Abstract
Fibronectin (FN) is supposed to play important roles in various aspects of hematopoiesis through binding to very late antigen 4 (VLA4) and VLA5. However, effects of FN on hematopoietic stem cells are largely unknown. In an effort to determine if FN had a growth-supporting activity on hematopoietic stem cells, human CD34+/VLA4bright/VLA5dullhematopoietic stem cells and a murine stem cell factor (SCF)-dependent multipotent cell line, EML-C1, were treated with or without FN in a serum and growth-factor–deprived medium, and then subjected to clonogenic assay in the presence of hematopoietic growth factors. The pretreatment of the CD34+ cells with FN gave rise to significantly increased numbers of granulocyte-macrophage colony-forming units (CFU-GM), erythroid burst colony-forming units, and mixed erythroid-myeloid colony-forming units. In addition, the numbers of blast colony-forming units and CFU-GM that developed after culture of EML-C1 cells with SCF and the combination of SCF and interleukin-3, respectively, were augmented by the pretreatment with FN. The augmented colony formation by FN was completely abrogated by the addition of CS1 fragment, but not of GRGDSP peptide, suggesting an essential role of FN-VLA4 interaction in the FN effects. Furthermore, the effects of various FN fragments consisting of RGDS-containing cell-binding domain (CBD), heparin-binding domain (HBD), and/or CS1 portion were tested on clonogenic growth of CD34+ cells. Increased colony formation was induced by CBD-CS1 and CBD-HBD-CS1 fragments, but not with other fragments lacking CBD or CS1 domains, suggesting that both CS1 and CBD of FN were required for the augmentation of clonogenic growth of hematopoietic stem/progenitor cells in vitro. In addition to the in vitro effects, the in vivo administration of CBD-CS1 fragment into mice was found to increase the numbers of hematopoietic progenitor cells in bone marrow and spleen in a dose-dependent manner. Thus, FN may function on hematopoietic stem/progenitor cells as a growth-supporting factor in vitro and in vivo.
HEMATOPOIETIC CELLS receive critical signals for proliferation and differentiation from bone marrow (BM) microenvironment, including cytokines, stromal cells, and extracellular matrix (ECM).1-3 In long-term BM cultures, hematopoietic stem cells are preferentially found within the adherent cell layers. Blood cell formation in these cultures was completely blocked by inhibition or modulation of interactions between stromal and hematopoietic cells.4-6
ECM is composed of a variety of molecules such as fibronectin (FN), collagens, laminin, and proteoglycans. ECM in BM is not merely an inert framework; it also mediates specialized functions.7-9 Some components of ECM have been shown to bind to growth factors produced by stromal cells and to immobilize them around cells, resulting in giving spaces where hematopoietic cells and growth factors colocalize. For example, heparan-sulfated proteoglycans can bind to tumor growth factor-β, basic fibroblast growth factor, interleukin-3 (IL-3), granulocyte/macrophage colony-stimulating factor (GM-CSF), and IL-7.10-13 Osteonectin can immobilize platelet-derived growth factor.14 In addition, ECM can bind to cell surface glycoproteins. FN, collagens, and laminin are ligands of integrins which not only control anchorage, spreading, and migration of hematopoietic cells but also induce signal transduction pathway in these cells.7,8,15,16 Hyaluronan is a ligand of CD44, and in several systems, facilitates cell-cell adhesion and cell migration.9,17 18
FN, one of the major ECM components, is a disulfide-linked dimeric glycoprotein consisting of two similar but not identical 220-kD monomers. Each monomer is composed of type I-III domains, identified as repeating amino acid motifs in the primary structure.19 It contains several functional sites with cell-binding properties.20,21 The CS1 portion which is alternatively spliced and located near the COOH-terminal of heparin-binding domain (HBD) is recognized by very late antigen 4 (VLA4). The central cell-binding domain (CBD) which includes the minimal essential sequence, RGD(S), is recognized by VLA5. It has been reported that hematopoietic cells change requirement of interactions between FN and integrins along with their maturation. For example, primitive hematopoietic stem cells, long-term BM culture initiating cells (LTC-IC), and multi-lineage CFU-Mix progenitors adhere to the CS-1 portion, but significantly less to the CBD of FN.22Progenitors of erythroid or lymphoid lineages have been shown to attach to FN, but differentiated cells of these lineages become unable to adhere to FN because of their reduction of FN-receptor expression.23-25 In addition, a number of studies have shown that FN functions as not only an adhesion molecule but also a signal inducer via its binding to integrins.15,16,26,27Monoclonal antibodies (MoAbs) to VLA4 inhibited lympho-hematopoiesis in long-term BM cultures5 and erythropoiesis in fetal mice,28,29 suggesting that FN-VLA4 interaction has crucial roles in hematopoiesis. Adhesion of FN to VLA5 induced apoptosis on human myeloid leukemia cell lines and terminal differentiation on human mature monocytes,30 31 suggesting possible involvement of FN-VLA5 interaction in negative regulation of hematopoiesis.
Although proliferation and maturation of hematopoietic cells are suggested to be regulated in part by alterations in FN-integrin interaction, the precise role of the FN-integrin interaction in hematopoiesis remains unclear. Furthermore, little is known about effects of FN on the growth of primitive hematopoietic stem cells. In this study, we have investigated the effects of FN on clonogenic growth of human CD34+ cells purified from umbilical cord blood (UCB) and a murine multipotent hematopoietic cell line. Here, we show that FN has a growth-supporting activity on hematopoietic stem/progenitor cells in vitro and in vivo, and that both CS1 portion and CBD are structurally important for the growth-supporting effects of FN, whereas the FN effects seem to be mediated mainly through the interaction between FN(CS1) and VLA4 integrin.
MATERIALS AND METHODS
Reagents and antibodies.
Endotoxin-free human plasma fibronectin (FN) was purchased from Collaborative Biomedical Products (Bedford, MA). Endotoxin-free bovine serum albumin (BSA) and human serum albumin were purchased from Sigma (St Louis, MO) and Fujisawa Pharmaceutical Co Ltd (Osaka, Japan), respectively. FN fragments, C274, CH271, H296, C-CS1, and CH296 were generous gifts of Takara Shuzo Co Ltd (Siga, Japan). The concentrations of endotoxin of FN fragments were less than 0.1 endotoxin units/mL as tested by an E-TOXATE kit (Sigma). Synthetic peptides GRGDSP and GRGESP were purchased from Iwaki Glass (Tokyo, Japan), and CS1 from Sigma. A synthetic peptide composed of 24 amino acids within the cytoplasmic portion of human integrin β3 subunit (β3 721-744; IHDRKEFAKFEEERARAKWDTANN) was a generous gift from Toyobo Co Ltd (Osaka, Japan) and used as a control peptide for CS1. Iron-saturated human transferrin was purchased from Sigma. Human regular insulin was a kind gift of Yamanouchi Pharmaceutical Co Ltd (Tokyo, Japan). Recombinant murine (rm) stem cell factor (SCF) and rm IL-3 were kindly provided by Kirin Brewery Co Ltd (Tokyo, Japan). Murine MoAbs reactive with human integrin α4 subunit (SG17), α5 subunit (KH72), and β1 subunit (SG19) were kind gifts of Dr K. Miyake (Department of Immunology, Saga Medical School, Japan). LM142, a murine MoAb recognizing human αv subunit, was a generous gift from Dr D. Cheresh (The Scripps Research Institute, La Jolla, CA). M-KID2, recognizing human α3 subunit, was purchased from Southern Biotec (Birmingham, AL), and anti-human CD34 MoAb conjugated to phycoerythrin (PE) from Becton Dickinson (Mountain View, CA).
Source of cells.
UCB, obtained from normal single deliveries after their parents' informed consent, was used as a source of human hematopoietic progenitor cells. UCB mononuclear cells (MNC) were isolated by Ficoll-Hypaque density gradient centrifugation (Lymphoprep; Nycomed, Oslo, Norway). To enrich CD34+ cell population, UCB MNC were negative-selected by immunomagnetic beads conjugated with anti-CD3 and anti-CD11b MoAbs, followed by positive-selection using immunomagnetic beads conjugated with an anti-human CD34 MoAb (Miltenyi Biotec, Berguisch Gladbach, Germany). In our series, more than 96% of the purified cells always expressed CD34 and viability of them was higher than 98%.
EML-C1 cells32 were routinely grown in Iscove's modified Dulbecco's medium (IMDM; GIBCO, Grand Island, NY) supplemented with 20% heat-inactivated horse serum (HS; ICN Biomedical Inc, Costa Mesa, CA) and 10 ng/mL rmSCF.
Flow cytometry.
Surface expression of integrins on human UCB CD34+ cells was analyzed by two-color flow cytometry as previously described.6 Briefly, UCB MNC suspended in phosphate-buffered saline (PBS) containing 1% BSA and 0.1% sodium azide were incubated with 0.2% human Ig for 15 minutes at 4°C to reduce nonspecific binding. Cells were then stained with mouse anti-human α3, α4, α5, αv, or β1 MoAb, followed by incubation with goat anti-mouse IgG conjugated with fluorescein isothiocyanate (FITC). After washing, they were stained with PE-conjugated mouse anti-human CD34 MoAb. Cell surface immunofluorescence was evaluated by flow cytometer (FACScan; Becton Dickinson).
Sorted CD34+ cells were stained with PE-conjugated anti-human CD34, FITC-conjugated anti-human α4, or FITC-conjugated anti-human α5, and subsequently analyzed by single-color flow cytometry.
The binding of soluble FN to sorted CD34+ cells was also assessed by single-color flow cytometry. In brief, sorted CD34+ cells were incubated with 100 μL of Hanks' balanced salt solution (Ca2+, 1.26 mmol/mL; Mg2+, 0.90 mmol/mL) containing 50 μg/mL biotin-labeled soluble FN for 15 minutes in the presence or absence of a 20-fold excess of unlabeled FN. Subsequently, they were stained with FITC-conjugated avidin (Vector Laboratories, Inc, Burlingame, CA) and analyzed by flow cytometer. All staining and washing steps were performed in Hanks' balanced salt solution at 37°C.
Clonal cell cultures.
To assess the clonogenic ability, methylcellulose progenitor assays was performed as previously described.33 Briefly, human CD34+ cells preincubated in diverse conditions were plated at low cell density (3-5 × 102/mL) in methylcellulose media (Veritas, Vancouver, Canada) consisting of IMDM, 0.9% methylcellulose, 10−4 mol/L 2-mercaptoethanol, 2 mmol/L L-glutamine, 30% fetal calf serum (FCS), 1% deionized crystallized BSA, 3 U/mL recombinant human (rh) erythropoietin, 50 ng/mL rh SCF, 10 ng/mL rh GM-CSF, 10 ng/mL rh G-CSF, and 10 ng/mL rh IL-3. Cultures were set up in duplicate or triplicate and incubated in a humidified atmosphere at 37°C and 5% CO2 for 16 days. The numbers of granulocyte-macrophage colony-forming units (CFU-GM), erythroid burst colony-forming units (BFU-E), and mixed erythroid-myeloid colony-forming units (CFU-GEMM) were scored on days 12 to 16 of culture with an inverted microscope according to established criteria.33 In some experiments, types of colonies were determined by differential counting of May-Grunwald-Giemsa-stained preparations.
The clonogenic ability of EML-C1 cells was also assessed in methylcellulose culture. The cells were preincubated with 100 μg/mL of BSA, FN, or FN fragments in IMDM at 37°C for 1 hour and subsequently cultured at the density of 1.0 × 103/mL in methylcellulose media containing 50 ng/mL rmSCF alone (for blast colony-forming units [CFU-Blast]) or 50 ng/mL rmSCF + 2 ng/mL rmIL-3 (for CFU-GM). Cultures were established in triplicate or quardricate in 12-well plates. The number of CFU-Blast and CFU-GM were assessed on day 14 and day 7 of cultures, respectively.
Cell proliferation assay.
To quantitate the proliferation of cells, MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] (Sigma) rapid colorimetric assay was used as previously reported.34 In brief, quadricate aliquots of EML-C1 cells (3.0 × 104 cells suspended in serum-free IMDM containing 20 ng/mL rmSCF) were cultured in 96-well flat-bottom microtiter plates for an indicated period at 37°C in the presence of 100 μg/mL BSA or FN. MTT (10 μL of 5 mg/mL solution in PBS) was added for the final 4 hours of cultures, and 100 μL of acid isopropanol (0.04 N HCL in isopropanol) was added and mixed. The optical density was then measured on the Microelisa plate reader (Corona Electric, Ibaragi, Japan) with a test wavelength of 540 nm.
Cell adhesion assay.
Adhesion assays were performed as previously described with minor modification.35 Briefly, 50 μg/mL FN and FN fragments in PBS were adsorbed to wells of 24-well plates (Iwaki Glass) overnight at 4°C. Nonspecific bindings were blocked with 1 mg/mL BSA in PBS for the following 2 hours at 37°C. EML-C1 cells were labeled with FITC, suspended in IMDM, and then added to the coated wells (2.5 × 105 cells/well). After 2 hours of incubation at 37°C, the nonadherent cells were removed by three gentle washings with warm IMDM, and then 1.0 mL lysate buffer (10 mmol/L Tris, 150 mmol/L NaCl, 1.5 mmol/L MgCl2, 0.2% NP-40) was added to each well (fluorescence intensity [FI] Adhesion). Lysate buffer (1 mL) was also added to 2.5 × 105 FITC-labeled EML-C1 cells (FI Whole cell). Cell lysates were centrifuged for 10 minutes to remove nuclei and insoluble debris, and then FI of the supernatants was assessed on a fluorescence spectrophotometer (Hitachi F3000, Tokyo, Japan). To calculate the percentage of adhesion, the following formula was used: % Adhesion = FI Adhesion/FI Whole cell × 100.
Mice and in vivo treatment with FN fragments.
Female BALB/C mice at the age of 8 to 10 weeks were purchased from Nippon SLC (Shizuoka, Japan). The mice were injected intravenously with the indicated doses of FN fragments daily for 4 days. On day 5, 24 hours after the last injection, peripheral blood, BM, and spleens of the mice were obtained and subjected to methylcellulose colony assay. The methylcellulose media for murine cells consisted of IMDM, 0.9% methylcellulose, 30% FCS, 10−4 mol/L 2-mercaptoethanol, 2 mmol/L L-glutamine, 1% deionized crystallized BSA, 3 U/mL rh erythropoietin, 100 ng/mL rm SCF, 3 ng/mL rm IL-3, and 10 ng/mL rh IL-6. On days 10 to 16 of culture, colonies were counted on the basis of morphological criteria as previously described.33
RESULTS
Expression of integrins on human cord blood CD34+cells.
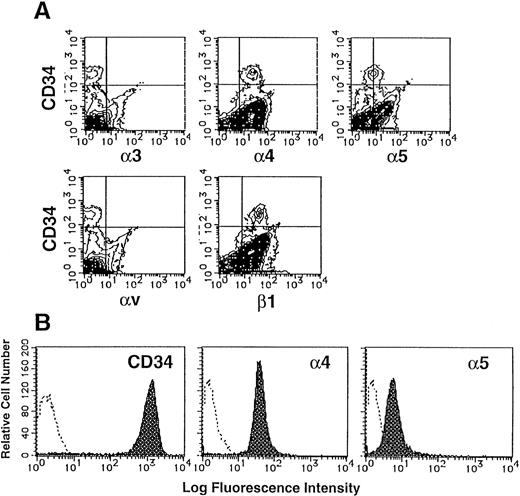
The large integrin superfamily includes many potential ligands for components of ECM. The expression of integrins on CD34+populations of UCB MNC was examined by two-color flow cytometry analysis. As shown in Fig 1A, 0.53% of UCB MNC expressed CD34, a marker of human hematopoietic stem cells. These CD34+ populations were stained strongly with anti-human α4 MoAb (mean fluorescence intensity [MFI], 28.15) and anti-human β1 MoAb (MFI, 40.87), and faintly with anti-human α5 MoAb (MFI, 8.81). In contrast, α3 and αv subunits were not detected on their surface.
Expression of integrins on human CD34+cells. (A) UCB MNC were stained with MoAbs specific for human integrin α3, α4, α5, αv, or β1 subunit and FITC-conjugated goat anti-mouse IgG, and then with PE-conjugated anti-human CD34 MoAb. The stained cells were analyzed by flow cytometry. Quadrants are indicated to show levels of background staining observed with appropriate irrelevant control antibodies. (B) Purified CD34+ cells were stained with anti–CD34-PE, anti–α4-FITC or anti–α5-FITC (shaded histograms). The levels of background staining with negative control antibodies (open histograms) are shown in each panel for direct comparison.
Expression of integrins on human CD34+cells. (A) UCB MNC were stained with MoAbs specific for human integrin α3, α4, α5, αv, or β1 subunit and FITC-conjugated goat anti-mouse IgG, and then with PE-conjugated anti-human CD34 MoAb. The stained cells were analyzed by flow cytometry. Quadrants are indicated to show levels of background staining observed with appropriate irrelevant control antibodies. (B) Purified CD34+ cells were stained with anti–CD34-PE, anti–α4-FITC or anti–α5-FITC (shaded histograms). The levels of background staining with negative control antibodies (open histograms) are shown in each panel for direct comparison.
The purity of sorted CD34+ cells was 98.0% (Fig 1B). These sorted cells expressed almost the same levels of α4 and α5 subunits (VLA4brightVLA5dull) as original CD34+ cells, indicating that the levels of VLA4 and VLA5 expression on CD34+ cells were scarcely influenced by our sorting method.
FN augments the clonogenic efficiency of human CD34+cells.
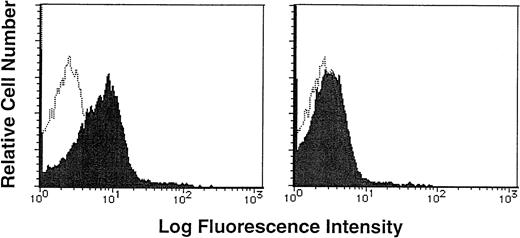
To determine if human CD34+/VLA4bright/VLA5dull cells had adhesive interaction with FN, human purified CD34+ cells were incubated with biotin-labeled soluble FN in the presence or absence of a 20-fold excess of unlabeled FN, and then stained with FITC-conjugated avidin. Flow cytometric analysis showed that CD34+ cells were stained with biotin-labeled soluble FN and FITC-conjugated avidin (MFI, 13.72; MFI of negative control, 2.84), and the addition of excessive unlabeled FN significantly blocked the staining (MFI, 3.83; Fig 2),suggesting the specific binding of FN to CD34+ cells.
Binding of soluble FN to human CD34+ cells. Purified CD34+ cells were incubated with biotin-labeled soluble FN in the presence (right panel, shaded histogram) or absence (left panel, shaded histogram) of a 20-fold excess of unlabeled FN, and then stained with FITC-conjugated avidin. The stained cells were analyzed by a single-color flow cytometry. The level of background staining with unlabeled FN and FITC-conjugated avidin (open histogram) is shown in each panel.
Binding of soluble FN to human CD34+ cells. Purified CD34+ cells were incubated with biotin-labeled soluble FN in the presence (right panel, shaded histogram) or absence (left panel, shaded histogram) of a 20-fold excess of unlabeled FN, and then stained with FITC-conjugated avidin. The stained cells were analyzed by a single-color flow cytometry. The level of background staining with unlabeled FN and FITC-conjugated avidin (open histogram) is shown in each panel.
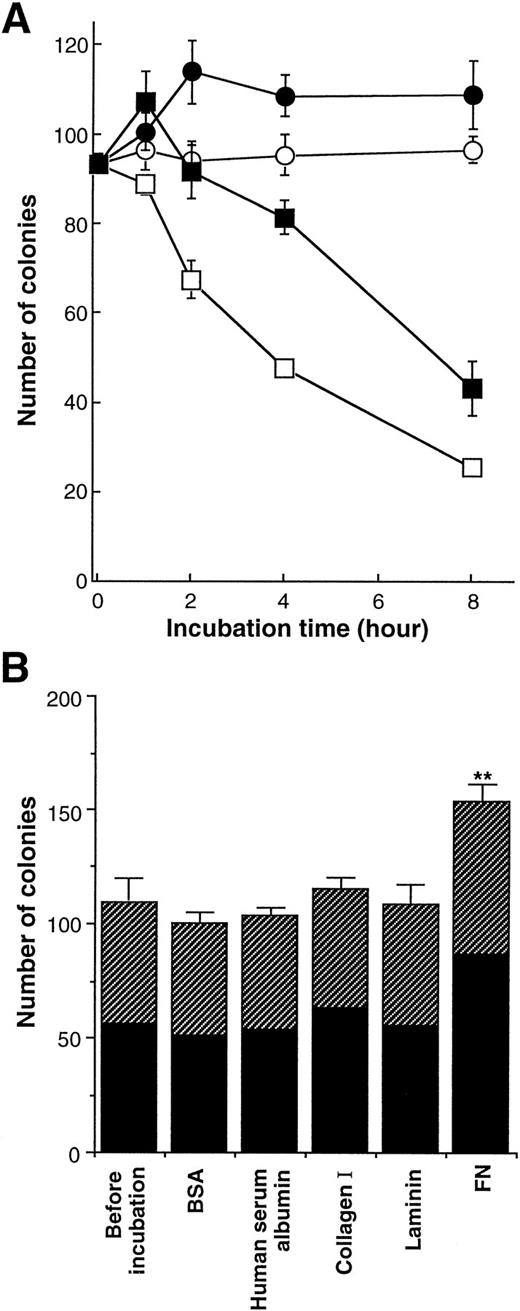
We next investigated the effects of FN on the clonogenic growth of human purified CD34+ cells. The cells were preincubated with FN or BSA (100 μg/mL) for the indicated periods in IMDM without iron-saturated human transferrin and human insulin, and then the cells were subjected to methylcellulose colony assay (Fig3A). CD34+ cells rapidly lost their clonogenic ability in the absence of FN. Preincubation with BSA alone for 4 hours led to approximately 50% of decrease in the number of colonies, whereas trypan blue dye-exclusion revealed that approximately all of cells were alive for at least 8 hours. In the presence of FN, by contrast, CD34+ cells generated increased number of colonies after 1 hour incubation, and maintained their clonogenic ability to the same level of fresh cells even after 2 hours incubation.
Effect of FN on the clonogenic capacity of human hematopoietic progenitor cells. (A) The human CD34+ cells were incubated with 100 μg/mL of BSA (○, □) or FN (•, ▪) in IMDM (□, ▪) or in IMDM containing 200 μg/mL transferrin and 10 μg/mL insulin (○, •) at 37°C for the indicated periods. The preincubated cells (5.0 × 102) were subsequently cultured in methylcellulose media to assess their clonogenic growth. All of the results are shown as mean ± SD of triplicate cultures. (B) Human CD34+ cells were incubated with 100 μg/mL of BSA, human serum albumin, collagen type I, laminin, or FN in serum-free IMDM with no other proteins at 37°C for 1 hour and subsequently were cultured in methylcellulose media at 5.0 × 102/mL to assess their clonogenic growth. The data represent mean ± SD of triplicate cultures. Statistically significant difference from a control (before incubation) value is indicated by two (P < .01) asterisks. Similar results were obtained in three independent experiments. (▨), BFU-E; (▪), CFU-GM.
Effect of FN on the clonogenic capacity of human hematopoietic progenitor cells. (A) The human CD34+ cells were incubated with 100 μg/mL of BSA (○, □) or FN (•, ▪) in IMDM (□, ▪) or in IMDM containing 200 μg/mL transferrin and 10 μg/mL insulin (○, •) at 37°C for the indicated periods. The preincubated cells (5.0 × 102) were subsequently cultured in methylcellulose media to assess their clonogenic growth. All of the results are shown as mean ± SD of triplicate cultures. (B) Human CD34+ cells were incubated with 100 μg/mL of BSA, human serum albumin, collagen type I, laminin, or FN in serum-free IMDM with no other proteins at 37°C for 1 hour and subsequently were cultured in methylcellulose media at 5.0 × 102/mL to assess their clonogenic growth. The data represent mean ± SD of triplicate cultures. Statistically significant difference from a control (before incubation) value is indicated by two (P < .01) asterisks. Similar results were obtained in three independent experiments. (▨), BFU-E; (▪), CFU-GM.
When CD34+ cells were preincubated with BSA in IMDM containing iron-saturated human transferrin and human insulin, they maintained their clonogenic ability to the same level of fresh cells for at least 8 hours even in the absence of FN. When preincubated with FN in the medium, CD34+ cells generated approximately 1.3-fold increased number of colonies after 2 hours of incubation and preserved their clonogenic ability for the following 6 hours. Although the difference between the two medium conditions in the numbers of colonies implied the importance of transferrin and/or insulin in supporting clonogenic ability of CD34+ cells, FN had the enhancing effects on the clonogenic growth of CD34+ cells in both conditions. In subsequent assays, preincubation with FN, its fragments, or other ECM molecules was performed in serum-free IMDM without transferrin and insulin to examine their direct effects on hematopoietic stem/progenitor cells without influence of any serum components or growth factors.
Because FN was found to influence colony formation of CD34+cells, we further examined the effects of FN as well as other ECM molecules on clonal growth of myeloid and erythroid progenitor cells. Purified CD34+ cells were preincubated with BSA, human serum albumin, collagen type I, laminin, or FN in IMDM for 1 hour, and then cells were subjected to methylcellulose colony assay. As shown in Fig 3B, 109 colonies consisting of 53 BFU-E and 56 CFU-GM were generated from freshly isolated 500 CD34+ cells (before incubation). When CD34+ cells were preincubated with each type of ECM molecule for 1 hour, collagen type I or laminin did not significantly affect the numbers and types of colonies. By contrast, treatment with FN resulted in increased number of total colonies up to 153, and this supportive effect of FN was observed in both BFU-E and CFU-GM colonies (elevation by 25% in BFU-E and 55% in CFU-GM). Moreover, although the proportion of CFU-GEMM was very low, the number of CFU-GEMM colonies was also increased by FN treatment (the mean number of CFU-GEMM: before incubation, 3.3; 1 hour treatment, 5.3). In addition to colony numbers, size of colonies generated with FN treatment was bigger than that of colonies developed without FN treatment when scored on days 7 to 10, although FN itself could not support colony formation in the absence of any added growth factors (data not shown). These effects of FN were observed at a concentration of 50 μg/mL as well as 100 μg/mL, but not statistically obvious at a concentration of 10 μg/mL (data not shown).
Influence of GRGDSP and CS1 peptides on the effect of FN.
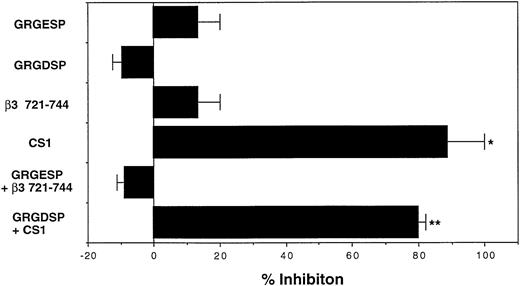
Human CD34+ cells of UCB MNC expressed both VLA4 and VLA5 as FN receptors (Fig 1). To determine which integrin was involved in the supportive effects of FN, we evaluated the effects of GRGDSP and CS1 peptides, which respectively block the FN-VLA5 and FN-VLA4 interactions,20 21 on the numbers of colonies developed after treatment with or without FN. In this series of experiments, CD34+ cells that were preincubated with FN for 1 hour generated 1.3-fold of colonies in number as compared with those preincubated with BSA. The supportive effect of FN was completely abrogated by CS1 peptide, whereas GRGDSP peptide had little effect (Fig4). These results suggested that the enhancing effect of FN on the clonogenic efficiency of human CD34+ cells was exhibited mainly via the attachment of CS1 portion, but not GRGDSP region, of FN to cell surface integrins.
Involvement of FN-integrin interaction in augmentation of clonogenic growth of human hematopoietic progenitor cells. The human CD34+ cells were incubated with 100 μg/mL of BSA or FN in serum-free IMDM at 37°C for 1 hour in the presence or absence of the indicated peptides (10 μg/mL) and subsequently cultured in methylcellulose media at 5.0 × 102/mL to assess their clonogenic growth. GRGESP and β3 721-744 were used as control peptides for GRGDSP and CS1, respectively. The reduction in colony numbers was used to calculate the percentage inhibition relative to the FN-increased colony number. The data represent mean percent inhibition ± SD of triplicate cultures. Statistically significant differences from respective control values are indicated by one (P < .05) or two (P < .01) asterisks. Similar results were obtained in three independent experiments and when the peptides were used at 100 μg/mL.
Involvement of FN-integrin interaction in augmentation of clonogenic growth of human hematopoietic progenitor cells. The human CD34+ cells were incubated with 100 μg/mL of BSA or FN in serum-free IMDM at 37°C for 1 hour in the presence or absence of the indicated peptides (10 μg/mL) and subsequently cultured in methylcellulose media at 5.0 × 102/mL to assess their clonogenic growth. GRGESP and β3 721-744 were used as control peptides for GRGDSP and CS1, respectively. The reduction in colony numbers was used to calculate the percentage inhibition relative to the FN-increased colony number. The data represent mean percent inhibition ± SD of triplicate cultures. Statistically significant differences from respective control values are indicated by one (P < .05) or two (P < .01) asterisks. Similar results were obtained in three independent experiments and when the peptides were used at 100 μg/mL.
Effects of FN fragments on the clonogenic efficiency of human CD34+ cells.
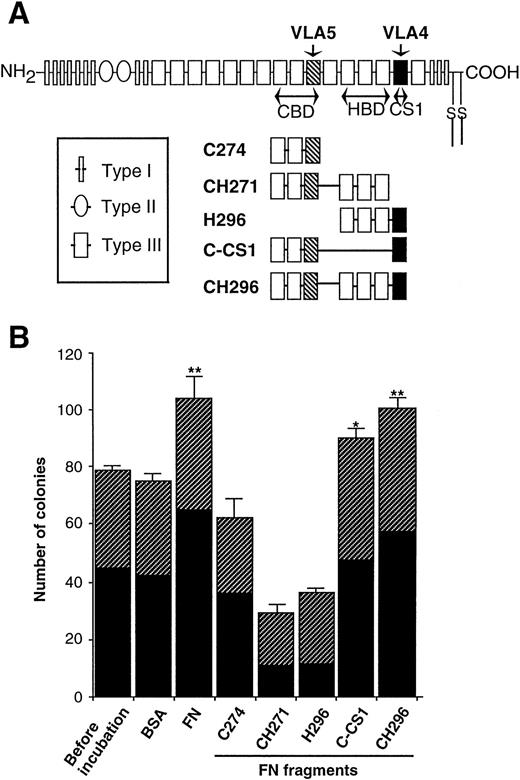
To understand structural and minimal sequence requirement of FN for the supportive effects on progenitor cells, several fragments of FN (illustrated in Fig 5A) as well as native form of FN were added to preincubations of CD34+ cells. As shown in Fig 5B, CH296 (CBD-HBD-CS1) increased the number of colonies at an almost similar level to native form of FN, and C-CS1 (CBD-CS1) was also effective (39% elevation with FN, 34% elevation with CH296, and 20% elevation with C-CS1). In contrast, C274 (CBD alone), CH271 (CBD-HBD), and H296 (HBD-CS1) did not increase the number of colonies, but rather suppressed colony formation. It was therefore suggested that both CS1 and CBD may be required for augmenting clonogenic growth of hematopoietic progenitor cells, and the combination of three domains, CBD-HBD-CS1, may substitute for native FN.
Effects of FN fragments on the clonogenic capacity of human hematopoietic progenitor cells. (A) Schematic representation of FN molecules and FN fragments. FN is made up of a series of repeats termed Type I, II, and III. Regions of FN with proved cell-binding activity are shown as the RGD-containing CBD, the nonintegrin-dependent HBD, and the LDV-containing CS1 region (CS1). The portions adhere to VLA4 (▪) and VLA5 (▧) are also indicated. (B) The human CD34+ cells were incubated with 0.5 nmol/mL of FN or each FN fragment in serum-free IMDM at 37°C for 1 hour and subsequently cultured in methylcellulose media at 3.0 × 102/mL to assess their clonogenic capacity. BSA was used as a control protein. The data are shown as mean ± SD of duplicate cultures. Statistically significant differences from a control (before incubation) value are indicated by one (P < .05) or two (P < .01) asterisks. Similar results were obtained in three independent experiments. (▨), BFU-E; (▪), CFU-GM.
Effects of FN fragments on the clonogenic capacity of human hematopoietic progenitor cells. (A) Schematic representation of FN molecules and FN fragments. FN is made up of a series of repeats termed Type I, II, and III. Regions of FN with proved cell-binding activity are shown as the RGD-containing CBD, the nonintegrin-dependent HBD, and the LDV-containing CS1 region (CS1). The portions adhere to VLA4 (▪) and VLA5 (▧) are also indicated. (B) The human CD34+ cells were incubated with 0.5 nmol/mL of FN or each FN fragment in serum-free IMDM at 37°C for 1 hour and subsequently cultured in methylcellulose media at 3.0 × 102/mL to assess their clonogenic capacity. BSA was used as a control protein. The data are shown as mean ± SD of duplicate cultures. Statistically significant differences from a control (before incubation) value are indicated by one (P < .05) or two (P < .01) asterisks. Similar results were obtained in three independent experiments. (▨), BFU-E; (▪), CFU-GM.
Effects of FN on a murine multipotent cell line, EML-C1.
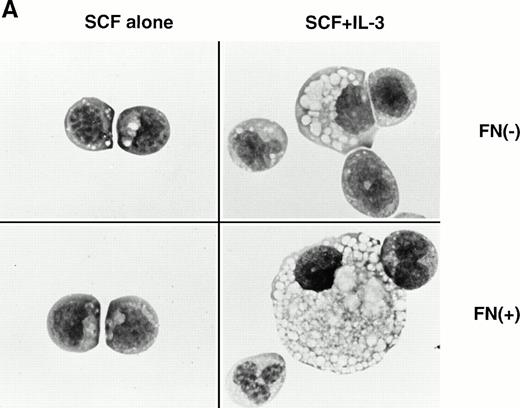
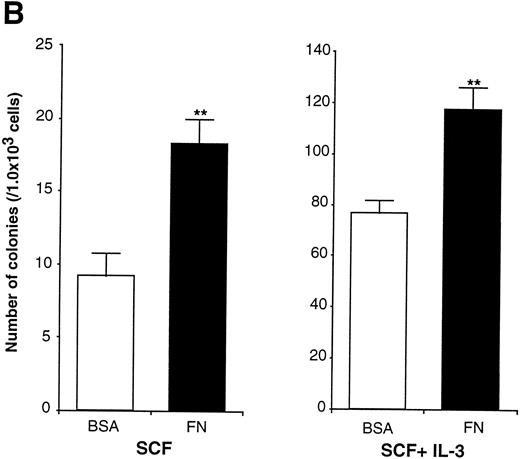
In the experiments using human CD34+ cells, FN treatment resulted in increased numbers of committed progenitors like CFU-GM and BFU-E. However, it was difficult to evaluate colony formation of more primitive and self-renewing progenitors, CFU-Blast, because of their rarity. EML-C1 cells, a murine multipotent cell line,32 was used for this purpose because they generated CFU-Blast colonies responding to SCF and generated CFU-GM colonies by costimulation with SCF and IL-3 in methylcellulose media. These observations were also reproducible when EML-C1 cells were preincubated with or without FN (Fig 6A). Flow cytometry analysis revealed that EML-C1 cells showed VLA4bright/VLA5dull phenotype in the same manner as human CD34+ cells (data not shown). In accordance with the findings on human CD34+ cells, EML-C1 cells preincubated with FN generated more colonies of CFU-Blast and CFU-GM than those preincubated with BSA (2-fold elevation in CFU-Blast and 1.5-fold elevation in CFU-GM; Fig 6B).
Effects of FN on the clonogenic efficiency of EML-C1 cells. (A) Cytocentrifuged preparations of the colonies generated in the presence of SCF alone or SCF + IL-3, with or without FN, respectively, were photographed after Giemsa staining. (B) After exposure to FN, EML-C1 cells (1.0 × 103) were plated in methylcellulose media containing SCF alone or SCF + IL-3. The number of CFU-Blast and CFU-GM were assessed on day 14 and day 7 of culture. The data represent mean ± SD of triplicate cultures and statistically significant differences from control (BSA) values are indicated by two (P < .01) asterisks. Similar results were obtained in three independent experiments.
Effects of FN on the clonogenic efficiency of EML-C1 cells. (A) Cytocentrifuged preparations of the colonies generated in the presence of SCF alone or SCF + IL-3, with or without FN, respectively, were photographed after Giemsa staining. (B) After exposure to FN, EML-C1 cells (1.0 × 103) were plated in methylcellulose media containing SCF alone or SCF + IL-3. The number of CFU-Blast and CFU-GM were assessed on day 14 and day 7 of culture. The data represent mean ± SD of triplicate cultures and statistically significant differences from control (BSA) values are indicated by two (P < .01) asterisks. Similar results were obtained in three independent experiments.
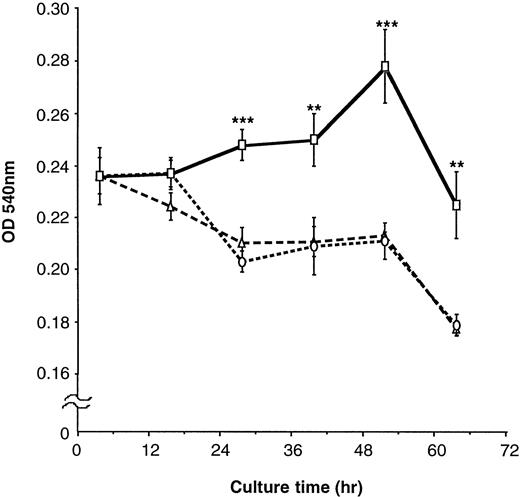
To further clarify the effects of FN on proliferation of EML-C1 cells, they were cultured with or without 100 μg/mL FN in serum-free IMDM containing SCF for the indicated periods, followed by measurement of cell proliferation by MTT rapid colorimetric assay. In IMDM without FN, EML-C1 cells could not respond to SCF and reduced their viable cell number (Fig 7). In contrast, EML-C1 cells could respond to SCF and grew gradually for at least 60 hours in the presence of FN. EML-C1 cells did not grow in the absence of SCF, regardless of treatment with FN (data not shown).
Effects of FN on the proliferation of EML-C1 cells. EML-C1 cells (3 × 104) were incubated with or without FN in IMDM containing SCF (20 ng/mL) for the indicated times. Cell proliferation was measured using an MTT colormetric assay. The results are shown as mean ± SD of quardricate cultures. Statistically significant differences from control (BSA) values are indicated by two (P < .01) or three (P < .001) asterisks. (▵), Medium; (○), BSA; (□), FN.
Effects of FN on the proliferation of EML-C1 cells. EML-C1 cells (3 × 104) were incubated with or without FN in IMDM containing SCF (20 ng/mL) for the indicated times. Cell proliferation was measured using an MTT colormetric assay. The results are shown as mean ± SD of quardricate cultures. Statistically significant differences from control (BSA) values are indicated by two (P < .01) or three (P < .001) asterisks. (▵), Medium; (○), BSA; (□), FN.
Effects of FN and its fragments on the clonogenic ability and adhesion function of EML-C1 cells.
In several cell systems, cell attachment and cell spreading have been proved to be critical in survival and division of cells.36 37 To investigate whether the growth-supporting activity of FN paralleled the adhesion function, the effects of FN as well as its fragments on the adhesion and growth of EML-C1 cells were examined. As shown in Table 1, FN, CH296 (CBD + HBD + CS1), and C-CS1 (CBD + CS1) supported the CFU-GM production of EML-C1 cells, whereas other tested fragments did not have supporting function. These findings were comparable with those obtained from human CD34+ cells. In adhesion assay, EML-C1 cells were found to adhere to CH296 and H296 (HBD + CS1) at the highest level; the cells showed the lesser level of adhesion to FN, C274 (CBD alone), CH271 (CBD + HBD), and C-CS1, and the percents of adhesion to these molecules were almost similar. In cell-shape observation using an inverted-contrast microscope, EML-C1 cells were found to attach and spread on the growth-supporting FN fragments as well as the FN fragments that did not stimulate CFU-GM production (data not shown). Thus, there seemed to be no apparent correlation between the growth-supporting activity and the adhesion function of the tested FN molecules.
Adhesion and Proliferative Response of EML-C1 Cells to FN and Its Fragments
| Proteins . | % Adhesion* . | CFU-GM Production-151 . |
|---|---|---|
| BSA | 8.3 ± 2.6 | 67 ± 3 |
| FN | 24.9 ± 2.8 | 92 ± 4 |
| C274 | 20.0 ± 0.8 | 59 ± 5 |
| CH271 | 23.4 ± 0.2 | 17 ± 3 |
| H296 | 42.9 ± 1.4 | 58 ± 4 |
| C-CS1 | 28.2 ± 0.6 | 83 ± 4 |
| CH296 | 41.7 ± 0.8 | 89 ± 5 |
| Proteins . | % Adhesion* . | CFU-GM Production-151 . |
|---|---|---|
| BSA | 8.3 ± 2.6 | 67 ± 3 |
| FN | 24.9 ± 2.8 | 92 ± 4 |
| C274 | 20.0 ± 0.8 | 59 ± 5 |
| CH271 | 23.4 ± 0.2 | 17 ± 3 |
| H296 | 42.9 ± 1.4 | 58 ± 4 |
| C-CS1 | 28.2 ± 0.6 | 83 ± 4 |
| CH296 | 41.7 ± 0.8 | 89 ± 5 |
*FITC-labeled EML-C1 cells were incubated at 37°C for 2 hours on the plates precoated with the indicated proteins, and the proportions of adherent cells were measured by a fluorescence spectrophotometer. Adhesion (%) was calculated as described in Materials and Methods. The results are shown as mean ± SD of triplicate cultures.
EML-C1 cells preincubated with the indicated proteins were plated at 1.0 × 103/mL in methylcellulose media containing SCF + IL-3, and the numbers of CFU-GM were assessed on day 7 of culture. The results (the numbers of GM colonies/1 × 103 EML-C1 cells) are expressed as mean ± SD of quardricate cultures.
Effects of in vivo treatment of FN fragments on the number of hematopoietic progenitors.
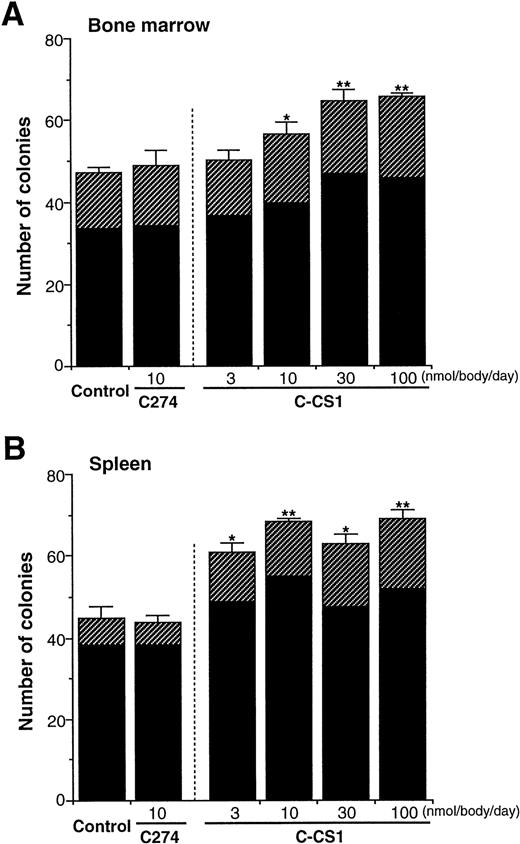
As all of the above results were obtained from in vitro experiments, we examined whether FN truly acts on progenitor cells in vivo. FN fragments, C274 and C-CS1, were injected intravenously to mice daily for 4 days, and then peripheral blood, BM, and spleen cells of the mice were obtained and subjected to methylcellulose colony assay. No significant changes were observed in total cell numbers of BM and spleen among mice after injection of these fragments (data not shown). However, C-CS1 injection for 4 days led to an increase in the numbers of colony-forming cells (CFC) in both BM (Fig 8A) and spleen (Fig8B) in a dose-dependent manner; the effects on CFC numbers were evident at 3 nmol/body/d and maximal at 30 to 100 nmol/body/d. These enhancing effects were not observed after injection of C274 (10 nmol/body/d) in either organs. The increased numbers of colonies were observed in both BFU-E and CFU-GM (at a dose of 100 nmol/body/d of C-CS1; 1.5- and 1.4-fold elevation in BM BFU-E and CFU-GM, 2.6- and 1.4-fold elevations in spleen BFU-E and CFU-GM, respectively). Unexpectedly, the number of CFC in peripheral blood did not increase, but rather decreased 24 hours after injection of C-CS1 (normal control, 120 ± 13/mL of blood; C274 10 nmol/body/d, 99 ± 11/mL; C-CS1 10 nmol/body/d, 51 ± 6/mL; C-CS1 100 nmol/body/d, 46 ± 6/mL). However, because the proportion of CFC in the peripheral blood is very small in the whole body, C-CS1 injection at a dose of 100 nmol/body led to an approximately 1.3-fold increase in the estimated number of total CFC. These results indicate that particular structure of FN may not only influence the distribution of hematopoietic progenitor cells but also support their growth in vivo.
Influences of FN fragments on hematopoiesis in vivo. BALB/C mice were intravenously injected with C274 or C-CS1 fragment of FN daily for 4 days at the indicated doses (n = 5 in each dose). The mice were killed at day 5, and subsequently the numbers of CFC in BM cells (1 × 104) (A) and spleen cells (2 × 105) (B) were assessed. The data are shown as mean ± SD. Control mice received an equal volume of PBS. Statistically significant differences from control values (shown at the left sides in each panel) are indicated by one (P < .05) or two (P < .01) asterisks. The figure shows one of two similar experiments. (▨), BFU-E; (▪), CFU-GM.
Influences of FN fragments on hematopoiesis in vivo. BALB/C mice were intravenously injected with C274 or C-CS1 fragment of FN daily for 4 days at the indicated doses (n = 5 in each dose). The mice were killed at day 5, and subsequently the numbers of CFC in BM cells (1 × 104) (A) and spleen cells (2 × 105) (B) were assessed. The data are shown as mean ± SD. Control mice received an equal volume of PBS. Statistically significant differences from control values (shown at the left sides in each panel) are indicated by one (P < .05) or two (P < .01) asterisks. The figure shows one of two similar experiments. (▨), BFU-E; (▪), CFU-GM.
DISCUSSION
Blood cell formation is controlled by a complex set of events, including interactions between ECM and hematopoietic cells.1-3 Multiple cell types interact within a confined space in hematopoietic organs to deliver and receive critical signals for proliferation and differentiation. In this report, we have shown that FN augments in vitro and in vivo growth of hematopoietic stem cells. Attachment of FN to VLA-4, not to VLA-5, is essential, while both CBD and CS1 domains of FN are structurally required for its optimal effects.
Treatment of purified human CD34+ cells with FN increased the number of colonies of CFU-GEMM, CFU-GM, and BFU-E. Increased number of CFU-Blast colonies was also observed when EML-C1 cells were pretreated with FN. Therefore, FN seemed to promote colony formation of both committed and uncommitted progenitors, and these effects of FN were seen on a wider stage of differentiation than that reported by Weinstein et al.38 In addition to the number of colonies, FN treatment increased the sizes of colonies when observed on days 7 to 10 of cultures. FN also augmented proliferation of EML-C1 cells in the presence of SCF. These results suggested that FN might collaborate or synergize functionally with cytokines to induce proliferation of progenitor cells.
Verfaillie39 has previously shown that BM stromal cells produce molecules that augment the cytokine-induced proliferation and maturation of hematopoietic progenitors. Our results suggest that FN may be one of such stromal cell molecules. Regarding the cooperation of integrins and cytokine receptors, Lévesque et al40 41have reported that signals mediated through cytokine receptors modulate the activation of β1 integrins, thereby leading to generation of secondary signals from FN-integrin interaction, which may synergize and/or complete the initial signals from cytokines. Our results provide additional support for signal coordination of integrin and cytokine receptors, and raise the possibility that because pretreatment of FN could augment cytokine-induced clonogenic growth of hematopoietic progenitor cells in our system, FN may function to trigger progenitor cells to become more responsive to cytokines by changing expression of cytokine receptors, and/or by modifying signal pathway through cytokine receptors.
VLA4 has been known to play pivotal roles in interactions between hematopoietic stem/progenitor cells and stromal cells.42,43Antibodies against VLA4 completely blocked production of hematopoietic cells in long-term BM cultures.5 The primitive hematopoietic stem cells can adhere to CS1 site in the C-terminal fragment of FN.22 In this study, CS1 fragments could almost completely abrogate the effects of FN, suggesting that attachment of FN to VLA4 may be essential for the supportive effects of FN on hematopoietic progenitors. By contrast, the supportive effects of FN were not significantly affected by the addition of GRGDSP peptides that could inhibit the interaction between CBD and VLA5, suggesting little involvement of CBD/VLA5 interaction. However, the CBD-HBD-CS1–connected or CBD-CS1–connected fragments had growth-supporting activities almost similar to native FN, whereas little or no activity was observed in HBD-CS1 that had approximately comparable size with CBD-CS1. Furthermore, it was noted that there was no significant correlation between the adhesion function and growth-supporting activity of the FN molecules tested. It has been shown that signal transduction in response to several growth factors is affected by integrins when they are both aggregated and occupied by ligands.44 It was therefore possible that the aggregation and occupancy of integrins on hematopoietic stem/progenitor cells may require both CBD and CS1 as a minimal functional structure of FN, and also that the adhesion function of the FN fragments may not necessarily correlate with their proliferative activity. Furthermore, because the structural importance of FN has been reported for VLA5-mediated cell adhesion and/or the downstream signal transduction,45 structural requirement may also exist in the FN-VLA4 interaction.
Recent studies have shown that ligation of FN to integrins stimulate a variety of signaling events, including tyrosine phosphorylation, cytoplasmic alkalization, calcium influx, accumulation of cytoskeletal molecules at sites of cell adhesion, and altered gene expression.15,16,26,27,46 Attachment of epithelial and endothelial cells to ECM is essential for their survival both in vitro and in vivo because they undergo apoptosis by the inhibition of these interactions.47 In addition, several lines of evidence suggest that signal transduction events through integrin-ligand engagement are involved in the suppression of apoptosis in anchored cells. For example, attachment of epithelium to ECM via integrins regulated expression of IL-1β converting enzyme, a protein associated with apoptosis.48 Apoptosis of detached endothelial cells was suppressed by the addition of sodium vanadate, a protein tyrosine phosphatase inhibitor.49 Among the signals via integrins, pp125 focal adhesion kinase (pp125FAK) is particularly of interest because constitutive activation of pp125FAK was sufficient to rescue a epithelial cell line, MDCK, from apoptosis.50 Moreover, injection of an anti-pp125FAK antibody into rounded fibroblasts resulted in the rapid onset of apoptosis.51 It is also known that pp125FAK directly associates with β subunit of integrins, and its tyrosine phosphorylation and kinase activity are upregulated by the binding of FN to integrins.52-54 It seems it would be of interest to evaluate roles of pp125FAK in apoptosis of EML-C1 cells, a multipotent hematopoietic cell line. Further analysis will be necessary to reveal the signal transduction pathway and structural basis of FN for promoting cell survival and growth.
The system to expand CFC in vitro is currently being investigated by many research groups.55-57 Successful in vitro expansion of primitive stem cells would promise invaluable contributions to clinical medicine such as stem cell transplantation and gene therapy. A number of studies have shown that multipotential hematopoietic progenitors like CFU-GM, CFU-GEMM, and LTC-IC can be expanded in vitro after exposure to combinations of various cytokines, including SCF, IL-1, IL-3, IL-6, IL-11, GM-CSF, erythropoietin, interferon, and leukemia inhibitory factor.55-57 However, transplantation experiments have shown that hematopoietic progenitors expanded by cytokines do not have potential to support the long-term hematopoietic reconstitution.58 59 Because our results show that FN can promote proliferation of self-renewing CFU-Blast stem cells in cooperation with cytokines, this glycoprotein may be practicable for the in vitro expansion of stem cells without impairing their long-term reconstituting ability.
In addition to the in vitro effect, the intravenous administration of CBD-CS1 fragment of FN into mice was found to cause an increase of CFC in the BM and spleen. The mechanism underlying the growth-supporting activity of CBD-CS1 fragment in vivo may not be simple because ample growth factors and serum proteins are present in vivo. However, our data suggest that FN may be functional in terms of supporting growth of hematopoietic progenitors in vivo, possibly in cooperation with growth factors and/or serum components. Interestingly, in agreement with our preliminary observation, it has recently been shown that the number of CFC in peripheral blood and hematopoietic organs is not influenced by CS1 treatment.60 These findings suggest that although CS1 peptide could abrogate the FN effect in vitro, other VLA4 ligands like VCAM1 may substitute for FN in vivo. Recently, the FN-, VLA4-, VLA5-, or β1-deficient mice have been generated by gene targeting.61-65 However, precise roles of the FN-integrin interactions in the in vivo hematopoiesis remain unclear because of embryonic lethality of the gene-targeted mice. Because mechanisms of hematopoiesis have been explored using in vitro embryonic stem cell differentiation systems that may mimic early hematopoiesis in vivo,66 67 it will be interesting to clarify when and how FN-integrin interaction acts on early events of hematopoiesis by using these systems. Clear and decisive information from such studies should not only lead to a more precise understanding of mechanisms underlying regulation of hematopoiesis, but also suggest novel therapeutic procedures for patients with hematologic disorders.
ACKNOWLEDGMENT
We thank Drs Tohru Kanzaki, Hajime Ogata, and Shinji Sogo for their help in collecting fresh cord blood and sorting CD34+cells. We also thank Dr Ikunoshin Kato for generously providing FN fragments.
Supported in part by grants from the Ministry of Education, Science, and Culture; the Inamori Foundation; Senri Life Science Foundation; and Mochida Memorial Foundation.
Address reprint requests to Takafumi Yokota, MD, The Second Department of Internal Medicine, Osaka University Medical School, 2-2 Yamada-oka, Suita, Osaka 565, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal