Abstract
We assessed the clonality of duodenal mucosal T cells in patients with celiac disease and controls. Fifteen adult patients were studied. Four patients had a complicated celiac disease, 3 did not respond to a gluten-free diet, and 2 had an ulcerative jejunitis (including 1 patient with nonresponsive celiac disease). Seven patients had an untreated celiac disease responsive to a gluten-free diet. Histological examination of duodenal biopsies of these 11 patients showed benign-appearing celiac disease without evidence of lymphoma. Four patients with nonulcer dyspepsia and normal duodenal biopsies served as controls. TCRγ gene rearrangements were analyzed by multiplex polymerase chain reaction on DNA extracted from duodenal biopsies. Major clonal rearrangements of the T-cell receptor were found in 4 cases, all with complicated celiac disease. Monoclonality was confirmed by DNA sequencing of the junctional region in 3 cases and by hybridization with clone-specific oligoprobes. Patients with celiac disease responsive to gluten-free diet had mainly a polyclonal pattern, with 1 of them having an oligoclonal rearrangement. An oligoclonal pattern was also observed in 2 control patients. Three patients with complicated celiac disease evolved to T-cell lymphoma with liver (n = 2) or bone marrow (n = 1) invasion. Identical clones were found in the enteropathic duodenojejunum and peripheral blood in the patient with large-cell lymphoma with bone marrow invasion. This study suggests that complicated celiac disease is a cryptic T-cell lymphoma.
FOR MANY YEARS it has been recognized that patients with celiac disease (CD) have an increased risk of developing intestinal lymphoma.1-3 Diagnosis of celiac associated lymphoma is often difficult and survival is generally poor.4-7 Earlier recognition of celiac-associated lymphoma could be the first step in the improvement of prognosis.
In the past several years the understanding of celiac-associated lymphoma has improved. Isaacson and Wright8 have shown that celiac-associated lymphomas were of single histogenetic type and probably derived from histiocytes. Subsequent immunohistochemical and molecular studies indicated that these tumors are of T-cell origin.9,10 Wright et al11 later described a patient with CD, jejunal ulcerations, and a clonal rearrangement of the TCRγ and TCRβ chain gene together with karytotype abnormalities of jejunal T cells. From this case report, they hypothesized that adult-onset CD could be a low-grade lymphoma of intraepithelial T lymphocytes.11 However, other studies performed on isolated γδ T-cell clones12 or fresh biopsy material13 from duodenojejunal mucosa of CD patients have found no evidence of the expansion of one or more T-cell clones in CD.
We hypothesized that complicated CD (either ulcerative jejunitis or CD unresponsive to gluten-free diet [GFD]) was a low-grade T-cell lymphoma, whereas uncomplicated CD was not. We therefore assessed the clonality of mucosal T cells in complicated and uncomplicated CD, using polymerase chain reaction (PCR) analysis of TCRγ clone-specific junctional sequences.
MATERIALS AND METHODS
Patients
Patient no. 1 prompted us to undertake a prospective study. Eleven untreated patients who underwent an upper gastrointestinal endoscopy for suspected CD between November 1993 and August 1997 were included. Four patients with nonulcer dyspepsia who underwent an upper gastrointestinal endoscopy and duodenal biopsies served as controls. Relevant clinical data are summarized in Table 1.
Clinical Details
| Patient No. . | Age/Sex . | Relevant Clinical Data . | Clinical Outcome . |
|---|---|---|---|
| Complicated CD | |||
| 1 | 49/M | Adult-onset CD unresponsive to GFD. | Liver TCL 12 mo after diagnosis of CD. Died 6 mo later. |
| 2 | 44/F | Diarrhea since childhood, ulcerative jejunitis. | Liver TCL 4 mo after diagnosis of CD. Died in remission 10 mo later. |
| 3 | 68/F | Adult-onset CD unresponsive to GFD, cavitation of mesenteric lymph nodes, pancreatic cysts, bronchectasia, autoimmune hypothyroidism. | Symptomatic remission with low-dose steroids for 11 yrs. Ulcerations of the colon (small lymphocyte infiltration). Died of pulmonary embolism. |
| 4 | 46/F | Adult-onset CD. GFD not followed for 2 yrs, subsequently unresponsive to GFD, ulcerative duodenitis. | Hemophagocytic syndrome and large-cell lymphoma of the bone marrow diagnosed 31 mo after CD. Died soon thereafter. |
| Untreated CD responsive to GFD | |||
| 5 | 33/M | Adult-onset CD. | Clinical and biological remission under GFD/39 mo of follow-up. |
| 6 | 73/M | Adult-onset CD. | Clinical and biological remission under GFD/43 mo of follow-up. |
| 7 | 24/M | Relapse of childhood-onset CD. | Clinical and biological remission under GFD/49 mo of follow-up. |
| 8 | 18/F | Relapse of childhood-onset CD. | Clinical, biological, and histological remission under GFD/26 mo of follow-up. |
| 9 | 25/F | Childhood-onset CD. | Clinical and biological remission under GFD/30 mo of follow-up. |
| 10 | 38/R | Adult-onset CD. | Clinical, biological, and histological remission under GFD/49 mo of follow-up. |
| 11 | 23/F | Adult-onset CD. | Clinical, biological, and histological remission under GFD/48 mo of follow-up. |
| Controls | |||
| 12 | 24/M | Nonulcer dyspepsia. | |
| 13 | 52/F | Nonulcer dyspepsia. | |
| 14 | 43/M | Nonulcer dyspepsia. | |
| 15 | 27/M | Nonulcer dyspepsia. |
| Patient No. . | Age/Sex . | Relevant Clinical Data . | Clinical Outcome . |
|---|---|---|---|
| Complicated CD | |||
| 1 | 49/M | Adult-onset CD unresponsive to GFD. | Liver TCL 12 mo after diagnosis of CD. Died 6 mo later. |
| 2 | 44/F | Diarrhea since childhood, ulcerative jejunitis. | Liver TCL 4 mo after diagnosis of CD. Died in remission 10 mo later. |
| 3 | 68/F | Adult-onset CD unresponsive to GFD, cavitation of mesenteric lymph nodes, pancreatic cysts, bronchectasia, autoimmune hypothyroidism. | Symptomatic remission with low-dose steroids for 11 yrs. Ulcerations of the colon (small lymphocyte infiltration). Died of pulmonary embolism. |
| 4 | 46/F | Adult-onset CD. GFD not followed for 2 yrs, subsequently unresponsive to GFD, ulcerative duodenitis. | Hemophagocytic syndrome and large-cell lymphoma of the bone marrow diagnosed 31 mo after CD. Died soon thereafter. |
| Untreated CD responsive to GFD | |||
| 5 | 33/M | Adult-onset CD. | Clinical and biological remission under GFD/39 mo of follow-up. |
| 6 | 73/M | Adult-onset CD. | Clinical and biological remission under GFD/43 mo of follow-up. |
| 7 | 24/M | Relapse of childhood-onset CD. | Clinical and biological remission under GFD/49 mo of follow-up. |
| 8 | 18/F | Relapse of childhood-onset CD. | Clinical, biological, and histological remission under GFD/26 mo of follow-up. |
| 9 | 25/F | Childhood-onset CD. | Clinical and biological remission under GFD/30 mo of follow-up. |
| 10 | 38/R | Adult-onset CD. | Clinical, biological, and histological remission under GFD/49 mo of follow-up. |
| 11 | 23/F | Adult-onset CD. | Clinical, biological, and histological remission under GFD/48 mo of follow-up. |
| Controls | |||
| 12 | 24/M | Nonulcer dyspepsia. | |
| 13 | 52/F | Nonulcer dyspepsia. | |
| 14 | 43/M | Nonulcer dyspepsia. | |
| 15 | 27/M | Nonulcer dyspepsia. |
Abbreviation: TCL, T-cell lymphoma; CD, celiac disease; GFD, gluten-free diet.
Patients With Complicated CD
Four patients were studied, all of whom had serum antiendomysial antibodies before the start of GFD or during periods of poor dietary compliance.
Patients no. 1, 3, and 4 had nonresponsive CD. Patient no. 1 failed to respond to GFD after diagnosis. His clinical course was characterized by a relentless decline with severe malabsorption and cachexia despite strictly followed GFD, antibiotics, corticosteroids, and total parenteral nutrition. Patient no. 3 initially had a clinical remission under GFD, albeit with persisting hypocalcemia and hypomagnesemia; diarrhea recurred after 3 years of well-followed GFD. Clinical remission was maintained for 8 years with low-dose corticosteroids. Patient no. 4 did not follow GFD during the 2 years after diagnosis of CD. Subsequently, her clinical course was characterized by a relentless decline with severe malabsorption and cachexia despite strictly followed GFD, elemental diet, antibiotics, and corticosteroids. She also had ulcerative duodenitis.
Patient no. 2 had complained of intermittent diarrhea since her early childhood. At first admission in our unit, ulcerative jejunitis was diagnosed. Clinical improvement was obtained with total parenteral nutrition and corticosteroids and maintained under GFD.
Three of 4 patients (patients no. 1, 2, and 4) evolved to histologically overt lymphoma within 4 to 31 months and utimately died. Patient no. 3 had a long standing evolution. Eleven years after diagnosis of CD, she had diarrhea and rectal bleeding. Colonoscopy disclosed multiple colonic ulcerations. She died of massive pulmonary embolism.
Patients With CD Responding to GFD
There were 4 female and 3 male patients 18 to 73 years of age at the time of biopsies. Five patients had a newly discovered CD, although 1 patient had failure to thrive and diarrhea since childhood. Two patients had a relapse of CD diagnosed in childhood due to interruption of GFD. All patients had serum antiendomysial and/or antigliadin antibodies. All patients responded clinically and biologically to GFD and none developed lymphoma.
Control Patients With Nonulcer Dyspepsia
There were 1 female and 3 male patients 24 to 52 years of age who underwent upper gastrointestinal endoscopy for dyspepsia.
Pathology
Perendoscopic duodenojejunal biopsies, liver biopsies, and surgical specimens were fixed in Bouin’s fluid or in 10% neutral buffered formalin, embedded in paraffin, and routinely processed.
Tissues
Duodenal biopsies for TCR rearrangement were taken in the same area as those for morphological analysis. Mucosal samples were stored frozen at −80°C until used. TCR analysis was performed in a blinded fashion.
Molecular Analysis of TCRγ Gene Rearrangements
DNA was extracted by standard procedures and PCR Vγ-Jγ analysis was performed as described.14,15 Briefly, multiplex PCR was performed using oligonucleotides specific for VγI (primer VγI cons), II (Vγ9), III (Vγ10), IV (Vγ11), and Jγ1, Jγ2 (Jγ2S2), JγP, JγP1, JγP2 (JγP1/2). The size of Vγ-Jγ junctional regions was analyzed by electrophoresis on 6% polyacrylamide gels after ethidium bromide staining, allowing discrimination between oligoclonal and monoclonal pattern.15 16
Junctional region was sequenced in 3 patients with a monoclonal pattern and available material. Oligonucleotides recognizing the junctional sequences were constructed and used as clone-specific probes in hybridization experiments.14 For cases no. 1 and 4, Vγ2, Vγ3, Vγ4, Vγ5, and Vγ8 primers were used with the Jγ primers to determine the Vγ and the Jγ segments involved in the rearrangement. The cloned PCR fragment was eluted and directly sequenced14 using the relevant Vγ primer. The same strategy was used for case no. 3, using Vγ9, Vγ10, and Vγ11 primers and Jγ primers. A Vγ9 rearrangement was found.
RESULTS
Pathological Analysis of Biopsies
Patients With Complicated CD (Figs1 and2)
Duodenal biopsies.
The 4 patients with complicated CD had duodenal biopsies consistent with untreated CD; total villous atrophy, intraepithelial lymphocytosis, crypt hypertrophy, and inflammation of lamina propria with benign-appearing lymphocytes, plasma cells, and eosinophils were observed. There was no histological evidence of lymphoma. Patient no. 4 also had biopsies performed at the border of duodenal ulcerations that showed epithelial erosions without histological evidence of lymphoma.
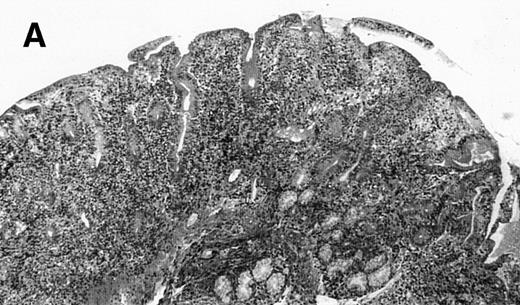
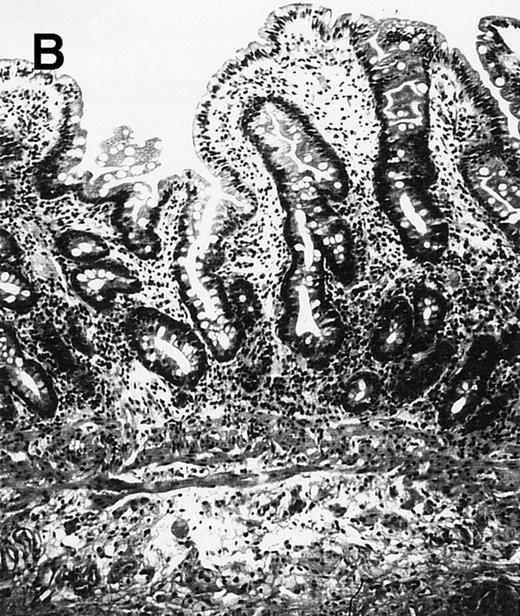
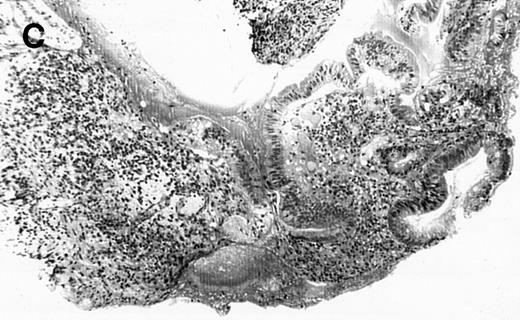
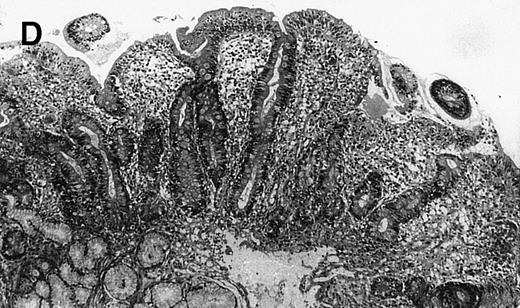
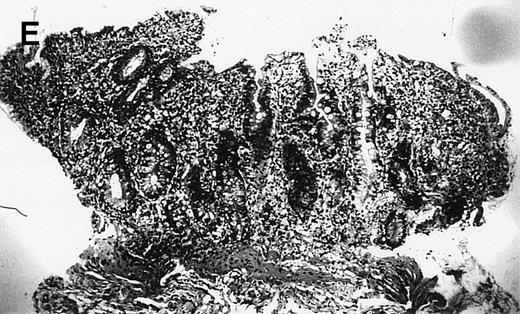
(A) Case no. 1. (B and C) Case no. 2. (D) Case no. 3. (E) Case no. 4. (A, B, D, and E) Duodenal biopsies showing total villous atrophy with crypt hyperplasia and infiltration of lamina propria by nonatypical inflammatory cells (Hematoxylinandeosin; original magnification × 10). (C) Jejunal biopsy showing villous atrophy and ulcerations with regenerative epithelial changes on the border of ulcerations; no evidence of lymphoma.
(A) Case no. 1. (B and C) Case no. 2. (D) Case no. 3. (E) Case no. 4. (A, B, D, and E) Duodenal biopsies showing total villous atrophy with crypt hyperplasia and infiltration of lamina propria by nonatypical inflammatory cells (Hematoxylinandeosin; original magnification × 10). (C) Jejunal biopsy showing villous atrophy and ulcerations with regenerative epithelial changes on the border of ulcerations; no evidence of lymphoma.
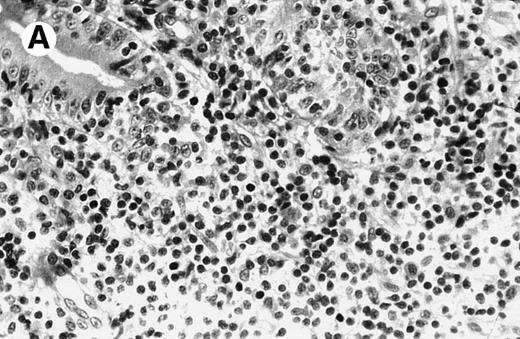
Case no. 2, original magnification × 25. (A) Mononuclear infiltrate in duodenal lamina propria without cellular atypia. (B) Same magnification of liver biopsy 4 months later. Note complete destruction of liver parenchyma by atypical large cells signaling non-Hodgkin anaplastic lymphoma.
Case no. 2, original magnification × 25. (A) Mononuclear infiltrate in duodenal lamina propria without cellular atypia. (B) Same magnification of liver biopsy 4 months later. Note complete destruction of liver parenchyma by atypical large cells signaling non-Hodgkin anaplastic lymphoma.
Jejunal biopsies.
Jejunal biopsies were performed in patient no. 1 (whole thickness jejunal biopsy performed under laparotomy), patient no. 2, and patient no. 4 (upper enteroscopy). In patients no. 1 and 4, the jejunum displayed the same histological pattern as in the duodenum. In patient no. 2, jejunal biopsies showed ulcerations with regenerative epithelial changes on their borders and partial villous atrophy. No jejunal biopsy displayed histologically overt lymphoma. Immunohistological examination of jejunal samples in patient no. 1 showed that small lymphocytes in the lamina propria and within the epithelium stained positively for CD2, CD3, CD7, CD45, CD103, TcR αβ and negatively for CD5, CD8, CD25, CD30, CD22, CD37, CD56, and TcRγδ. Some of these cells were weakly positive for CD4.
Others.
A liver biopsy was performed in patients no. 1 and 2. In patient no. 1, liver biopsy showed periportal and lobular infiltration with small lymphocytes with pleomorphic nuclei and multiple indentation that stained positively for CD3 and CD4. Electron microscopic examination showed dense cytoplasmic granulations with an outer membrane. The diagnosis of T-cell lymphoma of the liver was made. In patient no. 2, percutaneous biopsy of liver nodules showed an anaplastic lymphoma (Fig1) that stained positively for CD30 and CD3 and negatively for CD20. Patient no. 1 had pathological examinations of accessory spleen and mesenteric lymph nodes that were normal. Patient no. 4 had a bone marrow aspiration that showed hemophagocytic syndrome and large-cell lymphoma. Peripheral blood smears showed the presence of large abnormal cells.
Patient no. 3 had colonic biopsies that showed a dense and diffuse infiltration of the mucosa and upper part of submucosa with small lymphocytes (CD3+, CD43+, CD20−). There was no destruction of cryptic glands. Postmortem examination of mesenteric lymph nodes showed that normal architecture was preserved. There was an infiltration with small lymphocytes (CD3+, CD43+, CD20−) extending into the surrounding fat tissue and disrupting the capsula. Numerous lipophagic macrophages were seen at the border of mesenteric lymph nodes. Thoracic lymph nodes appeared normal.
Patients With CD Responding to GFD
These 7 patients had duodenal biopsies showing total villous atrophy. Crypt hyperplasia, intraepithelial lymphocytosis, and inflammation of the lamina propria with lymphocytes, plasma cells, and eosinophils were also observed. Three of these patients had duodenal biopsies under GFD, showing normal villous architecture in 2 and mild, partial villous atrophy in 1.
Control Patients
Duodenal biopsies showed normal villous architecture in all patients.
Molecular Analysis of TCRγ Gene Rearrangements
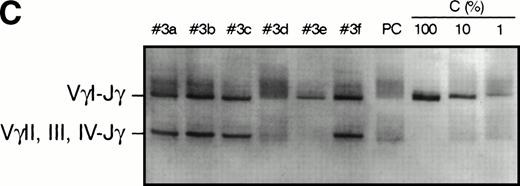
The results of T-cell clonality analysis of the 15 patients are summarized in Table 2 and shown in Fig 3. A major clonal rearrangement of the T-cell receptor was found in 4 cases, all with complicated CD. In case no. 1, the same monoclonal rearrangement was evidenced in the jejunal mucosa, mesenteric lymph nodes, and accessory spleen. Additionally, a clonal TCRβ gene rearrangement was also detected in jejunal samples by Southern blotting using Cβ probe (data not shown). In patient no. 2, a biallelic VγI-Jγ monoclonal rearrangement was detected. The same rearrangement was evidenced on two occasions in the atrophic mucosa of duodenum and 108 days later, in the mid jejunum, whereas there was no morphological evidence of lymphoma. Case no. 3 had a major clonal biallelic rearrangement of TCRγ (involving VγI on one allele and VγII, III, or IV on the other allele); the same pattern was observed in small bowel and colon samples obtained postmortem 30 months later. Interestingly, the same VγI-Jγ rearrangement was found in the mesenteric lymph nodes, but the VγII, III, IV-Jγ rearrangement was lost (Fig 3C). A polyclonal pattern was found in the thoracic lymph nodes that appeared histologically normal. Case no. 4 also had a major VγI clonal rearrangement; the same monoclonal rearrangement was found in the enteropathic duodenum and jejunum at a 30-day interval and 64 days later in peripheral blood when the bone marrow was invaded with large-cell lymphoma.
Molecular Analysis of T-Cell Clonality
| Patient No. . | Samples . | Histology . | TCR Status . |
|---|---|---|---|
| Complicated CD | |||
| 1 | Duodenum and jejunum | Consistent with untreated CD | C (Vγ3-Jγ)* |
| MLN/Accessory spleen | Normal | C (Vγ3-Jγ)* | |
| 2 | Duodenum | Consistent with untreated CD | C (VγI-Jγ) |
| Jejunum (108 days later) | Ulcerative jejunitis | C (VγI-Jγ) | |
| 3 | Duodenum | Consistent with untreated CD | C (VγI-Jγ; Vγ9-Jγ)* |
| Colon (30 months later) | Small lymphocyte infiltration | C (VγI-Jγ; Vγ9-Jγ)* | |
| MLN (30 months later) | Small lymphocyte infiltration | C (VγI-Jγ) | |
| 4 | Duodenum | Consistent with untreated CD + ulcerations | C (Vγ8-Jγ)* |
| Jejunum (30 days later) | Consistent with untreated CD | C (Vγ8-Jγ)* | |
| Peripheral blood (64 days later) | Large-cell lymphoma | C (Vγ8-Jγ)* | |
| Untreated CD responsive to GFD | |||
| 5 | Duodenum | Consistent with untreated CD | P |
| 6 | Duodenum | Consistent with untreated CD | P |
| 7 | Duodenum | Consistent with untreated CD | P |
| 8 | Duodenum | Consistent with untreated CD | P |
| 9 | Duodenum | Consistent with untreated CD | P |
| 10 | Duodenum | Consistent with untreated CD | P |
| 11 | Duodenum | Consistent with untreated CD | O (faint bands) |
| Controls | |||
| 12 | Duodenum | Normal villous architecture | P |
| 13 | Duodenum | Normal villous architecture | O (strong bands) |
| 14 | Duodenum | Normal villous architecture | O (faint bands) |
| 15 | Duodenum | Normal villous architecture | P |
| Patient No. . | Samples . | Histology . | TCR Status . |
|---|---|---|---|
| Complicated CD | |||
| 1 | Duodenum and jejunum | Consistent with untreated CD | C (Vγ3-Jγ)* |
| MLN/Accessory spleen | Normal | C (Vγ3-Jγ)* | |
| 2 | Duodenum | Consistent with untreated CD | C (VγI-Jγ) |
| Jejunum (108 days later) | Ulcerative jejunitis | C (VγI-Jγ) | |
| 3 | Duodenum | Consistent with untreated CD | C (VγI-Jγ; Vγ9-Jγ)* |
| Colon (30 months later) | Small lymphocyte infiltration | C (VγI-Jγ; Vγ9-Jγ)* | |
| MLN (30 months later) | Small lymphocyte infiltration | C (VγI-Jγ) | |
| 4 | Duodenum | Consistent with untreated CD + ulcerations | C (Vγ8-Jγ)* |
| Jejunum (30 days later) | Consistent with untreated CD | C (Vγ8-Jγ)* | |
| Peripheral blood (64 days later) | Large-cell lymphoma | C (Vγ8-Jγ)* | |
| Untreated CD responsive to GFD | |||
| 5 | Duodenum | Consistent with untreated CD | P |
| 6 | Duodenum | Consistent with untreated CD | P |
| 7 | Duodenum | Consistent with untreated CD | P |
| 8 | Duodenum | Consistent with untreated CD | P |
| 9 | Duodenum | Consistent with untreated CD | P |
| 10 | Duodenum | Consistent with untreated CD | P |
| 11 | Duodenum | Consistent with untreated CD | O (faint bands) |
| Controls | |||
| 12 | Duodenum | Normal villous architecture | P |
| 13 | Duodenum | Normal villous architecture | O (strong bands) |
| 14 | Duodenum | Normal villous architecture | O (faint bands) |
| 15 | Duodenum | Normal villous architecture | P |
Parentheses indicate the oligonucleotides with which monoclonal rearrangements were demonstrated.
The Vγ segments used were determined using Vγ specific PCR experiments and confirmed by sequencing.
Abbreviations: C, monoclonal rearrangements; P, polyclonal pattern; O, oligoclonal pattern; MLN, mesenteric lymph nodes.
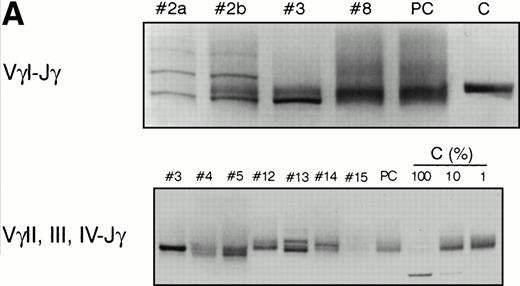
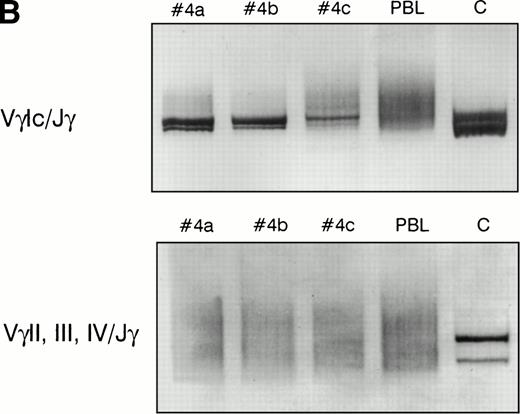
PCR analysis of TCR Vγ-Jγ genes in duodenal biopsies of patients with complicated CD, untreated CD responsive to GFD, and dyspeptic controls. (A) Representative experiments using VγI-Jγ (top) and VγII, III, IV-Jγ (bottom) specific oligonucleotides. Sample numbers refer to patient numbers in Tables 1 and 2. Samples no. 2 and 3 demonstrate a clonal pattern. In patient no. 2, an identical VγI-Jγ biallelic rearrangement was found in (a) duodenal mucosa and in (b) jejunal mucosa 108 days later. In case no. 3, a monoallelic VγI-Jγ and a monoallelic VγII, III, IV-Jγ rearrangement were detected. PC, polyclonal control; C, clonal control. Dilution of the clonal control into peripheral blood lymphocytes polyclonal DNA (from 100% to 1%) has been included in the experiments and is shown for the VγII, III, IV-Jγ experiments. (B) Analysis of patient no. 4 DNAs. (Top) VγI-Jγ rearrangements. A clonal rearrangement was found in the 3 patients’ samples. (a) Duodenal biopsy at entry; (b) duodenal biopsy 30 days later; (c) peripheral blood specimen obtained 64 days later at the moment of overt lymphoma. PBL, peripheral blood lymphocytes from a polyclonal control. (C) Analysis of patient no. 3 DNAs by multiplex PCR. (a) Duodenal biopsy; (b through f) postmortem samples obtained 30 months later. (b) Small bowel; (c) colon; (d) thoracic lymph nodes; (e) mesenteric lymph nodes; (f) colonic mucosa. PC, polyclonal control; C, dilution of the clonal control into peripheral blood lymphocytes polyclonal DNA (from 100% to 1%).
PCR analysis of TCR Vγ-Jγ genes in duodenal biopsies of patients with complicated CD, untreated CD responsive to GFD, and dyspeptic controls. (A) Representative experiments using VγI-Jγ (top) and VγII, III, IV-Jγ (bottom) specific oligonucleotides. Sample numbers refer to patient numbers in Tables 1 and 2. Samples no. 2 and 3 demonstrate a clonal pattern. In patient no. 2, an identical VγI-Jγ biallelic rearrangement was found in (a) duodenal mucosa and in (b) jejunal mucosa 108 days later. In case no. 3, a monoallelic VγI-Jγ and a monoallelic VγII, III, IV-Jγ rearrangement were detected. PC, polyclonal control; C, clonal control. Dilution of the clonal control into peripheral blood lymphocytes polyclonal DNA (from 100% to 1%) has been included in the experiments and is shown for the VγII, III, IV-Jγ experiments. (B) Analysis of patient no. 4 DNAs. (Top) VγI-Jγ rearrangements. A clonal rearrangement was found in the 3 patients’ samples. (a) Duodenal biopsy at entry; (b) duodenal biopsy 30 days later; (c) peripheral blood specimen obtained 64 days later at the moment of overt lymphoma. PBL, peripheral blood lymphocytes from a polyclonal control. (C) Analysis of patient no. 3 DNAs by multiplex PCR. (a) Duodenal biopsy; (b through f) postmortem samples obtained 30 months later. (b) Small bowel; (c) colon; (d) thoracic lymph nodes; (e) mesenteric lymph nodes; (f) colonic mucosa. PC, polyclonal control; C, dilution of the clonal control into peripheral blood lymphocytes polyclonal DNA (from 100% to 1%).
In the 7 CD patients who responded to GFD, the PCR showed that T cells were polyclonal in these cases. However, in 1 patient, oligoclonal bands appeared on the polyacrylamide gel analysis of Vγ-Jγ PCR products.
Among the 4 dyspeptic patients, the PCR analysis showed the polyclonal nature of the T cells in 2 patients. Two patients had oligoclonal TCRγ rearrangements.
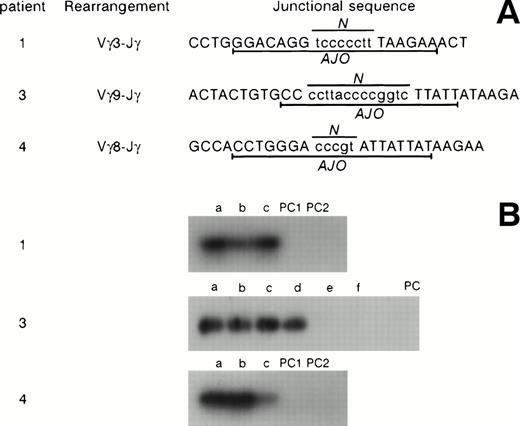
To further analyze the clonal rearrangements detected in patients no. 1, 3, and 4 (no material was available in patient no. 2), direct sequencing of the TCRγ junctional regions was performed, demonstrating a clonal patient-specific sequence (Fig 4). This was confirmed by hybridization experiments using clone-specific primers designed from the junctional sequences. These hybridization experiments demonstrated the clonal relationship between the cells found in the jejunum, mesenteric lymph nodes, and accessory spleen in patient no. 1. In case no. 3, the same clone was present in duodenal biopsies and postmortem samples of small bowel and colon 30 months later. Moreover, in case no. 4, the same clone was evidenced in the duodenum, in the jejunum 30 days later, and 64 days later in peripheral blood at the moment of large-cell lymphoma with bone marrow invasion.
Clones TcR γ junctional sequences in patients no. 1, 3, and 4. (A) The sequences, the Vγ segments involved, and the clone-specific antijunctional oligonucleotides (AJO) are shown. N, N regions. (B) Hybridization experiments using patient-specific AJO probes. (1) Patient no. 1; (a) jejunum; (b) accessory spleen; (c) mesenteric lymph nodes. (3) Patient no. 3; (a) duodenal biopsy; (b through f) postmortem samples obtained 30 months later. (b) Small bowel; (c) sigmoid colon; (d) colon; (e) thoracic lymph nodes; (f) mesenteric lymph nodes. (4) Patient no. 4; (a) duodenal biopsy; (b) jejunal biopsy 30 days later; (c) peripheral blood specimen obtained 64 days later at the time of overt lymphoma. PC, polyclonal controls.
Clones TcR γ junctional sequences in patients no. 1, 3, and 4. (A) The sequences, the Vγ segments involved, and the clone-specific antijunctional oligonucleotides (AJO) are shown. N, N regions. (B) Hybridization experiments using patient-specific AJO probes. (1) Patient no. 1; (a) jejunum; (b) accessory spleen; (c) mesenteric lymph nodes. (3) Patient no. 3; (a) duodenal biopsy; (b through f) postmortem samples obtained 30 months later. (b) Small bowel; (c) sigmoid colon; (d) colon; (e) thoracic lymph nodes; (f) mesenteric lymph nodes. (4) Patient no. 4; (a) duodenal biopsy; (b) jejunal biopsy 30 days later; (c) peripheral blood specimen obtained 64 days later at the time of overt lymphoma. PC, polyclonal controls.
DISCUSSION
This study describes the PCR analysis of TCR Vγ-Jγ genes in duodenal biopsies of patients with CD. None of the patients studied had histological evidence of lymphoma. We found that a minority of patients had a monoclonal rearrangement and a majority of patients had a polyclonal (8 cases) or oligoclonal (3 cases) pattern. Monoclonal pattern was associated with complicated CD, whereas other patterns were associated with uncomplicated CD, responsive to GFD. The present study suggests that some patients with adult onset CD may have a cryptic low-grade T-cell lymphoma and may be at higher risk for the development of a subsequent overt enteropathy-associated T-cell lymphoma (EATL).
It has long been recognized that ulcerative jejunitis and EATL are related conditions associated with CD. In some patients with ulcerative jejunitis, malignant cells can be found within the ulcers,8whereas others have inflammatory benign-appearing jejunal ulcers, despite careful examination of pathological specimens.17Isaacson and Wright18 have advocated the view that these patients have cryptic lymphoma. Recently, Ashton-Key et al19 have studied the T-cell clonality of microdissected benign-appearing jejunal ulcers of ulcerative jejunitis. They found dominant bands implying monoclonal rearrangements in all cases of ulcerative jejunitis, although, within the same patient, some ulcers were monoclonal, whereas others exhibited a polyclonal pattern. In the present study, cases no. 2 and 4 had an ulcerative jejunitis and both were found to have monoclonal rearrangements of Vγ-Jγ within the enteropathic duodenojejunal mucosa.
Nonresponsive CD is a heterogeneous entity.20 The most common cause of failure to respond to GFD is dietary noncompliance or inadvertent gluten intake. Careful dietary assessments showed that the 3 patients with nonresponsive CD strictly adhered to GFD; moreover, 2 of them did not improve under elemental diet or total parenteral nutrition. The diagnosis of CD can be questioned in patients who have primary unresponsiveness to GFD. This diagnosis can be inferred from detection of serum antiendomysial antibodies in the 3 patients with nonresponsive CD. In some cases, nonresponsive CD may correspond to EATL, but histological or radiological evidence can be difficult to obtain.21 In the present study, 3 patients with nonresponsive CD had monoclonal rearrangements of Vγ-Jγ TCR genes, without overt malignancy at the moment of biopsies. The monoclonal T-cell population found in ulcerative jejunitis and nonresponsive CD seems stable over time and distributed along the intestines, as showed in cases no. 2, 3, and 4, in whom the same TCR rearrangement was evidenced at two distant sites of the small intestine, at 108, 900, and 30 days interval, respectively.
Taken together, these data suggest that benign-appearing ulcerative jejunitis and nonresponsive CD may be low-grade T-cell lymphomas. Three patients with monoclonal CD developed overt EATL, whereas none of the polyclonal CD did so. It appears likely that EATL arose from monoclonal T cells of the duodenal mucosa. Moreover, in case no. 4, DNA sequencing showed that identical clones were present in the duodenojejunal mucosa and, subsequently, in the peripheral blood when the bone marrow was invaded with large-cell lymphoma. Murray et al22 have shown that, within the same patient, benign-appearing enteropathic bowel and EATL exhibit the same monoclonal T-cell receptor gene rearrangements. These data suggested that the enteropathic bowel was a low-grade lymphoma from which high-grade EATL was derived. However, it could not be excluded that the tumor had undergone an intramucosal spread that accounted for monoclonal rearrangement found in the enteropathic bowel. Ashton-Key et al19 found that, in 1 patient, the dominant band amplified from benign-appearing ulcers was identical to that found in the subsequent cutaneous T-cell lymphoma. Taken together, these data suggest that overt EATL may arise from the dominant clone found in the benign-appearing duodenojejunal mucosa.
In this study, uncomplicated CD was associated mainly with a polyclonal pattern. Studies performed on isolated γδ T-cell clones12 or fresh biopsy material13 from duodenojejunal mucosa of patients with uncomplicated CD have also found no evidence of the expansion of one or more clones expressing particular types of γδ T-cell receptor. It may be suggested that monoclonal T cells arose from a polyclonal infiltrate after long years of untreated, asymptomatic CD.23 Studies performed in human normal colon and jejunum have shown oligoclonality of IEL bearing αβ phenotype24-26 and δ TCR-bearing cells.27 28 It was thus not unexpected that some patients with uncomplicated CD and controls had an oligoclonal pattern. However, in patients with complicated CD, a dominant clone was evidenced, indicating a preferential expansion of a T-cell clone among a highly cellular inflammatory mucosa.
In conclusion, this study suggests that complicated CD is associated with a monoclonal T-cell infiltrate. Additionally, patients with complicated CD and a monoclonal T-cell rearrangement may be at higher risk of subsequent overt EATL. In patients with complicated CD, molecular analysis of clonality may be used to assist in earlier diagnosis of lymphoma by detecting a predominant T-cell clone before a tumor mass is evident. However, a word of caution is needed in the interpretation of PCR assays, because oligoclonal rearrangements yielding usually faint bands may be noted in normal as well as CD mucosa. Within the limits of our study that included a small sample of patients, the finding of a major rearranged band was indicative of complicated CD and was associated with a higher risk of evolution to overt EATL.
ACKNOWLEDGMENT
The authors thank Anne Lavergne-Slove, MD, and Philippe Gaulard, MD, for their help.
Address reprints requests to Franck Carbonnel, MD, Service de Gastroentérologie et Nutrition, Hôpital Rothschild, 33 Boulevard de Picpus, 75012 Paris, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal