly granulocytes. Marked proliferation accompanied the differentiation process with both cytokine treatments (data not shown). The addition of M-CSF or GM-CSF + G-CSF to control GTS cultures failed to produce any mono/granulocyte progeny or proliferative events. Thus, on day 10, myeloid progenitors cells that are responsive to both lineage- and nonlineage-restricted myeloid cytokines persist in the anti-TNF–treated GTS cultures, but not in the control GTS cultures.
Institution of the mono-DC pathway from progenitors present on day 10.
Because of the switch to the mono-DC pathway in the anti-TNF–treated GTS cultures, we investigated whether the myeloid progenitors present on day 10 also exhibited DC developmental potential. In these experiments cells were removed from anti-TNF–treated GTS cultures on day 10 and recultured with a combination of DC-restricted cytokines (GM-CSF + IL-4 + SCF or GTS). Expansion into the CD14-dependent DC pathway occurred with this treatment, as determined by morphological, cell surface, and functional characterization. A distinct cellular progression was noted, as shown in Fig 7. Three to five days after the addition of GTS or GM-CSF + IL-4 + SCF (days 13-15), twofold to threefold increases in CD14+CD1a+ DC precursors were observed, when compared with the parent anti-TNF GTS cultures. Upon further differentiation on days 17-18, CD1a expression and CD14 CD1a coexpression decreased dramatically, suggesting the differentiation of the CD14+ CD1a+ and CD1a+ cells into mature DCs. As early as day 13 there was a dramatic decrease in CD14 single expression with GM-CSF + IL-4 + SCF treatment, further indicating the development of CD14-dependent DC pathway (data not shown).2 27-30 Morphological assessment showed typical DC clusters and veiled cells in anti-TNF–treated GTS cultures receiving cytokines inducing DC development between days 15-18 (data not shown). In contrast, DC-associated differentiation events were rare when anti-TNF–treated GTS cultures were untreated or when anti-TNF–treated GTS cells were removed from culture and exposed to NHS/RPMI only (data not shown).
Institution of the CD14-dependent DC pathway from progenitors present on day 10 in anti-TNF–treated GTS cultures. (□), Anti-TNF–treated GTS cultures; (▪), anti-TNF–treated GTS cells exposed to GTS on day 10; (▨), anti-TNF–treated GTS cells exposed to GM-CSF + SCF + IL-4 on day 10. Results represent one of two similar experiments.
Institution of the CD14-dependent DC pathway from progenitors present on day 10 in anti-TNF–treated GTS cultures. (□), Anti-TNF–treated GTS cultures; (▪), anti-TNF–treated GTS cells exposed to GTS on day 10; (▨), anti-TNF–treated GTS cells exposed to GM-CSF + SCF + IL-4 on day 10. Results represent one of two similar experiments.
Assessment of T-cell stimulatory activity revealed the development of potent APC functions consistent with DC development, as shown in Fig8A. Only anti-TNF–treated GTS cells that had been subjected to the differentiating effects of DC-restricted cytokines on day 10 induced a potent MLR on day 18; untreated anti-TNF GTS cells were weak stimulators of a MLR on day 18, as were anti-TNF GTS cells treated with either M-CSF or G-CSF. Control GTS cultures also exhibited poor MLR potential on day 18, consistent with the loss of DCs.13
Potent MLR stimulatory function is achieved only after mature DCs develop from myelodendritic progenitors present in the anti-TNF–treated GTS cultures. (A) On day 18, cells that had been treated with GM-CSF + SCF + IL-4 on day 10 produced potent T-cell responses. Cells that were left untreated (GTS, anti-TNF on day 3), control GTS cultures, or cells that were treated on day 10 with GM-CSF + G-CSF or M-CSF showed weak stimulatory potential on day 18. Results represent one of three typical experiments. (B) Progenitor cells exhibiting DC differential potential are preserved in anti-TNF–treated GTS cultures beyond day 18. Cells removed from anti-TNF–treated cultures on day 18 and exposed to GM-CSF + IL-4 acquired MLR stimulatory capacity by day 26. Cells obtained from untreated anti-TNF GTS cultures and control GTS cultures on day 26 produced weak T-cell responses, as did anti-TNF GTS cells exposed to GM-CSF + G-CSF or M-CSF on day 18. Results represent one of two experiments.
Potent MLR stimulatory function is achieved only after mature DCs develop from myelodendritic progenitors present in the anti-TNF–treated GTS cultures. (A) On day 18, cells that had been treated with GM-CSF + SCF + IL-4 on day 10 produced potent T-cell responses. Cells that were left untreated (GTS, anti-TNF on day 3), control GTS cultures, or cells that were treated on day 10 with GM-CSF + G-CSF or M-CSF showed weak stimulatory potential on day 18. Results represent one of three typical experiments. (B) Progenitor cells exhibiting DC differential potential are preserved in anti-TNF–treated GTS cultures beyond day 18. Cells removed from anti-TNF–treated cultures on day 18 and exposed to GM-CSF + IL-4 acquired MLR stimulatory capacity by day 26. Cells obtained from untreated anti-TNF GTS cultures and control GTS cultures on day 26 produced weak T-cell responses, as did anti-TNF GTS cells exposed to GM-CSF + G-CSF or M-CSF on day 18. Results represent one of two experiments.
These data show that the progenitor cell population expanded by anti-TNF treatment of GTS exhibits the capacity to yield three distinct lineages, namely, monos, granulocytes, and CD14-dependent DCs. As expected, only the CD14-dependent DCs exhibited potent APC functions. The increased stimulatory capacity expresssed by these cells always correlated with increased levels of cells coexpressing DR and CD86 antigens (data not shown).
Induction of mature DCs from progenitors preserved in anti-TNF–treated GTS cultures beyond day 18.
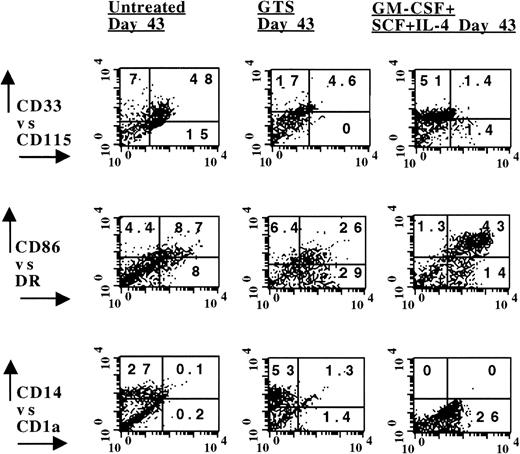
The advancement of the CD14-dependent DC pathway from the progenitors present in the anti-TNF–treated GTS cultures on day 10 was strictly regulated by DC cytokines. Notwithstanding, significant proliferation was observed in the anti-TNF GTS cultures on days 13 and 18 (P≤ .03; Fig 2), even in the absence of additional cytokine supplementation. Although the distribution of CD14+CD1a+ and CD14−CD1a+ DC precursors had decreased in the anti-TNF–treated GTS cultures by day 18 (Fig 7), CD115/CD33 coexpression was still increased approximately seven times in these cultures, compared with control GTS cultures. These observations led to the speculation that the capability to exhibit very late DC differentiating potential might be associated with the long-term preservation of the myeloid progenitors. The data in Figs 8B and 9 support this assumption. Although cells derived from day 26 anti-TNF–treated GTS cultures were incapable of stimulating an MLR, cells previously exposed to GM-CSF + IL-4 + SCF on day 18 demonstrated increased MLR capacity on day 26 (Fig8B). Even later (day 35), treatment of cells obtained from anti-TNF–treated GTS cultures with GM-CSF + IL-4 or GTS produced CD14-dependent DCs, as revealed by the downregulation of CD115 and the distribution of DR, CD1a, CD14, and CD86 antigens (Fig 9).
Cells present in anti-TNF–treated GTS cultures on day 35 developed into DCs when exposed to GTS or GM-CSF + SCF + IL-4, whereas untreated anti-TNF GTS cells remained in an undifferentiated state. Phenotype analysis was performed on day 40. The numbers in the quadrants depict percent positive cells, after subtracting negative controls. Results are representative of four separate experiments.
Cells present in anti-TNF–treated GTS cultures on day 35 developed into DCs when exposed to GTS or GM-CSF + SCF + IL-4, whereas untreated anti-TNF GTS cells remained in an undifferentiated state. Phenotype analysis was performed on day 40. The numbers in the quadrants depict percent positive cells, after subtracting negative controls. Results are representative of four separate experiments.
The equivalent of the anti-TNF–treated GTS progenitors could not be shown in the control GTS cultures. This is not unexpected because after day 18, viability is greatly decreased and greater than 40% of the cells are undergoing apoptosis.13 By day 35, few viable cells remained in the control GTS cultures. These were large, long-lived macrophages, as revealed by Wright stain, NSE, and flow analysis (data not shown). Although control GTS cells expressed DR antigens on day 35, they were unresponsive to DC restricted cytokines.
DISCUSSION
In this study we reveal a new role for TNF in DC development and a novel strategy for obtaining large numbers of CD14-dependent DCs from immature myeloid progenitor cells. Our findings indicate that while TNF is required to ensure the onset of DC hematopoiesis from CD34+ progenitor cells, its effect on a later progenitor serves to limit the development of the CD14-dependent DC pathway.
Three days after exposure of CD34+ progenitors to GTS, cultures contain committed myeloid progenitors expressing CD13, CD33, and DR, as previously noted.13,30,31 If GTS cultures progress without interruption, these committed myeloid progenitors undergo tremendous expansion into both CD14-dependent and -independent DC pathways between days 3 and 7, as judged by the greater than 80-fold increase of DR+CD13+CD33+ DC intermediates and the distribution of CD14 and CD1a antigens during this period.4,13 Although both pathways were induced, the percentage of CD14+CD1a+ precursors did not exceed 10%, implying that the CD14-dependent DC pathway exists as a secondary DC pathway when hematopoiesis is instituted from CD34+ progenitors with GTS.10
Treatment of GTS cultures with anti-TNF on day 3 promoted approximately sevenfold increases in CD14+CD1a+mono-DC precursors by day 10, indicating the selective amplification of the CD14-dependent DC pathway. When compared with day 10 untreated GTS cultures, the anti-TNF–treated GTS cultures exhibited a superior capacity to stimulate T cells, which correlated with increases in class II MHC antigens (DR) and costimulatory molecules (CD86). Anti-TNF also decreased the percentage of CD14+cells and the fluorescence intensity of CD14, while increasing the percentage of CD1a+cells. These changes are similar to those previously reported to accompany the development of mono-derived DCs from CD14+precursors isolated from either peripheral blood or cord blood GTS cultures.2-5 10 However, because CD14+ cells were nearly absent (<0.5%) at the time we added anti-TNF (day 3, Fig1), our results indicate that the amplification of the CD14-dependent DC pathway can be achieved from an earlier, CD14−progenitor.
Although distinct nonproliferating precursors for the CD14-dependent and -independent DC pathways can be detected in GTS cultures on days 5-7, the inability to clearly identify an earlier progenitor that is common to both DC pathways has led some investigators to speculate that the CD14-independent and -dependent DC pathways stem from separate CD34+ progenitors.1 32 We cannot discount the existence of separate progenitors in this study. However, because both DC pathways arose from day 3 cell populations that lacked CD14 and CD1a, and CD13, CD33 expression was not altered by anti-TNF, the possibility that common progenitors exist must also be considered. Strategies for manipulating the selective amplification of both DC pathways from putative common DC (CDC) progenitors are currently being investigated in our laboratory.
In addition to expanding CD14+CD1a+ DC precursors, the neutralization of TNF activity on day 3 produced a rebound growth effect that was related to increases in CD115+ CD33+ cells. Although CD115 may be present on nonproliferating mono-DC and mono precursors,27-29 the persistence of proliferation in the anti-TNF GTS cultures beyond day 10 suggested the preservation of an immature CD115+ myeloid progenitor cell committed to the granulomonocytic lineages.24-26 Assessment of differentiation potential in methylcellulose and liquid cultures showed that these progenitors in fact expressed multilineage potential. When exposed to the appropriate cytokine combination, cells obtained from anti-TNF GTS cultures on day 10 exhibited the capacity to yield monos, granulocytes, and CD14-dependent DCs. Of the lineages produced, only the CD14-dependent DCs exhibited the capacity to yield potent APC function. This function was strictly dependent on the induction of mature DCs from the myeloid progenitors with either GM-CSF + TNF + SCF or GM-CSF + SCF + IL-4. Thus, anti-TNF supports the expansion of an intermediate myeloid progenitor characterized by CD115, CD33, DR positivity, and CD14-dependent DC differential potential. We refer to these progenitors as “myelodendritic,” so as to reflect their trilineage potential.
Other investigators have recently described the distribution of DC progenitors of myeloid origin in bone marrow and lymphoid tissue that share several features with the myelodendritic progenitors we describe.33 The progenitors studied by these investigators exhibited CD115 and DR antigens and did not require TNF to proliferate. When subjected to the appropriate cytokine treatment, these progenitors differentiated into monos, granulocytes, and mono-derived DCs.
The undifferentiated myelodendritic progenitors provoked by neutralization of anti-TNF activity on day 3 were remarkably long-lived and persisted for weeks, even in the absence of additional cytokine supplementation. Although anti-TNF–treated GTS cultures contained few DC precursors on day 18, as judged by the distribution of CD14 and CD1a antigens and poor MLR stimulatory potential (Figs 7 and 8), significant increases in CD115+CD33+ cells were still noted in these cultures. When removed from culture on day 18 and exposed to the appropriate cytokine treatment, these cells were still capable of differentiating into monos, granulocytes, and CD14-dependent DCs. Again, only cells treated with DC restricted cytokines on day 18 developed into mature DCs that showed MLR stimulatory activity on day 26. Even as late as day 35, anti-TNF–treated GTS cultures were still capable of yielding DC progeny.
Although the mechanisms of TNF action are clearly complex, we have developed a hypothesis to explain how TNF may be involved in regulating the CD14-dependent and -independent DC pathways. Although TNF and GM-CSF are both required for DC hematopoiesis to proceed from CD34+ progenitors, we and others have determined that TNF signaling must occur first.11,12 TNF rapidly upregulates GM-CSF receptors on the developing progenitors, which in turn optimizes responsiveness to GM-CSF.11 In light of the fact that TNF also downregulates CD115 and GM-CSF upregulates CD115,27,34 it is conceivable that the priority in TNF signaling also preferentially yields progenitor cells lacking CD115. Subsequent differentiation events would yield predominantly CD14 (mono) independent DCs, such as those developing in GTS cultures. When TNF activity is neutralized, GM-CSF signaling would dominate to yield CD115+ progenitors exhibiting the capacity to produce CD14-dependent DCs. The downregulation of CD115 would then be mediated by TNF during the process of DC maturation, as previously shown.27-29 In support of this hypothesis, we noted that GTS cultures exhibited decreased expression of CD115+ on day 7, as well as day 10, when compared with the anti-TNF–treated GTS cultures (data not shown). Our previous correlations between TNF-mediated apoptosis and downregulation of the myelogranulocytic pathway during the critical day 3-7 period may reflect yet another consequence of CD115 inhibition during the development of the CD14-independent DC pathway.13
The addition of anti-TNF to GTS cultures on days 5 and day 10 produced only a modest expansion of the CD14-dependent DC pathway, as shown by increases in proliferation and the distribution of CD14+CD1a+ DC precursors (data not shown). Therefore, TNF-sensitive progenitors for the CD14-dependent DC pathway were still present on these days, but as a minority population.
The recent realization that distinct DC subsets exist has prompted much scientific and clinical interest. It has been suggested that DC subtypes are restricted to activating either TH1 or TH2 subsets and that local and systemic DC/T-cell abnormalities associated with specific diseases such as psoriasis and atopic asthma may reflect the predominance of a particular DC type.35,36 In line with our current study, we have recently described that synovial fluid and serum from patients with rheumatoid arthritis (RA) also yield the preferential expansion of the CD14-dependent DC pathway from CD34+ progenitors.37 These effects were correlated with high levels of soluble TNF receptors, which are biologically related to anti-TNF. The function of such naturally occurring TNF antagonists in vivo would conceivably include neutralization of TNF activity that is generated during the inflammatory process in RA, including regulation of DC subtypes. Further elucidation of such regulatory mechanisms would no doubt be useful in the advancement of therapeutic TNF antagonists that are currently in development for the treatment of RA and other inflammatory diseases.
ACKNOWLEDGMENT
The authors gratefully acknowledge the cooperation of the staff in Labor and Delivery, Department of Obstetrics and Gynecology at Winthrop University Hospital, in the collection of cord blood, in particular Joanne Pastore. We also thank Dr Donald Coppock for review of the manuscript and the Department of Radiation Oncology at Winthrop-University Hospital for assistance with cell irradiation.
Address reprint requests to Frances Santiago-Schwarz, PhD, Division of Rheumatology, Allergy and Immunology, 222 Station Plaza North Suite 430, Winthrop University Hospital, Mineola, NY 11501.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.








This feature is available to Subscribers Only
Sign In or Create an Account Close Modal