Abstract
Recently, there has been an increasing interest in the expression pattern and biological significance of the CD45 molecule in myeloma cells. In this study, we have further defined the phenotypic pattern of CD45 expression on myeloma cells. Using a panel of myeloma cell lines, we showed that CD45 showed a remarkably heterogeneous pattern of expression. Whereas some cell lines were CD45+ and others were CD45−, the U-266 cell line, although predominantly CD45−, still had a considerable subpopulation of CD45+ cells. Among the myeloma cell lines examined, there was a direct correlation between interleukin-6 (IL-6) dependency and CD45 positivity. Moreover, we showed that IL-6 stimulation led to the induction of expression of CD45 and cellular proliferation. Using independent experimental approaches, we could show that the IL-6–induced effects were closely linked to CD45 expression. First, sorting out CD45+ and CD45− subsets of U-266 cell line followed by IL-6 stimulation, only the CD45+ cells showed a proliferative advantage after IL-6 stimulation. Second, IL-6 stimulation of sorted CD45−cells was gradually followed by phenotypic conversion to CD45+ cells that started after 2 days as judged by the detection of CD45 mRNA by reverse transcription polymerase chain reaction (RT-PCR) and immunophenotypic analysis by flow cytometry. Withdrawal of IL-6 from the medium led to gradual loss of CD45 expression in CD45+ flow-sorted U-266 cells. Third, the use of vanadate, a potent inhibitor of protein tyrosine phosphatase (PTP), abrogated the IL-6–induced proliferation in the CD45+ myeloma cells. On the other hand, cellular proliferation induced by IL-6 was not affected by the serine-threonine phosphatase inhibitor okadaic acid. Our data show that the expression pattern of CD45 in myeloma cell lines is heterogeneous and show for the first time that CD45 expression can be induced by IL-6 stimulation. Finally, these data shed some light on the biological role of CD45 in myeloma by determining the proliferative population among myeloma cells.
THE CD45 MOLECULE IS A transmembrane glycoprotein expressed on the surface of all hematopoietic cells and their precursors except for mature erythrocytes and platelets.1 Differential usage of three variable exons tentatively called A-, B-, and C-encoding amino acids near the N-terminus of the molecule results in the generation of at least 5 alternatively spliced isoforms. CD45 isoforms reactive with exon 4-, 5-, and 6-specific monoclonal antibodies are referred to as CD45RA, CD45RB, and CD45RC, respectively.2 In humans, the lowest molecular weight isoform of CD45 (CD45RO) was found to be selectively expressed on CD34+ progenitor cells with functional erythroid colony–forming activity.3 The cytoplasmic portion of CD45 molecule contains two tyrosine phosphatase domains in which activity has been shown to be crucial for both T- and B-cell activation through the corresponding antigen receptor.4Mice homozygous for CD45-exon 6 mutation had a block in thymocyte maturation at the transitional stage from double-positive to single-positive stage and a functional B-cell defect in the form of abrogated Ig μ-induced proliferation.5
The initial signal transduction events essential for eliciting an immune response are controlled by concerted action of protein tyrosine kinases and protein tyrosine phosphatases (PTPs). Distinct enzymes can be positive or negative regulators of a molecular reaction, and in certain circumstances a single enzyme may have both functions. The biological functions of two PTPs, the src homology region 2–containing protein tyrosine phosphatase and CD45, are considered to serve to illustrate the dichotomy by which such class of enzymes regulate immune responses.6 Another role for CD45 in regulation of B-cell–negative and –positive selection has been shown by experiments crossing CD45-deficient mice with mice carrying Ig transgenes specific for hen egg lysozyme.7
The expression of CD45 molecule in primary myeloma cells and cell lines is quite variable, where in some studies myeloma cells were described as CD45−/dim mature cells,8-12whereas in others they were described as CD45+/++ immature cells.13-16 The biological functions of CD45 in myeloma are still largely unknown.
Interleukin-6 (IL-6) had been originally characterized as a B-cell differentiation factor, but it also has a variety of biological functions in various cells and tissues, including the hematopoietic cells, hepatocytes, and nerve cells.17 In myeloma cells, IL-6 had been shown to play a role as an autocrine growth factor.18 IL-6 is the original member of a family of cytokines that share gp130 as a critical component for signal transduction. The ubiquitous expression of gp130 explains the basis for the extensive function of IL-6 family in hematopoietic, cardiovascular, and nervous tissues. After IL-6 stimulation, gp130 homodimers or heterodimers activate associated cytoplasmic tyrosine kinases and subsequently modify downstream transcription factors.19 In this work, we examined the expression pattern and the biological significance of CD45 in a panel of myeloma cell lines. The observation of concordant correlation between IL-6 dependency and CD45 positivity led us to proceed to investigate the possible correlation between IL-6 and CD45 expression.
MATERIALS AND METHODS
Cell culture.
The IL-6–independent KMS-5,20 U-266, and IL-6–dependent (ILKM-2 and ILKM-3) human myeloma cell lines (kindly provided by Dr S. Shimizu, Shimane Prefectural Hospital, Matsue, Japan) were maintained in RPMI 1640 medium (Nissui, Tokyo, Japan) supplemented with 10% fetal calf serum (M.A. Bioproducts, Walkersville, MD). IL-6 was added to the culture of IL-6–dependent cell lines and in other experiments as appropriately mentioned in a final concentration of 2 ng/mL. Cell counts were performed both manually on a hemocytometer and automatically by harvesting the whole culture into 0.5 mL and counting cells at a constant flow rate for 1 minute using the cell sorter (Epics Elite ESP; Coulter, Hialeah, FL).
Monoclonal antibodies and flow cytometry.
Labeled monoclonal antibodies fluorescein isothiocyanate (FITC)-CD38, phyroerythrin (PE)-CD45, PE-Cy5-CD45, PE-CD45RO, and PE-CD45RA were purchased from Immunotech (Marseille, France), and R-PE-CD45B was purchased from Pharmingen (San Diego, CA). Cells were incubated with 20 μg of each appropriate antibody, left to react for 30 minutes on ice, and washed twice in phosphate-buffered saline (pH 7.4) with 200 μg/mL bovine serum albumin and 0.01% sodium azide. Cells were then analyzed for surface immunofluorescence, cell count, and viability by the cell sorter.
Cell sorting.
For cell sorting, the cells were stained with PE-CD45 and FITC-CD38 as described above under sterile conditions. Either CD45+ or CD45−U-266 subpopulations were isolated via the cell sorter (Epics Elite ESP; Coulter) for subsequent in vitro culture.
Single-cell assay.
U-266 cells were extensively washed and cultured without exogenous IL-6 to minimize the number of CD45+ cells. The CD45− subpopulation was sorted out and plated at 1 cell per well in a 96-well plate and cultured for 28 days. Forty-eight wells received exogenous IL-6 (2 ng/mL), and the other 48 wells were maintained without exogenous IL-6. The number of wells that received one cell were verified microscopically on the second day of plating. Wells with significant proliferating cells were analyzed by flow cytometry after staining with PE-CD45 monoclonal antibody, as described above.
Semiquantitative RT-PCR.
Total cellular RNA was collected by guanidine thiocyanate and phenol/chloroform extraction and reverse transcribed by Superscript II (GIBCO-BRL, Gaithersburg, MD) at 37°C for 60 minutes as recommended by the manufacturer. The primers used were as follows: β-actin forward, ATC TGG CAC CAC ACC TTC TAC A AT GAG CTG CG; β-actin reverse, CGT CAT ACT CCT GCT TGC TGA TCC ACA TCT GC; IL-6 forward, ATG AAC TCC TTC TCC ACA AGC GC; IL-6 reverse, GAA GAG CCC TCA GGC TGG ACT G; CD45 forward, AAC AGT GGAGAA AGG ACA CA; and CD45 reverse, TGT GTC CAG AAA GGC AAA GC. Thermal cycling was performed for 30 cycles: denaturation at 94°C for 1 minute, primer extension at 72°C for 1 minute, and primer annealing for 1 minute at 65°C for β-actin and IL-6 gene and at 55°C for CD45 gene. For the semiquantitative polymerase chain reaction (PCR), complementary DNA load was standardized in preliminary experiments by making serial dilution and amplifying β-actin for 20 cycles to produce just detectable signal, thus keeping the PCR condition within the linear range of amplification before reaching saturation.
CD45 phosphatase blocking experiments.
CD45+ subpopulations of U-266 cells were extensively washed in medium and subsequently plated in 24-well plates at 5 × 104/well. The cells were incubated for 2 days in medium alone: rIL-6 at a concentration of 2 ng/mL; IL-6 and sodium orthovanadate (Na3VO4; Sigma, St Louis, MO); IL-6 and okadaic acid (Wako Pure Chemicals, Osaka, Japan); or IL-6 and dimethyl sulfoxide (DMSO). The PTP inhibitor Na3VO4 was dissolved in sterile water just before use and added to the culture at 50 μmol/L final concentration. Preliminary experiments were performed using a range of 12.5 μmol/L to 100 μmol/L to determine the optimum concentration for blocking CD45 phosphatase. Okadaic acid was dissolved in DMSO to 0.25 mmol/L and stocked at −80°C; for control experiments, okadaic acid was diluted in Tris-HCl buffer and used at a concentration of 10 ng/mL.
RESULTS
Expression of CD45 was heterogeneous and possibly correlated with IL-6 dependency.
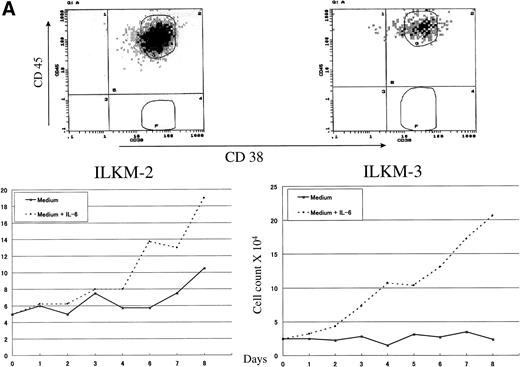
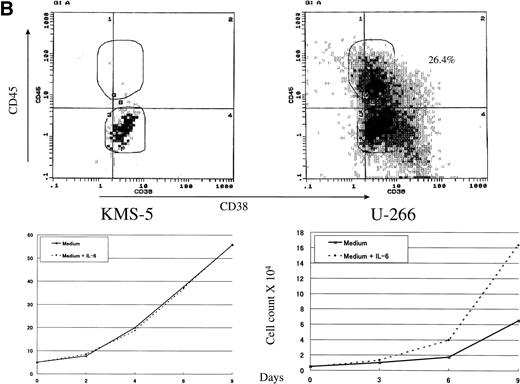
To study the role and biological importance of CD45 expression in myeloma cells, we first examined its pattern of expression by flow cytometry on a panel of myeloma cell lines. The expression pattern of CD45 showed a remarkable heterogeneity between the various myeloma cell lines (Fig 1). Whereas some cell lines were strongly positive (ILKM-2 and ILKM-3; Fig 1A), others, such as KMS-5, were almost completely CD45− (Fig 1B). U-266 cells showed a peculiar pattern of CD45 expression, in which a relatively high percentage of cells (>25%) were CD45+ (Fig 1B). ILKM-2 and ILKM-3 were strictly IL-6–dependent cell lines, and they could not be grown in culture without the addition of exogenous IL-6 (2 ng/mL). They had a duplication time of 4 and 3 days, respectively (Fig 1A, bottom). On the other hand, KMS-5 was completely IL-6–independent and had a duplication time of less than 2 days (Fig 1B, bottom). Again, U-266 cell line showed a peculiar pattern in which it grew satisfactorily in cultures without exogenous IL-6, yet the addition of IL-6 to the culture enhanced its growth by about 30% (Fig 1B, bottom).
CD45 was heterogeneously expressed on myeloma cells and correlated with IL-6 dependency. (A) IL-6–dependent myeloma cell lines ILKM-2 and ILKM-3 were stained for 30 minutes by PE-Cy5-CD45 (forward scatter) and FITC-38 (side scatter) and analyzed by cell sorter (top panels), and growth curves are shown of the cells cultured in medium alone (solid lines) or in the presence of IL-6 (2 ng/mL; dotted lines) for the indicated period of time (bottom panels). (B) CD45 expression (top) and growth curves (bottom) of the IL-6–independent cell lines KMS-5 and U-266.
CD45 was heterogeneously expressed on myeloma cells and correlated with IL-6 dependency. (A) IL-6–dependent myeloma cell lines ILKM-2 and ILKM-3 were stained for 30 minutes by PE-Cy5-CD45 (forward scatter) and FITC-38 (side scatter) and analyzed by cell sorter (top panels), and growth curves are shown of the cells cultured in medium alone (solid lines) or in the presence of IL-6 (2 ng/mL; dotted lines) for the indicated period of time (bottom panels). (B) CD45 expression (top) and growth curves (bottom) of the IL-6–independent cell lines KMS-5 and U-266.
Next, we have extended our analyses to delineate the expression pattern of CD45 isoforms in myeloma cells. Within the CD45+ lines, all of them expressed the CD45RO isoform and all were CD45RA− and CD45RB+ (data not shown). These data showed that the expression pattern of CD45 was heterogeneous in myeloma cells, not only in the context of line-to-line variation, but also within a given cell line. It also showed a concordant correlation between CD45 expression and IL-6 dependency and raised the possibility that the CD45+ populations were those that responded to IL-6 stimulation in the form of proliferation.
CD45 expression was induced by IL-6 and was lost again upon its withdrawal.
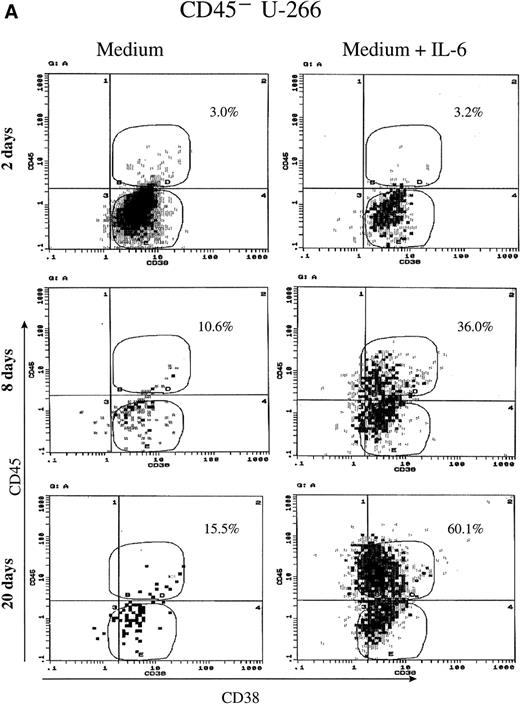
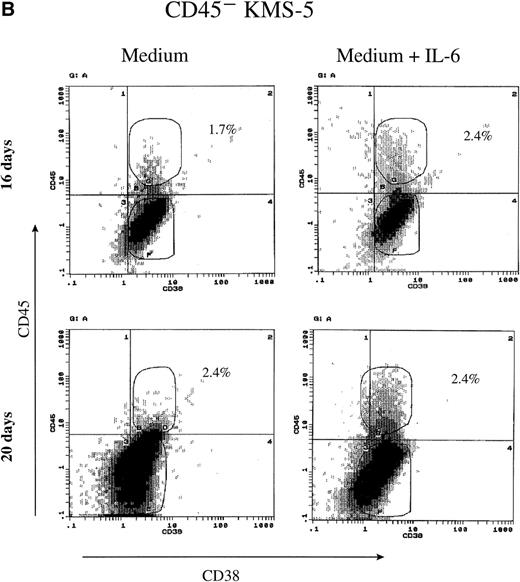
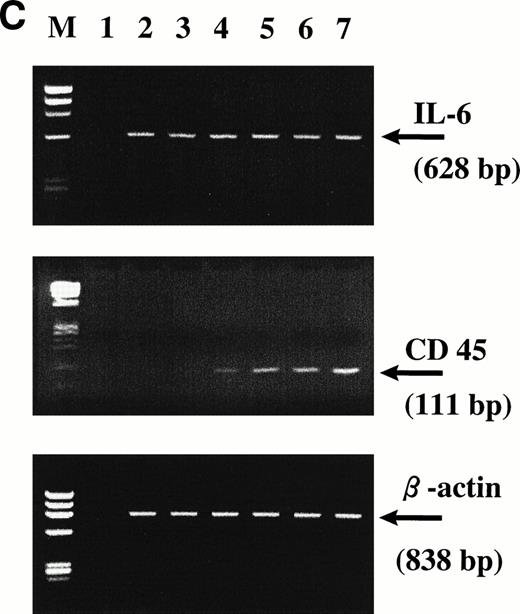
To further analyze the relationship between IL-6 dependency and the expression of CD45, we have sorted out both CD45+ and CD45− subpopulations from U-266 and KMS-5 cell lines. Sorted cells were maintained in culture either in the presence or absence of IL-6 for about 20 days and were analyzed at different time points by flow cytometry and reverse transcription polymerase chain reaction (RT-PCR) for the expression of CD45. Surprisingly, more than 35% of the CD45− U-266 cells underwent phenotypic conversion to CD45+ after 8 days, and more than 60% underwent phenotypic conversion after 20 days of culture with IL-6 (2 ng/mL) compared with a minor fraction of 15% of cells kept in medium without IL-6 (Fig2A). Conversely, the sorted CD45− fraction from the IL-6–independent KMS-5 line did not show significant phenotypic conversion, even after culture for 20 days with IL-6 (Fig 2B). Using RT-PCR to detect CD45 mRNA in U-266 cells, we observed that although it was hardly detectable after 2 days (Fig 2C, lane 3), it was clearly detectable after 4 days (Fig 2C, lane 4) of IL-6 stimulation. PCR analysis showed that the level of endogenous IL-6 mRNA did not change during the course of IL-6 treatment and that both CD45−and CD45+ cells produced endogenous IL-6 of comparatively the same level. In the culture supernatant of U-266 cell line, endogenous IL-6 was produced at a concentration of 10 pg/mL/1 × 106 cells, as previously reported.21
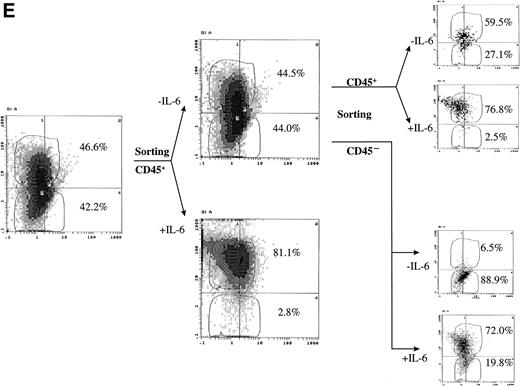
CD45 expression was induced by IL-6 and lost on its withdrawal. (A) Flow-sorted CD45− U-266 cells were cultured in medium alone (left) or in medium + IL-6 (2 ng/mL; right), and cells were analyzed by flow cytometry after staining with PE-Cy5-CD45 and FITC-CD38. The percentage of CD45+ cells is indicated on each flow cytogram. (B) CD45−IL-6–independent KMS-5 cells were cultured with or without exogenous IL-6 (2 ng/mL) and analyzed after staining by PE-Cy5-CD45 and FITC-CD38. (C) RT-PCR analysis of β-actin, CD45, and IL-6 of CD45− U-266 cells cultured with IL-6. Lane M, ◊ X174/Hae III digest DNA size marker; lane 1, negative control without RT product; lanes 2, 3, 4, 5, and 6, IL-6–treated CD45− cells at day 0, 2, 4, 6, and 8, respectively; lane 7, CD45+ U-266 cells. (D) Flow-sorted CD45+ U-266 cells were incubated in medium without IL-6. Cells were stained with PE-CD45 and FITC-CD38 and subjected to flow cytometric analysis as described in Materials and Methods. The percentage of CD45+ cells is indicated on each flow cytogram. (E) Flow-sorted CD45+ U-266 cells were cultured without exogenous IL-6 for 2 weeks (left), and only CD45+ fraction was sorted out and incubated with or without IL-6 (2 ng/mL) for another 2 weeks (middle). Both CD45+ and CD45− fractions were separately sorted out and cultured for 5 days with and without IL-6 (right). The percentages of both CD45+ and CD45− fractions are indicated on each flow cytogram.
CD45 expression was induced by IL-6 and lost on its withdrawal. (A) Flow-sorted CD45− U-266 cells were cultured in medium alone (left) or in medium + IL-6 (2 ng/mL; right), and cells were analyzed by flow cytometry after staining with PE-Cy5-CD45 and FITC-CD38. The percentage of CD45+ cells is indicated on each flow cytogram. (B) CD45−IL-6–independent KMS-5 cells were cultured with or without exogenous IL-6 (2 ng/mL) and analyzed after staining by PE-Cy5-CD45 and FITC-CD38. (C) RT-PCR analysis of β-actin, CD45, and IL-6 of CD45− U-266 cells cultured with IL-6. Lane M, ◊ X174/Hae III digest DNA size marker; lane 1, negative control without RT product; lanes 2, 3, 4, 5, and 6, IL-6–treated CD45− cells at day 0, 2, 4, 6, and 8, respectively; lane 7, CD45+ U-266 cells. (D) Flow-sorted CD45+ U-266 cells were incubated in medium without IL-6. Cells were stained with PE-CD45 and FITC-CD38 and subjected to flow cytometric analysis as described in Materials and Methods. The percentage of CD45+ cells is indicated on each flow cytogram. (E) Flow-sorted CD45+ U-266 cells were cultured without exogenous IL-6 for 2 weeks (left), and only CD45+ fraction was sorted out and incubated with or without IL-6 (2 ng/mL) for another 2 weeks (middle). Both CD45+ and CD45− fractions were separately sorted out and cultured for 5 days with and without IL-6 (right). The percentages of both CD45+ and CD45− fractions are indicated on each flow cytogram.
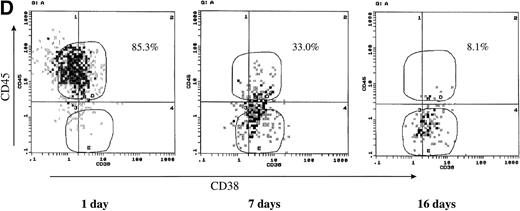
To further confirm the effect of IL-6 on CD45 expression, we have cultured CD45+ U-266 in the absence of exogenous IL-6. Withdrawal of IL-6 led to the loss of CD45 expression, with gradual conversion of the CD45+ cells into CD45−. More than 65% of the cells lost CD45 after 7 days, and more than 90% lost CD45 after 16 days of culture in IL-6 free medium (Fig 2D). To further confirm that U-266 cells can freely change from the CD45+ to the CD45− phenotype and vice versa depending on IL-6 and that this phenotypic conversion is not a one-way process, we have performed the following experiment. Flow-sorted CD45+ cells were maintained in culture without exogenous IL-6 for approximately 2 weeks. After this period of time, more than 40% of the cells had a CD45− phenotype (Fig 2E, left). The CD45+ fraction of these cells was sequentially sorted and then cultured with and without exogenous IL-6 (2 ng/mL; Fig 2E, middle). After culture for another 2 weeks, both the CD45+and the CD45− fractions that appeared in culture without IL-6 were again sorted and then cultured with and without IL-6 for 5 days. These cultures (Fig 2E, right) had a strikingly heterogeneous pattern of CD45 expression despite all originating from flow-sorted CD45+ cells. These data showed that CD45 expression could be induced by IL-6 stimulation at least in the U-266 cell line and that CD45 mRNA is apparently upregulated by IL-6 and precedes the surface expression of CD45. Phenotypic conversion from CD45− to CD45+ and vice versa occurred freely in both directions and was highly dependent on the IL-6 level to which the cells were exposed.
Single-cell assay confirmed that induction CD45 are IL-6–induced effects and not due to contaminating CD45+ cells.
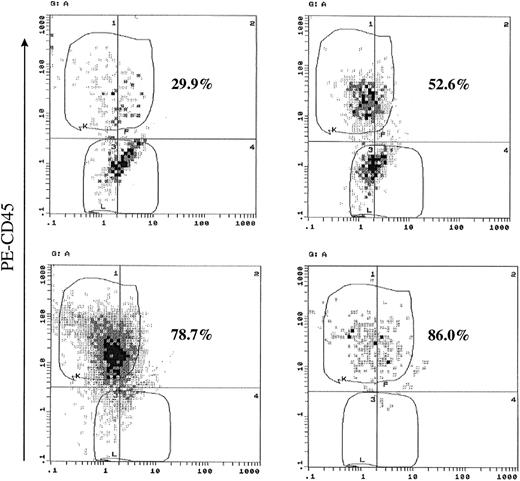
To address the question of a possible sorting defect that could result in a small number of contaminating CD45+ cells, which could proliferate and result in the observed phenotypic conversion, we have performed a single-cell assay by limiting dilution of flow-sorted CD45− cells. Of 45 wells cultured in the presence of exogenous IL-6 (2 ng/mL), 35 wells (77.7%) had proliferating cells after 28 days of culture. We have analyzed 14 (40%) of those clones for the expression of CD45. All of the analyzed clones without exception showed CD45+ cells with varying percentages ranging from 29.9% to 86% (Fig 3 and Table1). On the other hand, the number of wells containing proliferating cells cultured without exogenous IL-6 were considerably less than those cultured with IL-6. Most of those wells had a considerably lower cell number (<100 cells/well), and only 4 wells had about 200 cells. When those wells were analyzed, they showed low percentages of CD45+ cells (0.9% to 8.9%), accounting for their ability to proliferate depending only on endogenous IL-6 production (Table 1). The results of this experiment confirm that phenotypic conversion of CD45− to CD45+ in U-266 cells is an IL-6–induced effect rather than caused by contamination by CD45+ cells during cell sorting. It also shows that the proliferative capacity is confined to the CD45+ population and much enhanced by IL-6.
Single-cell assays confirmed that CD45 expression and proliferation are IL-6–induced effects. CD45− U-266 were flow-sorted, diluted, and plated at 1 cell per well in 96-well plate. Cells were cultured for 28 days with or without IL-6 (2 ng/mL). At the end of the culture period, cells were harvested from wells containing enough cells for flow cytometric analysis, stained with PE-CD45 monoclonal antibody, and analyzed. Representative flow cytograms from cells cultured with IL-6 are shown. The percentage of CD45+ cells is shown on each flow cytogram.
Single-cell assays confirmed that CD45 expression and proliferation are IL-6–induced effects. CD45− U-266 were flow-sorted, diluted, and plated at 1 cell per well in 96-well plate. Cells were cultured for 28 days with or without IL-6 (2 ng/mL). At the end of the culture period, cells were harvested from wells containing enough cells for flow cytometric analysis, stained with PE-CD45 monoclonal antibody, and analyzed. Representative flow cytograms from cells cultured with IL-6 are shown. The percentage of CD45+ cells is shown on each flow cytogram.
Summary of Single-Cell Assays
| . | Medium . | +IL-6 . |
|---|---|---|
| No. of wells containing single cell at the start of culture | 46 | 45 |
| No. of wells containing cells by the end of culture (28 days) | 24 | 35 |
| 1-100 cells/well | 20 | 0 |
| 100-200 cells/well | 4 | 0 |
| >500 cells/well | 0 | 35 |
| No. of analyzed clones | 4 | 14 |
| No. of wells containing CD45+ cells | 4 | 14 |
| Percentage of CD45+ cells in analyzed clones (range) | 0.9%-8.9% | 29.9%-86.0% |
| . | Medium . | +IL-6 . |
|---|---|---|
| No. of wells containing single cell at the start of culture | 46 | 45 |
| No. of wells containing cells by the end of culture (28 days) | 24 | 35 |
| 1-100 cells/well | 20 | 0 |
| 100-200 cells/well | 4 | 0 |
| >500 cells/well | 0 | 35 |
| No. of analyzed clones | 4 | 14 |
| No. of wells containing CD45+ cells | 4 | 14 |
| Percentage of CD45+ cells in analyzed clones (range) | 0.9%-8.9% | 29.9%-86.0% |
Myeloma cells expressing CD45 proliferated in response to IL-6 stimulation.
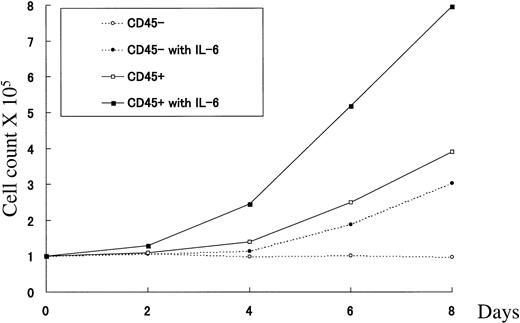
To assess the proliferative activity of U-266 CD45+ and CD45− subpopulations in response to IL-6 stimulation, flow-sorted CD45+ and CD45− cells were cultured separately either in the presence or absence of IL-6 (2 ng/mL), and cell counts were performed at different time points. As shown in Fig4, CD45+ cells showed a rapid response that led to a 2.4-fold increase in cell number after 4 days compared with a 1.4-fold increase in the absence of exogenous IL-6. This increase scaled up to 5.1- and 7.9-fold after 6 and 8 days of culture with IL-6, respectively, compared with only 2.5- and 3.9-fold increases in the absence of IL-6. On the other hand, sorted CD45− cells did not show any increase after 4 days of culture with IL-6, but after 6 and 8 days, 1.9- and 3.0-fold increases, respectively, were observed. This proliferative activity is probably attributed to emergence of CD45+ cells during culture with IL-6. The CD45− fraction cultured without IL-6 remained consistently unchanged during the whole culture period as the medium was frequently changed to remove any endogenously produced IL-6. These results showed that the CD45+ fraction of U-266 constituted the continuously proliferating fraction in response to IL-6 stimulation, and this response was largely dependent on the level of IL-6 available in culture.
CD45+ cells proliferated in response to IL-6 stimulation. Growth curves of flow-sorted CD45−cells (dotted lines) cultured in medium alone (○) or in the presence of IL-6 (2 ng/mL; •), and CD45+ cells (solid lines) cultured in medium alone (□) or in the presence of IL-6 (▪). Cells were cultured for the indicated period of time, and cell counts were performed as described in Materials and Methods.
CD45+ cells proliferated in response to IL-6 stimulation. Growth curves of flow-sorted CD45−cells (dotted lines) cultured in medium alone (○) or in the presence of IL-6 (2 ng/mL; •), and CD45+ cells (solid lines) cultured in medium alone (□) or in the presence of IL-6 (▪). Cells were cultured for the indicated period of time, and cell counts were performed as described in Materials and Methods.
IL-6–induced effects were abolished by the PTP inhibitor sodium vanadate.
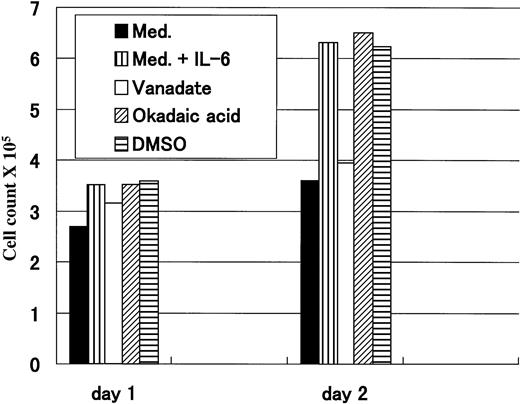
To verify whether the IL-6–induced proliferation could be mediated through CD45, we have used Na3VO4, which is known to be a potent PTP inhibitor, to block the action of CD45 phosphatase. Okadaic acid, a serine-threonine phosphatase inhibitor, was used as a control, and because it was dissolved in DMSO, another experiment using DMSO at the same concentration was the control. After 1 day of treatment, no remarkable difference was observed, but on day 2 cells treated with medium alone and those treated with vanadate and IL-6 (2 ng/mL) had a similar proliferative pattern that was clearly less active compared with that of cells grown in medium with IL-6. Conversely, cells treated with IL-6 together with either okadaic acid or DMSO had an almost identical proliferative pattern to that of cells treated with IL-6 alone (Fig 5). These data suggested that the IL-6–induced cellular proliferation could be mediated through the CD45 molecule, because this effect could only be abolished by PTP inhibitor (vanadate), but not by serine-threonine phosphatase inhibitor (okadaic acid). However, because we have used vanadate in this experiment, the possibility of involvement of other PTPs cannot be ruled out.
IL-6–induced proliferation was abrogated by vanadate. Flow-sorted CD45+ U-266 cells were cultured in medium alone; in medium +IL-6 (2 ng/mL); or in medium + IL-6 + either 50 μmol/L Na3VO4; 10 ng/mL okadaic acid; or DMSO. Cell counts were performed after 1 and 2 days after treatment. Only vanadate could abrogate IL-6–induced proliferation after 2 days.
IL-6–induced proliferation was abrogated by vanadate. Flow-sorted CD45+ U-266 cells were cultured in medium alone; in medium +IL-6 (2 ng/mL); or in medium + IL-6 + either 50 μmol/L Na3VO4; 10 ng/mL okadaic acid; or DMSO. Cell counts were performed after 1 and 2 days after treatment. Only vanadate could abrogate IL-6–induced proliferation after 2 days.
DISCUSSION
In this study, we showed that CD45 expression in myeloma cell lines was quite heterogeneous, not only regarding the presence of positive and negative lines, but also within a given cell line such as U-266, in which both positive and negative subpopulations did exist (Fig 1A and B). This is in agreement with the existence of different phenotypes for myeloma cells regarding CD45 expression, in which it is both reported as CD45−/dim,8-12 and as CD45+/++in other studies.13-16 Our finding that CD45 expression could be induced by IL-6 can explain the different patterns of expression that appear in the literature. Different microenvironmental levels of IL-6, either endogenously produced by myeloma cells (autocrine loop) or exogenously supplied by the bone marrow stroma cells, may modulate the pattern of CD45 expression in different cases. Alterations in the level of IL-6 in a given case may result in different CD45 phenotypes when analyzed at different time points. Indeed, different myeloma cell lines produce variable levels of endogenous IL-6, and it is quite high in some and barely detectable in others (unpublished data).
The isoform expression pattern in CD45+ myeloma cells is much more uniform in that almost all CD45+ myeloma cell lines predominantly express the CD45RO and CD45RB isoforms (data not shown). The biological significance of isoform expression is still ill understood, but it could be speculated that RO and RB isoforms are involved in positive regulation of cellular proliferation in myeloma cells.
We also showed that CD45 expression could be induced by exogenous IL-6 (2 ng/mL) stimulation in sorted CD45−U-266 cells and that this phenotypic conversion occurred spontaneously but at a much lower rate in the absence of exogenous IL-6 (Fig 2A). This spontaneous conversion might be caused by the effect of endogenously produced IL-6 (10 pg/mL; Fig 2C). Because immature CD45+ cells are generally considered to differentiate into mature CD45−, it would be argued that a contamination with a small fraction of CD45+ cells during sorting could be responsible for those observations. Given that the sorting purity was greater than 97% and that the doubling time for U-266 was definitely more than 2 days, if contamination with CD45+ cells was the cause, we should not have seen such a high percentage of CD45+ cells (3.2% to 36%) within 8 days (Fig 2A). This result instead can be explained if some CD45− convert to CD45+ that continue to proliferate. Single-cell assays from flow-sorted CD45−cells unequivocally confirmed that IL-6 induces phenotypic conversion (Fig 3 and Table 1). Single-cell assays also showed clear difference in the rate of emergence of CD45+ cells depending on the availability of IL-6 in culture (0.9% to 8.9% in response to endogenous IL-6 compared with 29.9% to 86.0% with exogenous IL-6) and show the proliferative advantage of cells expressing CD45. The limited proliferation of cells incubated without exogenous IL-6 (Table 1) is not surprising, because we observed spontaneous conversion to CD45+ phenotype depending only on endogenous IL-6 production (Fig 2A). Moreover, withdrawal of IL-6 from the medium led to gradual loss of the CD45 from sorted CD45+ cells (Fig2D).
Sequential culture and sorting of CD45+ cells further confirms the ability to modify CD45 expression by IL-6 (Fig 2E). This indicates that induction of expression and loss of the CD45 is an exogenous IL-6 effect and that this effect is a dose-dependent one, because both CD45− and CD45+ subpopulations produce similar levels of endogenous IL-6 (10 pg/mL; Fig 2C). Also, the CD45− fraction is a nonproliferative one, and it starts to proliferate only after expressing CD45 in response to IL-6. Conversely, the CD45+ cells are continuously proliferating, and their proliferation is much enhanced in response to exogenously added IL-6 (Fig 4).
The underlying mechanism by which IL-6 modulates CD45 expression is still not fully known, but apparently IL-6 has a positive regulatory role on CD45 gene transcription as determined by RT-PCR (Fig 2C). Despite the characterization of upstream22 and intragenic23 promoters of CD45, nothing is known aboutcis- and trans-acting elements. Examination of the upstream promoter region shows a putative STAT consensus sequence, but it is still unknown whether IL-6 stimulation is followed by binding of gp130 heterodimers with a STAT member to that site. Because we observe a lag time between IL-6 stimulation and the appearance of CD45 mRNA, we speculate that an indirect mechanism is more likely in IL-6–induced CD45 transcriptional activation. Work is going on in our laboratory to delineate downstream signaling events of IL-6 using CD45+and CD45− U-266 cells.
Finally, to verify that IL-6–induced proliferation could be mediated through CD45, we have performed a blocking experiment in which we used a potent PTP inhibitor (Na3VO4). As shown in Fig 5, vanadate could abolish the IL-6 enhancement of proliferation; on the other hand, okadaic acid, which is an unrelated phosphatase inhibitor that acts specifically on serine-threonine phosphatases, had almost no effect.
In conclusion, in this study we showed that CD45 had a remarkably heterogeneous pattern of expression in myeloma cell lines. We show that CD45 expression could be acquired or lost depending on the availability of IL-6 in culture. Our observations indicate that CD45 may act as a cellular growth regulatory molecule rather than solely as a differentiation molecule. Finally, we also showed that the IL-6–induced cellular proliferation in U-266 cells was attributed to the CD45+ population of cells in an IL-6 dose–dependent manner, and it seems plausible that this effect could be mediated through the CD45 molecule.
ACKNOWLEDGMENT
The authors thank Dr S. Shimizu for his generous gift of the IL-6–dependent myeloma cell lines, Dr H. Asaoku (Hiroshima Red Cross Hospital, Hiroshima, Japan) for providing bone marrow samples, and N. Aoki for her secretarial help in preparing the manuscript.
Supported in part by grants from the Japanese Ministry of Education, Science, and Culture.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Michio M. Kawano, MD, Department of Immuno-hematology, Yamaguchi University School of Medicine, 1144 Kogushi, Ube, Yamaguchi 755-8505, Japan.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal