Abstract
Human neutrophils have a short half-life and are believed to die by apoptosis or programmed cell death both in vivo and in vitro. We found that caspases are activated in a time-dependent manner in neutrophils undergoing spontaneous apoptosis, concomitant with other characteristic features of apoptotic cell death such as morphologic changes, phosphatidylserine (PS) exposure, and DNA fragmentation. The treatment of neutrophils with agonistic anti-Fas monoclonal antibodies (MoAbs) significantly accelerated this process. However, in cells treated with the potent neutrophil activator phorbol 12-myristate 13-acetate (PMA), caspase activity was only evident after pharmacologic inhibition of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. Similarily, inhibition of the NADPH oxidase in constitutive and Fas/APO-1–triggered apoptosis resulted in increased rather than suppressed levels of caspase activity, suggesting that reactive oxygen species may prevent caspases from functioning optimally in these cells. Moreover, oxidants generated via the NADPH oxidase were essential for PS exposure during PMA-induced cell death, but not for neutrophils undergoing spontaneous apoptosis. We conclude that caspases are an important component of constitutive and Fas/APO-1–triggered neutrophil apoptosis. However, these redox sensitive enzymes are suppressed in activated neutrophils, and an alternate oxidant-dependent pathway is used to mediate PS exposure and neutrophil clearance under these conditions.
THE PIVOTAL ROLE of apoptosis, or programmed cell death, in the regulation of the immune system is well-established.1,2 Apoptosis, which is distinct from necrotic cell death occuring in response to various noxious stimuli, takes place in a predictable and well-choreographed sequence of morphological events, which ultimately results in the death of the cell and removal by professional phagocytes.1 Several lines of evidence indicate that the activation of aspartic acid-specific cysteine proteases, known as caspases, plays a central role in the execution of the apoptotic process.3,4 Overexpression of various caspase family members induces apoptosis in cultured mammalian cells.3 Furthermore, caspase inhibitors such as the cowpox virus protein crmA and certain peptide methyl ketones and peptide aldehydes have been shown to be potent inhibitors of apoptosis induced by a variety of different stimuli.3 In addition, recent studies have identified a number of proteins, including poly (adenosine 5′-diphosphate [ADP]-ribose) polymerase and nuclear lamin, that are specifically cleaved by caspases in cells undergoing apoptosis.3
Reactive oxygen species have been implicated as the final common mediator of apoptosis in a variety of systems,5 yet the precise role of reactive oxygen species in the modulation of caspase activity remains unresolved. The evidence for a role of oxidative stress in the induction of apoptosis comes mainly from the numerous observations that antioxidants inhibit or delay the onset of apoptotic cell death in different systems.6,7 However, this does not imply that reactive oxygen species are absolutely required as mediators of all types of apoptosis. Several groups have demonstrated that apoptosis can occur independently of ambient oxygen tension,7 and recent studies demonstrate that oxidants are also able to inhibit apoptosis under certain conditions,8-10 thereby adding to the complexity of the current model of redox regulation in apoptosis. In this study, we have focused on neutrophils, which are known to rapidly undergo apoptosis and are also prolific generators of oxidants.
Human neutrophils constitute an important line of host defense against invading microorganisms, and the production of reactive oxygen species in these cells is an essential step in the killing of ingested bacteria.11 Neutrophils are known to have the shortest life span among leukocytes and they spend only 6 to 10 hours in circulation before marginating and entering tissues where they survive for 1 to 2 days.12 They rapidly undergo spontaneous apoptosis upon in vitro culture, and the exposure of surface factors such as phosphatidylserine (PS) labels the cells for engulfment by macrophages.13 The apoptotic death of activated neutrophils is also proposed to be a critical component in the resolution of the inflammatory process.14 Activated neutrophils are known to generate extremely high amounts of reactive oxygen species, but these are normally targeted at pathogens inside intracellular phagosomes, thereby reducing damage to surrounding tissue. The major source of reactive oxygen species in neutrophils is the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, a multicomponent enzyme that catalyzes the transfer of electrons from NADPH to molecular oxygen.15 Chronic granulomatous disease (CGD) is a rare hereditary disease characterized by severe, protracted, and potentially fatal bacterial and fungal infections.16 The basic defect is an inability of phagocytic cells, including neutrophils, to produce superoxide and hydrogen peroxide, as a result of a genetic deficiency in any one of the components of the NADPH oxidase.17 A recent study suggests that neutrophil apoptosis may be severely curtailed in these individuals.18
In recent years, Fas/APO-1 and the corresponding Fas/APO-1 ligand have emerged as key regulators of the apoptotic response, particularily within the immune system.19 Fas/APO-1 is a widely expresssed type I membrane protein belonging to the tumor necrosis factor/nerve growth factor (TNF/NGF) receptor family19 and mediates apoptosis upon cross-linking by either specific monoclonal antibodies (MoAbs) or the endogenous ligand itself. Other investigators have recently shown that neutrophils are susceptible to Fas/APO-1–induced cell death,20,21 and interactions between Fas/APO-1 and its ligand have been suggested to represent a key element in controlling constitutive apoptosis in these cells.20 However, the downstream events involved in this signalling cascade have not been extensively studied in neutrophils. Furthermore, the role of reactive oxygen species in the Fas/APO-1–triggered apoptotic pathway remains controversial.22-26
We sought to determine whether caspases are involved in neutrophil apoptosis and to examine the possible role of reactive oxygen species in the regulation of caspase activity. For this purpose, we studied two different models of neutrophil cell death, the spontaneous apoptosis model of resting neutrophils, which is accelerated upon ligation of the Fas/APO-1 molecule by specific antibodies, and the activation-induced model in which treatment of the cells with phorbol 12-myristate 13-acetate (PMA) results in the rapid generation of reactive oxygen species and subsequent cell death. Our data clearly demonstrates the activation of caspases in neutrophils undergoing spontaneous and Fas/APO-1–triggered apoptosis, and we found that reactive oxygen species derived from the NADPH oxidase were not involved in this process. On the other hand, PS exposure after PMA treatment was blocked both in neutrophils treated with diphenylene iodonium (DPI) and in neutrophils from CGD patients, which lack the NADPH oxidase. PS exposure in this model was caspase-independent, and it was not until the NADPH oxidase was inhibited that caspase activity could be measured. These findings indicate that the high level of reactive oxygen species generated in activated neutrophils actually prevents caspases from functioning, and an alternate death pathway therefore appears to operate in these cells.
MATERIALS AND METHODS
Reagents.
The agonistic anti-Fas IgM MoAb (clone CH-11) and the antagonistic anti-Fas IgG1 MoAb (clone ZB4) were purchased from Medical & Biological Laboratories, Ltd (Nagoya, Japan). Ac-Asp-Glu-Val-Asp-aldehyde (DEVD-CHO) and z-Val-Ala-Asp-chloromethylketone (zVAD-cmk) were purchased from Enzyme Systems Products (Dublin, CA), and Ac-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin (DEVD-AMC) was obtained from Peptide Institute, Inc (Osaka, Japan). The Apoptest-fluorescein isothiocyanate (FITC) kit containing annexin V-FITC was from Nexins Research B.V. (Hoeven, The Netherlands). Ultrapure agarose was purchased from Life Technologies (Paisley, Scotland). Propidium iodide (PI), DPI, and PMA were from Sigma (St Louis, MO).
Isolation and culture of human neutrophils.
Peripheral neutrophils were isolated from heparinized blood from healthy adult blood donors and also from three adult CGD patients27 by a method of dextran sedimentation and density gradient centrifugation as previously described.28 Residual erythrocytes were removed by hypotonic lysis. The CGD diagnosis was established by the lack of chemiluminescence (CL) reaction and a negative nitroblue tetrazolium reduction test.27 Isolated neutrophils (1 × 106/mL) were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 2 mmol/L glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycin in 24-well flat-bottomed plates (Costar, Cambridge, MA) at 37°C in a humidified atmosphere containing 5% CO2. For PMA-treated cultures, a 1% trypsin solution was used to detach adherent cells. Preliminary experiments showed that trypsinization of neutrophils undergoing spontaneous apoptosis did not significantly affect the subsequent measurement of DEVD-AMC cleavage (data not shown).
Assessment of apoptotic morphology.
Apoptosis was assayed by estimating hematoxylin/eosin-stained cytocentrifuge preparations of neutrophils for morphologic changes characteristic of apoptosis as previously described.14 A minimum of 400 cells were scored for each sample and the percentages of apoptotic neutrophils were determined.
Agarose gel electrophoresis of fragmented DNA.
DNA fragmentation was assessed by one-stage agarose gel electrophoresis as described by Sorenson et al.29 Briefly, 2 × 106 cells were washed and resuspended in sample buffer containing RNase (10 mg/mL) for 20 minutes. Samples were then loaded onto a 1.8% agarose gel with a digestion gel (0.8% Ultrapure agarose, 2% sodium dodecyl sulfate (SDS), and 0.6 mg/mL proteinase K) at the upper end. The gel was run at 20 V overnight and electrophoresis was then continued for 3 hours at 90 V to separate the DNA fragments. The gel was subsequently stained with ethidium bromide, visualized under 305 nm ultraviolet (UV) illumination, and photographed using Polaroid 665 positive/negative film (Polaroid Corp, Cambridge, MA).
Measurement of PS exposure.
PS exposure was measured by the binding of annexin V-FITC using the protocol outlined in the Apoptest-FITC kit. Cells were also stained with PI (100 μg/mL) before analysis with a FACScan flow cytometer (Becton Dickinson, San Jose, CA) equipped with a 488 nm argon laser. Ten thousand events were collected and analyzed using the CellQuest software (Becton Dickinson). Low-fluorescence detritus was gated out before analysis. Data are presented as dot plots showing the change in mean fluorescence intensity of annexin V-FITC. For clarity, the fluorescence profiles of 25% of the analyzed events are shown.
Determination of DEVD-AMC cleavage.
The measurement of DEVD-AMC cleavage was performed in a fluorometric assay modified from Nicholson et al.30 Cell lysates and substrate were combined in a standard reaction buffer (100 mmol/L HEPES, 10% sucrose, 5 mmol/L dithiothreitol, 10−4% octylphenoxy polyethoxy ethanol [NP-40], and 0.1% 3-[(3-cholomidopropyl) dimethylammonio] propane-1-sulphonic acid [CHAPS], pH 7.25) and added to a microtiter plate. The cleavage of the fluorogenic peptide substrate DEVD-AMC (50 μmol/L) was monitored by AMC liberation in a Fluoroscan II plate reader (Labsystems, Stockholm, Sweden) using 355 nm excitation and 460 nm emission wavelengths. Fluorescence was measured every 70 seconds during a 30-minute period, and fluorescence units were converted to pmol of AMC using a standard curve generated with free AMC. Data from duplicate samples were then analyzed by linear regression.
Statistics.
Means and standard deviations (SD) were calculated from at least three independent experiments.
RESULTS
PS exposure, DNA laddering, and characteristic morphologic changes in constitutive and Fas/APO-1–triggered apoptosis.
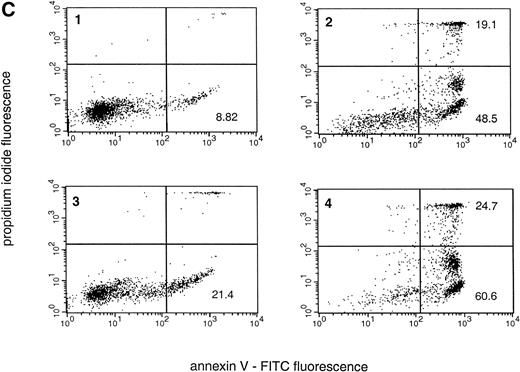
We determined apoptosis in freshly isolated neutrophils from healthy donors by morphologic evaluation. Typical apoptotic cells, displaying diminution in cell volume, increased cytoplasmic staining, and nuclear pyknosis, were readily observed in our cultures, and the appearance of such cells also correlated with a time-dependent increase in DNA fragmentation (Fig 1A and B and Table 1). One important mechanism for the recognition of apoptotic cells is the exposure of PS on the outer leaflet of the plasma membrane.31 This PS exposure was evident in neutrophils by 3 hours, and the level increased to 48.5% ± 4.3% at 24 hours, in keeping with the assessment of morphologic and biochemical end points of apoptosis that we measured (Fig 1C and Table 1). There was a concomitant increase in the apoptotic (lower right quadrant) and necrotic (upper right quadrant) populations at 24 hours, indicating that at later time points many of the apoptotic cells have proceeded into secondary necrosis (Fig 1C).
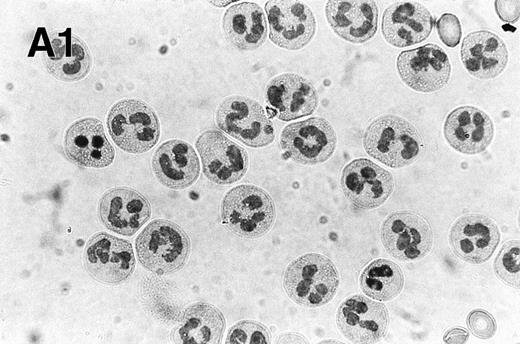
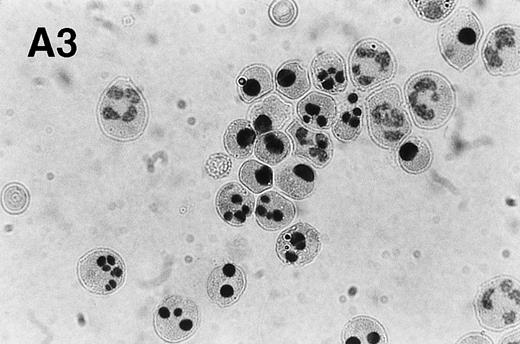
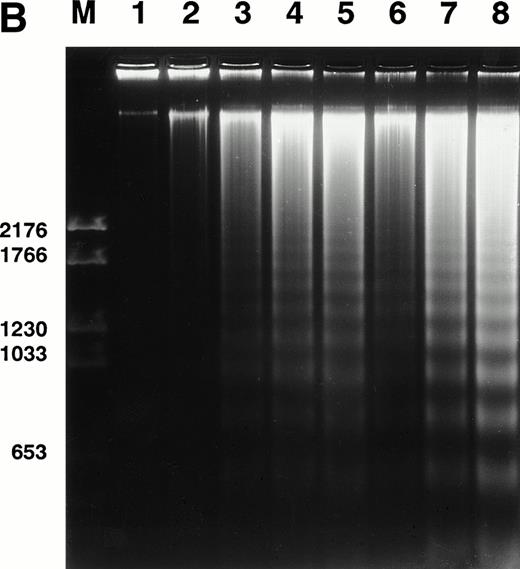
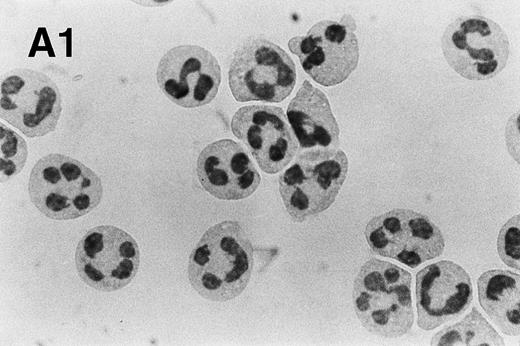
Spontaneous and Fas/APO-1–mediated apoptosis of freshly isolated human neutrophils. (A) Cytospin preparations of neutrophils were stained with hematoxylin/eosin and apoptotic cells were quantified morphologically: (1) medium alone 0 hour, (2) medium alone 6 hours, (3) anti-Fas MoAb 250 ng/mL 6 hours, (4) medium alone 24 hours. Bold arrows indicate typical cells in the intermediate stages of apoptosis. Percentages of apoptotic cells are reported in Table 1. (B) Agarose gel electrophoresis of DNA showing a time-dependent increase in the formation of oligonucleosomal DNA fragments in spontaneous (lane 1, 0 hour; lane 2, 3 hours; lane 3, 6 hours; lane 4, 12 hours; lane 5, 24 hours) and Fas/APO-1–triggered (lane 6, 3 hours; lane 7, 6 hours; lane 8, 12 hours) apoptosis. Lane M represents molecular weight marker (kB). (C) Flow cytometric analysis of annexin V binding in neutrophils undergoing spontaneous (panel 1, 6 hours; panel 2, 24 hours) and Fas/APO-1–mediated (panel 3, 6 hours; panel 4, 24 hours) apoptosis. The percentage of PS positive cells in each sample is indicated. Similar results were obtained in four separate donors (see Table 1).
Spontaneous and Fas/APO-1–mediated apoptosis of freshly isolated human neutrophils. (A) Cytospin preparations of neutrophils were stained with hematoxylin/eosin and apoptotic cells were quantified morphologically: (1) medium alone 0 hour, (2) medium alone 6 hours, (3) anti-Fas MoAb 250 ng/mL 6 hours, (4) medium alone 24 hours. Bold arrows indicate typical cells in the intermediate stages of apoptosis. Percentages of apoptotic cells are reported in Table 1. (B) Agarose gel electrophoresis of DNA showing a time-dependent increase in the formation of oligonucleosomal DNA fragments in spontaneous (lane 1, 0 hour; lane 2, 3 hours; lane 3, 6 hours; lane 4, 12 hours; lane 5, 24 hours) and Fas/APO-1–triggered (lane 6, 3 hours; lane 7, 6 hours; lane 8, 12 hours) apoptosis. Lane M represents molecular weight marker (kB). (C) Flow cytometric analysis of annexin V binding in neutrophils undergoing spontaneous (panel 1, 6 hours; panel 2, 24 hours) and Fas/APO-1–mediated (panel 3, 6 hours; panel 4, 24 hours) apoptosis. The percentage of PS positive cells in each sample is indicated. Similar results were obtained in four separate donors (see Table 1).
Percentages of Cells Displaying Morphologic Characteristics of Apoptosis and PS Exposure in Untreated and Anti-Fas MoAb-Treated Neutrophils
| Treatment . | % Apoptotic Morphology . | % Annexin V-FITC Fluorescence . |
|---|---|---|
| Medium alone | ||
| 3 h | 2.1 ± 1.1 | 5.3 ± 2.0 |
| 6 h | 8.4 ± 2.2 | 12.3 ± 4.1 |
| 24 h | 48.5 ± 4.3 | 55.0 ± 6.2 |
| Anti-Fas MoAb (250 ng/mL) | ||
| 3 h | 4.4 ± 1.8 | 6.8 ± 3.2 |
| 6 h | 17.2 ± 4.5 | 22.7 ± 4.8 |
| 24 h | 68.2 ± 8.0 | 72.9 ± 9.4 |
| Treatment . | % Apoptotic Morphology . | % Annexin V-FITC Fluorescence . |
|---|---|---|
| Medium alone | ||
| 3 h | 2.1 ± 1.1 | 5.3 ± 2.0 |
| 6 h | 8.4 ± 2.2 | 12.3 ± 4.1 |
| 24 h | 48.5 ± 4.3 | 55.0 ± 6.2 |
| Anti-Fas MoAb (250 ng/mL) | ||
| 3 h | 4.4 ± 1.8 | 6.8 ± 3.2 |
| 6 h | 17.2 ± 4.5 | 22.7 ± 4.8 |
| 24 h | 68.2 ± 8.0 | 72.9 ± 9.4 |
Freshly isolated neutrophils were evaluated for morphology and degree of PS exposure as outlined in the legend to Fig 1. Data are shown as mean ± SD (n = 4).
Neutrophils have been demonstrated to express the apoptosis-mediating surface molecule Fas/APO-1, and incubation of neutrophils with the agonistic anti-Fas MoAb significantly accelerates the apoptotic process in these cells.20 21 We treated neutrophils with 250 ng/mL of anti-Fas MoAb (clone CH-11) and observed a marked increase in apoptosis, thus corroborating previous observations (Fig 1A through C and Table 1). Neutrophils were also preincubated with the antagonistic anti-Fas MoAb ZB4 (1 μg/mL) for 1 hour and then treated with the agonistic antibody as before (Table 2). These results clearly show that the ZB4 MoAb effectively inhibits anti-Fas MoAb-induced PS exposure in cultured neutrophils, yet fails to block constitutive apoptosis.
Effect of Various Inhibitors on PS Externalization and Caspase-3–Like In Vitro Activity in Constitutive and Fas/APO-1–Triggered Apoptosis
| Treatment . | PS Exposure (% annexin V-FITC fluorescence) . | DEVD-AMC Cleavage (pmol/min/106 cells) . |
|---|---|---|
| Medium alone | 22.5 ± 5.4 | 20.2 ± 4.8 |
| + zVAD-cmk (10 μmol/L) | 5.0 ± 3.2 | 10.7 ± 2.2 |
| + DPI (10 μmol/L) | 35.2 ± 7.4 | 36.2 ± 12.5 |
| + ZB4MoAb (1 μg/mL) | 20.4 ± 4.1 | 22.5 ± 4.8 |
| Anti-Fas MoAb (250 ng/mL) | 25.4 ± 6.2 | 35.8 ± 6.1 |
| + zVAD-cmk (10 μmol/L) | 5.5 ± 2.5 | 5.1 ± 2.8 |
| + DPI (10 μmol/L) | 55.2 ± 10.5 | 51.8 ± 11.2 |
| + ZB4MoAb (1 μg/mL) | 13.6 ± 5.3 | 12.4 ± 4.9 |
| Treatment . | PS Exposure (% annexin V-FITC fluorescence) . | DEVD-AMC Cleavage (pmol/min/106 cells) . |
|---|---|---|
| Medium alone | 22.5 ± 5.4 | 20.2 ± 4.8 |
| + zVAD-cmk (10 μmol/L) | 5.0 ± 3.2 | 10.7 ± 2.2 |
| + DPI (10 μmol/L) | 35.2 ± 7.4 | 36.2 ± 12.5 |
| + ZB4MoAb (1 μg/mL) | 20.4 ± 4.1 | 22.5 ± 4.8 |
| Anti-Fas MoAb (250 ng/mL) | 25.4 ± 6.2 | 35.8 ± 6.1 |
| + zVAD-cmk (10 μmol/L) | 5.5 ± 2.5 | 5.1 ± 2.8 |
| + DPI (10 μmol/L) | 55.2 ± 10.5 | 51.8 ± 11.2 |
| + ZB4MoAb (1 μg/mL) | 13.6 ± 5.3 | 12.4 ± 4.9 |
Freshly isolated neutrophils were maintained in culture with anti-Fas MoAb (clone CH-11) or medium alone in the presence or absence of the inhibitors DPI and zVAD-cmk and harvested after 6 hours (anti-Fas–treated) and 12 hours (medium alone), respectively. The antagonistic anti-Fas MoAb ZB4 was added 1 hour before the addition of the CH-11 MoAb. PS exposure and DEVD-AMC cleavage was determined as described in Materials and Methods. Data are presented as mean ± SD (n = 3).
Caspases are activated in constitutive and Fas/APO-1–mediated apoptosis.
Having established a system of constitutive and Fas/APO-1–triggered apoptosis in neutrophils, we next sought to investigate whether the caspases are activated in these cells. Caspase activity is measured in vitro by the cleavage of the fluorogenic substrate DEVD-AMC in a continous fluorometric assay. DEVD-AMC is a specific substrate for caspase-3–like proteases and represents the common cleavage site for this class of enzymes.4 Freshly isolated neutrophils were incubated in the absence or presence of anti-Fas MoAb CH-11 (250 ng/mL) and the maximum linear rate of AMC release was measured in samples collected at various time points (Fig 2A). A clear induction of caspase activity, with peak values at 24 hours (38.8 ± 12.6; n = 3) was seen during constitutive apoptosis. Treatment of the cells with the agonistic anti-Fas MoAb markedly augmented and accelerated this process, with peak values exceeding those seen in the spontaneous model and reached at 12 hours after stimulation (54.9 ± 9.2; n = 3). DEVD-CHO (50 nmol/L), a specific inhibitor of caspase-3–like enzymes, completely blocked DEVD-AMC cleavage thereby confirming the specificity of the neutrophil lysates for DEVD-AMC (Fig 2B). Preincubation with the ZB4 MoAb (1 μg/mL) effectively blocked the increase in DEVD-AMC cleavage mediated by the Fas/APO-1–specific antibody (Table 2). On the other hand, DEVD-AMC cleavage in the constitutive model was unaffected by the addition of the antagonistic anti-Fas antibody. These findings are therefore indicative of the occurrence of both Fas/APO-1–dependent and -independent mechanisms of caspase activation in neutrophil apoptosis. Furthermore, Fas/APO-1–induced PS exposure in freshly isolated neutrophils was effectively inhibited by the general caspase inhibitor zVAD-cmk (Table 2). Similar results were obtained in the constitutive apoptosis model, thereby demonstrating that PS exposure is caspase-dependent in these two models of neutrophil apoptosis (Table2). As expected, the caspase inhibitor also blocked DEVD-AMC cleavage in cells undergoing spontaneous and Fas/APO-1–triggered apoptosis, although less efficiently at 12 hours (Table 2).
Caspase-3–like activity in neutrophils undergoing spontaneous and Fas/APO-1–mediated apoptosis, as measured by cleavage of the specific fluorogenic substrate DEVD-AMC (50 μmol/L). (A) Time course of caspase-3–like activation in spontaneous (⧫) and anti-Fas MoAb-triggered (250 ng/mL; ◊) apoptosis of neutrophils. The cells were lysed at various times (0 to 72 hours) after initiation of in vitro culture and the release of AMC was monitored. The maximum rate of AMC release (pmol/min) was estimated by linear regression (r2 > 0.99). Data shown are the mean and range of duplicate determinations from a representative experiment. (B) The competitive inhibitor DEVD-CHO (50 nmol/L) was added to cell lysates in vitro and shown to block DEVD-AMC cleavage in cells undergoing spontaneous apoptosis (control, ○; plus inhibitor, •). Cells (1 × 106) were incubated for 15 hours before the fluorometric assay. Each data point represents the average value of determinations performed in triplicate samples.
Caspase-3–like activity in neutrophils undergoing spontaneous and Fas/APO-1–mediated apoptosis, as measured by cleavage of the specific fluorogenic substrate DEVD-AMC (50 μmol/L). (A) Time course of caspase-3–like activation in spontaneous (⧫) and anti-Fas MoAb-triggered (250 ng/mL; ◊) apoptosis of neutrophils. The cells were lysed at various times (0 to 72 hours) after initiation of in vitro culture and the release of AMC was monitored. The maximum rate of AMC release (pmol/min) was estimated by linear regression (r2 > 0.99). Data shown are the mean and range of duplicate determinations from a representative experiment. (B) The competitive inhibitor DEVD-CHO (50 nmol/L) was added to cell lysates in vitro and shown to block DEVD-AMC cleavage in cells undergoing spontaneous apoptosis (control, ○; plus inhibitor, •). Cells (1 × 106) were incubated for 15 hours before the fluorometric assay. Each data point represents the average value of determinations performed in triplicate samples.
Role of the NADPH oxidase in spontaneous and Fas/APO-1–mediated apoptosis.
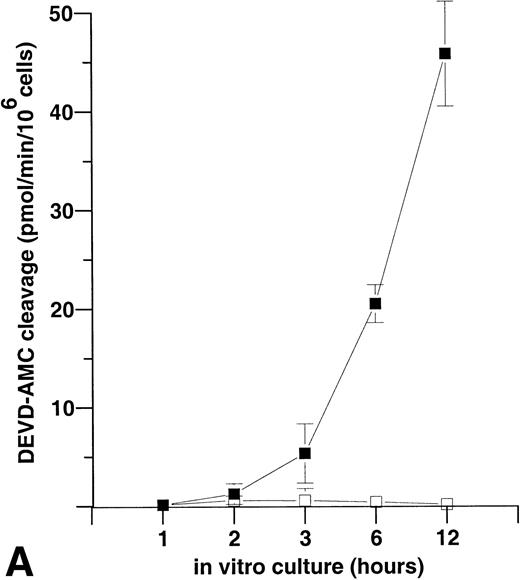
To examine whether reactive oxygen species are involved in caspase activation in neutrophils, the flavoprotein inhibitor, DPI, an inhibitor of the NADPH oxidase,32 was used. We found that treatment of freshly isolated neutrophils with 10 μmol/L DPI resulted in an increase in caspase activity, as determined by DEVD-AMC cleavage, both in the constitutive and Fas/APO-1–triggered models (Fig 3). Peak values for spontaneous DEVD-AMC cleavage were 36.1 ± 10.5 (without DPI) and 59.5 ± 8.3 (with DPI), and peak values for Fas/APO-1–triggered DEVD-AMC cleavage were 50.9 ± 7.6 (without DPI) and 66.2 ± 8.7 (with DPI) (mean ± SD; n = 3). In addition, DPI treatment enhanced PS exposure in these two model systems, consistent with the view that this event is caspase-mediated (Table 2). These results suggest that NADPH oxidase-derived reactive oxygen species are not involved in activating the caspases, and in contrast are partially suppressing caspase activity in neutrophils triggered to undergo apoptosis.
Treatment of neutrophils with the NADPH oxidase inhibitor DPI leads to an increase in caspase-3–like activity. Neutrophils undergoing spontaneous or Fas/APO-1–triggered apoptosis (▵ and □, respectively) were cultured in the absence or presence of DPI (10 μmol/L; ▴ and ▪) for various times (0 to 72 hours). Anti-Fas MoAb was used at 250 ng/mL. Determination of AMC release was performed in a fluorometric assay. The maximum rate of AMC release (pmol/min) was estimated by linear regression (r2 > 0.99). The mean and range of duplicate determinations from a representative experiment are shown. Solvent alone (ie, dimethyl sulfoxide [DMSO]) did not affect DEVD-AMC cleavage (data not shown).
Treatment of neutrophils with the NADPH oxidase inhibitor DPI leads to an increase in caspase-3–like activity. Neutrophils undergoing spontaneous or Fas/APO-1–triggered apoptosis (▵ and □, respectively) were cultured in the absence or presence of DPI (10 μmol/L; ▴ and ▪) for various times (0 to 72 hours). Anti-Fas MoAb was used at 250 ng/mL. Determination of AMC release was performed in a fluorometric assay. The maximum rate of AMC release (pmol/min) was estimated by linear regression (r2 > 0.99). The mean and range of duplicate determinations from a representative experiment are shown. Solvent alone (ie, dimethyl sulfoxide [DMSO]) did not affect DEVD-AMC cleavage (data not shown).
Reactive oxygen species promote PS exposure, but suppress caspase activity, in activated neutrophils.
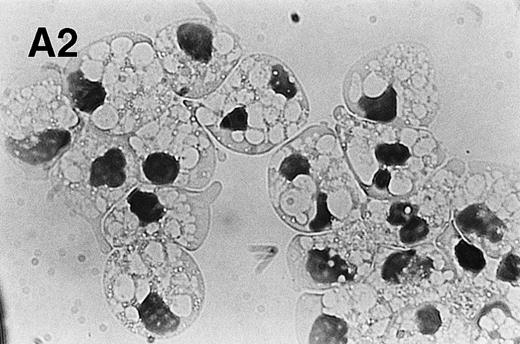
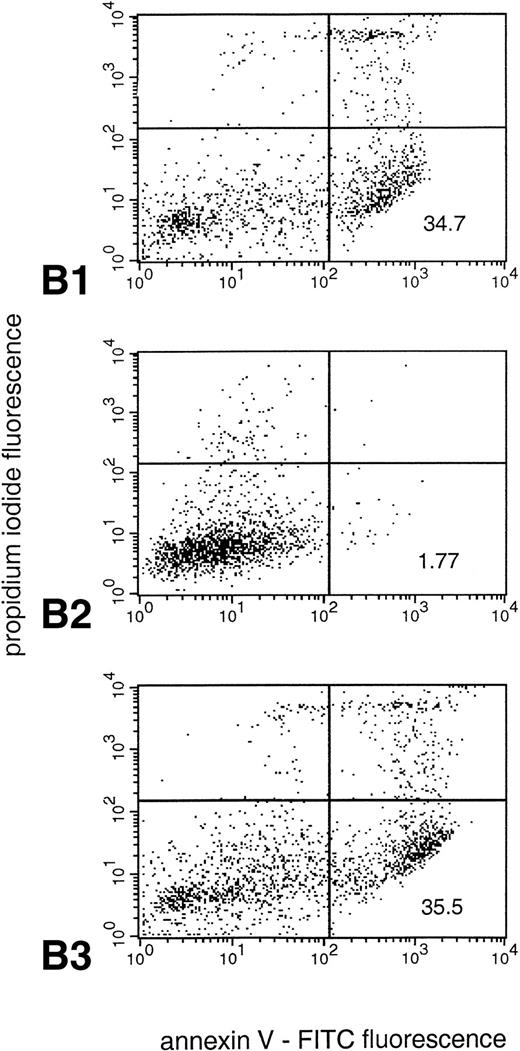
The protein kinase C activator, PMA, activates the NADPH oxidase in neutrophils, leading to a burst of reactive oxygen species similar to what is seen when neutrophils ingest invading pathogens.33,34 We found that PMA (200 nmol/L) rapidly induced morphologic changes, which were distinct from those observed in constitutive and Fas/APO-1–triggered apoptosis, with an increase in cell size and the appearance of numerous vacuoles throughout the cytoplasm (Fig 4A). Moreover, in agreement with a previous report by Takei et al,35 we were unable to detect any DNA fragmentation in PMA-treated cells (data not shown). However, PS exposure was evident at 3 hours and was markedly accelerated when compared with untreated neutrophils (untreated cells: 6.4 ± 3.1; PMA-treated cells: 38.5 ± 6.4; mean ± SD; n = 3; Fig 4B), demonstrating that changes similar to those observed in apoptotic cells are taking place.
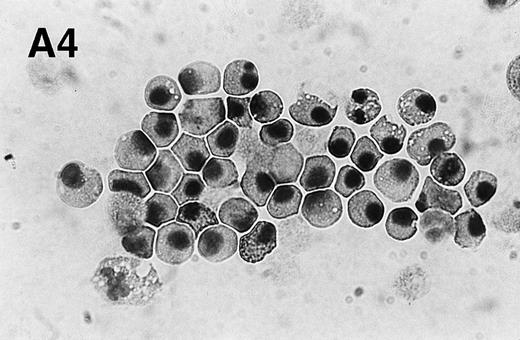
PMA treatment of neutrophils results in rapid morphologic changes accompanied by externalization of PS. (A) Cytospin preparation of neutrophils cultured in medium alone (1) or treated for 3 hours with 200 nmol/L PMA (2). Note the presence of numerous vacuoles in the cytoplasm and the coalescence of the nuclear lobes after PMA treatment. Original magnification × 100. (B) PS exposure in unstimulated (panel 1) and PMA-treated (panel 2, 200 nmol/L) neutrophils after 3 hours of culture. PS exposure was determined by flow cytometric analysis of annexin V binding as described in Materials and Methods. The percentage PS exposure is indicated. PMA treatment also caused a marked increase in forward scatter of these cells, indicative of an increase in cell size (data not shown). Similar results were obtained with three independent donors.
PMA treatment of neutrophils results in rapid morphologic changes accompanied by externalization of PS. (A) Cytospin preparation of neutrophils cultured in medium alone (1) or treated for 3 hours with 200 nmol/L PMA (2). Note the presence of numerous vacuoles in the cytoplasm and the coalescence of the nuclear lobes after PMA treatment. Original magnification × 100. (B) PS exposure in unstimulated (panel 1) and PMA-treated (panel 2, 200 nmol/L) neutrophils after 3 hours of culture. PS exposure was determined by flow cytometric analysis of annexin V binding as described in Materials and Methods. The percentage PS exposure is indicated. PMA treatment also caused a marked increase in forward scatter of these cells, indicative of an increase in cell size (data not shown). Similar results were obtained with three independent donors.
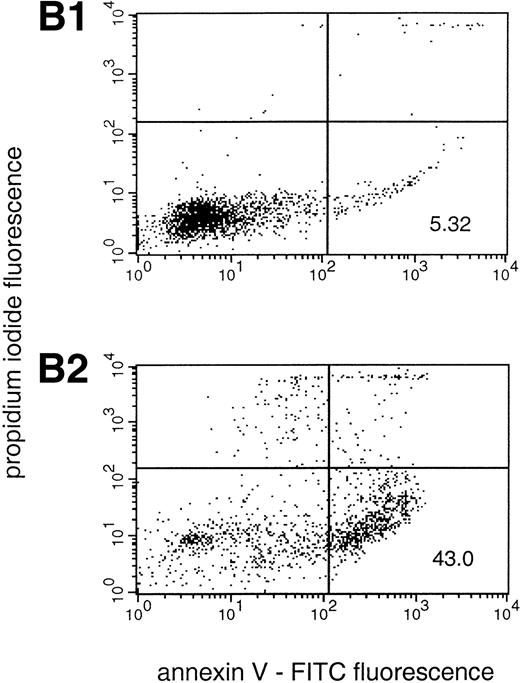
PMA-treated neutrophils were also assessed for caspase activity in vitro and were found to yield no detectable DEVD-AMC cleavage activity at any time point tested. However, when cells were pretreated with DPI (10 μmol/L), there was a time-dependent induction of caspase activity, as evidenced in the in vitro enzyme assay (Fig 5A). Interestingly, we found that DPI (10 μmol/L), but not zVAD-cmk (10 μmol/L) was capable of completely diminishing PS exposure in these cells (PMA alone: 38.5 ± 6.4; PMA plus DPI: 2.4 ± .1.1; PMA plus zVAD-cmk: 39.8 ± .4.7; mean ± SD; n = 3; Fig 5B), suggesting that caspase activity is not required for PS exposure under these conditions. Rather, the PMA-induced increase in the level of reactive oxygen species appears to be critical for externalization of PS. Taken together, these results suggest that reactive oxygen species are simultaneously inhibiting caspase activity and mediating PS exposure in PMA-activated neutrophils.
NADPH oxidase-derived oxygen metabolites inhibit caspase-3–like activity and promote PS exposure in PMA-activated neutrophils. (A) PMA-stimulated neutrophils were cultured for the indicated times in the presence (▪) or absence (□) of the NADPH oxidase inhibitor DPI (10 μmol/L), and the rate of AMC liberation was determined in a fluorometric assay. Treatment of the cells with DPI did not affect the morphologic changes induced by PMA (data not shown). Solvent alone (ie, DMSO) had no discernible effect on either morphology or DEVD-AMC cleavage (data not shown). Data are presented as the mean and SD of three separate experiments performed in duplicate. (B) PS exposure was determined in neutrophils by flow cytometry after in vitro culture for 3 hours. Panel 1 shows cells treated with PMA alone (200 nmol/L). As seen in panel 2, addition of the NADPH oxidase inhibitor DPI (10 μmol/L) competely blocks PS exposure, whereas the general caspase inhibitor zVAD-cmk (10 μmol/L) has no effect in this system (panel 3). Reproducible results were obtained in three separate donors.
NADPH oxidase-derived oxygen metabolites inhibit caspase-3–like activity and promote PS exposure in PMA-activated neutrophils. (A) PMA-stimulated neutrophils were cultured for the indicated times in the presence (▪) or absence (□) of the NADPH oxidase inhibitor DPI (10 μmol/L), and the rate of AMC liberation was determined in a fluorometric assay. Treatment of the cells with DPI did not affect the morphologic changes induced by PMA (data not shown). Solvent alone (ie, DMSO) had no discernible effect on either morphology or DEVD-AMC cleavage (data not shown). Data are presented as the mean and SD of three separate experiments performed in duplicate. (B) PS exposure was determined in neutrophils by flow cytometry after in vitro culture for 3 hours. Panel 1 shows cells treated with PMA alone (200 nmol/L). As seen in panel 2, addition of the NADPH oxidase inhibitor DPI (10 μmol/L) competely blocks PS exposure, whereas the general caspase inhibitor zVAD-cmk (10 μmol/L) has no effect in this system (panel 3). Reproducible results were obtained in three separate donors.
Absence of PS exposure in PMA-treated neutrophils from CGD patients.
To confirm the results obtained with DPI treatment of normal neutrophils, we isolated cells from three adult CGD patients, whose neutrophils do not generate any superoxide.27 These patients suffered from the autosomal recessive form of the disease with genetic defects in the cytosolic components of the NADPH oxidase, ie, p67phox (phox, phagocyte oxidase) and p47phox, respectively. Upon isolation, cells were treated with PMA (200 nmol/L) and evaluated morphologically and the levels of PS exposure and DEVD-AMC cleavage were determined. PMA treatment was found to induce similar morphological changes as those seen in neutrophils from healthy individuals, ie, an increase in cell size and the formation of intracellular vacuoles (data not shown). PS exposure, however, was completely absent at 3 hours (Table 3). Moreover, caspase-3–like activity was evident in PMA-treated CGD neutrophils at later time points (Table 3). The PS exposure evidenced in PMA-treated cells at later time points most likely results from the delayed caspase activation. These data therefore corroborate our findings obtained with DPI treatment of PMA-activated neutrophils from healthy individuals.
Caspase-3–Like Activity and PS Exposure in CGD Neutrophils
| Treatment . | DEVD-AMC Cleavage (pmol/min/106 cells) . | % Annexin V-FITC Fluorescence . | ||||
|---|---|---|---|---|---|---|
| 3 h . | 6 h . | 24 h . | 3 h . | 6 h . | 24 h . | |
| Medium alone | 1.7 ± 0.2 | 3.3 ± 0.5 | 27.8 ± 2.8 | 1.2 ± 0.4 | 2.5 ± 0.9 | 7.4 ± 2.8 |
| Anti-Fas MoAb (250 ng/mL) | 16.6 ± 4.7 | 36.0 ± 4.5 | 37.5 ± 3.9 | ND | 21.5 ± 2.7 | 65.9 ± 4.0 |
| PMA (200 nmol/L) | 0.9 ± 0.4 | 12.2 ± 9.4 | ND | 0.6 ± 0.2 | 9.6 ± 5.6 | ND |
| Treatment . | DEVD-AMC Cleavage (pmol/min/106 cells) . | % Annexin V-FITC Fluorescence . | ||||
|---|---|---|---|---|---|---|
| 3 h . | 6 h . | 24 h . | 3 h . | 6 h . | 24 h . | |
| Medium alone | 1.7 ± 0.2 | 3.3 ± 0.5 | 27.8 ± 2.8 | 1.2 ± 0.4 | 2.5 ± 0.9 | 7.4 ± 2.8 |
| Anti-Fas MoAb (250 ng/mL) | 16.6 ± 4.7 | 36.0 ± 4.5 | 37.5 ± 3.9 | ND | 21.5 ± 2.7 | 65.9 ± 4.0 |
| PMA (200 nmol/L) | 0.9 ± 0.4 | 12.2 ± 9.4 | ND | 0.6 ± 0.2 | 9.6 ± 5.6 | ND |
Freshly isolated neutrophils from a p67phox-defective and two p47phox-defective CGD patients were maintained in culture with anti-Fas MoAb (clone CH-11), PMA, or in medium alone for the times indicated. Measurement of DEVD-AMC cleavage was performed in an in vitro fluorometric assay (50 μmol/L DEVD-AMC). PS exposure was determined by flow cytometry as described in Materials and Methods. Data are presented as mean ± SD of determinations performed in duplicate in three patients.
Abbreviation: ND, not determined.
Spontaneous apoptosis of neutrophils from CGD patients has previously been reported to be impaired, and these cells were purportedly resistant to anti-Fas–mediated apoptosis.18 Contrary to these findings, we detected PS exposure and morphological changes indicative of apoptosis in CGD neutrophils undergoing spontaneous as well as Fas/APO-1–triggered apoptosis (Table 3 and data not shown), albeit with slower kinetics when compared with healthy individuals. In addition, caspase-3–like activity was detected under both constitutive and Fas/APO-1–triggered conditions (Table 3), indicating that resting neutrophils from CGD patients are not resistant to undergoing apoptosis.
DISCUSSION
Caspases have been ascribed an essential role in the execution of the apoptotic process. We were interested in evaluating the role of reactive oxygen species in the regulation of these cysteine proteases and chose to investigate human neutrophils, as these cells readily undergo spontaneous apoptosis and also have the capacity to generate a powerful oxidative burst upon activation. In this study, we show caspase activation in neutrophils undergoing constitutive and Fas/APO-1–mediated apoptosis, but no caspase activation in neutrophils stimulated with PMA. Pretreatment with the NADPH oxidase inhibitor, DPI, allowed the caspases to function in PMA-stimulated neutrophils, indicating that caspase activity is suppressed by NADPH oxidase-derived reactive oxygen species. These findings are therefore suggestive of a specialized caspase-independent pathway of cell death in activated neutrophils.
It is becoming increasingly apparent that caspases are redox-sensitive enzymes. They possess an active site cysteine, which is predicted to be susceptible to oxidation, and thiol reductants such as dithiothreitol are routinely included in experimental assay buffers. Previous work from our laboratory has demonstrated that dithiocarbamate disulfides are potent inhibitors of caspase-1 and caspase-3, the inhibitory effect being accomplished most likely through thiol-disulfide exchange.36 Similarily, Takahashi et al37recently reported the inhibition of caspases by phenylarsine oxide, a compound that is known to covalently modify vicinal thiol groups. We have also documented the ability of hydrogen peroxide to inhibit caspase activity in Jurkat T cells,8 and several investigators have recently shown the inhibitory effect of nitric oxide on caspase function.9 10 Our current results provide the first evidence that endogenous oxidant production may prevent the activation of caspases within the same cell.
While caspases may function optimally in a reducing environment, this does not preclude a role for low level oxidative stress in the initiation of an apoptotic response. Kasahara et al18showed an impairment in spontaneous and Fas/APO-1–mediated apoptosis in CGD neutrophils and concluded that oxidants have a critical role in the induction process. We could detect PS exposure and caspase-3–like activity in the CGD cells we tested, although the response was slower when compared with normal individuals; furthermore, there was a moderate increase rather than inhibition of caspase-3–like activity and PS exposure in normal cells in the presence of DPI. Therefore, we conclude that NADPH oxidase-derived reactive oxygen species do not play a significant role as mediators of spontaneous neutrophil apoptosis. Narayanan et al38 measured an increase in intracellular oxidants in unstimulated neutrophils and showed that while DPI had no effect, cyanide lowered oxidant production, implicating mitochondrial-derived reactive oxygen species in apoptosis. However, the dichlorofluorescein assay used for measuring intracellular hydrogen peroxide requires peroxidase activity for substrate oxidation, and cyanide is likely to act by inhibiting the peroxidase rather than the source of hydrogen peroxide. Thiol antioxidants39 and a hypoxic environment40 were reported to inhibit Fas/APO-1–triggered and spontaneous neutrophil apoptosis, respectively, again suggesting that reactive oxygen species play an important role in the process. However, hypoxia was proposed to act by altering the expression of various cellular antioxidants,40rather than lowering the production of oxygen metabolites per se. Thiol antioxidants have been shown to inhibit apoptosis in T lymphocytes,41 but rather than directly scavenging reactive oxygen species, they could act by altering the redox status of thiol enzymes, such as the caspases, in the pathway leading to apoptosis.
While evidence for reactive oxygen species involvement in spontaneous neutrophil apoptosis remains inconclusive, it seems clear that cell death after PMA treatment is dependent on oxidant production. PMA failed to trigger PS exposure in neutrophils obtained from the CGD patients, and PMA-induced PS exposure was completely prevented by DPI treatment of normal cells, thus demonstrating that the burst of oxygen metabolites after PMA treatment is coupled to the externalization of PS. Coxon et al42 have recently reported that phagocytosis of opsonized particles, mediated by the β2 integrin CD11b/CD18, causes activation-induced apoptosis in neutrophils, which is dependent on NADPH oxidase-derived reactive oxygen species. Similarily, neutrophils have been reported to undergo apoptosis after ingestion ofEscherichia coli.43 While inhibition of these events by reduced glutathione and N-acetylcysteine supports the involvement of reactive oxygen species,43 the nonspecific thiol effects described above mean that more conclusive studies with DPI or CGD neutrophils are required. Interestingly, neutrophils from the CGD patients in the present study were susceptible to spontaneous and Fas/APO-1–triggered apoptosis in vitro. Therefore, it appears reasonable to speculate that while neutrophils from these patients are not inherently resistant to the induction of apoptosis, their failure to externalize PS in response to activation may result in the inefficient clearance by macrophages from the site of inflammation, thus accounting for the formation of characteristic granulomatous lesions and subsequent tissue destruction. Further work is needed to evaluate the contribution of these events to the emergence of clinical symptoms in CGD patients.
Takei et al35 recently reported that PMA induces rapid killing of neutrophils with features distinct from typical apoptosis or necrosis. Because extensive intracellular vacuolation made it difficult to observe nuclear changes, our best indicator of PMA-induced cell death was the rapid externalization of PS. Although difficult to classify as conventional apoptosis solely on the basis of PS exposure, the expression of surface markers is critical for neutrophil clearance by macrophages,13 making this event an essential physiologic process. Cell death induced by PMA may therefore be considered a type of activation-induced death, akin to apoptosis, albeit occuring more rapidly. We have shown that the flavoprotein inhibitor, DPI, efficiently blocks PS exposure in PMA-treated neutrophils. On the other hand, PS exposure readily occurs in CGD neutrophils undergoing spontaneous and Fas/APO-1–mediated apoptosis, suggesting that different mechanisms for PS exposure operate in resting and activated neutrophils. Caspase activation has previously been shown to be necessary for PS exposure in certain cell types44 and Brown et al45 reported that PS exposure in spontaneous neutrophil apoptosis is blocked by zVAD-fmk. However, caspase-independent PS exposure in apoptotic cell death is not unprecendented. Work from our laboratory has shown that PS exposure in TNF-treated monocytic U937 cells is blocked by calpain inhibitors, but not by the caspase inhibitor zVAD-cmk.46 Fabisiak et al47 recently reported on the selective oxidation and externalization of PS in paraquat-induced apoptosis in the murine myeloid cell line 32D, thereby supporting the view that oxidative stress may directly initiate PS exposure. The mechanisms involved warrant further investigation.
Recent reports suggest that constitutive apoptosis of neutrophils is, at least in part, due to an autocrine or paracrine Fas/APO-1–mediated mechanism.20 This conclusion was based on the fact that neutrophils appeared to express low levels of the Fas/APO-1 ligand on their surface and that the antagonistic anti-Fas MoAb, ZB4, partially inhibited the decrease in cell viability of cells undergoing spontaneous apoptosis. However, we could not detect any effect of the ZB4 MoAb on caspase activity or PS exposure in constitutive neutrophil apoptosis. On the other hand, apoptosis triggered by the agonistic anti-Fas MoAb was efficiently blocked in our system. In a previous study, we defined the epitope targeted by the ZB4 and CH-11 antibodies,48 and by molecular modeling, we could show that this epitope most likely differed from the receptor-ligand interface.49 These findings would tend to suggest that the antagonistic MoAb may be less efficient in blocking the endogenous ligand. However, steric interference of ligand binding may conceivably occur despite the targeting of an epitope distinct from the receptor-ligand interface. Indeed, the ZB4 MoAb was recently shown to inhibit apoptosis induced by ligation of the T-cell receptor with anti-CD3, a treatment known to initiate death by upregulating Fas/APO-1 ligand expression.50 Therefore, our current data favors the view that constitutive neutrophil apoptosis is likely to proceed by Fas/APO-1–independent mechanisms.
In summary, our findings suggest that neutrophils possess two different modes of cell death. The constitutive mode of cell death, which is likely to be involved in the turnover of senescent neutrophils in vivo, appears to use a caspase-dependent pathway characteristic of apoptotic cell death in many other systems. While the majority of neutrophils die in this way, a small number are called on to destroy invading pathogens. It is critical that these cells, loaded with their potentially deleterious contents, are safely cleared from an inflammatory site with minimal damage to surrounding tissues. The present data indicates that the powerful burst of reactive oxygen species generated to kill the infectious agent will prevent the redox sensitive caspases from functioning, thereby necessitating a novel oxidant-dependent pathway of neutrophil death. Further work is needed to elucidate the mechanisms of this pathway and to determine if abnormalities in its functioning are involved in the pathology of various inflammatory conditions.
ACKNOWLEDGMENT
We would like to thank Afshin Samali for constructive advice during the course of this study and Boris Zhivotovsky, David Burgess, Isabella Pörn-Ares, and Dean Jones for valuable discussions. We are also grateful for the cooperation of the CGD patients (LNG, AA, and MA) in the study.
Supported by the Swedish Medical Research Council, the Children’s Cancer Foundation of Sweden and the Institute of Environmental Medicine at Karolinska Institutet. M.H. was supported by the New Zealand Foundation for Research, Science and Technology.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Mark B. Hampton, PhD, Pathology Research, Massachusetts General Hospital, 149 13th St, Charlestown, MA 02129.




![Fig. 3. Treatment of neutrophils with the NADPH oxidase inhibitor DPI leads to an increase in caspase-3–like activity. Neutrophils undergoing spontaneous or Fas/APO-1–triggered apoptosis (▵ and □, respectively) were cultured in the absence or presence of DPI (10 μmol/L; ▴ and ▪) for various times (0 to 72 hours). Anti-Fas MoAb was used at 250 ng/mL. Determination of AMC release was performed in a fluorometric assay. The maximum rate of AMC release (pmol/min) was estimated by linear regression (r2 > 0.99). The mean and range of duplicate determinations from a representative experiment are shown. Solvent alone (ie, dimethyl sulfoxide [DMSO]) did not affect DEVD-AMC cleavage (data not shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/12/10.1182_blood.v92.12.4808/5/m_blod42401003x.jpeg?Expires=1767727086&Signature=cyMAMDp-NSBvuP5iya5EgDccZsFV6UWSXNYWPCKKPImfrnoVoTI9VkrirAFcEJfWzGjZUG2pZWIJ4jOcbiPmntzfwFSDFPlPcTJ5ozl1mK2YhcHs3fS1SK7V3el4urbZRT~xEas8OdMrnP7gtrmnIksU5MDxRCeWQRqd7iVz95b2f3RyHsk-km57VWnfbwqlnGVPRaTqHjJ4LSIAi0p9bBpU4tFT-yJUVAPubxtUVeb2r6siGOsNqS~yNjp5dqe4XfZ6BPk~fsx1ZBfTTZFlhfkgtwLKsMV04EDbQV6SNv7Me2n98FCguvK8k10cmsw8RCsHK~EEfObyD~2AS2Ebwg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal