Abstract
Purified CD34+ and CD34+CD38− human umbilical cord blood (UCB) cells were transduced with the recombinant variant of Moloney murine leukemia virus (MoMLV) MFG-EGFP or with SF-EGFP, in which EGFP expression is driven by a hybrid promoter of the spleen focus-forming virus (SFFV) and the murine embryonic stem cell virus (MESV). Infectious MFG-EGFP virus was produced by an amphotropic virus producer cell line (GP+envAm12). SF-EGFP was produced in the PG13 cell line pseudotyped for the gibbon ape leukemia virus (GaLV) envelope proteins. Using a 2-day growth factor prestimulation, followed by a 2-day, fibronectin fragment CH-296–supported transduction, CD34+ and CD34+CD38− UCB subsets were efficiently transduced using either vector. The use of the SF-EGFP/PG13 retroviral packaging cell combination consistently resulted in twofold higher levels of EGFP-expressing cells than the MFG-EGFP/Am12 combination. Transplantation of 105 input equivalent transduced CD34+ or 5 × 103input equivalent CD34+CD38− UCB cells in nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice resulted in median engraftment percentages of 8% and 5%, respectively, which showed that the in vivo repopulating ability of the cells had been retained. In addition, mice engrafted after transplantation of transduced CD34+ cells using the MFG-EGFP/Am12 or the SF-EGFP/PG13 combination expressed EGFP with median values of 2% and 23% of human CD45+ cells, respectively, which showed that the NOD/SCID repopulating cells were successfully transduced. EGFP+ cells were found in all human hematopoietic lineages produced in NOD/SCID mice including human progenitors with in vitro clonogenic ability. EGFP-expressing cells were also detected in the human cobblestone area–forming cell (CAFC) assay at 2 to 6 weeks of culture on the murine stromal cell line FBMD-1. During the transduction procedure the absolute numbers of CAFC week 6 increased 5- to 10-fold. The transduction efficiency of this progenitor cell subset was similar to the fraction of EGFP+ human cells in the bone marrow of the NOD/SCID mice transplanted with MFG-EGFP/Am12 or SF-EGFP/PG13 transduced CD34+ cells, ie, 6% and 27%, respectively. The study thus shows that purified CD34+ and highly purified CD34+CD38− UCB cells can be transduced efficiently with preservation of repopulating ability. The SF-EGFP/PG13 vector/packaging cell combination was much more effective in transducing repopulating cells than the MFG-EGFP/Am12 combination.
EFFICIENT PROCEDURES for gene transfer into human immature hematopoietic cells with repopulating capacities after transplantation may in principle open new avenues for the treatment of a variety of hereditary and acquired diseases. Retroviral-mediated gene transfer to such cells, which is attractive by its simplicity and efficiency, has, however met with considerable difficulty, which is only partly understood.1,2 The availability of a rapid selectable marker, such as the green fluorescent protein (GFP), is thought to be of pivotal importance to study major variables influencing the efficiency of gene transfer, as well as to track the progeny of transduced cells after transplantation. In the present study we evaluated the use of the enhanced (E) recombinant variant of GFP to label immature human umbilical cord blood cells, using outgrowth in nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice1,3,4 as well as cobblestone area–forming cells (CAFC)5 6 as assays for immature cells with considerable hematopoietic reconstitution capacity.
The CAFC assay and the long-term culture-initiating cell (LTC-IC) assay allow for frequency analysis of cells capable of long-term repopulation in vitro.5,7 Murine studies have shown that the CAFC scored at week 2 are related to colony-forming unit-spleen (CFU-S) day 12, while CAFC week 5 strongly correlate with long-term repopulating cells in vivo.6,8 In human hemopoiesis the rare population with the primitive phenotype of CD34+CD38− is highly enriched for CAFC week 6. The primitive nature of CAFC week 6 is further illustrated by enrichment after incubation with 5-fluorouracil (5-FU), a drug cytotoxic for proliferating cells. The CAFC week 2, however, are absent in the CD34+CD38− population and more than 1 log reduced after 5-FU treatment. Based on these results, the CAFC week 6 have been proposed to be representative for cells with long-term repopulating ability in vivo in the human situation.9 On this basis, this assay is considered suitable to assess the effect of manipulation of human hematopoietic progenitor cell populations, such as by gene-transfer protocols.10 11
The efficiency of gene transfer to stem cells is limited by the inability of most retroviral vectors to integrate DNA into the cellular genome of quiescent cells.12-15 Stimulation of stem-cell cycling with hematopoietic growth factors (HGF) such as interleukin-3 (IL-3), IL-6, stem cell factor (SCF), or Flt3-L16 before and during virus exposure would seem to be essential to promote transduction,17,18 but may result in loss of repopulating ability of transduced cells as a result of differentiation.16,19 In addition, colocalization of target cells and virus on dishes coated with the recombinant fibronectin-fragment CH-296 has been shown to further increase gene transfer efficiency.20 21
For transduction of human hematopoietic cells, murine retroviruses based on the Moloney murine leukemia virus (MoMLV) are most commonly used. However, expression of functional receptors for the MoMLV envelope protein is presumably low, and pseudotyping the vector with the GaLV envelope protein resulted in higher transduction efficiencies in hematopoietic progenitor cells,22-24 which has been attributed to a higher expression of functional pseudotyped GaLV receptor (Pit-1) by the immature hematopoietic cells22,24than the amphotropic retroviral receptor (Pit-2).24-28 A study in which CD34+ cells were transduced by the GaLV-pseudotyped retroviral vector showed that CD34+ cells were efficiently transduced (21% to 33% transduction) as determined by culture in a colony-forming cell assay.2 It is not known to what extent the relative transduction inefficiency of the MoMLV type viruses is caused by a low Pit-2 expression on immature stem cells or by inefficient activation and provirus integration in quiescent cells. Transplantation of CD34+ or CD34+CD38− transduced cells in immunodeficient beige/nude/xid (bnx) mice showed that 8 of 10 mice transplanted with CD34+ transduced cells contained the retrovirally transduced bacterial neomycin phosphotransferase resistance (neo), gene whereas only 2 of 14 mice that had received CD34+CD38− cells contained low levels of transduced cells.2 The ability to engraft the bone marrow (BM) of NOD/SCID mice and provide multilineage outgrowth, which resides exclusively in the CD34+CD38-population,3 has been described as unsuccessful, in contrast to the LTC-IC or CAFC week 6, which were transduced with efficiencies ranging between 10% and 70%.1 These differences led to the suggestion that NOD/SCID repopulating cells are distinct from the LTC-IC or CAFC week 6.1 However, recent data obtained with vectors that contained the neo-gene show that transplantation of retrovirally transduced CD34+ UCB cells in NOD/SCID mice result in transduced human hematopoiesis in the NOD/SCID BM with transduction levels similar to those obtained for LTC-IC.29
Use of the GFP gene from the jellyfish Aequorea victoria as a retrovirally transduced marker allows rapid identification of transduced cells by fluorescence microscopy, flow cytometry, or culture in real time without additional staining steps in contrast to other genetic markers such as the neo-gene30-32 and the bacterial β-galactosidase gene (LacZ).33-36 As wild-type GFP produces a weak (but stable) green fluorescence signal, several GFP variants, such as EGFP, have been created which are better suited for detection of expression by fluorescence microscopy and flow cytometry.37,38 Studies with murine cells have shown that cells with the ability of in vivo reconstitution can be transduced with EGFP.39 Our ongoing studies show that high expression levels of EGFP could be detected in mouse BM, peripheral blood, spleen, and thymus for a current observation period of 6 months after transplantation and were retained in secondary recipient mice, indicating that long-term repopulating stem cells can be successfully transduced. Human cell lines and purified CD34+ cells were also transduced using EGFP-containing vectors.28 Therefore, retroviral vectors containing EGFP genes can be used to transduce a variety of cells, which can then be easily detected in vitro as well as in vivo.
To initiate an analysis directed at optimal vectors and transduction procedures, the MFG-EGFP retroviral vector produced by an amphotropic packaging cell line and the SF-EGFP vector pseudotyped for the GaLV envelope protein were used to transduce immature cell subsets in human umbilical cord blood (UCB). The potential of these vector/packaging cell combinations for transduction of purified CD34+ and CD34+CD38− UCB subsets was compared by assessing the ability of transduced cells to produce EGFP+cobblestone areas in the CAFC assay and to contribute to multilineage human hematopoiesis in NOD/SCID mice.
MATERIALS AND METHODS
Human UCB cells.
UCB samples were obtained from placentas of full-term normal pregnancies after informed consent in conformity with legal regulations in The Netherlands. Mononucleated cells were isolated by Ficoll density gradient centrifugation (1.077 g/cm2; Nycomed Pharma AS, Oslo, Norway), and were cryopreserved in 10% dimethylsulphoxide, 20% heat-inactivated fetal calf serum (FCS), and 70% Hanks’ Balanced Salt Solution (HBSS; GIBCO, Breda, The Netherlands) at −196°C as described40 before use. After thawing by stepwise dilution in HBSS containing 2% FCS, the cells were washed with HBSS containing 1% FCS and used for gene transduction experiments.
Viral vectors and packaging cell lines.
The amphotropic retroviral producer cell line, MFG-EGFP, was obtained by a 20-hour incubation of GP+envAm12 under standard culture conditions with supernatants containing ecotropic retrovirus from the GP+E-86/MFG-EGFP cell line and hexadimethrine bromide at 4 μg/mL (Sigma, St Louis, MO) as described.38 The pseudotyped retroviral producer cell line PG13/EGFP7 was developed by transducing the PG13 packaging cell line (kindly provided by D. Miller, Fred Hutchinson Cancer Research Center, Seattle, WA) with 0.45 μm filtered supernatant from PA317/EGFP cell cultures.28 EGFP expression was analyzed by flow cytometry and bright single cells were sorted on 96-well plates by using an EPICS Elite ESP flow cytometer coupled to an autoclone device (both from Coulter, Miami, FL). Single clones were cultured as previously described.28 The sorted clones were additionally selected for high virus titer. The viral titer of both the amphotropic and the pseudotyped producer cell line was in the order of 106 infectious particles per mL as determined by supernatant titration on cultured murine NIH 3T3 cells and human HeLa cells, respectively. Absence of replication-competent virus was verified by failure to transfer GFP expression from a transduced cell population to a secondary population. Additionally, for the SF-EGFP/PG13 vector/packaging cell combination pseudotransduction was tested on HeLa cells and found absent.
Subset purification.
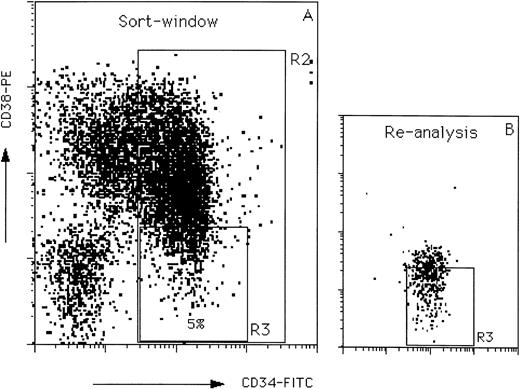
Purification of CD34+ cells was performed by positive selection using Variomacs Immunomagnetic Separation System as described41 (CLB, Amsterdam, The Netherlands). The percentage of CD34+ cells in the unseparated population (low-density UCB) and in the purified CD34+ and CD34− fractions was determined by fluorescence-activated cell sorting (FACS) analysis. For isolation of CD34+CD38− subsets, purified CD34+ cells were stained with fluorescein isothiocyanate (FITC) and R-phycoerythrin (PE) conjugated antibodies against human CD34 and CD38 (CD34-FITC, CD38-PE; Becton Dickinson, San Jose, CA) for 30 minutes on ice in HBSS, supplemented with 2% (wt/vol) bovine serum albumin (BSA; Sigma), 0.05% (wt/vol) sodium azide (Merck, Darmstadt, Germany) and 2% (vol/vol) normal human serum (NHS). After incubation, the cells were washed twice, resuspended in HBSS and CD34+CD38− cells, and the window set at 5% of the CD34+ population with the lowest CD38 expression levels (Fig 1) were sorted using a FACS Vantage flow cytometer (Becton Dickinson, San Jose, CA).
Flow cytometric profile used to define and sort the CD34+CD38− cell population (A). The window R3 was used to define CD34+CD38− cells for sorting and contains 5% of the CD34+ population (as defined by window R2) with the lowest CD38 antigen expression. Re-analysis of the sorted cells is shown in (B).
Flow cytometric profile used to define and sort the CD34+CD38− cell population (A). The window R3 was used to define CD34+CD38− cells for sorting and contains 5% of the CD34+ population (as defined by window R2) with the lowest CD38 antigen expression. Re-analysis of the sorted cells is shown in (B).
Retroviral transduction of UCB subsets.
Supernatants containing recombinant retrovirus were generated by culturing approximately 80% confluent producer cells for 12 hours in culture medium consisting of a serum-free enriched version of Dulbecco’s modified Eagle’s medium (DMEM; GIBCO, Gaithersburg, MD).3,39,42 Media for all cultures routinely included 100 U/mL of penicillin and 100 μg/mL of streptomycin. The cultures were maintained at 37°C with 10% CO2 (measured every 15 minutes with read-outs between 9.5% and 10%) in a humidified atmosphere. The culture supernatant was subsequently procured and passed through a 0.45-μm filter. To enhance the transfection efficiency, Falcon 1008 (35-mm) bacteriological culture dishes (Becton Dickinson, Plymouth, UK) were coated with the recombinant fibronectin fragment CH-296 (Takara Shuzo, Otsu, Japan) at a concentration of 10 μg/cm2 as described previously.21 UCB subsets (CD34+ or CD34+CD38−) were prestimulated for 2 days in either medium consisting of enriched Dulbecco’s medium (GIBCO, Gaithersburg, MD), or CellGroSCGM (Boehringer Ingelheim, Heidelberg, Germany). Different combinations of human recombinant HGF were added to the culture medium; IL-3 (20 ng/mL; Gist-brocades NV, Delft, The Netherlands), IL-6 (100 ng/mL; Ares-Serono SA, Genève, Switzerland), thrombopoietin (TPO; 10 ng/mL, kindly provided by Genentech, South San Francisco, CA), SCF (100 ng/mL), and Flt3-L (50 ng/mL; the latter two kindly provided by Amgen, Thousand Oaks, CA). The HGF combination of Flt-3L, TPO, IL-6, and SCF was used during the transduction procedure; in some initial experiments, as indicated in the legend of the figures and tables, the IL-3, IL-6, SCF combination was used. Before adding purified cord blood subsets to the fibronectin-coated dishes, the CH-296 fibronectin fragment was preincubated with supernatant containing the amphotropic MFG-EGFP or the pseudotyped SP-EGFP vector for 1 hour at 37°C.20 21Subsequently, nucleated cells were resuspended in the vector-containing supernatant supplemented with hematopoietic growth factors and added to the dishes. Over a period of 2 days, culture supernatant was once replaced completely by resuspending nonadherent cells into fresh retrovirus supernatant and HGF. Finally, the cells were obtained and used for FACS analysis, human granulocyte-macrophage CFU (GM-CFU) and erythroid burst-forming units (BFU-E) assays, CAFC assay, and transplantation into NOD/SCID mice.
Flow cytometry.
Cell samples were analyzed using a FACSCalibur flow cytometer (Becton Dickinson) as previously described.38 39 Immunophenotyping of EGFP-transduced cells was performed by staining with peridinin chlorophyll protein (PercP)-labeled anti-CD45 and cyanin-5–conjugated anti-CD34 (Cy5; Amersham, Buckinghamshire, UK) or PE-conjugated monoclonal antibodies against CD38, CD2, CD4, CD8, CD19, CD20, CD56, CD33 (Becton Dickinson). Mice were considered engrafted if the percentage CD45+ cells exceeded 1%.
Transplantation of transduced UCB subsets in immunodeficient mice.
Specific pathogen-free (SPF) NOD/LtSz-scid/scid (NOD/SCID) mice, 6 to 9 weeks of age, were bred and housed under SPF conditions in a laminar air flow unit and supplied with sterile food and acidified drinking water containing 100 mg/L ciprofloxacine (Bayer AG, Leverkusen, Germany) ad libitum. Housing, care, and all animal experimentation were done in conformity with legal regulations in The Netherlands, which include approval by a local ethical committee. All mice received total body irradiation (TBI) at 3.5 Gy, delivered by a 137Cs source adapted for the irradiation of mice (Gammacell, Atomic Energy of Canada, Ottawa), 2 to 4 hours before transplantation. The transplants were suspended in 200 μL HBSS containing 0.1% BSA and injected intravenously (IV) into a lateral tail vein. Transplanted cell numbers were 105 CD34+ cells and 5 × 103 CD34+CD38− cells. Thirty-five days after transplantation the mice were killed by CO2 inhalation followed by cervical dislocation, both femurs isolated, and BM cell suspensions prepared by flushing. After counting, the cells were cultured in colony assays and analyzed by flow cytometry to determine the percentage of human EGFP+ cells in the mouse BM.
In vitro colony assay.
Purified UCB cells, EGFP-transduced cells, and chimeric mouse BM samples were assayed for the presence of human GM-CFU and BFU-E by in vitro colony formation in viscous methylcellulose culture medium as previously described.3 42-44 The number of colonies was determined after 14 days of culture in a humidified atmosphere of 10% CO2 at 37°C. EGFP+ colonies were scored under excitation by UV light.
Stromal feeders and CAFC assay.
The contact inhibited FBMD-1 murine stromal cell line was used as described before.5 After 7 to 10 days of culture at 33°C and 10% CO2, the stromal layers had reached confluence and were overlaid with nontransduced or transduced CD34+ or CD34+CD38− UCB cells within the subsequent week. Confluent stromal layers of FBMD-1 cells in flat-bottom 96-well plates were overlaid with UCB cells in a limiting dilution setup. Input values of the CD34+CD38− population and the CD34+ were 25 nucleated cells and 500 nucleated cells per well in the first dilution, respectively. Twelve twofold serial dilutions were used for each sample with 15 replicate wells per dilution. The cells were cultured at 33°C and 10% CO2for 6 weeks with weekly half-medium changes. The percentage of wells with at least one phase-dark hematopoietic clone of at least five cells (ie, a cobblestone area) beneath the stromal layer was determined weekly with an inverted microscope. Green fluorescent cobblestone areas were screened in the same way but with a UV-light excitation source. Frequencies of total and green-fluorescent CAFC were calculated by using Poisson statistics as described previously.6 During the period of culture, no transfer of the EGFP gene to the stromal underlayer has been observed.
Statistical analysis.
Data are expressed as median (range). Statistical comparisons were performed according to Mann Whitney U-test. P values <.05, two-tailed, were considered significant.
RESULTS
Transduction efficiencies in purified cells with MFG-EGFP and SF-EGFP vectors.
Purified CD34+ and CD34+CD38−UCB cells (Fig 1) were prestimulated for 2 days and subsequently transduced with either MFG-EGFP/Am12 or SF-EGFP/PG13 vector/packaging cell combination, during 2 days of exposure to virus-containing supernatants in fibronectin fragment-coated bacterial dishes. Transduction efficiencies obtained by infection using the amphotropic MFG-EGFP producer cell line were compared to those obtained with the pseudotyped SF-EGFP cell line. The percentage EGFP+ cells was assessed by flow cytometry (Fig 2). The percentage of EGFP+ cells of the purified CD34+population transduced with the SF-EGFP/PG13 vector/packaging cell combination (median, 75% EGFP+) was more than twofold higher compared with MFG-EGFP/Am12–transduced CD34+ cells (median, 30%) (Table 1). Sorted CD34+CD38− cells were also transduced at a higher frequency using the SF-EGFP/PG13 combination (62%) than after transduction with the MFG-EGFP/Am12 combination (19%). On average, transduction frequencies were lower in the purified CD34+CD38- cells than in the CD34+cell fraction, but only for the MFG-EGFP/Am12–transduced cells the difference was statistically significant. The level of transduction of the CD34+CD38− subset within the purified CD34+ population obtained with the SF-EGFP/PG13 vector/packaging cell combination was more than 2.5-fold higher than with the MFG-EGFP/Am12 combination. The differences in transduction efficiency between the two vector/packaging cell combinations in these cell populations were significant (P < .025).
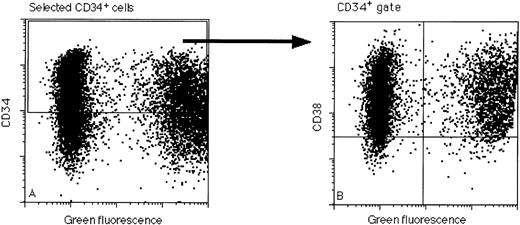
Flow cytometric analysis of a representative transfection of purified CD34+ cells with the amphotropic MFG-EGFP retroviral vector after 2 days of prestimulation and 2 days of supernatant infection in the presence of IL-3, IL-6, and SCF. This particular transduction resulted in efficiencies of 30% within the CD34+ population (A). In (B) CD34+ cells were gated and the CD38 distribution of the EGFP-transduced cells was studied. Also, CD34+CD38− cells expressed the EGFP gene with efficiencies similar to the total CD34+ population (30% EGFP+).
Flow cytometric analysis of a representative transfection of purified CD34+ cells with the amphotropic MFG-EGFP retroviral vector after 2 days of prestimulation and 2 days of supernatant infection in the presence of IL-3, IL-6, and SCF. This particular transduction resulted in efficiencies of 30% within the CD34+ population (A). In (B) CD34+ cells were gated and the CD38 distribution of the EGFP-transduced cells was studied. Also, CD34+CD38− cells expressed the EGFP gene with efficiencies similar to the total CD34+ population (30% EGFP+).
EGFP Expression of UCB Subsets
| Vector/Packaging Cell Line . | Purified CD34+Cells . | P Value* . | CD34+CD38−Population Within Purified CD34+ . | P Value† . | Purified CD34+CD38− . | PValue‡ . |
|---|---|---|---|---|---|---|
| MFG-EGFP/Am12 | 30 (8-51) (n = 13) | >.05 | 25 (15-55) (n = 9) | >.05 | 19 (8-21) (n = 4) | .02 |
| SF-EGFP/PG13 | 75 (53-84) (n = 7) | >.05 | 66 (58-81) (n = 5) | >.05 | 62 (21-71) (n = 4) | .12 |
| P value1-153 | .0001 | .003 | .02 |
| Vector/Packaging Cell Line . | Purified CD34+Cells . | P Value* . | CD34+CD38−Population Within Purified CD34+ . | P Value† . | Purified CD34+CD38− . | PValue‡ . |
|---|---|---|---|---|---|---|
| MFG-EGFP/Am12 | 30 (8-51) (n = 13) | >.05 | 25 (15-55) (n = 9) | >.05 | 19 (8-21) (n = 4) | .02 |
| SF-EGFP/PG13 | 75 (53-84) (n = 7) | >.05 | 66 (58-81) (n = 5) | >.05 | 62 (21-71) (n = 4) | .12 |
| P value1-153 | .0001 | .003 | .02 |
Results are expressed as percentages of EGFP+ cells and depicted as median (range). For statistical analysis the Mann-Whitney U-test has been used.
Comparison of the median of purified CD34+ cells and CD34+CD38− subset within the purified CD34+ population.
Comparison of the median of CD34+CD38−subset within the purified CD34+ population and purified CD34+CD38− cells.
Comparison of the median of purified CD34+ cells and purified CD34+CD38− cells.
Comparison of MFG-EGFP– and SF-EGFP–transduced cells.
Transduction efficiency of CAFC subsets.
The ability of transduced cells to form cobblestone areas was evaluated in long-term culture supported by FBMD-1 stromal cells. EGFP+ cobblestone areas were identified by fluorescence microscopy (Fig 3). The absolute numbers of CAFC at different culture periods increased as a result of the transduction procedure without significant differences between the target cells or vector used (Table 2). The absolute number of CAFC week 2 in the MFG-EGFP/Am12–transduced CD34+ UCB cells increased 5-fold, for the SF-EGFP/PG13–transduced CD34+ UCB cells the increase was 7-fold. The CAFC week 6 expanded 10-fold and 5-fold, respectively. For the CD34+CD38− UCB cells, similar results were obtained, 6-fold and 10-fold of CAFC week 6 after MFG-EGFP/Am12 and SF-EGFP/PG13 transduction, respectively. Consistent with the immaturity of the CD34+CD38− cell population, CAFC week 2 could not be detected in the CD34+CD38− cell fraction before transduction. These data show that the transduction protocol that has been used causes a modest expansion of both CAFC week 2 and week 6.
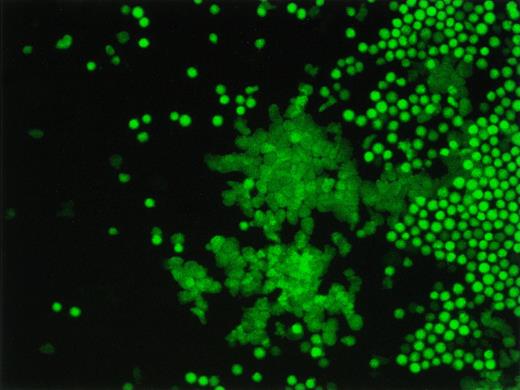
Fluorescence microscopic image of a representative EGFP+ cobblestone area. The bright green cells are the mature cells on top of the stromal layer and the dim green cells represents the EGFP+ cobblestone area.
Fluorescence microscopic image of a representative EGFP+ cobblestone area. The bright green cells are the mature cells on top of the stromal layer and the dim green cells represents the EGFP+ cobblestone area.
Absolute Numbers of CAFC Week 2 and Week 6 and Percentages of Green Fluorescent Cobblestone Areas After Transduction of 106 Selected UCB CD34+Cells or 35 × 103 CD34+CD38−Cells With the Vectors MFG-EGFP or SF-EGFP
| . | CAFC wk 2 . | CAFC wk 6 . | ||||||
|---|---|---|---|---|---|---|---|---|
| CD34+ . | % . | CD34+CD38− . | % . | CD34+ . | % . | CD34+CD38− . | % . | |
| Before transduction | 42 × 103 | — | ND | — | 4 × 103 | — | 0.3 × 103 | — |
| MFG-EGFP/Am12 | 218 × 103* | 26 | 2 × 103† | 15 | 41 × 103* | 6 | 2 × 103† | ND |
| SF-EGFP/PG13 | 315 × 103* | 60 | 2 × 103† | 24 | 22 × 103* | 27 | 3 × 103† | 25 |
| . | CAFC wk 2 . | CAFC wk 6 . | ||||||
|---|---|---|---|---|---|---|---|---|
| CD34+ . | % . | CD34+CD38− . | % . | CD34+ . | % . | CD34+CD38− . | % . | |
| Before transduction | 42 × 103 | — | ND | — | 4 × 103 | — | 0.3 × 103 | — |
| MFG-EGFP/Am12 | 218 × 103* | 26 | 2 × 103† | 15 | 41 × 103* | 6 | 2 × 103† | ND |
| SF-EGFP/PG13 | 315 × 103* | 60 | 2 × 103† | 24 | 22 × 103* | 27 | 3 × 103† | 25 |
Abbreviations: %, percentage of green fluorescent cobblestone areas expressed as median; ND, not detectable.
n = 2.
n = 1.
The transduction efficiency of the CAFC week 2 in MFG-EGFP/Am12 transduced CD34+ cells ranged between 23% and 30% with a median value of 26%, and in SF-EGFP/PG13–transduced CD34+cells the median value was 60% (46% to 74%) (Table 2). The transduction efficiency of the CAFC week 6 in MFG-EGFP/Am12–transduced CD34+ cells ranged between 0% and 11% with a median of 6% EFGP+ cobblestone areas. CAFC week 6 in SF-EGFP/PG13–transduced CD34+ cells showed transduction as high as 27%. CAFC week 6 in SF-EGFP/PG13–transduced CD34+CD38− cells showed a similar level of 25% transduction efficiency. Notably, highly purified CD34+CD38− cells transduced with the amphotropic cell line did not produce EGFP+ cobblestone areas week 6. These experiments clearly showed the superiority of SF-EGFP/PG13 over MFG-EGFP/Am12 in transducing late appearing CAFC, in concordance with the results obtained in phenotypically identified immature CD34+ subsets.
Repopulation of transduced subsets in NOD/SCID mice.
In parallel with analysis of cobblestone formation, the ability of transduced cells to reconstitute hematopoiesis in vivo was examined by transplantation of the equivalent of 105 noncultured CD34+ cells into sublethally irradiated NOD/SCID mice. After 35 days the level of chimerism and the percentage of EGFP+ cells in mouse BM were determined by flow cytometry (Table 3). Similar levels of engraftment were found in mice transplanted with noncultured or cultured CD34+ cells. After transplantation of noncultured CD34+ cells human cells were detected in all mice (n = 11) (median, 54% [range, 6% to 64%] CD45+cells). EGFP+ cells were found in 6 of 10 repopulated chimeric mice transplanted with MFG-EGFP/Am12–transduced CD34+ cells with a median percentage of EGFP+cells of 2% (Table 3). CD34+ cells transduced using the SF-EGFP/PG13 vector produced higher levels of EGFP+ cells (median, 23%) in the human population in all four mice transplanted. These data showed that the repopulating cells in the CD34+population can be transduced effectively and produce EGFP+progeny in transplanted NOD/SCID mice. In addition, SF-EGFP/PG13 was much more efficient in transducing the repopulating cells than MFG-EGFP/Am12.
Repopulation of EGFP-Transduced UCB Subsets in NOD/SCID Mice and CAFC Assay
| Vector/Packaging Cell Line . | UCB Subset . | Transduction Efficiency % EGFP . | EGFP+/Chimeric Mice3-150 . | Chimerism in NOD/SCID % CD45 . | EGFP+ on CD45+ Cells % . | CAFC wk 6 % EGFP . |
|---|---|---|---|---|---|---|
| MFG-EGFP/Am12 | CD34+ | 313-151 (29-51) | 6/10 | 12 (2-65) | 2 (0-18) | 6 (0-11) |
| SF-EGFP/PG13 | CD34+ | 663-151 | 4/4 | 8 (3-12) | 23 (2-41) | 27 (26-27) |
| P value | — | — | >.05 | .032 | .12 | |
| MFG-EGFP/Am12 | CD34+CD38− | 53-151 | 0/4 | 5 (1-24) | 0 | ND |
| SF-EGFP/PG13 | CD34+CD38− | 213-151 | 1/3 | 6 (4-9) | 3 | 253-151 |
| P value | — | — | >.05 | >.05 | — |
| Vector/Packaging Cell Line . | UCB Subset . | Transduction Efficiency % EGFP . | EGFP+/Chimeric Mice3-150 . | Chimerism in NOD/SCID % CD45 . | EGFP+ on CD45+ Cells % . | CAFC wk 6 % EGFP . |
|---|---|---|---|---|---|---|
| MFG-EGFP/Am12 | CD34+ | 313-151 (29-51) | 6/10 | 12 (2-65) | 2 (0-18) | 6 (0-11) |
| SF-EGFP/PG13 | CD34+ | 663-151 | 4/4 | 8 (3-12) | 23 (2-41) | 27 (26-27) |
| P value | — | — | >.05 | .032 | .12 | |
| MFG-EGFP/Am12 | CD34+CD38− | 53-151 | 0/4 | 5 (1-24) | 0 | ND |
| SF-EGFP/PG13 | CD34+CD38− | 213-151 | 1/3 | 6 (4-9) | 3 | 253-151 |
| P value | — | — | >.05 | >.05 | — |
Results are depicted as median (range) of 2 or 3 experiments. For statistical analysis the Mann-Whitney U-test has been used.
Abbreviation: ND, not detectable.
All transplanted mice engrafted with >1% CD45+ cells.
Insufficient data to perform statistical analysis.
Transplantation of noncultured CD34+CD38−cells and transduced CD34+CD38− resulted in chimerism levels of median 10% (range, 6% to 29%) for the noncultured cells and 5% (range, 1% to 24%) and 6% (range, 4% to 9%) for the MFG-EGFP/Am12– or SF-EGFP/PG13–transduced cells, respectively. In contrast to the results with purified CD34+ cells, CD34+CD38− cells transduced with MFG-EGFP/Am12 were not able to repopulate mouse BM with EGFP-expressing cells, although all four mice engrafted with human cells (Table 2); this parallels the absence of EGFP expressing CAFC week 6 in CD34+CD38− cells transduced with MFG-EGFP/Am12. Only one of three mice engrafted with SF-EGFP/PG13–transduced CD34+CD38−cells. EGFP+ could only be detected in 3% of the CD45+ cells produced. This is in contrast to the results with the CD34+ cells in that apparently most repopulating cells in the highly purified CD34+CD38−subset were not transduced efficiently or the transduced cells displayed a significant reduction in their engraftment potential compared with the cells that were not transduced during the procedure. Nevertheless, SF-EGFP/PG13 in these experiments was also apparently more efficient than MFG-EGFP/Am12.
Multilineage outgrowth of EGFP-transduced CD34+cells.
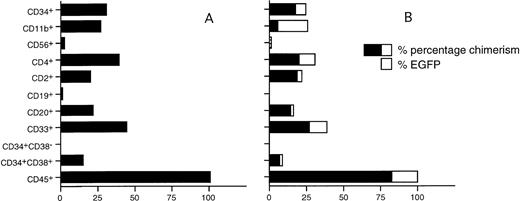
The composition of the EGFP+ human cell population in two mice was assessed by flow cytometry using a panel of lineage-specific markers (Fig 4). EGFP+ cells of the myeloid lineage (CD33, range, 31% to 39%; CD11b, range, 20% to 25%; CD4, range, 30% to 45%), T-lymphoid (CD2, range, 20% to 22%), B-lymphoid (CD20, range, 16% to 23%), and natural killer (NK) cells (CD56, 1%) were found in mice transplanted with EGFP-transduced CD34+ cells. Also, immature EGFP+CD34+ cells were present in the mouse BM (range, 1.1% to 6.8%) (Fig 5). Transduced cells and chimeric mice BM were also cultured in standard methylcellulose medium under conditions that selectively favor the outgrowth of human monomyeloid and erythroid progenitors3and fail to stimulate mouse progenitors. In both the graft and the chimeric mice BM, EGFP+ GM-CFU (15 of 39 in the graft and 3 of 23 in the mouse BM) and BFU-E (23 of 40 in the graft and 5 of 25 in the mouse BM) were identified by flow cytometry of isolated colonies or fluorescence microscopy of whole cultures.
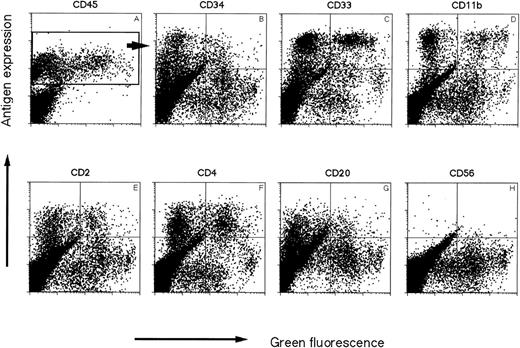
Representative immunophenotyping of chimeric NOD/SCID mouse BM 35 days after transplantation of MFG-EGFP/Am12 transduced, IL-3–, IL-6–, SCF–stimulated CD34+ UCB cells. BM (>10% CD45+) was stained with a panel of antibodies specific against different human blood cell lineages and CD45 as a marker for human cells. (A) The bright green autofluorescence on the x axes versus CD45. The window represents all human CD45+cells. The other dotplots shown are gated cells in this CD45+ window representing only human cells. Representative examples are shown for EGFP versus CD34 (B), EGFP versus CD33 (C), EGFP versus CD11b, (D) EGFP versus CD2 (E), EGFP versus CD4 (F), EGFP versus CD20 (G), and EGFP versus CD56 (H).
Representative immunophenotyping of chimeric NOD/SCID mouse BM 35 days after transplantation of MFG-EGFP/Am12 transduced, IL-3–, IL-6–, SCF–stimulated CD34+ UCB cells. BM (>10% CD45+) was stained with a panel of antibodies specific against different human blood cell lineages and CD45 as a marker for human cells. (A) The bright green autofluorescence on the x axes versus CD45. The window represents all human CD45+cells. The other dotplots shown are gated cells in this CD45+ window representing only human cells. Representative examples are shown for EGFP versus CD34 (B), EGFP versus CD33 (C), EGFP versus CD11b, (D) EGFP versus CD2 (E), EGFP versus CD4 (F), EGFP versus CD20 (G), and EGFP versus CD56 (H).
Representative chimerism and EGFP expression levels in chimeric NOD/SCID mouse BM 35 days after transplantation of nontransduced (A) and transduced (B) CD34+ UCB cells, relative to the numbers of human (CD45+) cells found.
Representative chimerism and EGFP expression levels in chimeric NOD/SCID mouse BM 35 days after transplantation of nontransduced (A) and transduced (B) CD34+ UCB cells, relative to the numbers of human (CD45+) cells found.
DISCUSSION
The versatile use of EGFP as a selectable marker of retroviral-mediated gene transfer in hematopoietic cells provides a basis to further optimize retroviral gene transfer to human repopulating stem cells and to evaluate the role of hematopoietic growth factors in activation and expansion of immature hematopoietic cells. This study focused on the development of optimal conditions for gene transfer to human CD34+ and CD34+CD38− UCB cells with the ability to reconstitute hematopoiesis in NOD/SCID mice and produce cobblestone areas for prolonged periods in stroma-supported long-term cultures.
Comparison of transduction frequencies of immunophenotypically characterized immature cells and those of SCID repopulating cells and CAFC may both demonstrate the relationship of these cell types as well as point to essential differences. In general, there was concordance between these assays, in that the GaLV-pseudotyped retroviral vector (SF-EGFP) transduction was much more efficient than the amphotropic retroviral vector (MFG-EGFP) transduction. Also, transduction frequencies of the immature CD34+CD38−subset within the CD34+ population related well to those obtained after transplantation of NOD/SCID mice and CAFC week 6. In addition, the study showed that repopulating cells in the highly purified CD34+CD38− cells were resistant to transduction in the absence of the CD38+ subset, particularly notable for MFG-EGFP/Am12 as demonstrated by the finding that the EGFP-transduced CD34+CD38−subset in general failed to produce EGFP+ progeny in NOD/SCID mice. One mouse transplanted with SF-EGFP/PG13–transduced sorted CD34+CD38− cells displayed 3% human EGFP+ cells, one order of magnitude less than the frequency of EGFP+ CAFC week 6 in the same sample.
The more prominent transduction efficiency of the EGFP gene into purified and highly purified immature UCB cells with the GaLV-pseudotyped SF-EGFP compared to the MFG-EGFP/Am12 retroviral packaging cell combination, is consistent with earlier studies where transduction of human hematopoietic progenitors was more efficient with a retroviral vector that uses the GaLV receptor.23-26 The lower transduction percentage obtained with the amphotropic vector may thus be primarily attributed to the low or absent expression of the amphotropic envelope-receptor on the target cells.45,46This was particularly corroborated by the absence of EGFP expression in MFG-EGFP/Am12–transduced sorted CD34+CD38− cells, both in the CAFC week 6 and after transplantation into NOD/SCID mice. Alternatively, UCB cells may be more efficiently transduced by the SF-EGFP/PG13 vector/packaging cell combination due to the use of the SFFV/MESV hybrid promoter, which has been designed to overcome transcriptional inefficiency and silencing associated with retroviral gene transfer into myeloid progenitors and hematopoietic stem cells.47 Other variables that obviously need to be further analyzed include differences in titer and the ability and efficiency of the vectors to transduce EGFP in hematopoietic cells. The titers of the two vectors used were comparable, but tested in different assays. The colocalization of vector and cells during transduction, using the CH-296 fibronectin fragment,21 makes it unlikely that differences in titer did heavily influence the results. This is even more so since preparative experiments (not shown) with the MFG-EGFP/AM12 retroviral vector showed that additional charges of the virus supernatant in the transduction protocol did not result in higher transduction frequencies, which indicated that the transduction system is sufficiently saturated with virus. Also, Hanenberg et al48 concluded that the amount of retroviral particles present in the supernatant was not a limiting factor for transduction of CD34+ BM cells on CH-296–coated plates. The higher efficiency of the SF-EGFP/PG13 combination when compared with the MFG-EGFP/AM12 combination should therefore not be considered as being caused by supernatant virus titer differences.
The observation that repopulating cells in the CD34+population can be transduced efficiently and produce transduced multilineage progeny in transplanted NOD/SCID mice, whereas repopulating cells in the highly purified CD34+CD38− subset are either not transduced effectively or do not develop in vivo, is of considerable interest for elucidation of mechanisms involved in successful transduction of immature hematopoietic cells. The transduction efficiency of the CD34+CD38− tended to be lower than that of the CD34+ cells,2 and was significantly so for the MFG-EGFP/Am12 combination, which may be related to the low or absent expression of the amphotropic receptor. Because repopulating cells are exclusively present in the small CD34+CD38− population, and CD34+CD38+ cells do not effectively engraft, the low levels of gene expression in the chimeric NOD/SCID BM after transplantation of transduced CD34+CD38−cells may indicate that the growth factors used during prestimulation and virus infection were not sufficiently effective for activation and stable virus integration of the NOD/SCID repopulating cells. The much higher frequency of EGFP expressing cells in the BM of NOD/SCID mice after transplantation of transduced stem cells from the less pure CD34+ fraction may indicate that stimuli provided by accessory CD34+ cells were responsible for the more efficient transduction of repopulating CD34+CD38- within the CD34+ cell fraction. Alternatively, these accessory cells may be needed to maintain the repopulating ability of stem cells during the transduction procedure of 4 days, eg, by preventing differentiation, or to promote the expansion and outgrowth of transduced stem cells after transplantation. We speculate that these accessory cells are related to the accessory CD34+CD38+ cells, which are involved in the maintenance and expansion of CD34+CD38− cells in immunodeficient mice transplanted with nontransduced human UCB subsets.3 Further identification of these accessory CD34+ cells and elucidation of the active principle may therefore be both relevant for stem cell expansion physiology and for the design of successful gene transfer strategies for immature hematopoietic cells.
The absolute numbers of CAFC produced after week 2 and week 6 of culture show a modest increase after transduction with the MFG-EGFP or SF-EGFP vectors. The frequency of EGFP+CAFC week 6 in SF-EGFP– or MFG-EGFP–transduced CD34+ UCB cells was similar to levels of EGFP+CD45+ cells found in NOD/SCID mice. The reason for the 10-fold discrepancy between the levels of transduction of the CAFC week 6 and the very low numbers of EGFP+CD45+ in NOD/SCID BM after transplantation of the SF-EGFP/PG13–transduced CD34+CD38− population is not clear. Studies with the murine ADA vector similarly yielded very low numbers of gene-marked human cells in the NOD/SCID mouse BM, in contrast to higher numbers of transduced LTC-IC and colony-forming cells (CFC), which was interpreted as evidence that the latter cell types are functionally distinct from NOD/SCID repopulating cells.1However, this distinction might be artificial if effectively transduced CD34+CD38− require the described CD34+ accessory cells for in vivo maintenance and expansion but not for in vitro cobblestone area forming ability.
We conclude that retroviral-mediated EGFP transduction in UCB cells, in combination with functional assays for repopulating cells, is a rapid tool to study essential gene transfer variables such as vector tropism and transduction conditions. In addition, the use of the GaLV-pseudotyped retroviral vector SF-EGFP resulted in highly efficient gene transfer in both late CAFC and NOD/SCID repopulating cells, the latter presently the most immature subset of human CD34+CD38− cells that can be approached by a functional assay. These results justify the expectation that the imminent analysis of variables promoting genetic marking of primitive, transplantable hematopoietic cells, such as further optimized transduction conditions and vector constructs, lead to protocols for clinically relevant levels of therapeutic gene transfer.
ACKNOWLEDGMENT
The authors thank Dr A.Th. Alberda and staff of the St Franciscus Hospital (Rotterdam, The Netherlands) for the collection of cord blood samples used in this study. We thank Alexandra de Koning and Sandra van Sluijs for excellent technical assistance, Joop Brandenburg for breeding the immunodeficient mice, and Els van Bodegom for excellent animal care.
P.B.v.H. and M.M.A.V. contributed equally to this manuscript.
Supported in part by grants of the Netherlands Organization for Scientific Research NWO, the Netherlands Cancer Foundation Koningin Wilhelmina Fonds, the Royal Netherlands Academy of Arts and Sciences, contracts of the Commission of the European Communities, and Spanish CICYT Grant No. SAF96-0130. J.A.C. is a recipient of a postdoctoral grant from the Areces Fund, Spain.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Gerard Wagemaker, PhD, Institute of Hematology, Room Ee1314, Erasmus University Rotterdam, Dr Molewaterplein 50, PO Box 1738, 3000 DR Rotterdam, The Netherlands; e-mail: wagemaker@hema.fgg.eur.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal