Abstract
We have previously demonstrated that human granulocyte-macrophage colony-stimulating factor fused to a truncated diphtheria toxin (DT388-GM-CSF) is toxic to patient acute myeloid leukemia progenitors bearing the GM-CSF receptor, but not normal marrow progenitors. We now report that exposure of mononuclear cells from five of seven (71%) juvenile myelomonocytic leukemia (JMML) patients and from 12 of 20 (60%) adult chronic myelomonocytic leukemia (CMML) patients to 10-9 mol/L DT388-GM-CSF for 48 hours in culture reduces the number of cells capable of forming colonies in semisolid medium (colony-forming units–leukemia) 10-fold to 300-fold (1 to 2.5 log decrease). In contrast, normal myeloid progenitors (colony-forming unit–granulocyte-macrophage) from six different donors treated and assayed under identical conditions were consistently insensitive to the same fusion toxin even when treated as highly purified CD34+ cells. The leukemic progenitors from the two other JMML patients showed intermediate sensitivity to DT388-GM-CSF and the leukemic progenitors from eight of the 20 (40%) CMML patients were not different from normal progenitors. Parallel measurements of the number and affinity of GM-CSF receptors on cells from the same samples showed no consistent differences between JMML, CMML, and normal light density or CD34+ bone marrow cells. The increased sensitivity of leukemic progenitors from all JMML progenitors and some CMML patients to the fusion toxin is therefore not likely to be explained by an increased density of GM-CSF receptors on these cells. We also examined the DT388-GM-CSF sensitivity of two murine cell lines transfected with cDNAs encoding varying portions of the human GM-CSF receptor and/or β chains. These studies showed that high-affinity ligand binding was sufficient for DT388-GM-CSF–induced toxicity, as this could occur even in the absence of functional signal transduction and that the background of the host cell had a major influence on the degree to which this decreased the toxicity of DT388-GM-CSF. The selective sensitivity to DT388-GM-CSF of leukemic progenitors from a majority of JMML and CMML patients suggests that this agent could have therapeutic potential for some patients with these diseases.
CHRONIC MYELOMONOCYTIC leukemia (CMML) is a distinct clonal hematopoietic disorder seen primarily in older men. It is characterized by peripheral monocytosis, dysmyelopoiesis, and minimal numbers of blasts in the marrow. Patients with CMML have a median survival of only 1 to 2 years.1 Death is usually due to infection, bleeding, or transformation of the disease to an acute leukemic phase. Intensive chemotherapy, low dose cytosine arabinoside, hydroxyurea, and ATRA have all proven ineffective in generating long-term remissions.2
Juvenile myelomonocytic leukemia (JMML), formerly termed juvenile chronic myelogenous leukemia, is another rare clonal myeloproliferative disease with similar features, but occurs in early childhood. Like CMML, it is characterized by an excessive in vitro proliferation of myeloid progenitors that show hypersensitivity to granulocyte-macrophage colony-stimulating factor (GM-CSF).3JMML is clinically manifested by failure to thrive, marked hepatosplenomegaly, adenopathy, skin rash, anemia, thrombocytopenia, leukocytosis with monocytosis, and less than 20% marrow blasts. Abnormalities in the neurofibromatosis and RAS genes have been found in 50% to 60% of patients’ hematopoietic progenitor cells, but chromosomal abnormalities are rare. While the use of allogeneic stem cell transplantation in JMML has resulted in some durable remissions, recurrent leukemia remains a significant problem, and the 5-year disease-free survival rate is in the range of 25%.4 Thus, novel therapeutics with unique mechanisms of action are needed for both of these myeloid disorders.
One class of therapeutics of potential interest are targeted toxins consisting of protein toxins covalently linked to peptide ligands. The ligand directs the molecule to the surface of specific cell types, and the toxin moiety then enters the cell and catalytically inactivates protein synthesis. We previously synthesized a fusion toxin composed of the first 388 amino acid residues of diphtheria toxin fused to human GM-CSF (DT388-GM-CSF) and tested its activity on acute myeloid leukemia (AML) blasts.5 6 These studies demonstrated a potent and selective cytotoxicity of DT388-GM-CSF on leukemic progenitors from AML patients with GM-CSF receptor-expressing blasts, whereas normal clonogenic progenitors were relatively insensitive to DT388-GM-CSF. In this report, we now show that the progenitors from a majority of CMML and JMML patients also show an increased sensitivity to DT388-GM-CSF and that this is not explained by an increased expression of GM-CSF receptors on their blasts by comparison to highly purified CD34+ marrow cells from normal marrow. In addition, investigation of two murine cell lines expressing different mutant human GM-CSF receptors showed that sensitivity to DT388-GM-CSF was variably affected when the ligand binding activity of the transduced receptor was retained, but the signaling function eliminated in the two different host cell types.
MATERIALS AND METHODS
JMML, CMML, and normal marrow cells.
With the approval of the respective Institutional Review Boards and after obtaining informed patient or parental consent, heparinized blood samples were obtained from seven patients with a diagnosis of JMML and 22 patients with CMML. Heparinized marrow aspirates were also obtained from three allogeneic bone marrow donors and from vertebral harvests of three normal cadaveric donors (Northwest Tissue Center, Seattle, WA). Blood and marrow cells were diluted 1:1 with RPMI 1640 medium and layered over 0.3 vol of Ficoll-Hypaque (1.070 g/mL; Pharmacia, Piscataway, NJ). After density gradient centrifugation at 2,000 rpm for 30 minutes, light density cells (<1.077 g/mL) were diluted threefold with RPMI 1640 and centrifuged again at 1,300 rpm for 10 minutes. Cells were then usually cryopreserved in 50% fetal calf serum (FCS) with 10% dimethyl sulfoxide (DMSO). Upon thawing, cells were suspended in RPMI 1640 medium with 15% FCS and 2 mmol/L L-glutamine (GIBCO-BRL, Grand Island, NY), 50 U/mL penicillin G (GIBCO), and 50 μg/mL streptomycin sulfate (GIBCO) with 1 mg/mL DNAse I (Sigma Chemicals, St Louis, MO). In some cases, normal CD34+ cells (≥99.9% pure) were isolated by immunoaffinity separation and fluorescence-activated cell sorting (FACS) as previously described.7,8 These methods enriched significantly for colony-forming unit–granulocyte-macrophage (CFU-GM). Cells were then counted and used for GM-CSF receptor measurements and studies of progenitor sensitivity to DT388-GM-CSF as described below. CD34+ selection was not performed on leukemic samples because of the known heterogeneity in leukemic progenitor phenotype.9
Cell lines.
The hematopoietic growth factor-dependent M1 mouse leukemia cell line10 and 32D mouse myeloid cell line (a gift of Dr J. Greenberger, University of Pittsburgh, Pittsburgh, PA) were maintained in RPMI 1640 with 15% FCS supplemented with 10% WEHI-3B conditioned medium.11 Both cell lines were transfected with cDNAs encoding the human wild-type GM-CSF receptor β chain, the term1 mutation of the human GM-CSF receptor α chain (truncated immediately after the transmembrane domain),12 and/or the α-2 variant of the human GM-CSF receptor α chain13 using previously reported methods.14 The α-2 variant is a normally occuring splice variant of the α chain and is fully biologically active for proliferation.13 The human GM-CSF receptor β chain cDNA contained in the mammalian expression plasmid pEF-BOS15 was a gift of Dr N. Nicola (Walter and Eliza Hall Institute, Melbourne, Australia). The full-length human GM-CSF receptor α-2 chain cDNA was ligated into the plasmid pLXSN,16 as was the term1 mutant α chain cDNA. pLXSN plasmids containing the α chain cDNAs were transfected along with a plasmid encoding the pol and env sequences into human 293 cells. Twenty-four hours later, supernatants containing recombinant retroviruses were used to infect cells of the amphotropic packaging line PA317. G418-resistant clones were then selected and analyzed for the production of α chain-transducing retroviruses. These were then used to transduce M1 and 32D cells with human GM-CSF receptors. In some cases, the β chain cDNA was introduced first, via electroporation, along with a puromycin resistance plasmid for selection. Expression of the β chain was confirmed by flow cytometry of puromycin-resistant clones, using a murine monoclonal antibody to an external domain of the β chain (AMRAD, Melbourne, Australia). Positive clones or M1 cells were then retrovirally infected (by cocultivation for 24 hours on the appropriate packaging cell line) to introduce either the α-2 chain or the truncated α chain (term1). Again, positive clones were selected by FACS analysis of G418-resistant clones, using a murine monoclonal antibody to the GM-CSF receptor α chain (Santa Cruz Biochemical, Santa Cruz, CA).
Fusion toxin.
DT388-GM-CSF was prepared and purified as previously described17 and stored as 830 μg/mL in phosphate-buffered saline (PBS) plus 1% human serum albumin (HSA) at −20°C. The material used in this study was found to kill human HL60 cells at an inhibitory concentration (IC)50 of 2 × 10−12 mol/L using a 48-hour thymidine incorporation assay to assess HL60 cell kill. The preparation at 10−9 mol/L reduced the clonogenic activity of HL60 cells in a semisolid medium by a factor of 3,000-fold.6
GM-CSF receptor density measurements.
Aliquots of 1 to 6 × 106 cells in RPMI 1640 plus 2.5% bovine serum albumin (BSA), 20 mmol/L Hepes, and 0.2% sodium azide were mixed with varying amounts of 125I Bolton-Hunter labeled human GM-CSF (80 to 120 μCi/μg, NEX249; DuPont, Boston, MA) with or without excess (1,500 ng) cold GM-CSF (Immunex, Seattle, WA) in a total volume of 150 μL in 1.5-mL Eppendorf tubes. Cells were incubated at 37°C for 30 minutes and then layered over a 200-μL oil phthalate mixture (1 part dioctylphthalate and 1.5 parts dibutylphthalate, Aldrich, Milwaukee, WI). After centrifugation at 12,000 rpm for 1 minute in a microfuge at room temperature, both pellets and supernatants were saved and counted in an LKB-Wallac 1260 Multi-gamma counter (Turku, Finland) gated for125I with 50% counting efficiency. Background cpm were calculated by linear extrapolation from incubations with excess cold GM-CSF. Scatchard plots of specific bound/free versus specific bound cpm were made. Experiments were performed in duplicate. Receptor number/cell was calculated by dividing the x intercept by (specific activity in μCi/μg times the cell number times 4.2 × 10−8); dissociation constant (kd) was calculated by multiplying the x-intercept by 2.7 × 10−13 divided by (the y-intercept times the specific activity). A statistical software package (Statsoft, Tulsa, OK) was used to perform linear regressions. Receptor densities of 0 were recorded when there was no specific 125I binding or when the kd values measured were negative.
Cellular sensitivity to DT388-GM-CSF.
Sensitivity to DT388-GM-CSF of progenitors in normal and leukemic samples was tested by exposing the cells in suspension culture for 48 hours and then assessing their residual ability to form colonies in semisolid cultures.6,18 For this, aliquots of 5 × 105 CMML, JMML, normal marrow light density, or purified CD34+ cells were incubated with different concentrations of DT388-GM-CSF (0 to 4 × 10−8 mol/L) in 150 μL of RPMI 1640 medium plus 15% FCS supplemented with 50 ng/mL G-CSF (Amgen, Thousand Oaks, CA) in 96-well flat-bottomed Costar plates at 37°C/5% CO2 in air. After 16 to 48 hours, 100-μL samples from each well were mixed with 3 mL of RPMI 1640 plus 15% FCS plus 50 ng/mL human G-CSF, 50 ng/mL human GM-CSF, 10% human 5637 bladder carcinoma cell line conditioned medium (5637 CM) and 0.3% agarose (SeaPlaque; FMC Bioproducts, Rockland, ME) and the mixture then poured into 35-mm gridded Petri dishes (Nunc, Naperville, IL) and allowed to solidify before incubation at 37°C/5% CO2in air and the number of colonies containing greater than 20 cells assessed 10 to 20 days later. Both of the concentrations of toxin reducing colony formation by 50% (IC50) and the maximal (log) cell kill values compared with control cells not exposed to toxin were calculated as previously described.6 All experiments were performed in duplicate, and control cells were treated identically to DT388-GM-CSF–treated cells, but with the absence of any toxin.
Receptor properties and DT388-GM-CSF sensitivity of mutant GM-CSF receptor cell lines.
The six different murine cell lines expressing different chains of the human GM-CSF receptor used in the present studies are listed (see Table4). Receptor affinity and density were measured as described above for patients’ cells. Their sensitivities to DT388-GM-CSF were determined using inhibition of 3H-leucine incorporation to assess effects on protein synthesis and 3H-thymidine incorporation to assess effects on proliferation after 48 hours incubation of the cells with varying concentrations of fusion proteins as previously described.5,19 These assays correlate well with measurements of clonogenicity for most myeloid cell lines.5 19
RESULTS
Clinical history of JMML and CMML patients studied.
Peripheral blood cells from 7 JMML and 22 previously untreated CMML patients were studied. The age, peripheral blood cell counts, percentage of blasts in the marrow at the time of collection of the samples, and the subsequent response of each patient to therapy are summarized in Table 1.
Clinical Data for the Patients Studied
| Patient . | Sex/Age (yr) . | Cytogenetics . | WBC ×109/L . | Hb g/dL . | Plat ×109/L . | Mono ×109/L . | BM %Bl . | Treatment . | Survival (mo) . |
|---|---|---|---|---|---|---|---|---|---|
| JMML | |||||||||
| AJ | M/3 | Normal | ND | ND | ND | ND | ND | ND | ND |
| NL | F/3 | Normal | 5 | 10 | 24 | ND | ND | BMT | 9+ |
| HA | M/2 | mono22 | 36 | 9 | 145 | 6 | 2 | None | 16+ |
| CF | M/3 | t(3;10) | 54 | ND | 7 | 3 | ND | HU | ND |
| PR | F/4 | ND | ND | ND | ND | ND | ND | ND | ND |
| MS | M/0.2 | Normal | 46 | 9 | 104 | 6 | 9 | BMT | 30+ |
| CB | M/0.2 | Normal | 86 | 8 | 46 | 4 | 1 | BMT | 11 |
| CMML | |||||||||
| G | M/76 | Normal | 67 | 8 | 78 | 7 | 1 | 5aza | 21 |
| CL | F/71 | Misc | 1 | 9 | 45 | 0.2 | 2 | Hycam | 9 |
| RS | M/71 | ND | 8 | 9 | 48 | 4 | 0 | Hycam | 22 |
| WS | M/70 | Misc | 99 | 8 | 57 | 50 | 2 | Topo/araC | 11 |
| AR | M/38 | Misc | 74 | 6 | 32 | 17 | 5 | Topo/araC | 1+ |
| JT | M/55 | Normal | 22 | 10 | 67 | 6 | 0.2 | Topo/araC | 4+ |
| CT | M/58 | Normal | 12 | 10 | 130 | 4 | 3 | None | 0+ |
| LS | F/73 | Normal | 43 | 10 | 580 | 14 | 16 | None | 0+ |
| RB | M/75 | Normal | 29 | 13 | 229 | 9 | 9 | None | 0+ |
| WB | M/65 | del 7,del19 | 49 | 10 | 71 | 14 | 4 | Interferon | 2 |
| FB | M/72 | ND | 36 | 11 | 130 | 14 | 21 | Etoposide | 1 |
| GN | M/ND | ND | 70 | ND | ND | 35 | 5 | None | 24+ |
| GW | M/78 | Normal | 85 | ND | 62 | 43 | 10 | HU | 24 |
| RM | M/56 | Normal | 33 | 14 | 25 | 7 | 1 | None | 24+ |
| EB | M/71 | Normal | 140 | 6.7 | 40 | 11 | 25 | HU | ND |
| KP | M/50 | Normal | 79 | 13 | 63 | 6 | 1 | HU, Spl | 24+ |
| SB | M/64 | Normal | 290 | 10 | 71 | 64 | 4 | HU | <1 |
| PP | M/61 | Normal | 52 | 11 | 23 | 28 | 17 | HU | 1 |
| DJ | M/30 | Normal | 73 | 12 | 43 | 64 | 22 | HU | 6 |
| ED | M/65 | Normal | 67 | 12 | 350 | 13 | 5 | HU | 18 |
| LP | F/79 | Normal | 65 | 11 | 98 | 22 | 29 | None | 12 |
| Patient . | Sex/Age (yr) . | Cytogenetics . | WBC ×109/L . | Hb g/dL . | Plat ×109/L . | Mono ×109/L . | BM %Bl . | Treatment . | Survival (mo) . |
|---|---|---|---|---|---|---|---|---|---|
| JMML | |||||||||
| AJ | M/3 | Normal | ND | ND | ND | ND | ND | ND | ND |
| NL | F/3 | Normal | 5 | 10 | 24 | ND | ND | BMT | 9+ |
| HA | M/2 | mono22 | 36 | 9 | 145 | 6 | 2 | None | 16+ |
| CF | M/3 | t(3;10) | 54 | ND | 7 | 3 | ND | HU | ND |
| PR | F/4 | ND | ND | ND | ND | ND | ND | ND | ND |
| MS | M/0.2 | Normal | 46 | 9 | 104 | 6 | 9 | BMT | 30+ |
| CB | M/0.2 | Normal | 86 | 8 | 46 | 4 | 1 | BMT | 11 |
| CMML | |||||||||
| G | M/76 | Normal | 67 | 8 | 78 | 7 | 1 | 5aza | 21 |
| CL | F/71 | Misc | 1 | 9 | 45 | 0.2 | 2 | Hycam | 9 |
| RS | M/71 | ND | 8 | 9 | 48 | 4 | 0 | Hycam | 22 |
| WS | M/70 | Misc | 99 | 8 | 57 | 50 | 2 | Topo/araC | 11 |
| AR | M/38 | Misc | 74 | 6 | 32 | 17 | 5 | Topo/araC | 1+ |
| JT | M/55 | Normal | 22 | 10 | 67 | 6 | 0.2 | Topo/araC | 4+ |
| CT | M/58 | Normal | 12 | 10 | 130 | 4 | 3 | None | 0+ |
| LS | F/73 | Normal | 43 | 10 | 580 | 14 | 16 | None | 0+ |
| RB | M/75 | Normal | 29 | 13 | 229 | 9 | 9 | None | 0+ |
| WB | M/65 | del 7,del19 | 49 | 10 | 71 | 14 | 4 | Interferon | 2 |
| FB | M/72 | ND | 36 | 11 | 130 | 14 | 21 | Etoposide | 1 |
| GN | M/ND | ND | 70 | ND | ND | 35 | 5 | None | 24+ |
| GW | M/78 | Normal | 85 | ND | 62 | 43 | 10 | HU | 24 |
| RM | M/56 | Normal | 33 | 14 | 25 | 7 | 1 | None | 24+ |
| EB | M/71 | Normal | 140 | 6.7 | 40 | 11 | 25 | HU | ND |
| KP | M/50 | Normal | 79 | 13 | 63 | 6 | 1 | HU, Spl | 24+ |
| SB | M/64 | Normal | 290 | 10 | 71 | 64 | 4 | HU | <1 |
| PP | M/61 | Normal | 52 | 11 | 23 | 28 | 17 | HU | 1 |
| DJ | M/30 | Normal | 73 | 12 | 43 | 64 | 22 | HU | 6 |
| ED | M/65 | Normal | 67 | 12 | 350 | 13 | 5 | HU | 18 |
| LP | F/79 | Normal | 65 | 11 | 98 | 22 | 29 | None | 12 |
Abbreviations: Bl, blasts; Spl, splenectomy; HU, hydroxyurea; ND, not determined; Misc, miscellaneous chromosomal defects; 5aza, 5-azacytidine; Hycam, hycamp-tamine; topo/araC, topotecan plus cytosine arabinoside; NL is patient 36 and HA is patient 38 of The Hospital for Sick Children; CF is patient J96 and PR is patient J95 from UAB.
Progenitor cells from some JMML and CMML patients show increased sensitivity to DT388-GM-CSF in vitro.
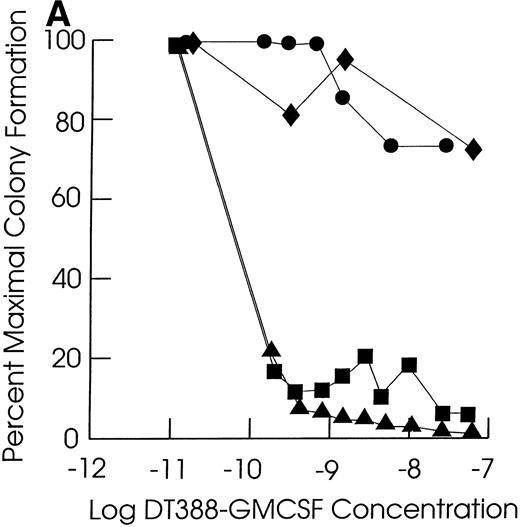
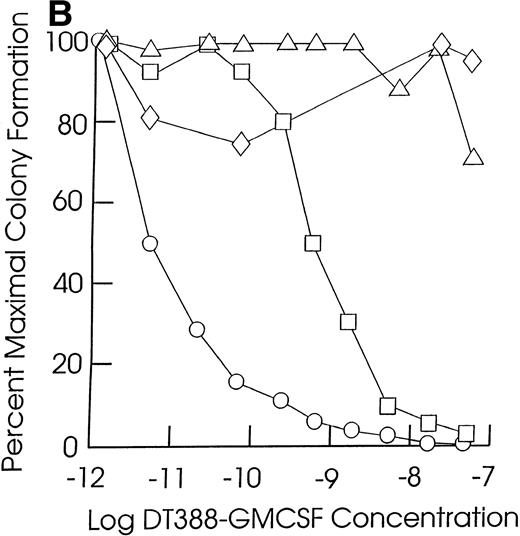
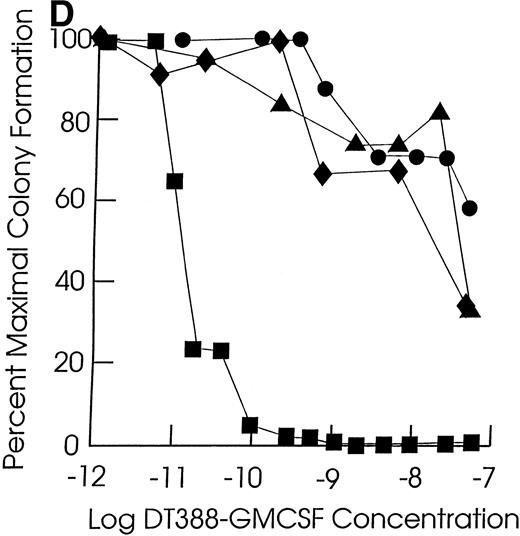
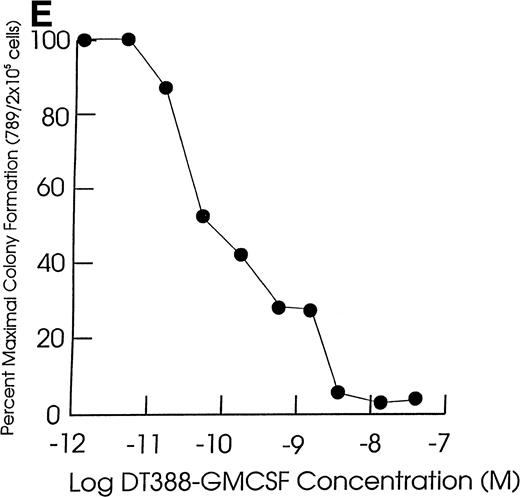
As shown in Table 2, all of the leukemic and normal samples showed colony growth in semisolid medium in the absence of fusion toxin. Exposure of JMML and CMML cells to DT388-GM-CSF for 48 hours resulted in a significant (P < .05, Student’s t-test) loss of progenitor activity in five of the seven JMML cases (71%) and in 12 of the 20 CMML cases studied (57%, Fig 1 and Table 2). The cells in the other two JMML cases showed intermediate sensitivities to DT388-GM-CSF with less than 1 log cell kill and an IC50 of 10−10 mol/L to 10−11 mol/L. In experiments with the other eight CMML samples, there was no significant loss of progenitor activity even in the presence of 4 × 10−8 mol/L DT388-GM-CSF. The DT388-GM-CSF sensitivity of the progenitors from two other CMML samples was not studied. A lack of sensitivity was exhibited by the clonogenic progenitors in six normal marrow samples treated and assessed under identical conditions. These included three light density normal marrow cell preparations, as well as three highly purified (>99%) CD34+ cell preparations isolated from another three normal donors.
Sensitivity of Myeloid Progenitors to DT388-GM-CSF In Vitro
| Patient . | Disease . | No. Colonies/ 2 × 105Cells . | IC50 (pmol/L) . | Maximal Log Cell Kill . |
|---|---|---|---|---|
| AJ | JMML | 790 | 160 | 1.3 |
| NL | JMML | 560 | 20 | 1.4 |
| HA | JMML | 2700 | 20 | 1.4 |
| CF | JMML | 26 | 10 | 1.4 |
| PR | JMML | 11 | 10 | 1.1 |
| MS | JMML | 90 | 4 | 0.8 |
| CB | JMML | 900 | 80 | 0.7 |
| G | CMML | 80 | 5 | 1.3 |
| CL | CMML | 210 | 60 | 1.5 |
| RS | CMML | 560 | 1500 | 0.7 |
| WS | CMML | 680 | 1 | 1.8 |
| AR | CMML | 81 | 3 | 1.9 |
| JT | CMML | 480 | 20 | 1.0 |
| CT | CMML | ND | ND | ND |
| LS | CMML | 31 | 40,000 | 0.3 |
| RB | CMML | 110 | >40,000 | 0.3 |
| WB | CMML | 130 | 8 | 2.2 |
| FB | CMML | 280 | 600 | 2.4 |
| GN | CMML | 17 | 20 | 1.3 |
| GW | CMML | 220 | 80 | 2.4 |
| RM | CMML | 120 | 70 | 2.2 |
| EB | CMML | 41 | 70 | 1.5 |
| KP | CMML | 20 | >40,000 | <0.3 |
| SB | CMML | 2600 | >40,000 | <0.3 |
| PP | CMML | 32 | >40,000 | <0.3 |
| DJ | CMML | 130 | 500 | 2.2 |
| ED | CMML | 3500 | >40,000 | <0.3 |
| LP | CMML | 120 | >40,000 | <0.3 |
| CAD25* | Normal | 200 | 1000 | 0.9 |
| CAD* | Normal | 2100 | 40,000 | 0.3 |
| CAD24D* | Normal | 2200 | 4000 | 0.6 |
| U11802 | Normal | 190 | >40,000 | <0.3 |
| U11811 | Normal | 260 | 40,000 | <0.3 |
| U11454 | Normal | 27 | 40,000 | <0.3 |
| Patient . | Disease . | No. Colonies/ 2 × 105Cells . | IC50 (pmol/L) . | Maximal Log Cell Kill . |
|---|---|---|---|---|
| AJ | JMML | 790 | 160 | 1.3 |
| NL | JMML | 560 | 20 | 1.4 |
| HA | JMML | 2700 | 20 | 1.4 |
| CF | JMML | 26 | 10 | 1.4 |
| PR | JMML | 11 | 10 | 1.1 |
| MS | JMML | 90 | 4 | 0.8 |
| CB | JMML | 900 | 80 | 0.7 |
| G | CMML | 80 | 5 | 1.3 |
| CL | CMML | 210 | 60 | 1.5 |
| RS | CMML | 560 | 1500 | 0.7 |
| WS | CMML | 680 | 1 | 1.8 |
| AR | CMML | 81 | 3 | 1.9 |
| JT | CMML | 480 | 20 | 1.0 |
| CT | CMML | ND | ND | ND |
| LS | CMML | 31 | 40,000 | 0.3 |
| RB | CMML | 110 | >40,000 | 0.3 |
| WB | CMML | 130 | 8 | 2.2 |
| FB | CMML | 280 | 600 | 2.4 |
| GN | CMML | 17 | 20 | 1.3 |
| GW | CMML | 220 | 80 | 2.4 |
| RM | CMML | 120 | 70 | 2.2 |
| EB | CMML | 41 | 70 | 1.5 |
| KP | CMML | 20 | >40,000 | <0.3 |
| SB | CMML | 2600 | >40,000 | <0.3 |
| PP | CMML | 32 | >40,000 | <0.3 |
| DJ | CMML | 130 | 500 | 2.2 |
| ED | CMML | 3500 | >40,000 | <0.3 |
| LP | CMML | 120 | >40,000 | <0.3 |
| CAD25* | Normal | 200 | 1000 | 0.9 |
| CAD* | Normal | 2100 | 40,000 | 0.3 |
| CAD24D* | Normal | 2200 | 4000 | 0.6 |
| U11802 | Normal | 190 | >40,000 | <0.3 |
| U11811 | Normal | 260 | 40,000 | <0.3 |
| U11454 | Normal | 27 | 40,000 | <0.3 |
For treatment conditions and assay details, see Materials and Methods.
Experiments performed with purified CD34+ cells from these samples.
Effect of exposing cells to DT388-GM-CSF in liquid culture for 48 hours followed by measurement of the remaining progenitor activity in semisolid assays. (A through C) Cells from representative CMML patients; (D) cells from normal marrow samples and HL60 cells (plated at 2 × 104 cells/dish); (E) cells from a representative JMML patient. (A) (⧫), KP; (▴), RM; (•), SB; (▪), EB. (B) (◊), ED; (▵), LP; (○), WB; (□), FB. (C) (⧫), PP; (▴), GW; (•), GN; (▪), DJ. (D) (⧫), U11811; (▴), U11454; (•), U11802; (▪), HL60; (E) (•), AJ.
Effect of exposing cells to DT388-GM-CSF in liquid culture for 48 hours followed by measurement of the remaining progenitor activity in semisolid assays. (A through C) Cells from representative CMML patients; (D) cells from normal marrow samples and HL60 cells (plated at 2 × 104 cells/dish); (E) cells from a representative JMML patient. (A) (⧫), KP; (▴), RM; (•), SB; (▪), EB. (B) (◊), ED; (▵), LP; (○), WB; (□), FB. (C) (⧫), PP; (▴), GW; (•), GN; (▪), DJ. (D) (⧫), U11811; (▴), U11454; (•), U11802; (▪), HL60; (E) (•), AJ.
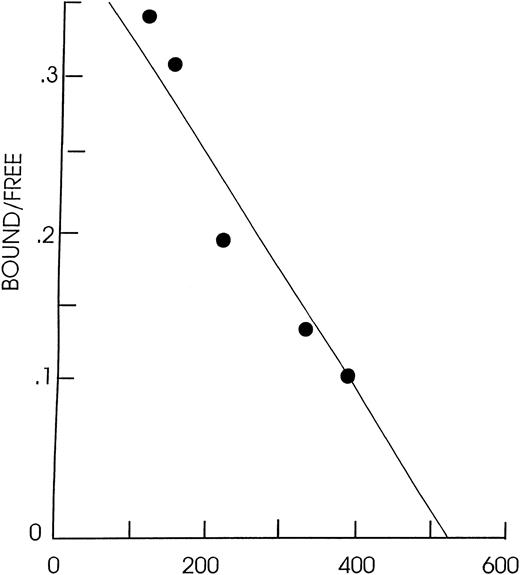
GM-CSF receptor numbers and affinities on JMML and CMML cells are normal.
To determine whether the increased DT388-GM-CSF sensitivity of the progenitors from all JMML and some CMML patients might reflect an increased expression of GM-CSF receptors, receptor densities (and affinities for GM-CSF) were determined by Scatchard analysis for most of the samples studied biologically. Representative Scatchard plots are shown in Fig 2. The results for five of the seven JMML patients’ samples and all 21 CMML patients’ samples and for cells from five normal marrow donors are shown in Table 3. The light density JMML and CMML cells all showed ≥120 high-affinity GM-CSF receptors/cell with a mean GM-CSF receptor density of 260 ± 90 (mean ± standard error of mean [SEM]) for the JMML cells and of 590 ± 100 for the CMML cells. The number of GM-CSF receptors per normal light density marrow cell was 340 ± 130 (n = 5). The average values for GM-CSF receptor numbers and affinities for light density JMML and CMML peripheral blood cells and normal marrow light density and CD34+ cells are not significantly different (P > .05, Student’st-test). There was no correlation between DT388-GM-CSF sensitivity (IC50) and the level of GM-CSF receptor expression among the JMML or CMML samples studied (r = .2). Thus, the lack of a toxic action of DT388-GM-CSF on leukemic cells from some patients with JMML or CMML cannot be attributed to a decreased level of GM-CSF receptor expression as appears to be the case in AML.6
Scatchard plot of JMML cells (patient CF) obtained using 1.3 × 106 cells per aliquot and125I-GM-CSF at a specific activity of 54 μCi/μg.r2 = .92 using 0.1 to 2 pmol125I-GM-CSF, kd = 6 × 10−12 mol/L and receptor density = 164 GM-CSF receptors/cell.
Scatchard plot of JMML cells (patient CF) obtained using 1.3 × 106 cells per aliquot and125I-GM-CSF at a specific activity of 54 μCi/μg.r2 = .92 using 0.1 to 2 pmol125I-GM-CSF, kd = 6 × 10−12 mol/L and receptor density = 164 GM-CSF receptors/cell.
GM-CSF Receptors on Light Density JMML and CMML Peripheral Blood and Normal Marrow Cells
| Patient . | Disease . | High-Affinity Receptors/Cell . | kd × 10−11 . |
|---|---|---|---|
| AJ | JMML | 141 | 1.2 |
| NL | JMML | ND | ND |
| HA | JMML | ND | ND |
| CF | JMML | 164 | 0.6 |
| PR | JMML | 119 | 0.6 |
| MS | JMML | 310 | 0.6 |
| CB | JMML | 539 | 2 |
| G | CMML | 636 | 2 |
| CL | CMML | 344 | 1.2 |
| RS | CMML | 624 | 1.1 |
| WS | CMML | 188 | 0.8 |
| AR | CMML | 384 | 1.4 |
| JT | CMML | 657 | 2 |
| CT | CMML | 1512 | 1.3 |
| LS | CMML | 268 | 0.6 |
| RB | CMML | 1720 | 1.3 |
| WB | CMML | 1100 | 2.9 |
| FB | CMML | 490 | 2.9 |
| GN | CMML | 310 | 0.5 |
| GW | CMML | 240 | 0.3 |
| RM | CMML | 990 | 2 |
| EB | CMML | 350 | 2.9 |
| KP | CMML | 170 | 2 |
| SB | CMML | 420 | 1 |
| PP | CMML | 410 | 2 |
| DJ | CMML | 330 | 1 |
| ED | CMML | 890 | 2.6 |
| LP | CMML | 340 | 1.5 |
| CAD253-150 | Normal | 525 | 0.4 |
| CAD3-150 | Normal | 159 | 1 |
| U11802 | Normal | 420 | 0.6 |
| U11811 | Normal | 580 | 1 |
| U11454 | Normal | 16 | 0.3 |
| Patient . | Disease . | High-Affinity Receptors/Cell . | kd × 10−11 . |
|---|---|---|---|
| AJ | JMML | 141 | 1.2 |
| NL | JMML | ND | ND |
| HA | JMML | ND | ND |
| CF | JMML | 164 | 0.6 |
| PR | JMML | 119 | 0.6 |
| MS | JMML | 310 | 0.6 |
| CB | JMML | 539 | 2 |
| G | CMML | 636 | 2 |
| CL | CMML | 344 | 1.2 |
| RS | CMML | 624 | 1.1 |
| WS | CMML | 188 | 0.8 |
| AR | CMML | 384 | 1.4 |
| JT | CMML | 657 | 2 |
| CT | CMML | 1512 | 1.3 |
| LS | CMML | 268 | 0.6 |
| RB | CMML | 1720 | 1.3 |
| WB | CMML | 1100 | 2.9 |
| FB | CMML | 490 | 2.9 |
| GN | CMML | 310 | 0.5 |
| GW | CMML | 240 | 0.3 |
| RM | CMML | 990 | 2 |
| EB | CMML | 350 | 2.9 |
| KP | CMML | 170 | 2 |
| SB | CMML | 420 | 1 |
| PP | CMML | 410 | 2 |
| DJ | CMML | 330 | 1 |
| ED | CMML | 890 | 2.6 |
| LP | CMML | 340 | 1.5 |
| CAD253-150 | Normal | 525 | 0.4 |
| CAD3-150 | Normal | 159 | 1 |
| U11802 | Normal | 420 | 0.6 |
| U11811 | Normal | 580 | 1 |
| U11454 | Normal | 16 | 0.3 |
Determined by Scatchard analysis between 0.6 pmol/L and 26 pmol/L125I-GM-CSF as described in the text. There were insufficient cells from patients NL, HA, CAD24D for receptor analyses.
CAD25 and CAD were CD34+ cells.
Investigations of the role of altered GM-CSF receptors on DT388-GM-CSF sensitivity.
To evaluate other types of alterations in GM-CSF receptor structure or signaling that might enhance cellular sensitivity to DT388-GM-CSF, several murine factor-dependent hematopoietic cell lines expressing different parts of the α and/or β chains of the human GM-CSF receptor were created as described in Materials and Methods. As expected, cell lines expressing only the α or β chain of the human GM-CSF receptor failed to bind human GM-CSF (Table 4). In contrast, all four cell lines expressing both the extracellular part of the α chain and the β chain of the human GM-CSF receptor did bind ligand and with a high-affinity. The numbers of ligand-binding receptors per transduced cell ranged from 2,700 to 9,400; ie, approximately 10-fold higher than seen with primary normal or JMML/CMML human hematopoietic cells (Table3). Interestingly, all four mouse cell lines capable of binding human GM-CSF were sensitive to DT388-GM-CSF at concentrations similar to those that kill primary human leukemia cells (Table 2 and Hogge et al6). However, the presence of an intracytoplasmically truncated α chain, which blocks GM-CSF–induced signaling did cause a fivefold reduction in sensitivity to DT388-GM-CSF in M1 cells and a 100-fold reduction in sensitivity in 32D cells exposed to the same agent. α Subunit alone expressed at high levels (10,000 to 20,000/cell) showed reduced but present sensitivity to DT388-GM-CSF. This suggests that the induction of cell kill in these cells is enhanced by the activation of internal receptor signaling events.
Properties of Cell Lines Expressing Mutant Human GM-CSF Receptors
| Cell Line . | GM-CSF Receptor Content . | kd (×10−11 mol/L) . | High- Affinity Receptors/ Cell . | Protein Synthesis IC50 (×10−12mol/L) . | Proliferation IC50 (×10−12mol/L) . |
|---|---|---|---|---|---|
| M1α2 | α alone | ND | 0 | >4,000 | >4,000 |
| M1β7 | β alone | ND | 0 | >4,000 | >4,000 |
| M1β7T1 | β plus term1 α | 4.5 | 2,700 | 10 | 10 |
| M1β7α2 | β plus α | 13 | 9,400 | 4 | 2 |
| 32Dα2 | α | ND | 0 | 120 | 80 |
| 32Dβ2 | β | ND | 0 | >4,000 | >4,000 |
| 32Dβ2T1 | β plus term1 α | 2 | 4,100 | 10 | 400 |
| 32Dβ2α2 | β plus α | 3 | 3,300 | 1 | 4 |
| Cell Line . | GM-CSF Receptor Content . | kd (×10−11 mol/L) . | High- Affinity Receptors/ Cell . | Protein Synthesis IC50 (×10−12mol/L) . | Proliferation IC50 (×10−12mol/L) . |
|---|---|---|---|---|---|
| M1α2 | α alone | ND | 0 | >4,000 | >4,000 |
| M1β7 | β alone | ND | 0 | >4,000 | >4,000 |
| M1β7T1 | β plus term1 α | 4.5 | 2,700 | 10 | 10 |
| M1β7α2 | β plus α | 13 | 9,400 | 4 | 2 |
| 32Dα2 | α | ND | 0 | 120 | 80 |
| 32Dβ2 | β | ND | 0 | >4,000 | >4,000 |
| 32Dβ2T1 | β plus term1 α | 2 | 4,100 | 10 | 400 |
| 32Dβ2α2 | β plus α | 3 | 3,300 | 1 | 4 |
M1β7 are M1 cells expressing the wild-type human GM-CSF receptor β chain.
Abbreviations: T1, term1 mutant human GM-CSF receptor α chain; α2, human GM-CSF receptor α chain; 32Dβ2, 32D cells expressing the wild-type human GM-CSF receptor β chain; M1α2, M1 cells expressing α2 α chain.
DISCUSSION
JMML and CMML are both heterogeneous disorders with variable cytogenetics and clinical courses generally affecting younger children (<5 years old) and older men (>50 years old), respectively.3,4,20,21 Nevertheless, for both, the prognosis without allogeneic bone marrow transplantation is dismal. Among the 22 CMML patients studied here, there was marked variability in the peripheral white blood cell (WBC) count, the extent of monocytosis, and the percentage of blasts in the marrow. Also, none showed the t(5;12) cytogenetic abnormality that has been associated with CMML. The majority were older men in agreement with the reported prevalence of the disease in this group.1 The WBC was, on average, higher and the platelet count, on average, lower than previously reported1; however, a skewing of these values may have been incurred by the biassed selection of peripheral blood samples suitable for cryopreservation.
The presence of high-affinity GM-CSF receptors was demonstrated on the light density cells present in both JMML and CMML blood, as well as light density and highly purified CD34+ cells from normal marrow samples. In all cases, the GM-CSF receptor numbers measured represent averages for heterogeneous mixtures of different cell types, only a fraction of which possess progenitor activity.9 The fact that the progenitors from a majority of the JMML and CMML patients (70% and 60%, respectively) that we studied were inactivated after exposure of the cells to DT388-GM-CSF confirms that these cells also express GM-CSF receptors. Similarly, mouse cells engineered to express a human GM-CSF–binding human GM-CSF receptor acquired sensitivity to DT388-GM-CSF, whereas those expressing only the human GM-CSF receptor β chain did not (Table 4) and, in human AML, sensitivity to DT388-GM-CSF was seen only in patients whose blasts showed evidence of GM-CSF receptor expression at normal (or higher) levels.6
GM-CSF receptor expression, although prerequisite for DT388-GM-CSF sensitivity, is, however, likely not to be the only factor involved. Normal human progenitors are known to express GM-CSF receptor mRNAs and respond to GM-CSF activation22,23 despite their insensitivity to DT388-GM-CSF,5,17,24,25 as confirmed here. Moreover, our assessment of GM-CSF receptor numbers on normal CD34+ cells (enriched for CFU-GM)7,8 suggests that similar values would be obtained for those with progenitor activity, despite their relative resistance to DT388-GM-CSF. However, we were not able to measure receptors quantitatively on individual progenitors. On the other hand, it is interesting to note that normal mouse CFU-GM have been found to be sensitive to a similar fusion toxin (DT389-mGM-CSF).26 Similarly, why some AML progenitors become much more sensitive to DT388-GM-CSF than their normal counterparts does not appear to be explained by differences in GM-CSF receptor expression.6 In the present study, an increased sensitivity to DT388-GM-CSF of JMML and CMML progenitors could also not be attributed to an abnormally elevated expression of GM-CSF receptors on their cells. Two studies have shown that some adult CMML patients display a hypersensitivity of their progenitors to GM-CSF stimulation in vitro, similar to JMML, although others have failed to show such GM-CSF hypersensitivity.1 27 It will thus be of interest to determine whether this feature is associated with DT388-GM-CSF sensitivity in a subgroup of CMML patients.
Progenitors that are not sensitive to DT388-GM-CSF may simply resemble their normal counterparts where a postbinding step involving, for example, receptor internalization or expression of antiapoptotic proteins5,6,28 may contribute to the observed resistance. Higher intracellular concentrations of antiapoptotic proteins in normal as compared with AML progenitors has been reported29 30 and could explain the failure of normal cells to undergo apoptosis after exposure to concentrations of DT388-GM-CSF that are sufficient to engage their GM-CSF receptors and which can effectively kill other cells with similar GM-CSF receptor densities. The fact that two cell lines expressing human GM-CSF receptors capable of binding ligand, but not signaling, were still susceptible to DT388-GM-CSF–induced killing, albeit with differently reduced sensitivities, further underscores the likelihood that other intracellular factors are important determinants. Additional studies with other mutant or transduced cell lines should help to identify what these are and their mechanisms of action.
JMML is a disorder with deregulated signal transduction through the Ras signaling pathway resulting in hypersensitivity to GM-CSF, but normal sensitivity to interleukin-3 and G-CSF.31Previous studies have demonstrated a normal GM-CSF receptor on JMML cells by fluorescence-labeled GM-CSF binding and by subunit sequence analysis.32 33 Hypersensitivity to GM-CSF appears to be a unifying characteristic in JMML patients. The present finding of an intermediate to high sensitivity toDT388-GM-CSF of the progenitors in all JMML patient samples tested suggests these two biological features may be mechanistically related.
Whatever the mechanisms that underlie the elevated DT388-GM-CSF sensitivity of some leukemic progenitors, its wide therapeutic index makes it an attractive therapeutic agent for consideration. The sensitivity of these rare myeloid leukemias resembled that of AML based on IC50 and log cell kill.6 13-cisretinoic acid demonstrates an overall 40% to 50% response rate in JMML, but does not appear to be sufficient to induce durable remissions.31,34 Hydroxyurea can prolong the survival of some CMML patients better than etoposide, but the median survival is still less than 20 months, and patients with this disease continue to die of bleeding, infection, and transformation to AML.35The use of topotecan in CMML has produced some complete remissions, but has also been found to cause severe mucositis, diarrhea, infections, and a poor overall median survival (<1 year).36 All trans retinoic acid (ATRA) significantly reduces CMML colony formation in vitro, but in vivo, the ATRA syndrome was seen and survival was not improved.37 Thus, in the absence of effective strategies for the treatment of either JMML or CMML, the experiments in this study underscore the need for further preclinical development of DT388-GMCSF as a new therapeutic agent that may have some promise for patients with a broad spectrum of poor prognosis leukemias.
ACKNOWLEDGMENT
We thank members of the Stem Cell Assay Service and the Division of Hematology of the B.C. Cancer Agency and Vancouver Hospital for provision of patient materials, normal marrow CD34+ cell purification, and clinical information. We thank Drs C. Chomienne and B. Cassinat (Laboratoire de Biologie Cellulaire Hematopoietique, University of Paris, Paris, France) for access to JMML sample AJ and Drs J. Prchal and R. Mayor (Birmingham, AL) for clinical information. We also thank J. Nicholson for photography and graphic analysis.
Supported by Leukemia Society of America Grant No. 6114-98 (to A.F.), NIH Grants No. R01CA76178 (to A.F.), CA45672 (to M.L.), U01CA60407 (to P.E.), and R01CA54116 (to E.T.) and grants from the National Cancer Institute of Canada (NCIC) (to C.E. and D.H.) with funds from the Terry Fox Run. C.E. is a Terry Fox Cancer Research Scientist of the NCIC.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Arthur E. Frankel, MD, Hanes 4046, Bowman Gray School of Medicine, Med Center Drive, Winston-Salem, NC 27157.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal