Abstract
We have previously shown that murine ELM erythroleukemia cells can only be grown in vitro in the presence of a stromal feeder layer, or alternatively stem cell factor (SCF), without which they differentiate. When grown in the presence of SCF, ELM cells can still differentiate in response to erythropoietin (Epo), but growth on stroma prevents this. We previously isolated a stroma-independent ELM variant, ELM-I-1, that is also defective in Epo-induced differentiation. We show here that this variant has an activating mutation in the Kit receptor, converting aspartic acid 814 to histidine. Expression of the mutant receptor in stroma-dependent ELM-D cells causes growth factor-independent proliferation and also gives the cells a selective advantage, in terms of proliferation rate and clonegenicity, compared with ELM-D cells grown in optimal amounts of SCF. Expression of the mutant receptor in ELM-D cells also prevents spontaneous differentiation, but not differentiation induced by Epo. Analysis of mitogenic signaling pathways in these cells shows that the mutant receptor induces constitutive activation of p42/p44 mitogen-activated protein kinases. It also selectively inhibits the expression of p66Shc but not the p46/p52 Shc isoforms (as did treatment of ELM cells with SCF), which is of interest, because p66Shc is known to play an inhibitory role in growth factor signaling.
OVERCOMING THE GROWTH factor dependence of cellular proliferation is an important step in the development of many cancers, and many oncogenes encode proteins that cause the abnormal activation of growth factor stimuli. Many growth factors act through cell surface receptors of the receptor tyrosine kinase (RTK) family that induce intrinsic tyrosine kinase activity and downstream intracellular signaling pathways upon binding of ligand.1Abnormal activation of RTK family members may be caused by mutations and is causally implicated in the development of human malignancies.2-6
The Kit receptor tyrosine kinase is the cellular receptor for stem cell factor (SCF) and a member of the type III RTK subfamily.7The study of Kit and SCF has been aided by the natural occurrence in mice of mutations in their corresponding genetic loci, W(dominant white spotting) and Sl (Steel), respectively.7 Loss of function of Kit or SCF leads to lack of skin and hair pigmentation, lack of intestinal pacemaker activity, sterility, and anemia. These effects correlate with the normal expression of Kit in melanocytes,8,9 the interstitial cells of Cajal in the gut,10 germ cells,8,11 and many hematopoietic cell lineages, including stem cells and erythroid progenitors,12 and demonstrate a requirement for Kit activity in the development of these cell lineages.
The potency of the growth signal induced in many cell types by the Kit/SCF interaction is confirmed by the frequent deregulation of this signal in cancer. The HZ4 feline sarcoma virus encodes the transforming oncogene, v-kit, a mutated and truncated viral homologue of c-kit.13 Also, activation of Kit through autocrine expression of SCF has been described in small cell lung cancers,14 colorectal carcinomas,15 breast carcinomas,16 gynecological tumors,17 and neuroblastomas.18 Finally, receptor mutations resulting in constitutive activation of Kit have been identified in mast cell leukemic cell lines and samples derived from patients with mastocytosis.19-23
Leukemia progression is generally associated with changes in growth factor requirements or responsiveness, differentiation arrest, and loss of stroma cell dependence. We have used the murine ELM erythroleukemia model to investigate the mechanisms of leukemia progression, because it is unusual in having a less abnormal phenotype than most other murine erythroleukemias, in that it has not progressed to stroma independent growth and retains the capacity to undergo erythroid differentiation in response to erythropoietin (Epo).24-26 We previously isolated variants that were able to grow without stroma. One of these variants, ELM-I-1, was not only growth factor independent, but had also lost the capacity to differentiate in response to Epo. We show here that ELM-I-1 posesses an activating mutation in c-kit and analyze how the mutant Kit receptor may have contributed to the phenotype of the ELM leukemic cells by testing its effects on the growth factor requirements, differentiation, and cellular signaling of erythroleukemic cells.
MATERIALS AND METHODS
Cells and tissue culture.
Unless otherwise specified, all reagents for tissue culture were supplied by GIBCO-BRL (Paisley, UK). Murine ELM leukemic cells and MS5 stromal cells were maintained in minimum essential medium α supplemented with 10% horse serum (Sigma, Poole, UK). Serum deprivation was conducted for 18 hours in 0.5% horse serum without added growth factors. Q2BN quail fibroblasts27were maintained in Dulbecco’s modified Eagle medium supplemented with 8% fetal bovine serum (TCS Biologicals, Buckingham, UK) and 2% chicken serum. Recombinant murine SCF (rmSCF) and recombinant human Epo (rhEpo) were purchased from R&D Systems (Abingdon, UK) and were routinely used at 10 ng/mL and 5 U/mL, respectively.
The growth factor-dependent ELM cells used were ELM-D6 cells,24,26,28 routinely passaged in coculture with the bone marrow-derived stromal cell line MS5.29,30 Leukemic cells were separated from stroma for experiments by vigorous agitation and three rounds of purification by settling and gentle agitation.28 Where specified, ELM-D6 subclone 20.5D28 was used and grown in 10 ng/mL rmSCF. The precise derivation of the growth factor-independent ELM-I cell lines ELM-I-1, I-2, and I-5 is described in Nibbs et al.26
Proliferation and colony assays.
Proliferation assays were performed over 7 days in 96-well microtiter plates, inoculating 200 cells into 100 μL medium with 0, 1, or 10 ng/mL SCF. Cell metabolic activity was assayed using the Promega MTT kit (Southampton, UK). An initial control plate (5,000 cells/well, 10 ng/mL SCF) was assayed after 4 hours to verify equal starting populations. Colony assays were performed using MethoCult M3230 methylcellulose colony medium (StemCell Technologies, Vancouver, British Columbia, Canada) supplemented with no growth factors, SCF (10 ng/mL), Epo (5 U/mL), or both SCF and Epo. Five hundred growing cells were inoculated into 1.5 mL medium in 30-mm dishes and scanned and counted unstained after 3 weeks. Scanned images were recorded using an Agfa Arcus PDI 420oe scanner (Agfa-Gevaert, Brentford, UK) and analyzed using the NIH Image program.
Transfections.
ELM-D6-20.5D cells were stably transfected by electroporation using a Bio-Rad gene pulser (Bio-Rad, Hemel Hemstead, UK). Cells (5 × 106) in 250 μL medium (20% serum + 10 ng/mL rmSCF) were mixed with 10 μg linearized expression vector in 4-mm electroporation cuvettes (Equibio, Kent, UK) and pulsed at 960 μF and 240 V. Cells were then diluted into 10 mL of fresh medium (10% serum and 10 ng/mL SCF) and, 48 hours after electroporation, transferred into microtiter wells with Geneticin Sulphate (800 μg/mL; GIBCO BRL) and SCF for isolation of transfected cell clones. Growth factor independence of transfectants was assessed by seeding 10,000 cells in 1 mL of medium without SCF into 24-well tissue culture dishes and providing fresh medium every 3 to 4 days. Quail fibroblasts were transiently transfected by calcium phosphate coprecipitation and maintained in fresh medium for 24 hours before lysis and Western blot analysis.
Antibodies and protein analysis.
Two antibodies against the murine Kit receptor were used, a rat monoclonal antibody (ACK231) for immunoprecipitation and a goat polyclonal antiserum (M-14; Santa Cruz, Santa Cruz, CA; used at 200 ng/mL) for immunoblotting. A polyclonal antiserum against Shc was purchased from Transduction Laboratories (Lexington, KY; used at 50 ng/mL), and antiphosphotyrosine antibodies PY20 and 4G10 (both used at 200 ng/mL) were purchased from Santa Cruz and Upstate Biotechnology (Lake Placid, NY), respectively. Analysis of the level of cellular MAPK activation used a polyclonal antibody preparation with specific activity against the dually phosphorylated forms of MAPK p44 and p42 (Promega; used at 50 ng/mL). An antibody against total p44 and p42 (sc-093; Santa Cruz; used at 50 ng/mL) was used as a loading control.
For immunoprecipitation of the Kit receptor, cells were lysed in buffer (1% NP40, 0.5% deoxycholate, 150 mmol/L NaCl, 50 mmol/L Tris, pH 8.0, 10% glycerol, 2 mmol/L EDTA, 1 mmol/L sodium orthovanadate, 1 mmol/L phenylmethyl sulfonyl fluoride [PMSF], 10 μg/mL leupeptin, 20 μg/mL aprotinin), precleared by centrifugation at 15,000 rpm for 1 hour, and incubated with 2 μg/mL ACK2 antibody. Immune complexes were collected with a rabbit antirat antibody (Sigma; 25 μg/mL) and protein A/G sepharose (Sigma). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting using polyvinylidene fluoride (PVDF) membrane (Millipore, Watford, UK) followed standard procedures and the manufacturer’s instructions. Proteins were detected using specific primary antibodies, horseradish peroxidase-conjugated secondary antibodies, and ECL chemiluminescence (Amersham, Little Chalfont, UK). Protein loading and transfer during blotting was finally verified by staining with Amido Black (Sigma).
The Kit receptor in vitro kinase assay was adapted from the method of Furitsu et al.19 The Kit receptor was immunoprecipitated as described above; washed at 4°C once each in lysis buffer, PBS, 500 mmol/L LiCl, and kinase buffer (10 mmol/L MnCl, 20 mmol/L Tris pH 7.4); and then resuspended in 30 μL kinase buffer containing 10μCi [γ-32P] ATP (Amersham) and 2 μmol/L unlabeled ATP, before incubation for 20 minutes at 25°C. Proteins were separated by SDS-PAGE and blotted onto PVDF membrane. Radioactive incorporation was determined by autoradiography and the relative amount of Kit receptor protein present in the immune complex was determined by immunoblotting.
RNA analysis.
Total cellular RNA was purified using the RNAzol B reagent and manufacturer’s protocol (Biogenesis, Poole, UK). Resolution of total RNA samples in formaldehyde-agarose gels and Northern blotting onto Hybond-N membrane (Amersham) was performed using standard protocols and manufacturers instructions. DNA probes were labeled using random primed DNA labeling (Boehringer Mannheim, Lewes, UK).
cDNA synthesis and sequencing.
Reverse transcriptase-polymerase chain reaction (RT-PCR) was performed using a Gene-amp RNA PCR Core Kit (Perkin Elmer, Norwalk, CT). The oligonucleotide primers used for the amplification of full-length c-kit cDNAs were KitS1 5′-GAGTCTAGCGCAGCCACC-3′ and KitA1 5′-CTGTTGGACTTGGGTTTCTG-3′. Oligonucleotide primers used for PCR sequencing were based on the published sequence of the murine c-kit gene.33 The amplification procedure used a Perkin Elmer Cetus DNA thermal cycler, including a manual hot-start, and 35 cycles of 1 minute of denaturation at 95°C, 1 minute of annealling at 58°C, and 2 minutes of synthesis at 72°C. The 72°C synthesis step of the cycle was extended by 4 seconds per cycle. Amplification products were purified from agarose gels using geneclean (Bio 101 Inc, La Jolla, CA) and ligated into the pCR2.1 cloning vector (Invitrogen, NV Leek, The Netherlands). All cloned cDNAs investigated further were obtained from separate PCR amplifications. These full-length cDNAs were then sequenced in both orientations using an Applied Biosystems 373A sequencer (Foster City, CA). Shorter fragments of the c-kit sequence were also amplified by RT-PCR using only 1 minute of synthesis cycles at 72°C, gel-purified, and sequenced directly.
Expression vector construction.
cDNAs of the full-length coding sequence of the wild-type murine c-kit gene and c-kitD814H were excised from the pCR2.1 vector with Xba I and Kpn I restriction enzymes and ligated into these sites of the expression vector pcDNA3 (Invitrogen). Expression vector cDNA inserts and ligation junctions were fully sequenced.
RESULTS
The ELM-I-1 cell line displays constitutive activation of the Kit receptor.
We initially set out to identify cellular changes associated with the conversion from stroma dependence to independence by comparison of stroma-dependent ELM erythroleukemia cells (ELM-D) and stroma-independent variants (ELM-I), particularly ELM-I-1.26
We have recently shown that growth of ELM leukemic cells on stromal cells relies on a complex array of signals provided by stroma, including integrin-mediated signaling, insulin-like growth factor-1 (IGF-1), and SCF.28 However, under optimal conditions, SCF alone (but not IGF-1) is able to replace stroma in supporting long-term cell growth without affecting its stroma/SCF dependence or ability to differentiate in response to Epo.28
Experiments were therefore performed to investigate the possible stimulation of ELM-I cells by autocrine growth factor production, either using blocking antibodies or detecting growth factor mRNAs by RT-PCR. This showed that none of the ELM-I cell clones investigated produced detectable quantities of autocrine growth factors for which they were known to express corresponding receptors, SCF, IGF-1, or Epo (O’Prey et al28 and data not shown). As a next step, we investigated the activation of tyrosine kinase signaling pathways known to be activated by many mitogenic growth factor signals and oncogenes. Initial experiments showed that several ELM-I cell lines displayed constitutive phosphorylation and activity of the p42/p44 mitogen-activated protein kinases (MAPKs) in the absence of stimulation, with levels being highest in the ELM-I-1 cell line (data not shown). The ELM-I-1 cell line was also found to exhibit constitutive tyrosine phosphorylation of the p46 and p52 Shc signaling proteins and a greatly reduced level of expression of the p66 isoform of Shc (data not shown). Because recent work has shown that, in contrast to the p46 and p52 Shc isoforms, p66 is able to play an inhibitory role in growth factor signaling,32 33 this may be significant.
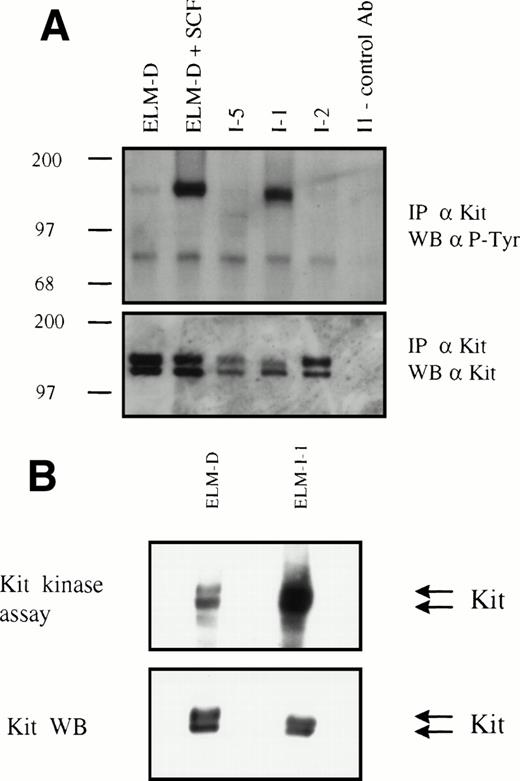
Having found activation of kinase signaling pathways in the ELM-I-1 cell line, antiphosphotyrosine Western blotting of whole cell lysates was performed to identify activated kinases. The pattern of protein tyrosine phosphorylation in unstimulated ELM-I-1 cells showed a protein of approximately 145 kD that comigrated with a protein phosphorylated in ELM-D cells stimulated with SCF and with the immunoprecipitated Kit receptor (data not shown). This suggested constitutive phosphorylation and activation of the Kit receptor in ELM-I-1 cells. This was confirmed directly by immunoprecipitation of the Kit receptor and Western blotting with antiphosphotyrosine and anti-Kit antibodies (Fig 1A) and also by an in vitro assay to detect Kit receptor kinase activity (Fig 1B). These experiments show that the Kit receptor is tyrosine phosphorylated in unstimulated serum-starved ELM-I-1 cells in contrast to two other ELM-I variants, ELM-I-2 and ELM-I-5 (Fig 1A), and that Kit receptor immunoprecipitates from ELM-I-1 cells have an elevated level of constitutive kinase activity (Fig 1B). We also found that, although ELM-I-1 cells express similar levels of Kit receptor protein as other ELM cells, there were changes in the relative abundance of the two receptor forms (immature and mature34) and in the apparent electrophoretic mobility of the larger mature form (Fig 1A and B and data not shown).
Tyrosine phosphorylation and kinase activity of the Kit receptor in serum-starved ELM-I-1 cells. (A) Kit protein was immunoprecipitated from serum-starved cells and from ELM-D cells subsequently stimulated with 10 ng/mL SCF for 10 minutes before cell lysis. Immunoprecipitated proteins were then analyzed by Western blotting using antiphosphotyrosine and anti-Kit receptor antibodies. A control antibody was used in the immunoprecipitation loaded into the right-hand lane. Size markers are shown in kilodaltons. (B) Kit protein was immunoprecipitated from unstimulated ELM-D and ELM-I-1 cells and its autophosphorylation activity was assayed in vitro as described in Materials and Methods.
Tyrosine phosphorylation and kinase activity of the Kit receptor in serum-starved ELM-I-1 cells. (A) Kit protein was immunoprecipitated from serum-starved cells and from ELM-D cells subsequently stimulated with 10 ng/mL SCF for 10 minutes before cell lysis. Immunoprecipitated proteins were then analyzed by Western blotting using antiphosphotyrosine and anti-Kit receptor antibodies. A control antibody was used in the immunoprecipitation loaded into the right-hand lane. Size markers are shown in kilodaltons. (B) Kit protein was immunoprecipitated from unstimulated ELM-D and ELM-I-1 cells and its autophosphorylation activity was assayed in vitro as described in Materials and Methods.
ELM-I-1 cells contain a point mutation in the c-kit gene in the region encoding the kinase domain.
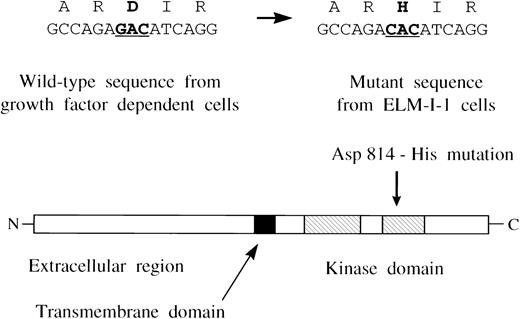
Because normal Kit receptor activation is believed to occur by ligand-induced dimerization and autophosphorylation and it is known that ELM-I-1 cells do not express detectable levels of SCF, these changes suggest that ELM-I-1 cells express a constitutively active mutant form of the Kit receptor. To investigate this possibility, Kit receptor mRNAs present in ELM-I-1 cells were cloned by RT-PCR and compared with those cloned from growth factor-dependent ELM-D cells. Each of the three independent cDNAs from ELM-I-1 cells sequenced showed a single G to C change at nucleotide 2468 compared with the sequence from growth factor-dependent ELM-D cells. This point mutation causes a change in the predicted amino acid sequence from aspartic acid to histidine at codon 814 (Fig 2). In addition, one cDNA from ELM-I-1 cells also contained a previously described 12 nucleotide insertion caused by differential splicing.35 Subsequent experiments using expression vectors with the mutant Kit cDNA without the insertion showed that it is not required for constitutive receptor activation; therefore, the effects of this insertion were not investigated further. There were also three previously described strain-specific polymorphisms36 in the c-kit nucleotide sequence of both ELM cell lines, derived from a C3H mouse, that differed from the published BALB/c mouse c-kitsequence.37 RT-PCR amplification products of the c-kit kinase domain region were also gel-purified and sequenced directly. These showed only the wild-type sequence expressed in ELM-D cells, but both wild-type and mutant sequences at nucleotide 2468 in ELM-I-1 cells, showing that ELM-I-1 cells retain expression of the wild-type Kit receptor in addition to the KitD814H mutant.
A mutation in c-kit expressed in ELM-I-1 cells. Nucleotide 2468 was a G both in each c-kit cDNA clone from ELM-D cells and the published wild-type c-kitsequence,37 but a C in each c-kit cDNA clone from ELM-I-1. This change results in an aspartic acid to histidine mutation at codon 814 in the predicted amino acid sequence of the mutant Kit receptor kinase domain. Direct sequencing of RT-PCR products shows that ELM-I-1 cells express both mutant and wild-type sequences.
A mutation in c-kit expressed in ELM-I-1 cells. Nucleotide 2468 was a G both in each c-kit cDNA clone from ELM-D cells and the published wild-type c-kitsequence,37 but a C in each c-kit cDNA clone from ELM-I-1. This change results in an aspartic acid to histidine mutation at codon 814 in the predicted amino acid sequence of the mutant Kit receptor kinase domain. Direct sequencing of RT-PCR products shows that ELM-I-1 cells express both mutant and wild-type sequences.
Expression of c-kitD814H abrogates the SCF dependence of ELM-D cells.
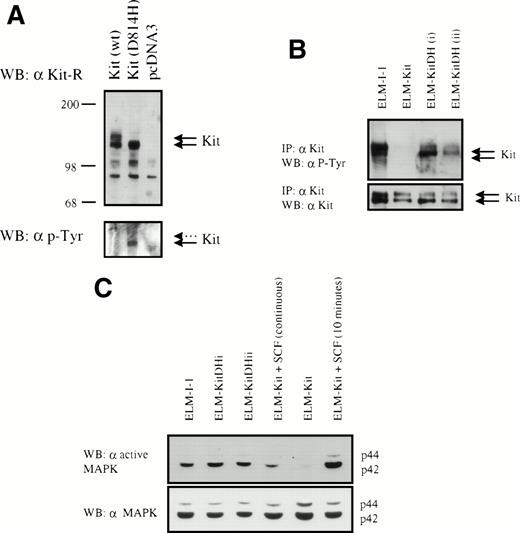
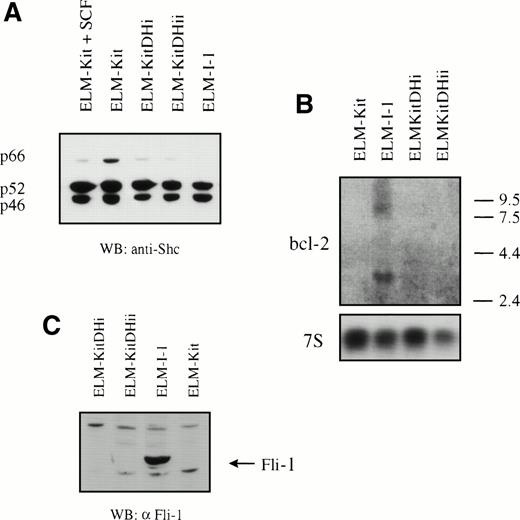
To determine whether the mutation in c-kit is responsible for the growth factor independence of ELM-I-1 cells, we introduced the c-kitD814H mutation into ELM-D cells. Expression constructs for c-kit and c-kitD814H were created using the vector pcDNA3 (Invitrogen). These were found to give efficient expression of the Kit receptor, as verified by Western blotting of transiently transfected Quail fibroblasts (Fig 3A). However, only the immature 125kD Kit glycoprotein was detected in cells transfected with the mutant c-kit cDNA, in contrast with those transfected with the wild-type vector, which expressed both 125- and 145-kD forms of Kit. As expected, the transiently expressed mutant Kit was found to be constitutively phosphorylated on tyrosine, unlike the wild-type receptor (Fig 3A).
Expression and tyrosine phosphorylation of Kit and MAPK in ELM cells expressing KitD814H and wild-type Kit. (A) pcDNA3 vector (Invitrogen) and expression constructs for c-kit and c-kitD814H based on pcDNA3 were transfected into the Q2BN Quail fibroblastic cell line and expression of Kit protein was analyzed by Western blotting with anti-Kit antibodies. This blot was then stripped and an antiphosphotyrosine antibody was used. (B) Phosphorylation of the Kit receptor in ELM-I-1 cells and ELM-D cells stably transfected with wild-type c-kit or c-kitD814H expression vectors. Phosphorylation of the Kit receptor in unstimulated serum-starved cells was examined by immunoprecipitation with anti-Kit antibodies and antiphosphotyrosine Western blotting. The membrane was stripped and an anti-Kit antibody was used to verify Kit immunoprecipitation. (C) MAPK phosphorylation was analyzed in serum-starved ELM-I-1, ELM-Kit, or two clones of ELM-KitDH cells (i and ii) and in serum-starved ELM-Kit cells either stimulated for 10 minutes with 10 ng/mL rmSCF before lysis or continuously maintained in 10 ng/mL rmSCF. Whole cell lysates were analyzed by Western blotting using an antibody against the activated form of MAPK and a nonspecific antibody against p42/p44.
Expression and tyrosine phosphorylation of Kit and MAPK in ELM cells expressing KitD814H and wild-type Kit. (A) pcDNA3 vector (Invitrogen) and expression constructs for c-kit and c-kitD814H based on pcDNA3 were transfected into the Q2BN Quail fibroblastic cell line and expression of Kit protein was analyzed by Western blotting with anti-Kit antibodies. This blot was then stripped and an antiphosphotyrosine antibody was used. (B) Phosphorylation of the Kit receptor in ELM-I-1 cells and ELM-D cells stably transfected with wild-type c-kit or c-kitD814H expression vectors. Phosphorylation of the Kit receptor in unstimulated serum-starved cells was examined by immunoprecipitation with anti-Kit antibodies and antiphosphotyrosine Western blotting. The membrane was stripped and an anti-Kit antibody was used to verify Kit immunoprecipitation. (C) MAPK phosphorylation was analyzed in serum-starved ELM-I-1, ELM-Kit, or two clones of ELM-KitDH cells (i and ii) and in serum-starved ELM-Kit cells either stimulated for 10 minutes with 10 ng/mL rmSCF before lysis or continuously maintained in 10 ng/mL rmSCF. Whole cell lysates were analyzed by Western blotting using an antibody against the activated form of MAPK and a nonspecific antibody against p42/p44.
The Kit expression constructs were then stably introduced by electroporation into stroma/SCF-dependent ELM-D cells that already express the wild-type Kit receptor. To obviate the need for isolating transfectants on antibiotic-resistant stroma, ELM-D cells were maintained in 10 ng/mL SCF instead of stroma, because SCF has been shown to support ELM cells without altering their growth factor dependence or differentiation capacity.28 After electroporation, antibiotic resistant clones were selected in the continuous presence of SCF, expression of introduced constructs was investigated by RT-PCR, and the SCF-dependence of the clones was tested by removal of SCF. As previously described,26 growth factor-independent variant cells arise spontaneously during selection: 1 of 12 or 2 of 16 ELM cell clones transfected with empty expression vector or the wild-type Kit cDNA expression vector, respectively, gave rise to cells capable of growth in medium without SCF. In contrast, 10 of 27 clones transfected with the KitD814H mutant cDNA were growth factor independent. However, only 8 of 18 of the latter clones tested showed detectable expression of the mutant KitD814H vector by RT-PCR (data not shown), and all of these 8 expressing clones continued to proliferate in the absence of SCF. Thus, in all cases, expression of KitD814H resulted in SCF-independent growth. Two of the expressing ELM-KitDH clones (i and ii) were chosen for further characterization: both showed constitutive phosphorylation of the Kit receptor, in contrast to cells expressing the wild-type receptor (ELM-Kit cells; Fig3B). As expected, direct sequencing of RT-PCR products obtained from one of these expressing clones using c-kit primers showed that both wild-type and mutant Kit receptors were expressed, as is the case also with ELM-I-1 cells (data not shown). These ELM-KitDH clones also showed a constitutively high level of MAPK phosphorylation after serum-starvation, in contrast to ELM-Kit cells (Fig 3C). The extent of constitutive MAPK activation in ELM-KitDH cells was similar to that in ELM-I-1 cells and, interestingly, consistently higher than that seen in ELM-Kit cells maintained in 10 ng/mL SCF (Fig 3C), the growth factor concentration shown to induce optimal growth of these cells in short-term assays (O’Prey et al28 and data not shown).
Effects of the c-kitD814H mutation on the proliferation and clonegenicity of ELM-D cells.
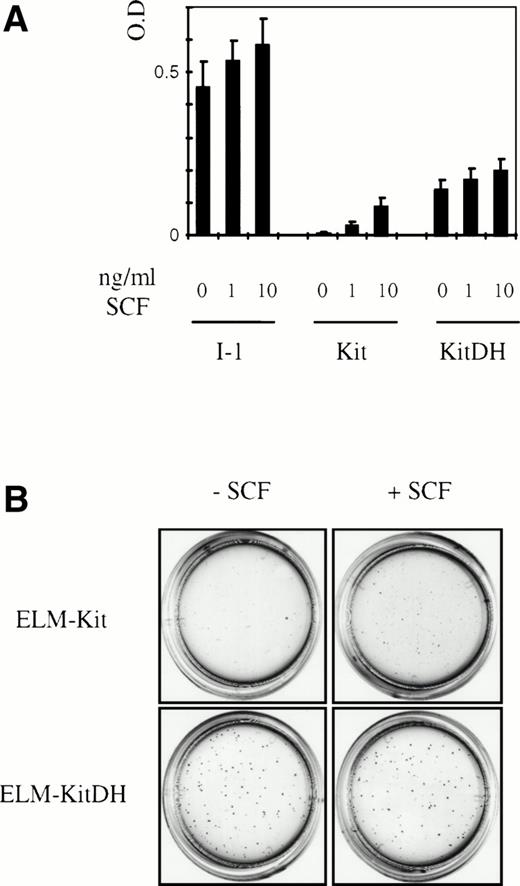
To determine the effects of the c-kitD814H mutation on the proliferation and survival of ELM cells, the growth of ELM-Kit, ELM-KitDH, and ELM-I-1 cells was compared in a microtiter well proliferation assay and a semisolid medium colony assay in the presence and absence of additional growth factors. These experiments showed that the KitD814H mutation not only allows ELM cells to continue to proliferate in the absence of SCF, but also gives ELM-KitDH cells a growth advantage compared with ELM-Kit cells maintained in the concentration of SCF we have previously shown to be optimal for ELM growth28 (10 ng/mL; Fig 4A). However, the proliferation of ELM-KitDH cells was not as great as that of ELM-I-1 cells (Fig 4A). These findings were supported by cell cycle analysis showing that ELM-Kit cells starved of SCF for 24 hours accumulate in the G0/G1 phase of the cell cycle, in contrast to ELM-KitDH cells, which maintain a large proportion of cells in S and G2/M phases in the presence and absence of SCF (data not shown). The ELM-KitDH cells also cloned at high efficiency without SCF, in contrast to ELM-Kit cells, and produced larger colonies than ELM-Kit cells cloned in the presence of optimal SCF concentrations (Fig 4B). Thus, the KitD814H mutation clearly confers a selective advantage over the wild-type Kit in terms of proliferation and clonegenicity.
Expression of c-kitD814H in ELM cells causes SCF-indpendence and enhanced proliferation and cloning efficiency. (A) The proliferation of ELM-Kit and ELM-KitDH cells over 7 days was compared after seeding 500 cells in 96-well plates in different concentrations of SCF. Cells were assayed using a colorimetric MTT proliferation assay and each data point shown is an average of 28 cultures, each read twice, with standard deviations. (B) Colony formation by ELM-Kit, ELM-KitDH, and ELM-I-1 cells in response to SCF. Five hundred cells were inoculated in the presence or absence of SCF (10 ng/mL) in semisolid medium.
Expression of c-kitD814H in ELM cells causes SCF-indpendence and enhanced proliferation and cloning efficiency. (A) The proliferation of ELM-Kit and ELM-KitDH cells over 7 days was compared after seeding 500 cells in 96-well plates in different concentrations of SCF. Cells were assayed using a colorimetric MTT proliferation assay and each data point shown is an average of 28 cultures, each read twice, with standard deviations. (B) Colony formation by ELM-Kit, ELM-KitDH, and ELM-I-1 cells in response to SCF. Five hundred cells were inoculated in the presence or absence of SCF (10 ng/mL) in semisolid medium.
Effects of the c-kitD814H mutation on the differentiation of ELM-D cells.
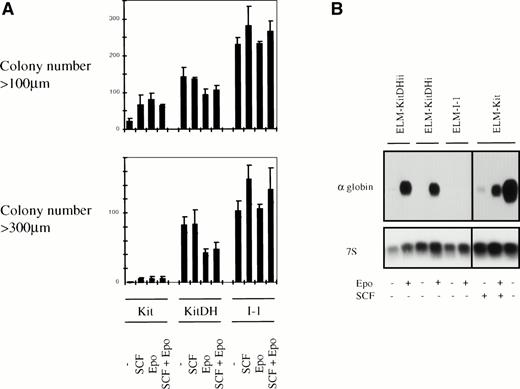
One of the obvious characteristics of the ELM-I-1 cells in which the KitD814H mutation was identified is that they do not undergo erythroid differentiation in response to Epo or upon removal from SCF or stroma, as ELM-D cells do.26 28 We therefore investigated the effects of the KitD814H mutation on the balance of proliferation and differentiation of ELM cells, either after removal of SCF or in response to Epo. The first approach adopted was to investigate the effects of SCF and/or Epo on colony formation of ELM-KitDH cells and ELM-Kit cells in clonogenic assays and measuring the colony size distributions by computer analysis (see Materials and Methods for details). Cloning in either SCF or Epo (or both) increased the total number of colonies of ELM-Kit cells, but few of them reached a size of 300 μm (a few hundred cells; Fig 5A). In contrast, a greater number of ELM-KitDH colonies were formed whether or not SCF or Epo were present, of which 40% to 50% of these grew to sizes greater than 300 μm (Fig 5A). However, the numbers of large colonies were reduced by treatment with Epo even in the presence of SCF (Fig 5A), suggesting that these ELM-KitDH cells can still differentiate in response to Epo, whereas ELM-I/1 cells are unaffected (Fig 5A).
The effect of KitD814H on the proliferation and differentiation of ELM cells in response to SCF and/or Epo. (A) ELM-KitDH or ELM-Kit cells were cloned in methocel in the presence or absence of 10 ng/mL SCF and/or 5 U/mL Epo as described in Fig4B and the colonies were analyzed after 2 weeks. To analyze the numbers and sizes of colonies, the dishes were recorded using a scanner, and colonies were counted and sized using the NIH image program. The representative plotted data are shown as the average colony number from triplicate plates, with error bars showing maximum and minimum numbers. (B) To examine the extent of erythroid differentiation, the cells were incubated for 3 days in the presence or absence of 2 U/mL Epo or 10 ng/mL SCF, as indicated. Total RNA was then analyzed by Northern blotting using labeled cDNA probes encoding -globin or 7S ribosomal RNA.
The effect of KitD814H on the proliferation and differentiation of ELM cells in response to SCF and/or Epo. (A) ELM-KitDH or ELM-Kit cells were cloned in methocel in the presence or absence of 10 ng/mL SCF and/or 5 U/mL Epo as described in Fig4B and the colonies were analyzed after 2 weeks. To analyze the numbers and sizes of colonies, the dishes were recorded using a scanner, and colonies were counted and sized using the NIH image program. The representative plotted data are shown as the average colony number from triplicate plates, with error bars showing maximum and minimum numbers. (B) To examine the extent of erythroid differentiation, the cells were incubated for 3 days in the presence or absence of 2 U/mL Epo or 10 ng/mL SCF, as indicated. Total RNA was then analyzed by Northern blotting using labeled cDNA probes encoding -globin or 7S ribosomal RNA.
To measure the differentiation responses directly, the expression of α-globin mRNA in ELM-KitDH and ELM-Kit cells was first determined after treatment with SCF and/or Epo (Fig 5B). Expression of globin mRNA correlated with the development of a red color in cell pellets. This experiment confirmed that ELM-KitDH cells did not undergo spontaneous differentiation upon removal of SCF, in contrast to ELM-Kit cells; nevertheless, ELM-KitDH cells still differentiated in response to Epo (Fig 5B).
Effects of the KitD814H mutation on expression of p66Shc, Fli-1, and Bcl-2.
Besides the c-kitD814H mutation, a number of other differences have been identified between ELM-I-1 and ELM-D cells: they express high levels of the ets protein Fli-126 and the regulator of apoptosis Bcl-2 (J. Qiu and P. Harrison, unpublished observations), and they also show greatly reduced levels of the p66 Shc protein (data not shown and Fig 6A). We therefore tested whether any of these other changes in ELM-I-1 cell gene expression are induced by expression of the c-kitD814H mutant.
Comparison of the effects of wild-type c-kit and c-kitD814H expression in ELM-D cells on (A) the pattern of Shc isoforms expression, (B) bcl-2 mRNA, and (C) Fli-1 protein. (A) Relative expression of Shc isoforms was investigated by Western blotting of whole cell lysates of serum-starved cells and ELM-Kit cells serum-starved in the presence of 10 ng/mL SCF. (B) Expression of bcl-2 mRNA in ELM-I-1, ELM-Kit maintained in 10 ng/mL SCF, and ELM-KitDH cells (clones i and ii) was analyzed by Northern blotting of total RNA from growing cells and probing with bcl-2 cDNA and 7S rRNA probes. Size markers are shown in kilobases. (C) Whole cell lysates from ELM-KitDH and ELM-I/1 cells or ELM-Kit cells maintained in SCF were analyzed by Western blotting with an anti-Fli-1 antibody.
Comparison of the effects of wild-type c-kit and c-kitD814H expression in ELM-D cells on (A) the pattern of Shc isoforms expression, (B) bcl-2 mRNA, and (C) Fli-1 protein. (A) Relative expression of Shc isoforms was investigated by Western blotting of whole cell lysates of serum-starved cells and ELM-Kit cells serum-starved in the presence of 10 ng/mL SCF. (B) Expression of bcl-2 mRNA in ELM-I-1, ELM-Kit maintained in 10 ng/mL SCF, and ELM-KitDH cells (clones i and ii) was analyzed by Northern blotting of total RNA from growing cells and probing with bcl-2 cDNA and 7S rRNA probes. Size markers are shown in kilobases. (C) Whole cell lysates from ELM-KitDH and ELM-I/1 cells or ELM-Kit cells maintained in SCF were analyzed by Western blotting with an anti-Fli-1 antibody.
Expression of p66Shc was compared in ELM-KitDH (clones i and ii), ELM-Kit, and ELM-I-1 cells. Western blotting of serum-starved cells showed that removal of SCF from ELM-Kit cells expressing the wild-type Kit receptor caused a marked increase in p66 Shc expression (Fig 6A). Starved ELM-KitDH cells expressed a low level of p66 Shc, similar to ELM-Kit cells grown in SCF, although this was not as low as in ELM-I-1 cells. No change in p46/p52 isoforms was evident. Thus, Kit signaling, either from the wild-type receptor and SCF or constitutively from the mutant KitDH receptor, suppresses the expression of p66 Shc. Finally, the expression of Bcl-2 and Fli-1 in ELM-Kit, ELM-KitDH, and ELM-I-1 cells was compared by Northern and Western blotting, respectively. However, neither wild-type Kit nor KitD814H induced expression of Bcl-2 or Fli-1 in ELM cells, compared with the ELM-I-1 control (Fig 6B and C).
DISCUSSION
We have identified an activating mutation in the Kit receptor of the ELM-I-1 growth factor-independent erythroleukemic cell line and, by expression of this mutant receptor in growth factor-dependent ELM cells, defined how it may have contributed to the phenotype of the leukemic cells in which it arose. Thus, we have demonstrated that this mutation gives the ELM cells a selective advantage in three respects: (1) SCF or stroma independence; (2) enhanced proliferation/cloning efficiency compared with an exogenous SCF signal; and (3) a reduction in spontaneous differentiation on stroma or SCF withdrawal. However, activation of Kit by mutation is obviously not the only mechanism whereby ELM leukemic cells acquire stromal independence, because two other stroma-independent ELM lines, ELM-I-2 and ELM-I-5, do not display constitutive Kit phosphorylation (Fig 1A). Collectively, these changes would be expected to increase the leukemogenic potential of the cells. We have tested whether expression of the mutant Kit receptor in stroma-dependent ELM-D leukemic cells enables them to grow in nonhematopoietic sites, eg, subcutaneously, as is the case with other aggressive retrovirus-induced erythroleukemia lines. However, none of the ELM-D, ELM-Kit, or ELM-Kit DH cell lines tested formed tumors after subcutaneous inoculation in nude mice after a period of 4 months (data not shown).
Because expression of the mutant Kit does not prevent ELM cells differentiating in response to Epo, this implies that some other genetic event is responsible for the total lack of response of ELM-I/1 cells to Epo (Fig 5B). Because ELM-I-1 cells also do not differentiate in response to the chemical inducers, dimethyl sulfoxide (DMSO) or N,N′-hexamethylene bisacetamide (HMBA; data not shown), it seems likely that this differentiation block is not simply caused by a defect in Epo signaling, but is more general. We have previously shown that growth of ELM cells on stroma, but not in soluble SCF, inhibits Epo-induced differentiation. This might be due to the stromal expression of the membrane bound form of SCF causing a more persistent intracellular Kit phosphorylation signal than the soluble form of the ligand.38 Our data here would argue against this, because the KitD814H mutation produces a constantly elevated level of receptor signaling and yet does not inhibit Epo-induced differentiation. This suggests that a different signal supplied by stroma inhibits the erythroid differentiation of ELM-D cells.
The activating point mutation in c-kit converts the aspartic acid at codon 814 to a histidine. Although a spontaneous D814H mutation has not been previously described, aspartic acid 814 has been identified as a hot spot for c-kit activating mutations, being mutated to a valine or a tyrosine in mast cell lines and mastocytosis.19-21 Mutation of aspartic acid 814 to tyrosine has also been shown to alter the substrate specificity of the Kit receptor kinase.39 Aspartic acid 814 lies within the activation loop of the cytoplasmic kinase domain. Studies with the insulin and fibroblast growth factor RTKs have suggested that this loop inhibits access to the active site for substrate peptides and ATP until tyrosine residues within the loop are phosphorylated upon ligand binding.40,41 It seems possible that mutation of aspartic acid 814 interferes with this autoinhibition, increasing accessibility of the active site. Mutagenesis has shown that many amino acids introduced in place of aspartic acid 814 allow some level of ligand-independent kinase activity.42 In these studies, the D814H mutation was described as modestly activating and displayed accelerated ligand-independent degradation relative to the wild-type receptor when expressed in kidney 293 cells. The identification of the KitD814H mutation in an erythroleukemia is also significant, because it appears that Kit codon 814 mutations are only transforming in some cell types, as KitD814V is unable to transform fibroblasts, in contrast to the wild-type receptor stimulated with SCF.43
Activating mutations have been shown to interfere with the normal cellular processing of the Kit receptor, normally present as 125-kD and 145-kD glycoproteins. We show that expression of mutant KitD814H in ELM cells results in the reduced expression of p145 and its complete absence when expressed in Quail fibroblasts. This correlates with the rapid ligand-independent receptor degradation and reduced cell surface expression seen in studies with other activating mutants,42,44 the rapid polyubiquitination and degradation of wild-type Kit after ligand stimulation,45,46 and the inefficient receptor maturation seen with some activated mutants of the related CSF-1-R.47
Activating mutations have also been identified in the kinase domain activation loop in other RTKs. For example, they have been identified in the Ret and Met receptors, associated with hereditary multiple endocrine neoplasia 2B (MEN2B) and papillary renal carcinoma, respectively,5,6 and in the CSF-1-R.44 Indeed, mutations in Met and CSF-1-R were found in the homologous aspartic acid residue to the Kit D814, at D1246 in Met, and at D802 in the CSF-1-R.
It appears that the Kit receptor mediates its cellular effects through association with a wide range of signaling molecules and activation of many different cellular signaling pathways, depending on cell type (eg, PKC isoforms,48 the JAK-STAT pathway,49Lyn,50 Vav,51 Chk,52CRKL,53 and Shp154). However, activation by Kit of PI3 kinase and the Ras-Raf-MAPK pathway is widespread.9,35,55 We have therefore analyzed the phosphorylation of p42/p44 MAPK as an example of a downstream target of Kit signaling believed to mediate profound effects in many cells. Our data show constitutively high levels of MAPK phosphorylation in unstimulated ELM cells expressing KitD814H and in the stroma-independent ELM-I-1 cells from which the mutant c-kitcDNA was cloned. This degree of activation was consistently higher than that induced by continuous incubation of cells expressing similar levels of the wild-type receptor in the optimal SCF concentration for growth of these cells. Because there is evidence that a quantitatively different level or duration of MAPK activation can result in a qualitatively different cellular response,56 the constant level of MAPK activation caused by the KitD814H mutation may be significant. This could generate a different range of responses in ELM cells to the short-term signal produced by SCF interacting with the wild-type Kit receptor.
Finally, our data show specific changes in the level of expression of the p66 isoform of the signaling molecule Shc in response to Kit signaling. Recent data show that, unlike the p46 and p52 Shc isoforms, p66 does not transform fibroblasts and inhibits, rather than enhances, EGF-induced activation of MAPK and the c-fospromoter.32,33 This implicates loss of p66 expression as a potential mechanism that may increase the sensitivity of cells expressing the KitD814H mutant to extracellular stimuli, and this may have been a factor in the selection of growth factor-independent ELM-I-1 cells during ELM erythroleukemia progression. Increased expression of p66Shc on SCF removal correlates with cells spontaneously differentiating, and a similar increase in p66 expression has been observed when myoblasts are induced to differentiate by serum starvation.57 However, increased p66Shc expression is not found in ELM cells induced to differentiate with Epo and, therefore, does not appear to be a marker of differentiation per se and may be more directly a consequence of growth factor withdrawal and cell cycle arrest.
ACKNOWLEDGMENT
The authors are grateful to Prof W. Ostertag (Heinrich-Pette Institute, Hamburg, Germany) and Dr K. Itoh (University of Kyoto, Kyoto, Japan) for the original gift of the ELM-D and ELM-I/1 cell lines and for many helpful discussions. We also thank Margaret Frame, Dave Gillespie, and John Wyke (all at the Beatson Institute) for their comments on this manuscript and Robert McFarlane and Peter McHardy for expert assistance with sequencing and FACS analysis, respectively.
Supported by the Cancer Research Campaign.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Paul R. Harrison, PhD, Beatson Institute for Cancer Research, CRC Beatson Laboratories, Garscube Estate, Switchback Road, Glasgow, G61 1BD, Scotland.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal