Abstract
Defensins are small, cationic antimicrobial peptides that are present in the azurophilic granules of neutrophils. Earlier studies have shown that defensins may influence complement activation by specific interaction with activated C1, C1q, and C1-inhibitor. In the present study, we show that the defensin human neutrophil peptide-1 (HNP-1) is able to inhibit activation of the classical complement pathway by inhibition of C1q hemolytic activity. The binding site for HNP-1 on C1q is most likely located on the collagen-like stalks, as a clear, dose-dependent binding of HNP-1 to either intact C1q or to the collagen-like stalks of C1q was demonstrated using enzyme-linked immunosorbent assay (ELISA). Besides binding of HNP-1 to C1q, also a limited binding to C1 and to a mixture of C1r and C1s was observed, whereas no binding to C1-inhibitor was found. Because binding of HNP-1 to C1-inhibitor has been suggested in earlier studies, we also assessed the binding of HNP-1 to mixtures of C1-inhibitor with either C1r/ C1s or C1. No binding was found. Using a competition ELISA, it was found that HNP-1, but not protamine, inhibited binding of biotin-labeled HNP-1 to C1q in a dose-dependent fashion. In the fluid phase, preincubation of HNP-1 with C1q resulted in complex formation of HNP-1 and C1q and generation of stable complexes. In conclusion, HNP-1 is able to bind to C1q in the fluid phase and inhibits the classical complement pathway. This mechanism may be involved in the control of an inflammatory response in vivo.
NEUTROPHIL DEFENSINS ARE cationic, small cysteine and arginine-rich peptides that comprise about 40% of the total protein content of azurophilic granules.1 Members of the α defensin subfamily of defensins include human neutrophil peptide (HNP) 1-3 and the less abundant HNP-4. Recently, the α defensins human defensin (HS)-5 and HD-6 were demonstrated to be present in Paneth cells.2 Two members of the human β defensins have been described to date: human beta-defensin (hBD)-1, expressed in various epithelia,3 and hBD-2 that was shown to be expressed in keratinocytes and also in the lung.4 In addition to the well established antimicrobial activity against bacteria, fungi, viruses, and parasites,5neutrophil defensins also display other activities such as the induction of histamine release by mast cells6 and chemotactic activity for monocytes and T cells.7,8 Also cytotoxic activity to various autologous cells has been described.9 Serum contains various defensin binding molecules including serpins, α2-macroglobulin and specific complement components that may inhibit the cytolytic activity.10-12With respect to complement, predominantly binding to the complex of the first component C1, C1q and C1-inhibitor was demonstrated.10,13 Complex formation between defensins and C1 not only may inhibit the cytolytic activity of defensins as suggested before,10 but potentially could also inhibit the activity of C1. C1, composed of C1q, C1r, and C1s, is not only able to initiate activation of the classical pathway of complement, but C1 subcomponents like C1q are also able to activate various cells via interaction with specific cellular receptors. Once C1, bound to an activator via the globular heads of C1q, is activated, C1-inhibitor is able to dissociate C1r/C1s from C1q.14 C1q, still bound to the activator, but now with a free collagen-like stalk, is able to activate different cell types via receptors that are specific for C1q (C1qR). Three types of C1qR have been described; the receptor for the globular domain of C1q (gC1qR15,16), the receptor for the collagen-like stalks of C1q, which has high homology with Calreticulin (cC1qR/CaR17-19), and the receptor for the collagen-like stalks that induces phagocytosis by neutrophils (C1qRp20-22). We have shown earlier that cC1qR/CaR inhibits C1q hemolytic activity by binding to the collagen-like stalks of C1q.23 In this report, we describe the capacity of HNP-1 to specifically bind to the collagen-like stalks of C1q, resulting in inhibition of C1q hemolytic activity. Because defensins are a major constituent of neutrophil azurophilic granules, we suggest that upon degranulation of neutrophils, inhibition of complement activity may occur in the microenvironment of the neutrophil.
MATERIALS AND METHODS
Isolation of HNP-1.
HNP-1 was isolated from neutrophils as described by Van Wetering et al.24 In short, neutrophils were disrupted by nitrogen cavitation and after centrifugation, granules were obtained from the supernatant. After extracting the granules with 5% acetic acid, the extracts were fractionated using gel filtration on Sephacryl S-200 HR columns (Pharmacia, Roosendaal, The Netherlands) and reverse phase high-performance liquid chromatography (HPLC) on C18 (Vydac, The Separations Group, Hesperia, CA). The preparations were analyzed by tricine sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), gold-PAGE (AU PAGE), and laser desorption mass spectrometry (Lasermat; Finnigan MAT, Hemel Hempstead, UK) and were shown to be devoid of other contaminating proteins.24 An aliquot of purified HNP-1 was biotinylated with Biotin-N-Hydroxysuccinimide ester (HNP-1biotin) according to the manufacturer’s protocol (Zymed Laboratories Inc, San Francisco, CA).
Isolation of C1 and C1q.
C1 and C1q were isolated as described earlier.23 C1 from human serum was precipitated with PEG-6000 (E. Merck, Amsterdam, The Netherlands) with a final concentration of 3% (wt/vol). The pellet was dissolved in Veronal-buffered saline, pH 7.4, conductivity was adjusted to 12 milliSiemens (mS), and EDTA to a final concentration of 2 mmol/L was added. After centrifugation, the solution was applied to a rabbit IgG-Sepharose column and after washing, bound C1q was eluted with a step gradient of 1 mol/L NaCl containing 2 mmol/L EDTA. Fractions with C1q hemolytic activity23 were pooled, concentrated, and fractionated on a Superdex 200 Hiload 26/60 FPLC gel filtration column (Pharmacia). Again, the fractions were tested for functional C1q activity and positive fractions were pooled, concentrated to 8 mg/mL and stored on ice. An aliquot was analyzed by SDS-PAGE and found to be devoid of other contaminating proteins. In enzyme-linked immunosorbent assay (ELISA), no detectable C1-inhibitor, C1r, or C1s was found.25
Preparation of C1q globular heads and C1q collagen-like stalks.
For the preparation of C1q globular heads, C1q was incubated for 20 hours at 37°C with collagenase type Ia (Sigma Chemical Co, St Louis, MO) in 50 mmol/L Tris-HCl with 10 mmol/L CaCl2, pH 7.2. The material was then fractionated by gel filtration HPLC on a TSK 3000 SW column (LKB Producter AB, Bromma, Sweden) and finally analyzed on SDS-PAGE. The 30-kD protein was found to be devoid of intact C1q.26
Effect of HNP-1 on complement activity.
To determine the effect of HNP-1 on complement activity, standard assays as described earlier27-30 for either CH50, AP50, or C3 were performed in the presence of increasing concentrations of HNP-1.
Effect of HNP-1 on C1q hemolytic activity.
To determine the effect of HNP-1 on C1q hemolytic activity, a hemolytic assay described earlier23 was used. In short, 1 × 107 sheep erythrocytes sensitized with rabbit antibodies (EA) were incubated for 1 hour at 37°C with 1/25 diluted C1q depleted serum (C1qD), a limiting dose of C1q, and increasing concentrations of HNP-1. After incubation for 1 hour at 37°C, the reaction was stopped by the addition of 1.5 mL phosphate-buffered saline (PBS). The percentage lysis was determined relative to a reagent blank and 100% lysis, expressed as units/mL (Z) and converted to percentage inhibition.
ELISAs for detection of binding of HNP-1 to complement proteins.
Ninety-six well microtiter plates (Greiner BV, Alphen a/d Rijn, The Netherlands) were coated overnight at room temperature with 1 μg amounts of C1q, C1q globular heads, C1q collagen-like stalks, or bovine serum albumin (BSA) in coating buffer (0.1 mol/L sodium carbonate, pH 9.6). All reaction volumes were 100 μL. Alternatively, plates were coated in the same buffer with increasing concentrations of either C1, C1q, mixtures of C1r and C1s, C1-inhibitor, or BSA. Also mixtures, consisting of different concentrations of either C1r/C1s and C1-inhibitor or C1 and C1-inhibitor were used as coating protein. After washing, various concentrations of HNP-1Biotin were added to the wells in PBS containing 0.05% (vol/vol) Tween and 1% delta-fetal calf serum (FCS). After 1 hour of incubation at 37°C, the wells were washed and bound HNP-1Biotin detected with streptavidin-horseradish peroxidase (HRP). After washing, ABTS (2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (Sigma Chemicals Co) was added as a substrate for HRP. The optical density at 415 nm was measured using a Microplate Biokinetics Reader EL 312e (Bio-tek Instruments Inc, Winooski, VT).
To determine binding specificity, a suboptimal concentration of biotinylated HNP-1 was preincubated for 30 minutes at 4°C with increasing concentrations of protamine or unlabelled HNP-1 in PBS/Tween before it was added to C1q-coated microtiter plates and incubated for 1 hour at 37°C. After washing, bound biotinylated HNP-1 was detected by a 1-hour incubation with streptavidin-HRP, washing, and subsequent staining with ABTS.
Generation of fluid phase C1q/HNP-1 complexes.
To determine whether interaction of C1q and HNP-1 resulted in the formation of complexes of these two proteins, 7 μg C1q and 50 μg HNP-1 were incubated for 30 minutes at 4°C in PBS and subsequently applied to a Sephadex G-150 column (Pharmacia Biotech, Roosendaal, The Netherlands) in PBS. As controls, C1q alone and HNP-1 alone were also run on the same column. In the fractions, C1q antigen and HNP-1 antigen were assessed by ELISA as described above.
RESULTS
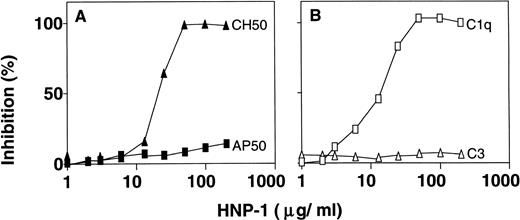
To determine whether HNP-1 had an effect on the classical complement pathway, EA in 0.5% (vol/vol) normal serum was incubated with various concentrations of HNP-1 for 1 hour at 37°C and assessed for hemolysis of EA. HNP-1 inhibited the CH50 activity of normal serum (2.72 U/mL) in a dose-dependent manner (Fig1A). To find out whether HNP-1 also influenced AP50 activity, rabbit erythrocytes, together with 5% (vol/vol) normal serum in the presence of various concentrations of HNP-1 were incubated for 1 hour at 37°C and assessed for hemolysis. HNP-1 had no detectable effect on AP50 activity. Taken together with the effect of HNP-1 on CH50 activity, the results suggested an effect of HNP-1 early in the classical pathway. Therefore, 0.5% (vol/vol) serum was incubated with various concentrations of HNP-1 for 1 hour at 37°C and subsequently analyzed for residual C1q and C3 hemolytic activity. The presence of HNP-1 resulted in a dose-dependent inhibition of C1q hemolytic activity, while no effect on C3 (Fig 1B) or on C2 and C4 was found (data not shown).
HNP inhibits C1q hemolytic activity and CH50. To determine the effect of HNP-1 on CH50, EA diluted in normal serum, were incubated with various concentrations of HNP-1 and assessed for hemolysis. Alternatively, AP50 was determined by incubation of rabbit erythrocytes in the presence of different concentrations of HNP-1 (A). Also, the effect of HNP-1 on specific complement components was determined (B). Dilutions of serum were incubated with increasing concentrations of HNP-1 and after incubation, the hemolytic activity of either C1q or C3 was determined. The data are expressed as the percentage inhibition of lysis, compared with values obtained in the absence of HNP-1.
HNP inhibits C1q hemolytic activity and CH50. To determine the effect of HNP-1 on CH50, EA diluted in normal serum, were incubated with various concentrations of HNP-1 and assessed for hemolysis. Alternatively, AP50 was determined by incubation of rabbit erythrocytes in the presence of different concentrations of HNP-1 (A). Also, the effect of HNP-1 on specific complement components was determined (B). Dilutions of serum were incubated with increasing concentrations of HNP-1 and after incubation, the hemolytic activity of either C1q or C3 was determined. The data are expressed as the percentage inhibition of lysis, compared with values obtained in the absence of HNP-1.
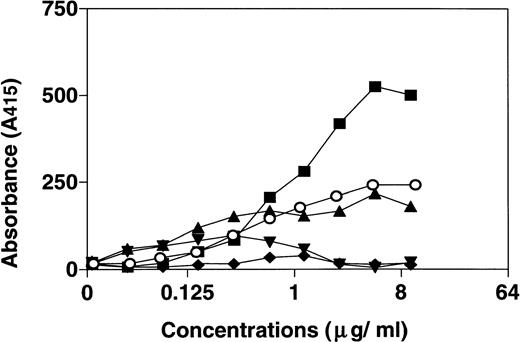
The results above indicated a direct interaction of HNP-1 with C1q. Therefore, ELISA wells were coated with fixed concentrations of C1q, C1q globular heads, C1q collagen-like stalks, or BSA and assessed for binding of HNP-1Biotin. The results (Fig 2) indicate that HNP-1 binds to intact C1q and to C1q collagen-like stalks in a dose-dependent fashion, whereas only minimal binding to C1q globular heads was observed.
Dose-dependent binding of HNP-1 to intact C1q (▪), C1q collagen-like stalks (○), and C1q globular heads (•). ELISA wells were coated with 1 μg of either C1q, C1q globular heads, C1q collagen-like stalks, or BSA (▴) and incubated with increasing concentrations of HNP-1Biotin. After incubation, bound HNP-1Biotin was determined by subsequent incubation with streptavidin-HRP and ABTS.
Dose-dependent binding of HNP-1 to intact C1q (▪), C1q collagen-like stalks (○), and C1q globular heads (•). ELISA wells were coated with 1 μg of either C1q, C1q globular heads, C1q collagen-like stalks, or BSA (▴) and incubated with increasing concentrations of HNP-1Biotin. After incubation, bound HNP-1Biotin was determined by subsequent incubation with streptavidin-HRP and ABTS.
Earlier studies have shown a specific interaction of HNP-1 with C1-inhibitor/C1r/C1s complexes and with C1-inhibitor/C1 complexes.10 Therefore, ELISA wells were coated with fixed concentrations of C1q, C1r/C1s, C1-inhibitor, or intact C1 and incubated with increasing concentrations of HNP-1Biotin. BSA was used as control. There was a dose-dependent binding of HNP-1Biotin to C1q (Fig 3) and, to a limited extent, to C1r/C1s and C1. The binding to the C1r/C1s preparation may be explained by a 1% (wt/wt) contamination of the preparation with C1q. No significant binding of HNP-1 to C1-inhibitor was observed.
Binding of HNP-1 to C1q (▪), C1r/C1s (▴), C1-inhibitor (▾), C1 (○), and BSA (⧫). Microtiter plates were coated with 1 μg C1q, C1r/C1s, intact C1, C1-inhibitor, or BSA and incubated with increasing concentrations of HNPBiotin. After washing, bound HNPBiotin was detected by subsequent incubation with streptavidin-HRP and ABTS. Optical density was measured and plotted.
Binding of HNP-1 to C1q (▪), C1r/C1s (▴), C1-inhibitor (▾), C1 (○), and BSA (⧫). Microtiter plates were coated with 1 μg C1q, C1r/C1s, intact C1, C1-inhibitor, or BSA and incubated with increasing concentrations of HNPBiotin. After washing, bound HNPBiotin was detected by subsequent incubation with streptavidin-HRP and ABTS. Optical density was measured and plotted.
In additional studies, HNP-1 was coated on ELISA wells and assessed for binding of C1q, C1r/C1s, C1-inhibitor, and intact C1 using HRP-conjugated polyclonal antibodies against these components. Also, in these experiments, significant binding of HNP-1 to C1q was seen, whereas no binding to C1-inhibitor was observed and only limited binding to C1r/C1s and C1 (data not shown).
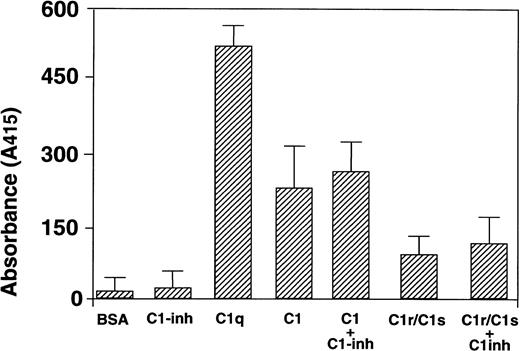
To investigate whether C1-inhibitor affects the binding of HNP-1 to C1 or its subcomponents, the following experiment was performed. ELISA wells were coated with PBS, BSA, C1q, C1 or C1r/C1s alone, or with mixtures containing C1-inhibitor. These mixtures were first preincubated in PBS for 30 minutes at 37°C to allow interaction of C1-inhibitor with the various components. After coating to ELISA wells, HNP-1Biotin was added and bound HNP-1Biotindetected with streptavidin-HRP (Fig 4). As before, clear binding of HNP-1 to C1q was observed with no change in binding in the presence of C1-inhibitor. Binding of HNP-1Biotin to C1-inhibitor was detectable, however, clearly less than to C1q.
Effect of complex formation with C1-inhibitor for HNP-1 binding. ELISA wells were coated with 1 μg BSA, C1-inhibitor, C1q, intact C1, or C1r/C1s. In addition, the same concentration of these proteins was coated after preincubation with 1 μg of C1-inhibitor. Subsequently, after washing, a fixed amount of HNP-1Biotinwas added and assessed for binding. After washing, bound HNP-1Biotin was determined by incubations with streptavidin-HRP and ABTS.
Effect of complex formation with C1-inhibitor for HNP-1 binding. ELISA wells were coated with 1 μg BSA, C1-inhibitor, C1q, intact C1, or C1r/C1s. In addition, the same concentration of these proteins was coated after preincubation with 1 μg of C1-inhibitor. Subsequently, after washing, a fixed amount of HNP-1Biotinwas added and assessed for binding. After washing, bound HNP-1Biotin was determined by incubations with streptavidin-HRP and ABTS.
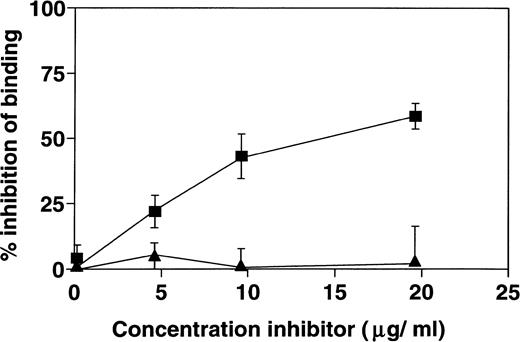
It is possible that the interaction of HNP-1 with C1q is dependent on charge interactions. Therefore, ELISA wells were coated with a fixed concentration of C1q and subsequently incubated with a fixed concentration of HNP-1Biotin in the presence of various concentrations of protamine or unlabelled HNP-1. While unlabelled HNP-1 dose-dependently inhibited the binding of HNP-1Biotin to C1q, no detectable effect of protamine was found (Fig 5).
Specific binding of HNP-1 to C1q. A fixed concentration of HNP-1Biotin was incubated with increasing concentrations of either unlabelled HNP-1 (▪) or Protamine (▴) for 30 minutes at 4°C, and then added to microtiter wells that were coated with 1 μg C1q. After incubation, bound HNP-1Biotin was detected as described. The optical density was measured and compared with binding of HNPBiotin that was not preincubated. The percentage inhibition of binding induced by HNP or protamine is depicted.
Specific binding of HNP-1 to C1q. A fixed concentration of HNP-1Biotin was incubated with increasing concentrations of either unlabelled HNP-1 (▪) or Protamine (▴) for 30 minutes at 4°C, and then added to microtiter wells that were coated with 1 μg C1q. After incubation, bound HNP-1Biotin was detected as described. The optical density was measured and compared with binding of HNPBiotin that was not preincubated. The percentage inhibition of binding induced by HNP or protamine is depicted.
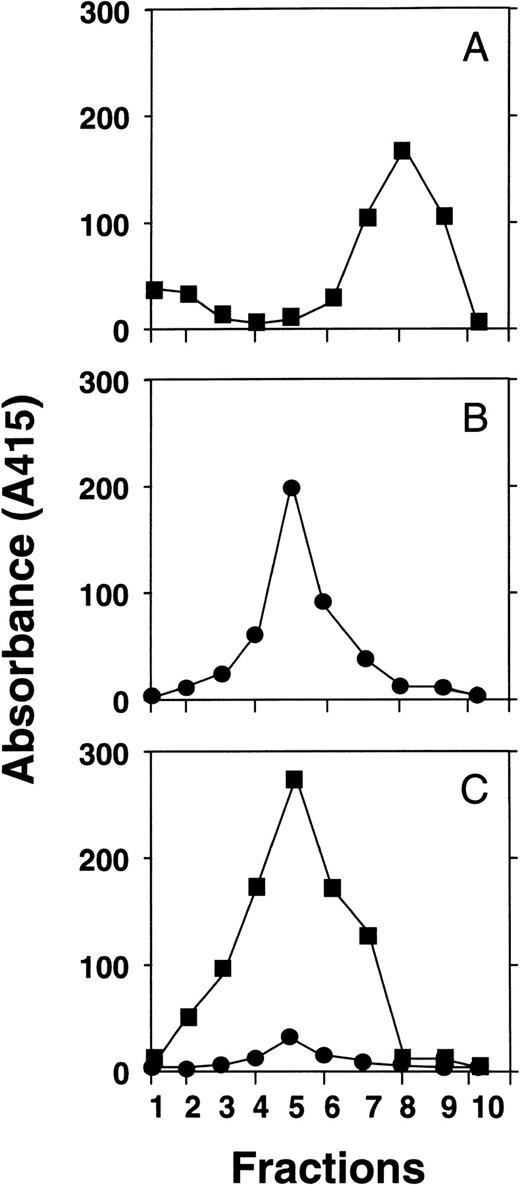
Although HNP-1 was shown to bind to solid phase bound C1q and vice versa, it is possible that binding to neoepitopes, which appear after coating of the protein, may be involved in the observed interaction. To demonstrate binding of C1q to HNP-1 in the fluid phase, the following experiment was performed. C1q and HNP-1 alone or HNP-1 preincubated with C1q were fractionated on a gel filtration column. In the fractions, HNP-1 and C1q were determined by specific ELISAs. The profiles of the gel filtration columns are shown in Fig 6. After filtration of HNP-1 alone, HNP-1 is mainly retrieved in the later fractions (fraction 8), whereas filtration of HNP-1, preincubated with C1q, was mainly retrieved in the early fractions. In the same early fractions, also C1q could be retrieved when filtered in the absence of HNP-1 and when preincubated with HNP-1. Colocalization of HNP-1 with C1q in the early fractions clearly indicates complex formation of HNP-1 with C1q in the fluid phase.
Binding of HNP-1 to C1q in the fluid phase. HNP-1 (A) or C1q (B) alone or a preincubated mixture of C1q and HNP-1 (C) was fractionated on Sepharose G150 columns. HNP-1 (▪) and C1q (•) were detected by ELISA.
Binding of HNP-1 to C1q in the fluid phase. HNP-1 (A) or C1q (B) alone or a preincubated mixture of C1q and HNP-1 (C) was fractionated on Sepharose G150 columns. HNP-1 (▪) and C1q (•) were detected by ELISA.
DISCUSSION
This report describes the binding of HNP-1 to the first subcomponent of complement C1q. Binding to C1q was dose-dependent, not solely dependent on charge interactions, and specific, as was demonstrated by competition studies. Two earlier studies described binding of HNP-1 to components of the C1-complex.10,13 Panyutich et al,10 using Western blotting of purified complement components, described that HNP-1 binds either to complexes of activated C1 and C1-inhibitor or to complexes of C1-inhibitor and activated C1s. No binding was observed to individual preparations of C1q, C1-inhibitor, or C1. In the present study, we determined binding of HNP-1 to purified C1, C1q, C1r/C1s, C1-inhibitor, and complexes of C1-inhibitor with C1 using ELISA. In agreement with the study of Panyutich et al, only limited binding of HNP-1 to either purified C1 or purified C1-inhibitor was detected. Also a limited binding of HNP-1 to C1r/C1s was found. In contrast with the study of Panyutich et al, however, marked binding of HNP-1 to highly purified C1q was observed. HNP-1 was shown to bind to immobilized C1q and vice versa. Also HNP-1/C1q complex formation in the fluid phase was clearly detected using gel filtration. These experiments show that the C1q subcomponent of C1, without denaturation or association to C1r/C1s or C1-inhibitor, is able to bind avidly to HNP-1. The discrepancy between our findings and the report of Panyutich et al may be explained by the interpretation of their results. C1q was not able to enter the gel upon native PAGE and therefore an interaction of HNP-1 with C1q could not be visualized. However, labelled protein was clearly present in the slots of the gels, which might reflect the presence of HNP-1/C1q precipitates.
C1q epitopes are fully exposed after C1q is dissociated from C1r and C1s present in the complex of activated C1. Dissociation of C1q occurs in vivo after inactivation of C1 by C1-inhibitor. The finding that inactivation of activated C1 by C1-inhibitor is necessary for binding to HNP-1, is in agreement with our findings on the binding of C1q to HNP-1.
More recently Prohaszka et al13 demonstrated that defensins, fixed on ELISA plates, are able to bind C1q in a dose-dependent fashion, confirming our findings. However in contrast to our report, they also describe defensin-induced activation of the classical pathway of complement, as assessed by deposition of C4b. The difference between our studies and those of Prohaszka et al is that in our studies we used defensins in the fluid phase while Prohaszka et al used defensins that had been immobilized on solid phase ELISA wells. It is possible that binding of defensins to a solid phase results in the exposure of C1q binding sites that are not available in fluid phase defensins and that are able to capture C1q in a different fashion than fluid phase defensin. Thus, captured C1q may still be able to activate the classical pathway of complement. Alternatively, immobilized defensins may have bound aggregates of C1q leaving C1q molecules in the aggregate that had not interacted with defensins available for complement activations. A number of findings in the study of Prohaszka et al, however, cannot be fully explained by the hypothesis that defensin induces classical pathway activation. For example 5 μg/mL of solid phase defensin was shown to induce marked C4b deposition in the presence of Mg/EGTA and even in heated human serum, in which also the alternative pathway is not operative. Therefore, at least part of the C4b deposition on defensin-coated microwells, as observed by Prohaszka et al, may not result from complement activation, but may result from other, possibly nonspecific, binding phenomena.
It has been described that receptors for the collagen-like domains of C1q can specifically inhibit the classical complement pathway by preventing the binding of C1r and C1s to C1q, which is required for formation of an intact C1 complex.23 Earlier we described a bacterial C1q binding protein (C1qBP) that is able to dissociate preformed C1.31 Inhibition of C1q hemolytic activity can also occur by binding of C1q to specific receptors for the globular heads of C1q (gC1qR). Inhibition of hemolytic activity in this case is based on the fact that C1q is no longer able to bind a complement activator with its globular head.15 The present report describes that the binding of HNP-1 to C1q also prevents activation of the classical pathway by interference at the level of C1q. Because the collagen-like stalks of C1q are the binding site for HNP-1 on C1q, this might explain the very limited extent of binding of HNP-1 to intact C1, in which the stalks of C1q are only partially exposed.32 In sepsis, characterized by massive neutrophil degranulation and high circulating defensin levels,33 this process might be responsible for the observed inactivation of the complement system.34-37
Complex formation of HNP-1 with C1q might also increase the turnover rate of both proteins. The activity of cytotoxic defensins in serum is known to be controlled by binding of HNP-1 to serum proteins including the F-form of α-2–macroglobulin11 and members of the serpin family of serine protease inhibitors.12 Despite the high concentrations of these proteins in full blood, HNP-1 at concentrations well below those observations in septic plasma, was able to inhibit C1q complement activity. Presumably this indicates that the association rate of HNP-1 with C1q is higher than that of HNP-1 with other plasma proteins. However, other mechanisms are also possible. Because of the presence of cC1qR/CaR on both lymphoid and nonlymphoid cells, it is possible that binding of HNP-1 to C1q facilitates clearance of cytotoxic HNP-1 via this receptor. Preliminary experiments by Panyutich et al10 in which uptake of HNP-1/C1s/C1-inhibitor complexes by the human hepatoma HepG2 cell line was shown, support this hypothesis. Another possibility however is that defensins might be cleared from the fluid phase by binding to the cell surface of, eg, macrophages or neutrophils, just because of their cationic nature. This has been described earlier for other cationic proteins derived from the azurophilic granules of neutrophils, such as myeloperoxidase and proteinase 3.38 39 Because C1qR of different types are expressed on the surface of various cell types, we hypothesize that HNP-1, in complex with C1q, is cleared from the circulation by binding to cell surface expressed C1qR.
In addition to inhibiting activation of the classical complement pathway as observed in the present study, defensins have also been reported to inhibit fibrinolysis.40 This effect was suggested to be the result of defensin-mediated shielding of plasminogen that is bound to fibrin from activation by tissue type plasminogen activation.41 Whether binding to C1q inhibits this or other effects of defensins, such as inactivation of serpins,12 remains to be determined. In conclusion, complex formation of HNP-1 and C1q can result either in inhibition of an inflammatory reaction by inhibiting the hemolytic activity of C1q or could result in clearance of HNP-1 via specific C1qR on the cell surfaces of many cell types. Together all of these mechanisms are involved in the downregulation of both complement and neutrophil activation. Therefore, C1q deficiency, in addition to α-1 proteinase inhibitor deficiency,12 might result in impaired regulation of defensin-mediated effects.
Supported by the Netherlands Organization for scientific research and by a grant from the Dutch Asthma Foundation (93.61).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Mohamed R. Daha, PhD, Department of Nephrology, Building 1, C3-P, Leiden University Hospital, PO Box 9600, 2300 RC Leiden, The Netherlands; e-mail:M.R.Daha@Nephrology.Medfac.Leidenuniv.nl.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal