Abstract
Telomerase is a ribonucleoprotein polymerase that synthesizes telomeric repeats onto the 3′ ends of eukaryotic chromosomes. Activation of telomerase may prevent telomeric shortening and correlates with cell immortality in the germline and certain tumor cells. Candidate hematopoietic stem cells (HSC) from adult bone marrow express low levels of telomerase, which is upregulated with proliferation and/or differentiation. To address this issue, we stimulated purified candidate HSC from human adult bone marrow with stem cell factor (SCF), interleukin-3 (IL-3), and Flt3-ligand (FL). After 5 days in culture, activity was detected in total cell extracts from IL-3–, SCF + FL–, SCF + IL-3–, FL + IL-3–, and SCF + IL-3 + FL–stimulated cultures, but not from cells cultured in SCF or FL alone. Within the CD34+fraction of the cultured cells, significant activity was found in the CD34+CD71+ fraction. In addition, PKH26 staining confirmed that detectable telomerase activity was present in dividing PKH26lo cells, whereas nondividing PKH26hi cells were telomerase negative. Because in these experiments no distinction could be made between cycling “candidate” stem cells that had retained or had lost self-renewal properties, fetal liver cells with a CD34+CD38− phenotype, highly enriched for cycling stem cells, were also examined and found to express readily detectable levels of telomerase activity. Given the replication-dependent loss of telomeric DNA in hematopoietic cells, these observations suggest that the observed telomerase activity in candidate stem cells is either expressed in a minor subset of stem cells or, more likely, is not sufficient to prevent telomere shortening.
THE ENDS OF all eukaryotic chromosomes are organized into telomeres that consist of tandem arrays of G-rich repeats and associated proteins. Telomeres protect chromosomes from degradation, prevent end-to-end fusions, and position chromosomes within the nucleus.1,2 In primary somatic cells such as fibroblasts,3,4 lymphocytes,5 or hematopoietic progenitors,6 telomeric DNA is gradually lost with each cell division presumably because conventional DNA polymerases cannot fully replicate the ends of linear chromosomes.7,8Progressive shortening of chromosome ends has been suggested to act as a mitotic clock that may contribute to cellular senescence and eventual cell mortality of normal mammalian somatic cells.9
On the other hand, cells of the germ line, such as sperm cells, have long telomeres of 10 to 20 kb that do not appear to shorten with aging of the organism.4 Such long telomeric ends are assumed to be maintained by telomerase, a ribonucleoprotein whose primary function is to synthesize telomeric DNA, thus counteracting losses at the chromosome termini with each round of replication. The telomerase RNA component contains a species-specific template for synthesis of telomeric repeats, and such RNA sequences have been cloned from ciliates,10 yeast,11,12 mouse,13and human.14 Recently, the gene encoding the reverse transcriptase catalytic subunit of telomerase from several species including humans has also been cloned.15,16 In humans, telomerase activity is readily detectable in testes and ovaries, but not in most somatic tissues.17,18 Telomerase activity is also elevated in carcinomas of the ovary,19breast,20 liver,21 lung,22prostate,23 and in neuroblastoma24 and hematological malignancies.25-27 In addition, the majority of immortal cell lines expresses telomerase, whereas most mortal cells lack this activity.17 Together, these data strongly suggest that telomerase may be involved in malignant transformation and cellular immortality.
The hematopoietic system replenishes the loss of mature blood cells via the recruitment of stem cells that have been defined as pluripotential cells with self-renewal properties. We and others have shown low levels of telomerase activity in normal bone marrow and peripheral blood cells, both in progenitors of the myeloid and lymphoid lineages as well as in terminally differentiated cells such as T and B cells.26-28 Furthermore, we and others found that “candidate” hematopoietic stem cells (HSC) upregulate telomerase activity upon stimulation in vitro.28,29 However, as the vast majority of stem cells are quiescent during steady-state hematopoiesis,30 the status of telomerase expression in cycling HSC has not yet been elucidated. This is an important issue because the self-renewal and replicative potential of the most primitive hematopoietic cells may depend on telomerase to maintain stable telomeres.31 In this report, we describe results of experiments designed to address the question of telomerase expression in cycling stem cells. For this purpose, we measured telomerase activity in extracts from purified candidate HSC from human adult bone marrow stimulated with different cytokines and in extracts from CD34+CD38− candidate HSC purified from human fetal liver. Our data indicated that telomerase activity is expressed in most if not all cycling stem cells but is repressed in quiescent stem cells.
MATERIALS AND METHODS
Purification of HSC from adult bone marrow and fetal liver.
Candidate HSC with the phenotype CD34+CD45RAloCD71lo were obtained from previously frozen cadaver marrow as previously described.32 Briefly, mononuclear cells retrieved from the interface after density separation were stained with 8G12-Cy5 (anti-CD34), 8d2-PE (anti-CD45RA), and OKT9-FITC (anti-CD71) for 30 minutes at 4°C. Cells were washed twice in Hanks' buffered saline with 0.2% BSA (HB) and stained with 2 μg/mL of propidium iodide (PI) before suspending in HB at a density of 5 × 106 cells/mL for sorting. Cells were sorted on a FACStarplus (Becton Dickinson, San Jose, CA) equipped with argon (488 nm) and helium-neon (633 nm) lasers.
CD34+CD45RAloCD71loCD38−(CD34+CD38−) cells were obtained from adult marrow and fetal liver in the 17th and 18th week of gestation according to previously described protocols.33 34 Cells were stained with 8G12-Cy5, 8d2-FITC, OKT9-FITC, and anti–CD38-phycoerythrin (PE; Becton Dickinson) for 30 minutes at 4°C. The washing and sorting procedures were performed as above.
Cell culture.
Sorted candidate HSC were cultured in serum-free medium consisting of Iscove's modified Dulbecco's medium (IMDM) supplemented with the following reagents: bovine serum albumin (BSA) at 2%, sodium bicarbonate at 0.1%, transferrin at 200 μg/mL, insulin at 10 μg/mL, 2-mercaptoethanol at 10−5 mol/L, low-density lipoprotein (Sigma, St Louis, MO) at 40 μg/mL, and penicillin-streptomycin at 100 U and 50 μg/mL, respectively.32 Both stem cell factor (SCF) and Flt3-ligand (FL) were used at a final concentration of 50 ng/mL, whereas interleukin-3 (IL-3) was used at 20 ng/mL. All growth factors were purchased from Pepro-tech (Rocky Hill, NJ).
Telomerase repeat amplification protocol (TRAP) assay.
Telomerase activity was measured by TRAP assay using an end-labeled telomerase substrate (TS) primer as described.17,27,28Briefly, cell extracts were prepared by lysing the cells in CHAPS extraction buffer17 at a concentration of 500 cells per μL of buffer, centrifuged at 1200g at 4°C, and 2 μL of these extracts were used in the assay.
The telomerase reaction was performed in 50 μL of TRAP reaction buffer containing 20 mmol/L tris-HCl (pH 8.3), 1.5 mmol/L MgCl2, 63 mmol/L KCl, 0.005% Tween-20, 1 mmol/L EGTA, 50 μmol/L deoxynucleotide triphosphates (Pharmacia, Uppsala, Sweden), 0.1 μg each of the labeled TS primer, ACX primer, U2 primer, 5 × 10−3 attamoles (amols) of an internal control primer (TSU2), 2 U of Taq DNA polymerase (Boehringer Mannheim, Laval, Quebec), and 2 μL of CHAPS extract. The primers for this reaction are obtainable through Oncor (Gaithersburg, MD). Reaction tubes were placed in a robocycler (Stratagene, La Jolla, CA) for 30 minutes at 30°C, followed by 27 cycles of polymerase chain reaction (PCR) at 94°C for 30 seconds and 72°C for 30 seconds. One half of the amplified products were resolved on a 12% polyacrylamide gel, dried, and visualized by autoradiography using BioMax films (Kodak, Rochester, NY) after 48 hours of exposure at room temperature. In some cases, RNase A (Boehringer Mannheim) at 6 μg/mL was added during the telomerase reaction to confirm the specificity of the telomerase products that disappeared in the presence of RNase A.
For semiquantitative analysis of telomerase activity, the radioactive bands were scanned by densitometer and determined using ImageQuant software (Molecular Dynamics, Sunnyvale, CA). The signal from individual test extract was normalized for the PCR efficiency and compared with that generated by 1 amol of an oligonucleotide M2R8, which contains the same sequence as the TS primer plus eight T2AG3 repeats using the following formula:
whereLB is the blank control containing 2 μL of CHAPS lysis buffer in lieu of cell extract.
Tracking proliferation by PKH26 staining.
The proliferative history of cells was followed using PKH26 labeling and analysis as described.30 Sorted candidate HSC were washed once in Hanks' buffered saline without BSA. The cell pellet was resuspended in 150 μL of dilutent C (Zynaxis Cell Science, Malvern, PA), mixed with an equal volume of PKH26-GL (2 × 10−6mol/L), and incubated for 5 minutes at room temperature. The reaction was terminated by the addition of an equal volume of 10% BSA in serum-free medium. Cells were washed twice and an aliquot was taken for analysis of PKH26 staining intensity on day 0 of culture. PKH26 was excited at 488 nm and the emission was measured with a 575/26 filter. The remainder of the cells was put in culture under serum-free conditions supplemented with SCF, IL-3, and FL for 8 days. After 8 days of culture, cells were reanalyzed and sorted for PKHhi and PKHlo cells using FACStarplus.
RESULTS
Telomerase activity in candidate stem cells from adult bone marrow.
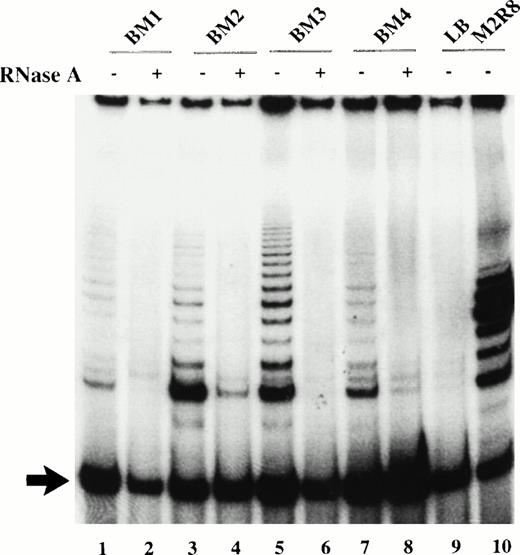
Cell extracts from adult marrow candidate HSC with the phenotype CD34+CD45RAloCD71lo were assayed for telomerase activity by a modified version of the PCR-based TRAP assay. In addition to the typical ladder of 6 bp repeats that correspond to the amplified product of the TS primer, an internal control (TSU2) is coamplified that yields a single lower band of 35 bp (Fig 1). By normalizing the signal intensity of the telomerase ladder to that of the internal control, sample to sample variation due to PCR amplification efficiency was minimized, thus allowing for semiquantitative analysis. Treatment with RNase obliterated the 6-bp ladder, indicating that telomerase was responsible for this reaction (Fig 1). This modified TRAP assay allowed us to detect telomerase activity in cell extracts obtained from 10 to 100 cell equivalent of 293 cells, a telomerase-positive immortalized kidney cell line.
Telomerase activity in candidate HSC before culture. TRAP products were generated from 2 μL of CHAPS extract (1,000 cell equivalents) in the presence (+) or absence (−) of RNase A. TRAP products generated from sorted cells on day 0 from 4 different cadaveric marrow (lanes 1 to 8); lane 9, no extract; lane 10, 1 amol M2R8 standard. Arrow indicates the position of the 35-bp amplified internal control.
Telomerase activity in candidate HSC before culture. TRAP products were generated from 2 μL of CHAPS extract (1,000 cell equivalents) in the presence (+) or absence (−) of RNase A. TRAP products generated from sorted cells on day 0 from 4 different cadaveric marrow (lanes 1 to 8); lane 9, no extract; lane 10, 1 amol M2R8 standard. Arrow indicates the position of the 35-bp amplified internal control.
Low levels of telomerase activity were detected in candidate HSC from four different marrow samples (Fig 1). The telomerase levels in the purified candidate HSC varied from 3% to 20% of that generated by 1 amol of M2R8, an oligonucleotide with 8 T2AG3repeats used as a quantitation standard, which translated to an equivalent of 0.06% to 0.4% of the activity of 293 cells when normalized on a per-cell basis.
Telomerase activity in candidate HSC after culture in cytokine combinations of SCF, FL, and IL-3.
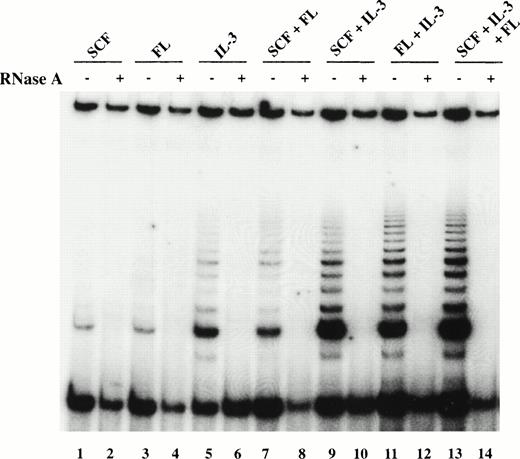
Because telomerase activity was reported to increase upon cellular activation,29 35-37 candidate HSC were cultured in the presence of SCF, IL-3, and FL to examine potential upregulation of telomerase activity. When the purified cells were cultured in SCF or FL alone for 5 days, no upregulation of telomerase activity was observed, whereas IL-3 by itself enhanced telomerase activity (Fig2). Among combinations of two cytokines, those containing IL-3 (SCF + IL-3 or FL + IL-3) were more effective in upregulating telomerase activity than the combination SCF + FL, giving rise to a twofold increase in telomerase activity (Fig 2). Total cell extracts derived from candidate HSC cultured in the presence of SCF + IL-3 + FL also showed enhanced telomerase activity (Fig 2). The relatively low levels of telomerase activity did not appear to result from inhibitory substances to the PCR reaction because the internal control was amplified as expected (Fig 2). Furthermore, mixing extracts from hematopoietic cells with those from 293 cells did not result in any significant decrease in 293 telomerase activity, further indicating that inhibitors of telomerase were unlikely to be present (data not shown).
Telomerase activity in candidate HSC after 5 days of culture. TRAP products using CHAPS extracts of total viable cells derived by culturing purified candidate HSC from BM4 for 5 days in SCF (lanes 1 and 2), FL (lanes 3 and 4), IL-3 (lanes 5 and 6), SCF + FL (lanes 7 and 8), SCF + IL-3 (lanes 9 and 10), FL + IL-3 (lanes 11 and 12), and SCF + FL +
IL-3 (lanes 13 and 14). TRAP products were resolved on a 15% polyacrylamide gel, dried, and exposed to film for 48 hours. The intensity of the signals was analyzed by a densitometer using ImageQuant program and normalized to that of the internal control. Cellular extracts from BM1 and BM3 yielded similar results.
Telomerase activity in candidate HSC after 5 days of culture. TRAP products using CHAPS extracts of total viable cells derived by culturing purified candidate HSC from BM4 for 5 days in SCF (lanes 1 and 2), FL (lanes 3 and 4), IL-3 (lanes 5 and 6), SCF + FL (lanes 7 and 8), SCF + IL-3 (lanes 9 and 10), FL + IL-3 (lanes 11 and 12), and SCF + FL +
IL-3 (lanes 13 and 14). TRAP products were resolved on a 15% polyacrylamide gel, dried, and exposed to film for 48 hours. The intensity of the signals was analyzed by a densitometer using ImageQuant program and normalized to that of the internal control. Cellular extracts from BM1 and BM3 yielded similar results.
Telomerase activity in cultured candidate HSC is restricted to cycling cells.
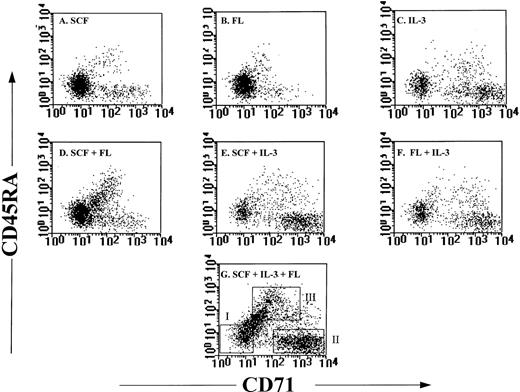
Because telomerase activity was upregulated in cultures containing IL-3, SCF + IL-3, FL + IL-3, and SCF + FL + IL-3, we next investigated whether increased levels of telomerase activity were restricted to cells of a particular phenotype. Flow cytometric analysis of purified candidate HSC after 5 days of culture in the seven different cytokine combinations revealed that in cultures with SCF or FL alone, the majority of cells retained the CD34+CD45RAloCD71lo phenotype (Fig3). On the other hand, in cultures containing IL-3, the percentage of CD34+CD45RAloCD71lo cells decreased to below 50% of total viable cells (Table1), and increased numbers of CD34−, CD34+CD45RAloCD71hi, and CD34+CD45RAhiCD71hi cells were observed (Fig 3, Table 1). Candidate HSC cultured for 5 days in SCF + FL + IL-3 were sorted into CD34−, CD34+, CD34+CD45RAloCD71lo, CD34+CD45RAloCD71hi, and CD34+CD45RAhiCD71hi cells. Semiquantitative analysis of telomerase activity indicated that CD34+ cells expressed fourfold higher levels than those in the CD34− cells (Fig 4).Among the CD34+ cells, most telomerase activity resided in the CD34+CD45RAloCD71hi and CD34+CD45RAhiCD71hipopulations, whereas CD34+CD45RAloCD71lo cells had negligible telomerase activity (Fig 4).
Phenotypic analysis of candidate HSC after culture. Candidate HSC were cultured for 5 days in (A) SCF, (B) FL, (C) IL-3, (D) SCF + FL, (E) SCF + IL-3, (F) FL + IL-3, and (G) SCF + FL + IL-3. After 5 days, cells were stained with antibodies against CD34, CD45RA, and CD71. Profiles shown were from events gated for low PI, low SCC, and high expression of CD34. In (G) boxes I, II, and III represent the windows used to sort for CD34+CD45RAloCD71lo cells, CD34+CD45RAloCD71hi cells, and CD34+CD45RAhiCD71hi cells, respectively. The same windows were applied to Fig 4 and Table 1.
Phenotypic analysis of candidate HSC after culture. Candidate HSC were cultured for 5 days in (A) SCF, (B) FL, (C) IL-3, (D) SCF + FL, (E) SCF + IL-3, (F) FL + IL-3, and (G) SCF + FL + IL-3. After 5 days, cells were stained with antibodies against CD34, CD45RA, and CD71. Profiles shown were from events gated for low PI, low SCC, and high expression of CD34. In (G) boxes I, II, and III represent the windows used to sort for CD34+CD45RAloCD71lo cells, CD34+CD45RAloCD71hi cells, and CD34+CD45RAhiCD71hi cells, respectively. The same windows were applied to Fig 4 and Table 1.
Cytokine Mixtures Used to Stimulate the Proliferation and Differentiation of Candidate HSC
| . | . | . | Percentages of Viable Cells With the Following Phenotype . | |||
|---|---|---|---|---|---|---|
| Cytokine Conditions . | Number of Expts . | Fold Increase* . | 34− . | 34+45lo71lo . | 34+45lo71hi . | 34+45hi71hi . |
| SCF | 3 | 0.27 ± 0.14-151 | 7 ± 4 | 79 ± 6 | 4 ± 2 | 1 ± 1 |
| FL | 2 | 0.21, 0.15-151 | 20, 11 | 62, 66 | 3, 13 | 1, 4 |
| IL-3 | 3 | 0.56 ± 0.17 | 46 ± 14 | 29 ± 15 | 19 ± 6 | 3 ± 2 |
| SCF + FL | 3 | 0.43 ± 0.17 | 9 ± 4 | 56 ± 14 | 14 ± 7 | 12 ± 6 |
| SCF + IL-3 | 4 | 1.48 ± 0.88 | 31 ± 17 | 47 ± 19 | 24 ± 9 | 8 ± 4 |
| FL + IL-3 | 2 | 2.9, 1.9 | 17, 57 | 31, 13 | 33, 22 | 14, 6 |
| SCF + IL-3 + FL | 5 | 1.96 ± 1.05 | 24 ± 14 | 19 ± 9 | 29 ± 10 | 23 ± 9 |
| . | . | . | Percentages of Viable Cells With the Following Phenotype . | |||
|---|---|---|---|---|---|---|
| Cytokine Conditions . | Number of Expts . | Fold Increase* . | 34− . | 34+45lo71lo . | 34+45lo71hi . | 34+45hi71hi . |
| SCF | 3 | 0.27 ± 0.14-151 | 7 ± 4 | 79 ± 6 | 4 ± 2 | 1 ± 1 |
| FL | 2 | 0.21, 0.15-151 | 20, 11 | 62, 66 | 3, 13 | 1, 4 |
| IL-3 | 3 | 0.56 ± 0.17 | 46 ± 14 | 29 ± 15 | 19 ± 6 | 3 ± 2 |
| SCF + FL | 3 | 0.43 ± 0.17 | 9 ± 4 | 56 ± 14 | 14 ± 7 | 12 ± 6 |
| SCF + IL-3 | 4 | 1.48 ± 0.88 | 31 ± 17 | 47 ± 19 | 24 ± 9 | 8 ± 4 |
| FL + IL-3 | 2 | 2.9, 1.9 | 17, 57 | 31, 13 | 33, 22 | 14, 6 |
| SCF + IL-3 + FL | 5 | 1.96 ± 1.05 | 24 ± 14 | 19 ± 9 | 29 ± 10 | 23 ± 9 |
*Represents the fold increase in cell number after 5 days in culture; calculated by dividing the number of viable cells recovered at day 5 by the number of cells seeded on day 0; a value less than 1 indicates a decrease in cell number.
Values represent the mean ± SD for experiments performed three times or more; for experiments performed only twice, both values are reported.
Telomerase activity in subpopulations of cells present after 5 days in cultures of purified candidate HSC from adult marrow. See also Fig 3G. (A) CHAPS extracts from BM4-derived cells (lanes 1 to 10): CD34− fraction (lanes 1 and 2), CD34+fraction (lanes 3 and 4), CD34+CD45RAloCD71lo fraction (lanes 5 and 6), CD34+CD45RAloCD71hi fraction (lanes 7 and 8), and CD34+CD45RAhiCD71hi cells and (lanes 9 and 10); CHAPS extracts from BM1-derived cells (lanes 11 to 14): CD34− fraction (lanes 11 and 12) and CD34+ fraction (lanes 13 and 14). All extracts were generated from 1,000 cells.
Telomerase activity in subpopulations of cells present after 5 days in cultures of purified candidate HSC from adult marrow. See also Fig 3G. (A) CHAPS extracts from BM4-derived cells (lanes 1 to 10): CD34− fraction (lanes 1 and 2), CD34+fraction (lanes 3 and 4), CD34+CD45RAloCD71lo fraction (lanes 5 and 6), CD34+CD45RAloCD71hi fraction (lanes 7 and 8), and CD34+CD45RAhiCD71hi cells and (lanes 9 and 10); CHAPS extracts from BM1-derived cells (lanes 11 to 14): CD34− fraction (lanes 11 and 12) and CD34+ fraction (lanes 13 and 14). All extracts were generated from 1,000 cells.
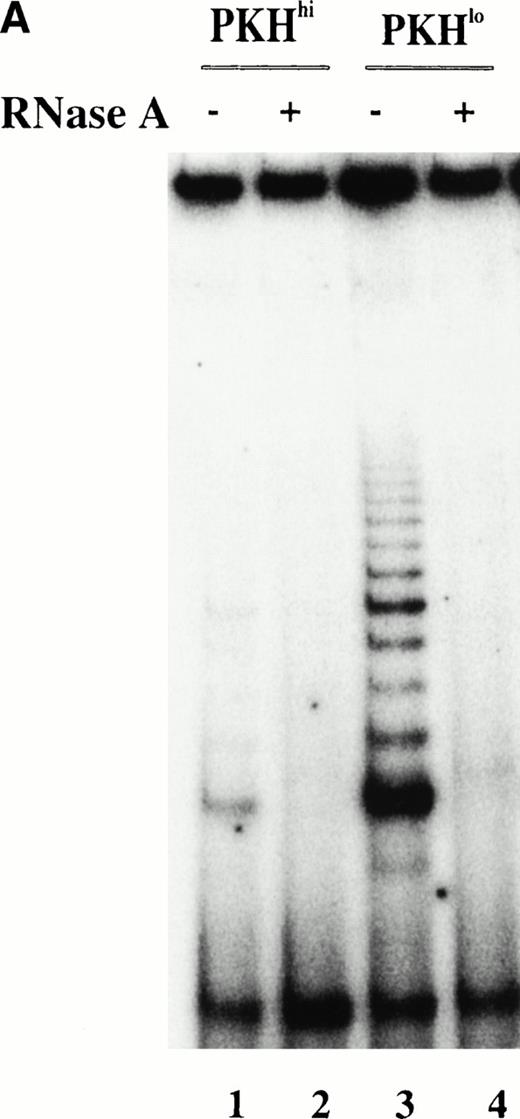
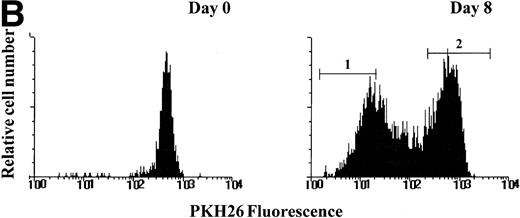
To further investigate the relationship between telomerase activity and cell proliferation in hematopoietic progenitors, we used PKH26 to track cell divisions at the level of single cells. Sorted candidate HSC were labeled with PKH26, a fluorescent dye that stably incorporates into the lipid bilayer and is diluted among the daughter cells with each successive cell division. After 8 days of culture in SCF + FL + IL-3, cells were sorted into PKHhi and PKHlofractions (Fig 5A). Telomerase activity in PKHlo cells was 5 to 10 times higher than the ones in PKHhi cells (Fig 5B), indicating that viable cells that remained quiescent in cytokine-stimulated cultures did not express detectable levels of telomerase.
PKH26 staining of purified candidate HSC before and after culture in SCF + IL-3 + FL. (A) PKH26 was incorporated into the lipid bilayer of purified candidate HSC at day 0. On day 8, PKH26 intensity was analyzed again and cells were sorted into PKHlo (gate1) and PKHhigh fractions (gate2) for TRAP. The cells in the PKHlo fraction have undergone several rounds of division, resulting in diminished dye fluorescence. (B) Telomerase activity in PKHhi and PKHlocells. PKHhi and PKHlo cells were sorted from BM1 after 8 days of culturing SCC in SCF + FL + IL-3. TRAP assay was performed on CHAPS extracts equivalent to 1,000 cells in the absence (−) and presence (+) of RNase A.
PKH26 staining of purified candidate HSC before and after culture in SCF + IL-3 + FL. (A) PKH26 was incorporated into the lipid bilayer of purified candidate HSC at day 0. On day 8, PKH26 intensity was analyzed again and cells were sorted into PKHlo (gate1) and PKHhigh fractions (gate2) for TRAP. The cells in the PKHlo fraction have undergone several rounds of division, resulting in diminished dye fluorescence. (B) Telomerase activity in PKHhi and PKHlocells. PKHhi and PKHlo cells were sorted from BM1 after 8 days of culturing SCC in SCF + FL + IL-3. TRAP assay was performed on CHAPS extracts equivalent to 1,000 cells in the absence (−) and presence (+) of RNase A.
Telomerase activity in fetal liver CD34+CD38− cells.
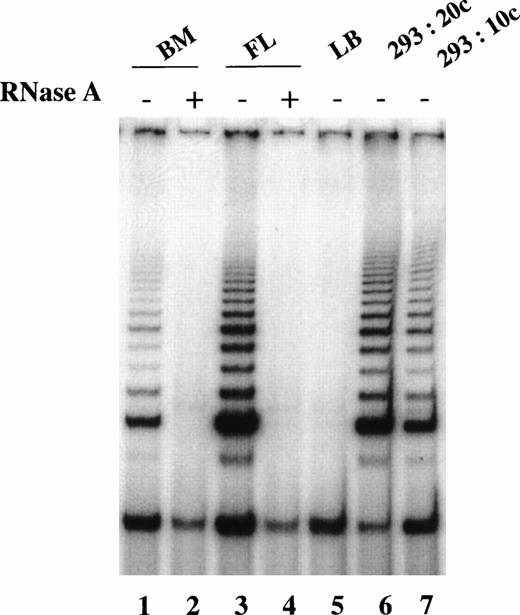
Although telomerase activity was upregulated in 5-day cultures of adult marrow candidate HSC stimulated by SCF, IL-3, and FL, the activity was found to reside predominantly in the proliferating cells containing mainly committed progenitors. To address the question of whether cycling stem cells express telomerase activity, we examined telomerase expression in CD34+CD38− candidate HSC from fetal liver. Higher telomerase activity was detected in the fetal liver CD34+CD38− cells than those from adult bone marrow (Fig 6). Pooled data from three different samples showed that the activity in fetal liver CD34+CD38− cells ranged from 0.5% to 1.5% of that found in 293 cells, whereas in adult bone marrow the activity was at most 0.3% of that present in 293 cells. These results suggest that either all or a subfraction of cycling fetal liver stem cells express telomerase activity.
Comparison of telomerase activity in freshly isolated CD34+CD38− cells from fetal liver and adult marrow. TRAP assay performed on CHAPS extracts equivalent to 1,000 cells in the absence (−) and presence (+) of RNase A. CD34+CD38− cells from adult marrow (lanes 1 and 2) and fetal liver (lanes 3 and 4). Lysis buffer (LB) served as the negative control (lane 5), whereas 293 cells at the equivalent of 20 cells (lane 6) and 10 cells (lane 7) served as the positive controls.
Comparison of telomerase activity in freshly isolated CD34+CD38− cells from fetal liver and adult marrow. TRAP assay performed on CHAPS extracts equivalent to 1,000 cells in the absence (−) and presence (+) of RNase A. CD34+CD38− cells from adult marrow (lanes 1 and 2) and fetal liver (lanes 3 and 4). Lysis buffer (LB) served as the negative control (lane 5), whereas 293 cells at the equivalent of 20 cells (lane 6) and 10 cells (lane 7) served as the positive controls.
DISCUSSION
The ability to induce or enhance telomerase activity may be important in maintaining the replicative potential of normal stem cells found in self-regenerating tissues such as those of the hematopoietic system.28,29 The studies reported here were aimed to investigate telomerase expression in the most primitive hematopoietic cells in humans. We confirmed our previous results regarding the low level of telomerase activity in freshly isolated “candidate” HSC with the CD34+CD45RAloCD71lophenotype from adult marrow.28 These sorted cells are highly enriched (several hundred-fold when compared with unpurified cells) in long-term culture-initiating cells (LTC-IC), arguably the best in vitro assay for human stem cells.38,39Unfortunately, most bone marrow cells with a CD34+CD45RAloCD71lo phenotype are unable to initiate long-term cultures, and rare committed progenitors from the adult bone marrow also share this phenotype.32 The latter, which are actively proliferating, could contribute partially or completely to the low but readily detectable levels of telomerase in purified candidate HSC before culture.
In the present study, we found that the level of telomerase activity in CD34+CD45RAloCD71lo cells sorted from cytokine-stimulated cultures was reduced as compared with that in freshly isolated cells with the same phenotype. On the other hand, telomerase activity was increased in cultured candidate HSC concomitantly with the upregulation of CD45RA and CD71 expression. By sorting various subpopulations after stimulation with SCF, FL, and IL-3 for 5 days, we found that telomerase activity was mainly confined to CD34+CD45RAloCD71hi and CD34+CD45RAhiCD71hi cells, which are known to be enriched in cycling progenitors committed to differentiate into the erythroid and myeloid lineages, respectively.32 In addition, tracking cellular division by PKH fluorescence confirmed that telomerase activity was confined to cells that had proliferated in culture. Cells with a CD34+CD45RAloCD71lo phenotype present after 5 days in cytokine culture could represent a population that failed to respond to SCF, FL, and IL-3 stimulation and remained quiescent or exited from the cell cycle. In both cases, telomerase activity is expected to be low in view of the data describing upregulation of telomerase activity upon entry into the cell cycle.35-37 40
Because cycling stem cells are extremely rare in the adult bone marrow,30,33 and because our culture conditions are unable to induce the selective self-renewal of adult bone marrow candidate stem cells, we next examined telomerase expression in CD34+CD38− cells from fetal liver. It has been shown that the proliferative potential of hematopoietic cells changes during ontogeny and that candidate HSC from fetal liver contain a very high proportion of cycling cells as compared with candidate HSC from bone marrow.33 Our finding that CD34+CD38− fetal liver cells express readily detectable levels of telomerase activity strongly suggests that such cycling candidate HSC express telomerase activity. In view of the loss of telomerase DNA in hematopoietic cells with age,6 this observation can be explained by assuming that the measurable telomerase activity is not preventing the overall telomere shortening in candidate HSC.31 Alternatively, telomerase could be expressed in only a proportion of the cells. If the latter hypothesis is correct, then identification and selective expansion of such telomerase positive clones could be useful in transplantation and gene transfer protocols because their progeny would possibly maintain a high proliferative potential. To address this issue, more information about telomerase expression in single CD34+CD38− cells from fetal liver and the in vivo of the telomerase levels detected by telomerase assays is urgently needed. Antibodies to the telomerase reverse transcriptase protein15 16 could possibly be used to address this issue.
In our study we found that in day-5 cultures, CD34+ cells expressed higher telomerase activity than CD34− cells. One possible explanation for this observation is that as CD34+ differentiate, their telomerase activity is downregulated together with their proliferative potential. Similarly, freshly isolated CD34− cells from adult marrow also have lower telomerase activity compared with CD34+71+ cells, and when the latter were placed in culture over a period of 10 days, their telomerase activity declined.28 One recent report also shows that leukemic cell lines lose telomerase activity when induced to differentiate.40 41
In a couple of transgenic mouse models, the reactivation of telomerase activity was correlated with tumorigenesis.42,43 However, the role of telomerase in normal somatic cells is still largely unknown. Telomerase activity is expressed in germline cells and is required to maintain telomere length and preserve the unlimited proliferative potential of these cells.44 In the hematopoietic system and skin epidermis,45,46 two other examples of self-renewing tissues, low levels of telomerase activity have been found. Possibly this activity could extend the proliferative potential of the stem cells in these tissues to a certain extent but not sufficient to confer immortality as telomeric DNA is still lost upon replication.47 In a similar manner, peripheral blood lymphocytes express low telomerase activity that is upregulated upon activation.29,35-37 Although the levels of telomerase activity once again appear insufficient to override telomeric decline, the enzyme activity could reduce the loss of telomeric DNA to allow repeated clonal expansion of immune cells upon antigenic stimulation.29
In a recent study, telomerase activity in murine hematopoietic “candidate” stem cells and various progenitors was assayed on a single-cell basis and found to be associated with “self-renewal” potential, with lower levels in committed progenitors than in their pluripotent precursors.48 In contrast, we and others have consistently detected higher telomerase levels in committed progenitors relative to those observed in “candidate” stem cells using human hematopoietic tissues27-29 and this study. This discrepancy suggests that telomerase expression is regulated differently in murine versus human hematopoietic cells, a phenomenon that was previously observed with other cell types from these two species.43 49
Because to date telomere length measurements are typically based on bulk DNA analysis using Southern hybridization, subtle changes in telomeric length on individual chromosomes could escape detection. This notion was recently confirmed in studies of cells from telomerase RNA knockout mice.44 On the basis of the distribution of telomere length in individual chromosomes of cultured hematopoietic cells using fluorescent in situ hybridization analysis, we proposed that telomerase in these cells may preferentially act on short telomeres to maintain a minimum number of repeats.50 If this is the case, the question of what telomere parameter, if any, is restricting the proliferative potential of adult hematopoietic cells becomes more pertinent. Possibly the low levels of telomerase that we measured are able to maintain the length of some but not many short telomeres. In this model, telomerase allows for a limited extension of the replicative lifespan of HSC. Studies in this general area using in situ hybridization to measure telomere length on individual chromosomes of clonally propagated hematopoietic cells in combination with assays of telomerase activity and protein expression should further clarify the role of telomerase in hematopoietic cells.
ACKNOWLEDGMENT
We thank Dr Nam Woo Kim (Geron Corperation) for generously sharing details on the modified TRAP assay before publication. Dr Mark Zijlmans is thanked for providing the fetal liver cells and Gayle Thornbury and Wieslawa Dragowska are thanked for their expertise in cell sorting.
Supported by National Institutes of Health Grant Nos. AI29524 and GM56162 and by a grant from Geron Corporation.
Address reprint requests to Peter M. Lansdorp, MD, PhD, Terry Fox Laboratory, British Columbia Cancer Agency, 601 W 10th Ave, Vancouver, BC, V5Z 1L3 Canada.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal