Abstract
Despite the fact that Hodgkin’s and Reed-Sternberg (H-RS) cells are morphological hallmarks of Hodgkin’s disease (HD), the nature of H-RS cells still remains to be resolved. Here we report that downregulation of CD99 (Mic2) leads to the generation of cells with an H-RS phenotype. IM9 and BJAB B-cell lines that were transfected with an antisense CD99 expression construct showed the morphological and immunological characteristics of H-RS cells such as multinuclearity, expression of CD15, decreased expression of major histocompatibility complex (MHC) class I and CD45RB, and deregulated secretion of cytokines. The reduced expression of CD99 was also confirmed in H-RS cells of patient’s lymph nodes and three HD-derived cell lines, L428, KM-H2, and HDLM-2. Moreover, features characteristic of H-RS cells were completely abolished by forced expression of CD99 and by a constitutively active form of Rac, which functions downstream of CD99. We suggest that CD99 molecules play a crucial role in regulating functions and morphology of cells through a Rac-Rho signaling pathway and that the loss of CD99 expression is a significant molecular event to generate H-RS cells.
HODGKIN’S DISEASE (HD) is a lymphoid neoplasm characterized by a low frequency of malignant tumor giant cells, known as Hodgkin’s and Reed-Sternberg (H-RS) cells, in an abundant background of nonneoplastic inflammatory cells.1,2The nature and origin of H-RS cells remain controversial even 160 years after the initial description of HD. Advances in the understanding of H-RS cell derivation have been made by studies that include immunophenotyping, genotyping, and cytokine production of H-RS cells in HD specimens or cell lines from HD tissues.3,4 In HD, unbalanced cytokine production elicits an abundance of reactive cells mainly comprising T cells, B cells, macrophages, and so on.5-9 However, it is intriguing that these infiltrates do not kill H-RS cells even though many H-RS cells contain Epstein-Barr virus (EBV) antigens.10,11 Indeed, HD patients show a severe impairment in their cellular immune responses.12 The frequent absence of major histocompatibility complex (MHC) class I expression in H-RS cells provides one explanation for the impaired CD8+ cytotoxic T-lymphocyte (CTL) response against EBV protein.13 14
CD99 (Mic2)15-17 is a 32-kD transmembrane glycoprotein that is involved in cell-cell adhesion during hematopoietic cell differentiation,18 apoptosis of immature thymocytes,19 and transport of transmembrane proteins.20 During investigation into the expression pattern of CD99 in various types of cells, we incidentally found out that spontaneously occurring H-RS–like multinuclear giant cells were devoid of CD99 expression. These findings led us to study CD99 expression in lymph nodes and cell lines from HD patients. In all cases examined, we were not able to see any expression of CD99 in H-RS cells. Subsequently, we investigated the relationship between downregulation of CD99 and generation of H-RS–like cells. This possibility was tested with a use of CD99-deficient IM9 and BJAB B-cell lines, which individually represent two extremes of B-cell differentiation. In this study, we were able to confirm that the enforced downregulation of CD99 generates H-RS–like cells. Because these cells also had immunological and functional features characteristic of H-RS cells, we suggest that CD99 downregulation is a significant molecular event to generate H-RS cells.
MATERIALS AND METHODS
Tissue and cells.
The lymph nodes were obtained from patients with HD. IM9 (Ig-secreting lymphoblast) and BJAB (Burkitt’s lymphoma) cell lines were obtained from American Type Culture Collection (ATCC; Rockville, MD). Three HD-derived cell lines (HDLM-2, L428, and KM-H2) were purchased from German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany). All cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) or RPMI 1640 media supplemented with 10% or 20% heat-inactivated fetal calf serum (FCS).
Gene constructs and transfection.
A full-length CD99 cDNA was inserted into the HindIII andXba I site of mammalian expression vector, RC-CMV, in the sense orientation or antisense orientation. Two constructs of Rac, constitutively active (L61) or partially active (L61F37A), were kind gifts of Allan Hall (University College London, London, UK). For the study of the relationship between Rac and CD99, CD99 cDNA was cloned at BamHI site in the antisense orientation and two forms of Rac were inserted at Hpa I site in the sense orientation of MTIN vector. LTR-driven transcripts for antisense CD99 and Rac genes are made as a polycistronic mRNA and the translation of Rac is guided by EMCV IRES sequence. Stable neomycin-resistant IM9 and BJAB transfectants were established after lipofectin-mediated (GIBCO-BRL, Gaithersburg, MD) gene transfer as described.18 Establishment and subcloning of stable cell lines were accomplished by culturing primary transfectants in the presence of 500 μg/mL of G-418 (GIBCO-BRL) for 1 month. All original and modified cell lines used in this study are summarized in Table 1.
Cell Lines Used in This Study
| Cell Lines Used . | Characteristics . |
|---|---|
| IM9 | A lymphoblastoid B-cell line that was transformed with EBV |
| Vec-TF IM9 | IM9 cells which were stably transfected with empty vector construct |
| AS-TF IM9 | IM9 cell lines which were stably transfected with antisense CD99 expression construct |
| Mut-IM9 | A spontaneous mutant of IM9 cells which was negative for CD99 expression |
| BJAB | A Burkitt’s lymphoma B-cell line that is free of EBV |
| AS-TF BJAB | BJAB cells lines which were stably transfected with antisense CD99 expression construct |
| HDLM-2 | Established from nodular sclerosis of HD, T lineage |
| L428 | Established from nodular sclerosis of HD, probable dendritic cell origin |
| KM-H2 | Established from mixed cellularity of HD, B lineage |
| Cell Lines Used . | Characteristics . |
|---|---|
| IM9 | A lymphoblastoid B-cell line that was transformed with EBV |
| Vec-TF IM9 | IM9 cells which were stably transfected with empty vector construct |
| AS-TF IM9 | IM9 cell lines which were stably transfected with antisense CD99 expression construct |
| Mut-IM9 | A spontaneous mutant of IM9 cells which was negative for CD99 expression |
| BJAB | A Burkitt’s lymphoma B-cell line that is free of EBV |
| AS-TF BJAB | BJAB cells lines which were stably transfected with antisense CD99 expression construct |
| HDLM-2 | Established from nodular sclerosis of HD, T lineage |
| L428 | Established from nodular sclerosis of HD, probable dendritic cell origin |
| KM-H2 | Established from mixed cellularity of HD, B lineage |
Flow cytometric analysis.
For staining, 106 cells were first incubated with relevant monoclonal antibodies (MoAbs) (1 μg/100 μL) in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) and 0.1% sodium azide for 30 minutes at 4°C. The cells were then washed twice with PBS, stained with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG antibody, and, after another washing with PBS, analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA). The antibodies used were purchased from Becton Dickinson (CD15, CD30, CD45RA, and CD45RB), Pharmingen (CD30 and CD69; San Diego, CA), Serotec (CD23, CD38, CD40, CD69, and CD80; Oxford, UK), Dako (CD19, CD21, and CD25; Carpinteria, CA), or obtained from hybridoma culture (MHC class I [W6/32, ATCC]). The MoAbs to MHC class II (YG18) and CD99 (DN16) were developed in this laboratory. The secondary antibody used was FITC-conjugated goat anti-mouse IgG Ab (Dako).
Confocal microscopic analysis of lymph node sections.
Lymph nodes were embedded in Tissue-Tec (Miles Inc, Elkhart, IN) and frozen on dry ice. Five-micrometer–thick frozen sections were cut and mounted onto poly-L-lysine-coated slides, permeabilized, fixed in cold acetone/methanol (50%/50% vol/vol) for 10 minutes, and blocked in 10% fetal bovine serum (FBS). The slides were incubated overnight with given MoAbs conjugated to FITC or biotin at a concentration between 10 and 20 μg/mL in PBS/1% BSA/0.02% sodium azide. After washing, they were incubated for 4 hours with streptoavidin-Texas Red (Caltag, Burlingame, CA) and mounted with PBS/azide/10% glycerol. The stained sections were examined by immunofluorescence confocal microscopy (BioRad 1024; BioRad Labs, Hercules, CA).
Northern blot analysis.
Cells were dissolved in TRIzol reagent (Life Technologies, Grand Island, NY) and RNA was extracted following the manufacturer’s instructions. Total RNA (20 μg) from each sample was separated by electrophoresis on a 1.0% agarose/formaldehyde gel and blotted onto nylon filters (Hybond-N+; Amersham International, Amersham, UK). The filters were hybridized with [α-32P-dCTP]–labeled cDNA fragments, then washed under stringent conditions (65°C for 30 minutes in washing buffer composed of 0.1× standard saline citrate [SSC] and 0.1% sodium dodecyl sulfate [SDS]), and detected by autoradiography. A full-length CD99 cDNA was used as a probe. The filters were stripped and rehybridized with a cDNA probe for β-actin as an internal control.
Cell-cycle analysis.
Asynchronous populations of IM9 transfectants in the log-phase of cell growth were fixed in 70% ethanol in PBS on ice, pelleted, incubated with RNase A (0.1 μg/mL) for 30 minutes at 37°C, and then stained with propidium iodide (40 μg/mL). The cell-cycle profiles (10,000 cells per sample) were analyzed on a FACScan machine.
Cytokine analysis.
IM9 and BJAB transfectants were cultured at 2 × 105/mL in 24-well microplates with either RPMI media alone or media supplemented with soluble CD40L (supernatant, 1:10 ratio). After a 48-hour incubation, supernatants were assayed for interleukin-10 (IL-10) or transforming growth factor β1 (TGF-β1) level by an ELISA kit (R & D, Minneapolis, MN). For the TGF-β1 assay, serum-free medium was used. Culture supernatant of soluble trimeric CD40L was a gift of Tae Ho Lee (Yonsei University, Seoul, Korea).
RESULTS
Lack of CD99 expression in H-RS cells from the lymph nodes of HD patients and in HD-derived cell lines.
In the course of long-term culture of IM9 cells, we noticed that spontaneously occurring H-RS–like multinucleated giant cells were consistently devoid of CD99 expression. This finding prompted us to examine whether CD99 is also downregulated in the H-RS cells from lymph nodes of HD patients. In 28 cases of HD studied, H-RS cells were consistently negative for CD99 expression by immunofluorescence and immunohistochemical analyses. In contrast, activated lymphocytes from lymph nodes of 15 cases of reactive lymphadenopathy were positive for CD99 and were taken as controls. The notion that CD99 is upregulated in activated lymphocytes was based on the flow cytometric finding that CD69+ cells expressed CD99 in a much higher level than did CD69− cells in these reactive lymph nodes (data not shown). Lack of CD99 expression in H-RS cells from HD patients was again shown with confocal microscopic analysis. Expression of CD99 was almost completely absent in H-RS cells, whereas a considerable number of surrounding activated lymphocytes showed strong reactivity (Fig 1A). CD99 expression in established HD-derived cell lines was almost identical to that of H-RS cells from lymph nodes of HD patients. Two HD-derived cell lines, L428 and KM-H2, showed no expression of CD99 as detected by Northern blot and flow cytometric analyses (Fig 2). In the case of HDLM-2 cell line, expression of CD99 was markedly diminished. These results strongly suggest that downregulation of CD99 expression is tightly correlated with the appearance of H-RS cells. For this reason, we addressed the question of whether forced downregulation of CD99 was able to produce similar H-RS–like cells.
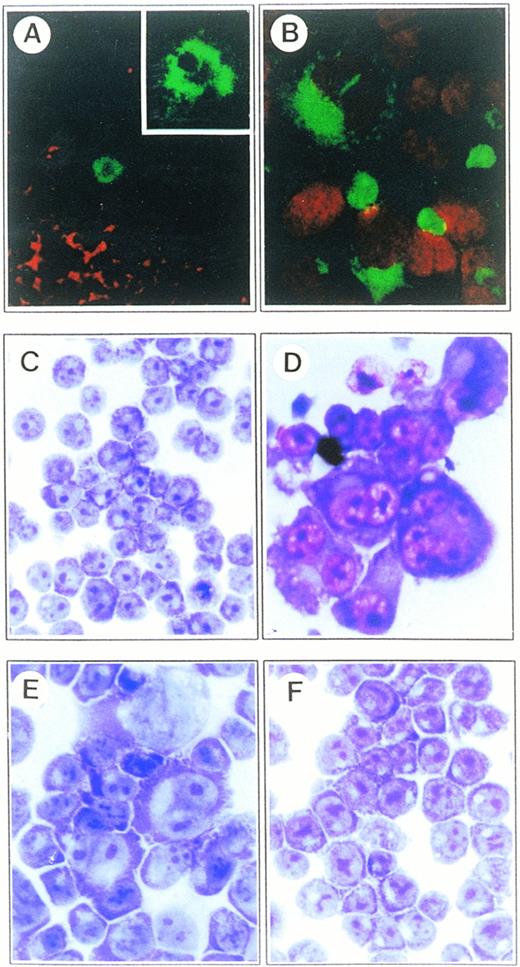
Microscopic analysis of H-RS cells from the lymph nodes of an HD patient and CD99-deficient B-cell lines. (A) Examination on the distribution of CD30 and CD99 molecules in lymph nodes of an HD patient by confocal microscopy. The frozen sections of lymph nodes were double-stained with FITC-conjugated anti-CD30 and biotinylated anti-CD99 antibodies. CD99 expression was identified by incubation with Texas Red–conjugated streptoavidin. CD99 molecule is absent in H-RS cells, whereas a considerable number of surrounding activated lymphocytes show strong reactivity (original magnification × 200). A CD30+ H-RS cell is shown at higher magnification (inset; original magnification × 1,000). (B) Localization of CD15 molecules in AS-TF IM9 cells with confocal microscopic analysis. AS-TF cells were stained with FITC-conjugated anti-CD15 antibody. Propidium iodide (PI) was included for nuclear staining. Most of large cells showed intense expression of CD15 in the Golgi and cytoplamic regions as well as on plasma membrane (original magnification × 630). Both (C) Vec-TF and (D) AS-TF IM9 cells were morphologically examined after Wright and Giemsa staining. AS-TF IM9 cells show typical H-RS morphology. (E and F) Restored cell morphology by exogenous expression of CD99 in Mut-IM9 cells. (E) Spontaneous CD99-deficient IM9 cells (Mut-IM9) and (F) CD99-TF Mut-IM9 cells were examined with Wright and Giemsa staining. All slides (C-F) were processed in parallel and photographed under identical magnification (40× objective).
Microscopic analysis of H-RS cells from the lymph nodes of an HD patient and CD99-deficient B-cell lines. (A) Examination on the distribution of CD30 and CD99 molecules in lymph nodes of an HD patient by confocal microscopy. The frozen sections of lymph nodes were double-stained with FITC-conjugated anti-CD30 and biotinylated anti-CD99 antibodies. CD99 expression was identified by incubation with Texas Red–conjugated streptoavidin. CD99 molecule is absent in H-RS cells, whereas a considerable number of surrounding activated lymphocytes show strong reactivity (original magnification × 200). A CD30+ H-RS cell is shown at higher magnification (inset; original magnification × 1,000). (B) Localization of CD15 molecules in AS-TF IM9 cells with confocal microscopic analysis. AS-TF cells were stained with FITC-conjugated anti-CD15 antibody. Propidium iodide (PI) was included for nuclear staining. Most of large cells showed intense expression of CD15 in the Golgi and cytoplamic regions as well as on plasma membrane (original magnification × 630). Both (C) Vec-TF and (D) AS-TF IM9 cells were morphologically examined after Wright and Giemsa staining. AS-TF IM9 cells show typical H-RS morphology. (E and F) Restored cell morphology by exogenous expression of CD99 in Mut-IM9 cells. (E) Spontaneous CD99-deficient IM9 cells (Mut-IM9) and (F) CD99-TF Mut-IM9 cells were examined with Wright and Giemsa staining. All slides (C-F) were processed in parallel and photographed under identical magnification (40× objective).
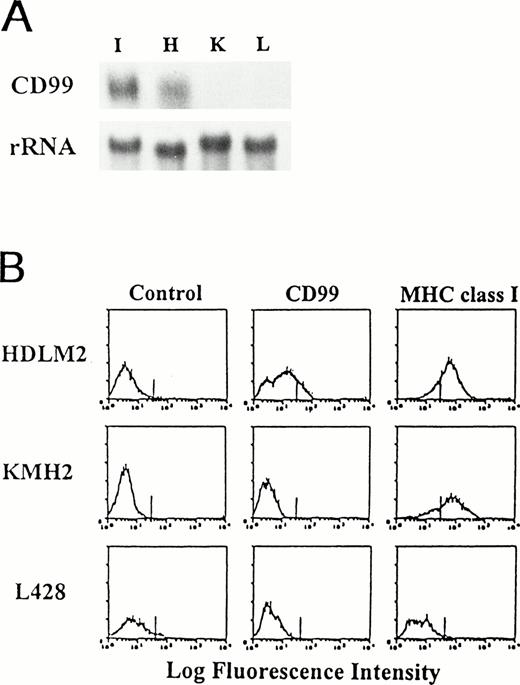
Lack of CD99 expression in HD-derived cell lines. (A) Northern blot analysis for CD99 expression in HD-derived cell lines. RNA samples were prepared from IM9 (I) and three Hodgkin’s cell lines, HDLM-2 (H), KM-H2 (K), and L-428 (L), and examined for the presence of CD99 transcripts. As a loading control, the blot was reprobed with rRNA. (B) Flow cytometric analysis of HD-derived cell lines.
Lack of CD99 expression in HD-derived cell lines. (A) Northern blot analysis for CD99 expression in HD-derived cell lines. RNA samples were prepared from IM9 (I) and three Hodgkin’s cell lines, HDLM-2 (H), KM-H2 (K), and L-428 (L), and examined for the presence of CD99 transcripts. As a loading control, the blot was reprobed with rRNA. (B) Flow cytometric analysis of HD-derived cell lines.
Production of H-RS–like cells by downregulation of CD99 in B-cell lines.
To generate CD99-deficient B-cell lines, we transfected IM9 B cells with antisense CD99 construct as described in Materials and Methods and obtained stable transfectants. As confirmed by immunoblot, Northern blot, and flow cytometric analyses (Fig 3), CD99 expression was abolished in the stable IM9 B cell transfectants (defined as AS-TF cells). Three independent stable AS-TF clones showed a substantial increase in cell size and severe morphological variations (Fig 1D) compared with control (Fig 1C). About 20% to 30% of the total cell culture population of AS-TF IM9 cells exhibited a typical H-RS cell morphology, which has been defined based on the morphologic criteria: abundant cytoplasm and bilobed or multilobate nuclei with amphophilic “owl-eyed” nucleoli (Fig 1D). We obtained similar results in spontaneous CD99-deficient mutant IM9 cells, which had been subcloned from IM9 cell culture (Mut IM9; Fig 1E), and AS-TF BJAB cells (data not shown). These results suggest that CD99 downregulation lead to generation of H-RS–like cells.
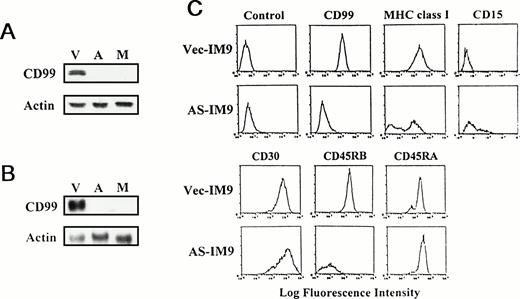
Generation of CD99-deficient IM9 cells and immunophenotyping. (A) Immunoblot and (B) Northern blot analyses of CD99-deficient IM9 cells. IM9 cells were transfected with either vector or antisense CD99 expression construct. The Vec-TF (V), AS-TF (A), and Mut-IM9 cells (M) were examined for CD99 expression. Actin was tested as an internal control. (C) Immunophenotypic analysis of AS-TF cells. The cells were first stained with control MoAb or with relevant primary MoAbs, and then with FITC-conjugated goat anti-mouse IgG antibody.
Generation of CD99-deficient IM9 cells and immunophenotyping. (A) Immunoblot and (B) Northern blot analyses of CD99-deficient IM9 cells. IM9 cells were transfected with either vector or antisense CD99 expression construct. The Vec-TF (V), AS-TF (A), and Mut-IM9 cells (M) were examined for CD99 expression. Actin was tested as an internal control. (C) Immunophenotypic analysis of AS-TF cells. The cells were first stained with control MoAb or with relevant primary MoAbs, and then with FITC-conjugated goat anti-mouse IgG antibody.
Immunological characteristics of H-RS–like cells.
We investigated whether H-RS–like cells generated by downregulation of CD99 have the immunophenotypic and functional characteristics of H-RS cells. Immunophenotypic features of AS-TF IM9 cells were compared with those of vector transfectant (Vec-TF) controls (Fig 3C). The current consensus is that a significant fraction of H-RS cells carry a high concentration of CD30 and CD15 in the absence of CD45RB on their surfaces, and this is the immunophenotypic criteria for the diagnosis of HD.21-27 Flow cytometric analysis showed a fairly constant high-level expression of CD30 in both types of IM9 cells tested, although AS-TF IM9 cells carried a twofold amount of CD30 compared with Vec-TF cells. For CD15, IM9 and Vec-TF IM9 cells did not reveal CD15 expression at all, but more than 20% of AS-TF cells that had a morphologic phenotype of H-RS cells contained a considerable amount of CD15 (Fig 3C). Confocal microscopic examination clearly showed a unique pattern of CD15 localization. As shown in Fig 1B, most of the large H-RS–like cells showed intense expression of CD15 in the Golgi and cytoplasmic regions, as well as on plasma membrane. In contrast, Vec-TF cells were completely negative for CD15 (data not shown). Regarding CD45RB expression, there was an almost complete downregulation of this molecule in AS-TF IM9 cells but not in Vec-TF cells, whereas expression of CD45RA remained high in both cell lines. In our study, CD99-deficient IM9 transfectants showed decreased MHC class I expression. The absence or reduced expression of MHC class I molecules was also found in three HD-derived cell lines (Fig 2B), again confirming that these H-RS–like AS-TF cells have immunological features comparable to those of H-RS cells in HD. Both AS-TF and Vec-TF cells expressed similar levels of other surface molecules such as CD19, CD21, CD23, CD25, CD40, MHC class II, ICAM-1, LFA-1a, LFA-3, and CD80 (data not shown). Both types of cells were negative for CD38 (data not shown).
H-RS cells express high levels of CD30 and CD40, which seem to share some common biological activities involved in the deregulated secretion of cytokines.28 The data obtained in the present study showed typical patterns of deregulated secretion of cytokines. Treatment of AS-TF cells with soluble trimeric CD40 ligand induced enhanced release of TGF-β1 from cultured AS-TF of both IM9 (1.5-fold) and BJAB cells (1.9-fold), and markedly diminished (9.5-fold) secretion of IL-10 in AS-TF BJAB cells by comparison with those in Vec-TF controls (Fig 4A).
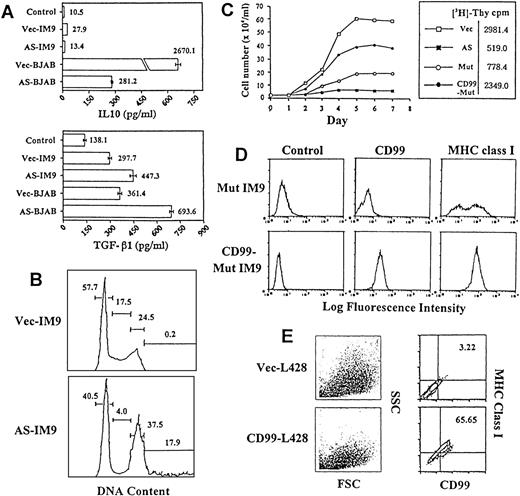
Immunologic characteristics of CD99 negative B cells and rescue from the H-RS phenotype by exogenous expression of CD99. (A) Deregulation of soluble CD40L-induced cytokine secretion in CD99-deficient B cells. Cells were cultured in RPMI media alone or in media supplemented with soluble CD40L (supernatant, 1:10 ratio). (B) Cell-cycle analysis. Flow cytometric analysis was performed on asynchronously proliferating Vec-TF (top) or AS-TF IM9 cells (bottom). The x-axis shows DNA content and the y-axis shows the number of cells. (C) Growth curve of CD99-deficient IM9 cells. The same number (5 × 104/mL) of cells was plated in 10% FCS-DMEM media at day 0. The total cell numbers were counted at the indicated days. The result of thymidine uptake is shown in the box. (D) Flow cytometric analysis of Mut-IM9 and CD99-TF Mut-IM9 cells. (E) Flow cytometric analysis of Vec-TF and CD99-TF L428 cells. Note the tight relation between the forward/side scatter profile and the expression of CD99 and MHC class I.
Immunologic characteristics of CD99 negative B cells and rescue from the H-RS phenotype by exogenous expression of CD99. (A) Deregulation of soluble CD40L-induced cytokine secretion in CD99-deficient B cells. Cells were cultured in RPMI media alone or in media supplemented with soluble CD40L (supernatant, 1:10 ratio). (B) Cell-cycle analysis. Flow cytometric analysis was performed on asynchronously proliferating Vec-TF (top) or AS-TF IM9 cells (bottom). The x-axis shows DNA content and the y-axis shows the number of cells. (C) Growth curve of CD99-deficient IM9 cells. The same number (5 × 104/mL) of cells was plated in 10% FCS-DMEM media at day 0. The total cell numbers were counted at the indicated days. The result of thymidine uptake is shown in the box. (D) Flow cytometric analysis of Mut-IM9 and CD99-TF Mut-IM9 cells. (E) Flow cytometric analysis of Vec-TF and CD99-TF L428 cells. Note the tight relation between the forward/side scatter profile and the expression of CD99 and MHC class I.
Defective cell-cycle progression and chromosome abnormality.
We investigated the growth characteristics of CD99-deficient cells. AS-TF IM9 cells showed slower kinetics of cell proliferation than did Vec-TF cells (sixfold decrease at day 5), which was further confirmed by [3H] thymidine-uptake assays (2981.4 → 519.0 cpm) (Fig 4C). We also obtained similar results in AS-TF BJAB cells (data not shown).
To evaluate the cell-cycle distribution and possible arrest or delay during a certain phase of the cell cycle, the DNA content of asynchronous cultures of Vec-TF and AS-TF IM9 cells was measured. All three AS-TF IM9 clones showed a marked accumulation of 4N cells (24.5% → 37.5%), a decrease in the number of S phase cells (17.5% → 4.0%), and many aneuploidy cells with larger than 4N (0% to → 8%) (Fig 4B) compared with Vec-TF cells. These results indicate that CD99-deficient cells are either arrested at the mitotic (M) phase of the cell cycle or are defective in cytokinesis, which was further confirmed with a comparative chromosomal analysis of CD99-deficient and control IM9 B cells. CD99-deficient cells showed numerical chromosomal changes (mostly tetrahypoploidy) and frequent structural abnormalities (data not shown).
Restoration of cell morphology from H-RS cells by forced expression of CD99.
In the course of long-term culture, we were able to obtain spontaneous CD99-deficient mutant IM9 cells (Mut-IM9) with an H-RS morphology (Fig1E), slow growth rate (Fig 4C), and CD15 positivity (data not shown) like AS-TF IM9 cells. Mut-IM9 cells were shown to be negative for CD99 expression by immunoblot and Northern blot analyses (Fig 3A and B), suggesting that lack of expression of CD99 on cell surfaces was attributable to a decrease in the synthesis of CD99 at transcriptional and translational levels. Because it was essential to test whether restoration of CD99 expression was able to rescue from the H-RS phenotype, we transfected the CMV-driven CD99 expression construct into Mut-IM9 and L428 cells and obtained stable transfectants (CD99-TF). With a recovery of CD99 expression, the characteristics of H-RS cells in Mut-IM9 cells were almost completely abolished and they regained a normal morphology (Fig 1F), growth rate, and surface phenotype (Fig 4C and D). It is notable that CD99-TF Mut-IM9 cells were completely negative for CD15 (data not shown). The CD99-TF L428 cells also acquired a reduced forward and side scatter profile on fluorescence-activated cell sorting (FACS) analysis, indicating monomorphism in the size and shape of cells. The frequency of cells with H-RS morphology was markedly diminished. The intensity of MHC class I expression was also proportional to that of CD99 expression (Fig 4E). All these results confirm the presence of a tight correlation between reduced expression of CD99 and generation of an H-RS phenotype.
Activation of Rho and Rac by engagement of CD99.
It is well established that the small GTP-binding proteins Rac and Rho participate in the control of cell morphology, cell aggregation, and cytokinesis.29,30 The findings that engagement of CD99 induces the aggregation of various types of lymphoid cells15,18 and diminished CD99 expression leads to defective cytokinesis led us to investigate whether stimulation of CD99 has an effect on the activities of Rac and Rho. Anti-CD99 MoAb triggered homotypic aggregation of IM9 cells within 1 hour (Fig 5A, a). This CD99-induced aggregation was blocked by pretreatment of IM9 cells with C3 transferase, a bacterial enzyme known to inactivate Rho through ADP ribosylation31 (Fig 5A, b). We also observed that phorbol myristate acetate (PMA)-induced aggregation was blocked by C3 pretreatment (positive control experiment, data not shown). To investigate whether Rac also participates in this particular signaling pathway, we generated two kinds of IM9 transfectants that contained either a constitutively active form of Rac (L61) or a mutant form of Rac (L61F37A) that is defective in the morphology signaling pathway.31 Despite the similar expression level of CD99 in both transfectants, only the L61F37A-TF cells showed reduced CD99-induced aggregation (Fig 5A, c and d). Expression of the transfected Rac genes was confirmed by the immunoblot analysis (data not shown). In this assay, we used isotype-matched MoAb as negative control. This control antibody induced very weak aggregation (data not shown). These results indicated that Rac and Rho were in the downstream of CD99 with respect to cell aggregation signaling pathway.
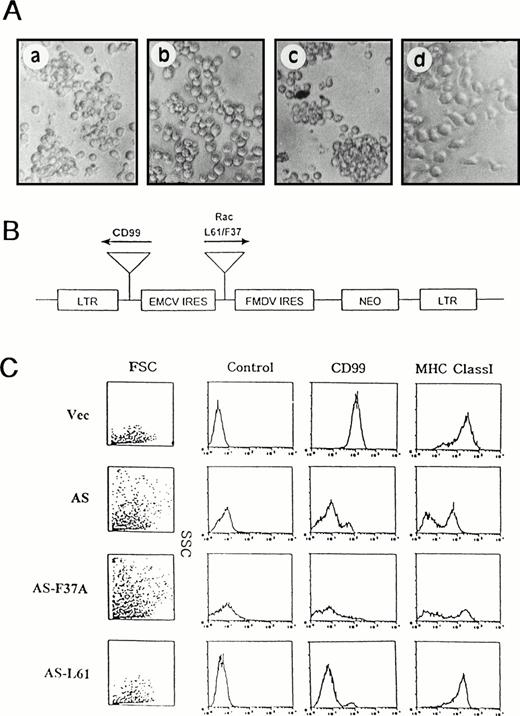
Function of Rac and Rho in CD99 signaling pathway. (A) Blocking of CD99 MoAb-induced aggregation of IM9 cells by C3 exoenzyme or L61F37A Rac mutant. Normal IM9 cells pretreated with (b) or without (a) the inhibitor of C3 transferase (20 μg/mL; UBI, Lake Placid, NY) for 36 hours were dispersed into single cells by repeated pipetting, incubated with control antibody or anti-CD99 MoAb for 1 hour, and assayed for cell aggregation. L61-TF (c) or L61F37A-TF (d) IM9 transfectants were stimulated with anti-CD99 MoAb for 1 hour and assayed for aggregation. (B) Gene constructs that contain L61 or L61F37A Rac in sense orientation and CD99 in antisense orientation. (C) Immunophenotypic analysis. All transfectants were examined for CD99 expression by flow cytometric analysis. L61 Rac rescues cell morphology from the H-RS phenotype, with a concomitant decrease in forward and side scatter and increased MHC class I expression, in CD99-deficient IM9 cells.
Function of Rac and Rho in CD99 signaling pathway. (A) Blocking of CD99 MoAb-induced aggregation of IM9 cells by C3 exoenzyme or L61F37A Rac mutant. Normal IM9 cells pretreated with (b) or without (a) the inhibitor of C3 transferase (20 μg/mL; UBI, Lake Placid, NY) for 36 hours were dispersed into single cells by repeated pipetting, incubated with control antibody or anti-CD99 MoAb for 1 hour, and assayed for cell aggregation. L61-TF (c) or L61F37A-TF (d) IM9 transfectants were stimulated with anti-CD99 MoAb for 1 hour and assayed for aggregation. (B) Gene constructs that contain L61 or L61F37A Rac in sense orientation and CD99 in antisense orientation. (C) Immunophenotypic analysis. All transfectants were examined for CD99 expression by flow cytometric analysis. L61 Rac rescues cell morphology from the H-RS phenotype, with a concomitant decrease in forward and side scatter and increased MHC class I expression, in CD99-deficient IM9 cells.
Rescue from the H-RS phenotype in CD99-deficient IM9 cells by expression of a constitutively active Rac.
Previous observation suggests that a constitutively active form of Rac might rescue from the H-RS phenotype in CD99-deficient IM9 B cells if basal activities of Rac and Rho are maintained via CD99. To address this issue, LTR-driven expression constructs were designed that contain CD99 gene in the antisense orientation alone (AS-TF) or with either L61 or L61F37A Rac gene in the sense orientation (AS-L61-TF or AS-F37A-TF) (Fig 5B). AS-TF cells acquired H-RS morphology, with a concomitant increase in forward and side scatter. In contrast, AS-L61-TF IM9 cells that express both L61 Rac and antisense CD99 showed normal morphology of IM9 cells, which was accompanied by the acquisition of proper MHC class I expression (Fig 5C), indicating that increased Rac activity rescued from the H-RS phenotype of CD99-deficient IM9 cells. In contrast, L61F37A-TF cells retained H-RS morphology.
DISCUSSION
In this study we have shown that downregulation of CD99 is tightly linked to generation of cells with the H-RS phenotype. Evidence supporting the presence of a relationship between reduced expression of CD99 and H-RS–like cell generation are presented here. In all 28 cases of HD examined, CD30+ H-RS cells in lymph nodes were negative for CD99 expression, while surrounding reactive lymphocytes from HD and activated lymphocytes from benign lymphadenopathy expressed a high level of CD99 molecule on their cell surfaces. In addition, established HD-derived cell lines showed markedly reduced or no expression of CD99 molecules. More convincing evidence was obtained from transfection studies. Downexpression of CD99 in B-cell lines led to the generation of H-RS–like cells irrespective of differentiation stages of cells. AS-TF cells exhibited most of the features characteristic of H-RS cells: (1) the typical H-RS cell morphology, characterized by abundant cytoplasm and bilobed or multilobated nuclei with amphophilic “owl-eyed” nucleoli, (2) the markedly diminished expression of MHC class I and CD45RB; (3) expression of CD15 and its unique localization in the Golgi region; (4) the typical pattern of deregulated cytokine secretion; and (5) defective cell-cycle progression and chromosomal abnormalities. Finally, cell morphology and proliferation activity were restored to normal by the forced expression of CD99 in Mut-IM9 and L428 cells.
The constitutive expression of CD30 and CD15 and the absence of CD45RB have been currently used as immunophenotypic criteria for diagnosis of HD.21-27 In the present study, even though high-level expression of CD30 was already detected in Vec-TF, CD30 expression was upregulated by twofold with downregulation of CD99. This result is in agreement with a previous report that CD30 can also be expressed in transformed or activated lymphocytes, rather than being H-RS cell-specific antigens.32 As for the CD15 molecule, its expression was confined to cells with H-RS morphology, which represent 20% of total AS-TF IM9 cells. This was further confirmed by confocal microscopic analysis. A large amount of CD15 was expressed in the cytoplasm of H-RS–like AS-TF cells with a less intense expression on their surfaces. Interestingly, this finding was in sharp contrast with that of Vec-TF cells and CD99-TF Mut IM9 cells where expression of CD15 was not visible. It is well known that expression of CD15 with its unique intracellular localization is one of the most important points for the differential diagnosis between HD and large cell lymphoma of anaplastic type.33-35 Therefore, based on present data regarding CD15 expression, it seems to be reasonable to say that AS-TF cells are more likely to be H-RS cells than other types of neoplastic lymphoid cells.
It has been reported that all HD cases examined showed a near-complete lack of expression of MHC class I molecules on the surfaces of H-RS cells.13,14 In this study, downregulation of CD99 was always accompanied by decreased expression of MHC class I molecule. Because the intensity of CD99 was proportional to the extent of MHC class I expression, we suggest that downregulation of MHC class I molecule may be a secondary event to the loss of CD99. Indeed, we have recently showed that CD99 ligation led to the marked upregulation of MHC class I and class II molecules on the cell surfaces.20The results obtained here are considered to have a biological relevance in vivo in the following aspects. The downregulation of MHC class I molecule can render cells resistant to CTLs and provide a selective growth advantage for H-RS cells in vivo. In this way, H-RS cells may escape from host immune surveillance and survive. However, the mechanism leading to this downregulation of MHC class I molecule has yet to be unraveled. With our previous study, it appeared that MHC class I and II molecules were regulated at the level of vesicular transport.20
H-RS cells express a high level of CD30 and CD40, which are known to be involved in the deregulated secretion of cytokines.28 This might provide a favorable condition for growth of H-RS cells and their surrounding reactive bystander cells. Typical deregulated secretion of cytokines was shown in AS-TF cells. We presume that increased TGF-β1 production by H-RS cells might cause decreased immune response in vivo by the suppression of the surrounding reactive bystander cells. Based on these findings, we suggest that CD99 molecules play a crucial role in controlling the interaction between H-RS cells and T cells in HD through a network of interactive signals generated by cytokine-mediated (CD40-CD40L) events.
CD99-deficient B cells exhibited slower kinetics of cell proliferation due to markedly decreased S phase with accumulation at mitotic (M) phase, a defect in cytokinesis. Furthermore, numerical chromosomal changes and frequent structural abnormalities were also found. These results are in support of the recent proposal that multinuclear RS cells are derived from originally mononuclear Hodgkin’s cells through nuclear division with disturbance of cytokinesis.36 We interpreted that cell-cycle blockage at M phase by CD99 downregulation is incomplete and typical H-RS cells are continuously provided by faster growing mononuclear variants, which may comprise a big proportion of bona fide tumor cells in the peripheral blood of HD patients.37 However, we thought that these CD99-deficient H-RS–like cells had been in cell cycle beyond restriction point, which might be an explanation for Ki67 positivity in H-RS cells from patients’ lymph nodes.
Experiments performed in this study support the idea that molecular signals generated by CD99 engagement are associated with the organization of the cytoskeleton. First, cell cycle and cytokinesis were disrupted by reduced expression of CD99. Second, the treatment of C3 exoenzyme completely abolished CD99-induced cell aggregation. Third, overexpression of a mutant form of Rac rendered cells resistant to aggregate-formation after CD99 engagement. Fourth, cell shape and MHC class I expression were restored to a normal state by increased Rac activity despite the absence of CD99 expression. Until recently, members of the Rho subfamily were believed to be involved in the regulation of cytoskeletal organization in response to extracellular signals, influencing the actin- and microtubule-based system.38 39 On the basis of these facts, it can be suggested that the activity of CD99 may be linked to the organization of the cytoskeleton or activation of cytoskeletal components.
Our findings suggest that the progression of HD may occur when a genetic alteration of H-RS precursor cells leads to the downregulation of CD99. At this point, the mechanism by which CD99 expression is downregulated is unknown. We suggest several possibilities for the diminished expression of CD99. One possibility is the transcriptional downregulation of CD99, because CD99 mRNA was undetectable in some of the HD-derived cell lines and in spontaneously occurring Mut-IM9 cells. Another possible causative event is the mutation of the CD99-encoding gene during tumorigenesis. According to the clinical studies, HD is frequently associated with immunosuppressive states such as viral infection40 that may allow the expansion of a lymphoid precursor of H-RS cells. Therefore, various viral gene products including LMP-1 might be candidates for the downregulation of CD99 expression.
In this report it is suggested that CD99 may play a major role in the maintenance of cell shape, cell integrity, and cell division through Rac-Rho signaling pathway. We suggest that the loss of CD99 expression is a significant molecular event for the generation of H-RS cells. This idea might be an adequate explanation for the actual presence of both EBV+ and EBV− HD cases.41
ACKNOWLEDGMENT
We are grateful to Dr A. Hall (Department of Biochemistry, University College London, London, UK) for providing us with L61Rac and L61F37A Rac genes, to Peter N. Goodfellow (SmithKline Beecham, Essex, UK) for helpful criticism and discussion, and to Sean Bong Lee (MGH Cancer Center, Boston, MA) for helpful comments on the manuscript.
S.H.K. and E.Y.C. contributed equally to this work.
Supported by a grant (HMP-96-M-2-1073) of the ’96 Good Health R&D Project, Ministry of Health & Welfare, Republic of Korea.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Seong Hoe Park, MD, Department of Pathology, Seoul National University College of Medicine, 28 Yongon-dong Chongno-gu, Seoul 110-799, Korea; e-mail:pshoe@plaza.snu.ac.kr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal