To the Editor:
In a recent report, Leith et al.,1 on behalf of the Southwest Oncology Group (SWOG), reported on the biologic peculiarities of acute myeloid leukemia (AML) in the elderly. They found that age greater than 55 years is associated with an increased frequency of unfavorable cytogenetics [complex karyotypes, t(9;22), anomalies of chromosome 5/7, abnormalities at 11q], MDR1, and CD34 expression. They conclude that such a pattern may well explain the poor response to therapy of these leukemias. In addition, striking similarities with secondary leukemias have been noted by the investigators, who therefore suggest that de novo and secondary AML in the elderly may share a common biologic mechanism different from that of younger patients. We would like to contribute by presenting our experience in 344 cases of de novo AML diagnosed at our Institution between January 1987 and June 1997. Cytogenetics, expression of MDR1, and CD34 were investigated in 201 patients greater than 55 years of age (median age, 66 years; range, 56 to 81 years) and 143 less than 55 years of age (median age, 41 years; range, 18 to 55 years). CD34 and MDR1 expression were demonstrated by flow cytometry using HPCA-2, phycoerythrin (PE)-conjugated (Becton Dickinson, Mountain View, CA) and C219 (CIS Diagnostici, Vercelli, Italy) monoclonal antibodies (MoAbs), respectively. In addition, in 103 cases, MDR1 expression was also tested using the MoAb MRK16; no significant differences were observed between C219 and MRK16 in terms of the number of positive cases and the percentage of positive cells (G. Del Poeta, manuscript in preparation). Sensitive (Lovo 109) and resistant (Lovo Dx) cells from the LoVo cell line were used as controls (generous gift from G. Zupi, Istituto Regina Elena, Rome, Italy). Finally, MDR1 was assessed on gated leukemic cells. Among 225 cases classified cytogenetically, abnormalities were found in 172 (76%). Karyotypic abnormalities were grouped into favorable, intermediate, and unfavorable.2-4 Fifty-eight of 116 evaluable (58%) patients with greater than 55 years of age were classified as unfavorable and 43 (37%) as intermediate, whereas only 15 (13%) showed a favorable karyotype. Among patients with less than 55 years of age, 40 (41%) had an unfavorable karyotype, 37 (46%) intermediate, and 32 (29%) favorable. The difference was statistically significant (P = .008; Table 1). MDR1 expression was assessed in 260 cases, and 136 (52%) were found positive. No differences were observed between the two age groups either in terms of the percentage of positive cases or in terms of intensity of fluorescence as quantified by flow cytometry. In fact, 56 of 113 (49%) evaluable patients less than 55 years of age expressed the MDR1 phenotype; among those greater than 55 years of age, 80 of 147 (54%) evaluable were MDR1 positive (Table 1). The distribution of karyotypic abnormalities among the 260 patients studied for MDR1 was the same as in the overall group of patients evaluated for cytogenetics. Furthermore, CD34 expression was not correlated with age and no significant differences were identified between the two groups (Table 1). Additional parameters such as CD7 and terminaldeoxynucleotidiltransferase (TdT) were investigated to verify whether they had prognostic implications and were possibly associated with age. Whereas CD7 and TdT were significantly associated with a lower rate of complete remission (CR), no significant correlation was demonstrated with age. In fact, regardless of age, 41% (40/97) of CD7+ patients achieved a CR as compared with 56% (115/207) of CD7−(P = .026). Likewise, 33% (26/78) of TdT+patients entered CR versus 58% (126/216) of those who were TdT− (P < .001). Notably, a significant correlation was found between the expression of MDR1protein, unfavorable cytogenetics, and the presence of CD7 and TdT. In fact, MDR1 phenotype and unfavorable cytogenetics were observed in 68% (61/90) and 56% (38/68), respectively, of CD7+ cases (P < .001 and P = .003, respectively; Fig 1). Similarly, they were found in 63% (42/67) and 66% (31/47), respectively, of TdT+ cases (P = .043 and P < .001, respectively; Fig 1). Overall, 89 of 134 (66%) patients less than 55 years of age assessable for response achieved a CR, whereas only 69 of 179 (40%) elderly patients did the same (P < .001; Table1). To evaluate the simultaneous impact of different variables on duration of survival (SV) and CR, a multivariate analysis was performed using the Cox regression model. A stepwise regression model was used to assess the effect of different variables on achievement of CR. Age (P = .001) emerged as an independent factor affecting CR achievement together with TdT (P = .018), white blood cell count (P = .001), and FAB (P = .012) karyotype (P < .001) and MDR1 (P < .001). In regards to survival, the independent role of MDR1(P = .001), FAB (P = .001), and unfavorable karyotype (P = .018) was confirmed. Unfavorable karyotype and CD7 were found to adversely affect duration of CR (P = .001 and .037, respectively). In conclusion, in our analysis, age greater than 55 years was significantly correlated with unfavorable cytogenetics but not with the expression of MDR1, which seems to occur at similar frequency in elderly and young patients. However, because in the multivariate model age was significantly and independently associated with a lower rate of CR, our results confirm that it represents by itself a poor prognostic factor or, alternatively, that in some elderly additional mechanisms other than karyotypic abnormalities may be operating. In addition, we have found that, regardless of age, the expression of unfavorable cytogenetics and MDR1 protein is significantly associated with that of CD7 and TdT. Thus, we speculate that the expression of TdT and/or CD7 may represent the phenotypic counterpart of cytogenetic and chemoresistance patterns that need to be searched for to deliver therapies as tailored as possible, even including MDR modulators, apoptosis-inducers, and stem cell transplantation.
Biological Features and Clinical Outcome by Age in 344 AML Patients
| . | <55 yr . | >55 yr . | P . |
|---|---|---|---|
| Favorable cytogenetics | 32/109 (29) | 15/116 (13) | .008 |
| Intermediate cytogenetics | 37/109 (34) | 43/116 (37) | .008 |
| Unfavorable cytogenetics | 40/109 (37) | 58/116 (50) | .008 |
| MDR1 | 56/113 (50) | 80/147 (54) | NS |
| CD34 | 83/136 (61) | 112/183 (61) | NS |
| CR | 89/134 (66) | 69/179 (40) | <.001 |
| . | <55 yr . | >55 yr . | P . |
|---|---|---|---|
| Favorable cytogenetics | 32/109 (29) | 15/116 (13) | .008 |
| Intermediate cytogenetics | 37/109 (34) | 43/116 (37) | .008 |
| Unfavorable cytogenetics | 40/109 (37) | 58/116 (50) | .008 |
| MDR1 | 56/113 (50) | 80/147 (54) | NS |
| CD34 | 83/136 (61) | 112/183 (61) | NS |
| CR | 89/134 (66) | 69/179 (40) | <.001 |
Values are the number of positive cases/number of cases evaluated (percentages in parentheses).
Abbreviation: NS, not significant.
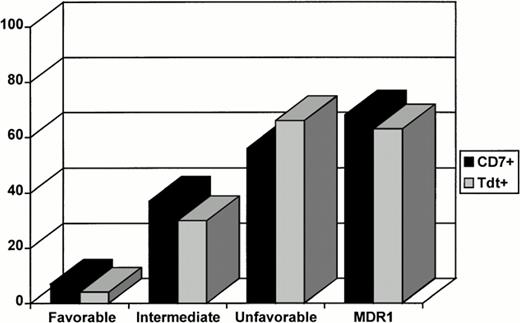
The correlation between CD7, TdT, cytogenetics, and MDR1 phenotype is shown. The expression of CD7 and/or TdT increases significantly from a near-lacking of positivity in favorable cytogenetics to almost 70% in unfavorable cytogenetics.
The correlation between CD7, TdT, cytogenetics, and MDR1 phenotype is shown. The expression of CD7 and/or TdT increases significantly from a near-lacking of positivity in favorable cytogenetics to almost 70% in unfavorable cytogenetics.
Biologic Features of Acute Myeloid Leukemia in the Elderly
We were very interested in the comments of Venditti et al on our study of MDR1 expression and cytogenetics in AML in elderly patients.1-1 From 344 cases of de novo AML diagnosed at their institution since 1987, they had the opportunity to assess MDR1 expression on 260 cases, including 103 cases in which they used two different monoclonal antibodies, and cytogenetics on 225 cases as well as CD7 and TdT expression in 304 and 294 cases, respectively. In our study on elderly patients, we found a very high frequency of expression of MDR1 (71%) by leukemic blasts using a highly specific and sensitive flow cytometric assay.1-1 1-2 In multivariate logistic regression analysis, MDR1 expression was found to be highly and independently correlated with achievement of complete remission (P = .0041) and of resistant disease (P = .0007). We thus postulated that the high frequency of MDR1 expression might be one reason for the poor response of elderly AML patients to therapy. In contrast to the high frequency of MDR1 expression found by us, Venditti et al found that only 54% of their elderly patients had MDR1+ blasts, a frequency similar to the 49% of younger patients they found to be MDR1+.
Both biologic and methodologic differences may account for the differences between the findings of our studies and those of Venditti et al. First, the patient populations studied may be different: the 211 patients we studied included some with secondary disease, whereas Venditti et al examined only de novo disease. Secondly, and surprisingly considering their patients all had de novo AML, Venditti et al's patients had a much higher frequency of unfavorable cytogenetics (58%) than that found in our study (32%). This frequency of unfavorable cytogenetics is significantly higher than what has been reported by several other groups.1-1,1-3,1-4 Thirdly, methodologic differences to detect MDR1 likely also play a critical role in the differences in frequency of MDR1 expression detected. Venditti et al used the cross-reactive antibody C219 to detect MDR1 expression. C219 is less specific for MDR1 than MRK16, because it cross-reacts with the related protein, MDR2, and various other proteins.1-5-1-7 In addition, because C219 is directed against a cytoplasmic epitope of MDR1, technical consistency may be more problematic than when an antibody directed against a surface epitope is used.1-8 Although Venditti et al state that C219 and MRK16 staining were highly correlated when compared on a subset of 103 patients, it is unclear how strong the correlation was or that MRK16 in their hands gives the same expression data as in ours.1-1,1-2,1-8A second methodologic factor may be the sensitivity of the assay; we use a duochrome reagent system to augment the often weak MDR1 expression signal in primary leukemic specimens.1-2 This step is essential to detect many MDR1+ primary AML samples; indeed, in our experience, without this reagent, many false-negative cases result. Lastly, we analyze the data using the KS statistic, rather than the percentage of positive cells, because we have found this, in our hands, to be more reliable in analyzing dimly positive cell populations. Such methodologic differences are well recognized and can lead to differences in results between laboratories.1-2,1-8They highlight the need for detailed descriptions of procedures to detect MDR1 and of using more than one assay (eg, functional studies) to better detect MDR1.1-2
Venditti et al found that MDR1 expression was similar among both younger and elderly patients with AML. Their experience is quite different from ours; in our recent studies on MDR1 expression among younger AML patients (median age, 43 years) using similar methodologies, we found that only 35% of patients were MDR1+, in contrast to the 71% found in our elderly patient group. In addition, unlike Venditti et al, we found that MDR1 expression frequency increased with patient age (P = .01).1-9
Despite these differences in results of MDR1 frequency, it is interesting that Venditti et al's studies also confirm the prognostic importance of MDR1 expression to clinical outcome in AML. Venditti et al's observations that CD7 and TdT expression appear to be phenotypic markers of AML cases with poor prognosis features such as unfavorable cytogenetics and MDR1 expression are interesting. Analysis of expression of these markers may thus be a useful initial indicator of unfavorable prognosis, pending the more lengthy cytogenetic analysis. Use of these markers to predict for MDR1 expression would be less helpful, because analysis of MDR1 expression and function can be performed quite rapidly with the rest of the immunophenotyping profile.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal