Abstract
Interleukin-18 (IL-18) is a costimulatory factor for interferonγ (IFNγ) production. Processing of pro–IL-18 by IL-1β–converting enzyme (ICE) leads to the release of bioactive IL-18. Compared with wild-type (WT) mice, splenocytes from ICE-deficient mice produced low IFNγ after lipopolysaccharide (LPS) or zymosan (50% and 80% reduction). In contrast, IFNγ production was unimpaired in ICE-deficient mice using Concanavalin A (Con A). Comparable results were obtained when endogenous IL-18 was blocked with a neutralizing antibody. LPS-induced IFNγ was also reduced by an ICE inhibitor. Exogenous IL-18 augmented zymosan-induced IFNγ production in WT mice. In ICE-deficient cells, IFNγ production was only partially restored by IL-18. The reduced levels of IFNγ in ICE-deficient mice were not due to a lack of IL-12, because zymosan induced IL-12 equally in WT and in ICE-deficient mice. IFNγ is an important regulator of cell proliferation. In accordance, splenocytes from ICE-deficient mice proliferated more when stimulated with LPS, but not with Con A. Furthermore, in ovalbumin-sensitized ICE-deficient mice, proliferation of lymph node cells in response to the specific antigen was not altered. Exogenous IFNγ inhibited, whereas blockade of endogenous IFNγ or IL-18 increased, LPS induced splenocyte proliferation both in WT and in ICE-deficient mice. Our results show that IL-18 is an IL-12–independent regulator of IFNγ production and of cell proliferation induced by microbial stimuli. However, ICE-dependent processing of IL-18 is not needed for response to mitogens or antigens.
INTERFERON γ (IFNγ)-inducing factor (IGIF or interleukin-18 [IL-18]) is a recently characterized cytokine that acts as a costimulatory factor for the production of IFNγ. IL-18 was initially purified and subsequently cloned from the liver of mice conditioned with Propionibacterium acnes and challenged with lipopolysaccharide (LPS);1,2 cloning of the human molecule has also been recently described.3 A critical role for IL-18 in LPS-induced toxicity has been shown. Anti–IL-18 antibodies protect P acnes-conditioned mice from liver injury after LPS.2 In addition, a role for IL-18 in the pathogenesis of insulin-dependent diabetes mellitus has recently been proposed.4
Similar to another IFNγ-inducing factor, IL-12, IL-18 is produced by monocytes/macrophages, but not by B or T cells.3 However, IL-18 induction of IFNγ is independent of IL-12 production. In fact, anti–IL-12 antibodies do not inhibit the increase in anti–CD3-stimulated IFNγ production induced by IL-18.2In addition to acting as a costimulus for IFNγ production, IL-18 enhances the production of granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-2,5 potentiates anti–CD3-induced T-cell proliferation,5 and increases Fas-mediated killing of natural killer (NK) cells by augmenting the expression of Fas ligand.6 However, unlike IL-12, IL-18 by itself, in the absence of another stimulus, is not a strong inducer of IFNγ.3
IL-18 is structurally related to IL-1β, with both cytokines having a unique, all-β–pleated structure.7 Also, similar to IL-1β, IL-18 is synthesized as a precursor lacking a typical signal peptide.3 Pro–IL-18, as pro–IL-1β, is devoid of biological activity and precursor amino acids must be cleaved to produce an active molecule.3 IL-1β–converting enzyme (ICE; caspase-1), which cleaves pro–IL-1β , also cleaves pro–IL-18 at aspartic acid in the P1 position, producing a mature, bioactive peptide that is readily released from the cell.8,9 When ICE-deficient mice are injected with LPS, with or without a preconditioning with P acnes, only low levels of IFNγ are detectable in the circulation compared with wild-type (WT) mice.8,9 The injection of IL-18 restores the LPS-induced IFNγ levels in ICE-deficient mice,8 supporting the concept that ICE is actually involved in the production of active IL-18.
To date, the role of endogenous IL-18 in the induction of IFNγ after stimuli other than LPS remains unknown. To help elucidate the role of IL-18 in the regulation of IFNγ production in response to various stimuli, we studied the in vitro production of IFNγ in splenocytes from ICE-deficient mice using two inflammatory stimuli, LPS and zymosan, and compared it with the response to an immune stimulus, the mitogen Concanavalin A (Con A). In addition, because IFNγ is an important factor regulating cell proliferation,10 11 we investigated the response of splenocytes obtained from WT and ICE-deficient mice to mitogenic concentrations of LPS and Con A, as well as to specific antigen.
MATERIALS AND METHODS
Materials.
LPS (a phenol-extracted preparation from Escherichia coli055:B5), zymosan, Con A, ovalbumin, and phenazine methosulfate (PMS) were purchased from Sigma Chemical Co (St Louis, MO); RPMI was from Cellgro (Waukesha, WI); fetal bovine serum (FBS) was from GIBCO (Pascagoula, MS); MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium, inner salt] was from Promega (Madison, WI); the reversible ICE inhibitor Ac-Tyr-Val-Ala-Asp-CHO was from Alexis (San Diego, CA); rat recombinant IFNγ was from Genentech (South San Francisco, CA); human recombinant IL-1Ra was a kind gift from Dr Daniel Tracey (Upjohn, Kalamazoo, MI); murine recombinant IL-18 was a kind gift from Dr Y. Stabinsky (Peprotech, Inc, Princeton, NJ). The anti–IL-18 antiserum was obtained from a New Zealand rabbit immunized by intradermal injection of murine recombinant IL-18 (Peprotech) in the presence of Hunter's Titermax adjuvant. After several booster injections, blood was collected and serum obtained. The anti-IFNγ monoclonal antibody (XMG1.2) was from Endogen Inc (Woburn, MA).
Animals.
Isolation and culture of spleen and lymph node cells.
Spleens were aseptically removed and cell suspensions were prepared according to standard procedures.14 Cells were washed twice and resuspended in RPMI supplemented with 10% FBS. For cytokine measurement, spleen cells were cultured at 5 × 106/mL in 24-well, flat-bottom culture plates in the presence or absence of various concentrations of LPS, zymosan, or Con A. When zymosan was the stimulus, 1% human serum was added to the culture to opsonize zymosan particles. Cultures were incubated at 37°C in a humidified atmosphere with 5% CO2. At the end of the incubation period, cultures were frozen at −70°C and subjected to 3 freeze-thaw cycles to obtain total cytokine levels. Before assaying, samples were centrifuged for 10 minutes at 10,000g to remove debris.
For proliferation assays, spleen cells were cultured in triplicate wells at 2.5 × 106/mL in 96-well, flat-bottom microtiter plates with increasing concentrations of either LPS (4, 20, and 100 μg/mL) or Con A (1, 3, and 10 μg/mL). Proliferation was measured using the MTS/PMS method, as previously described.15
For the evaluation of the proliferative response after ovalbumin, mice were injected at the base of the tail with a suspension of 50 mg of OVA in 100 μL complete Freund adjuvant (CFA). Fourteen days later, the draining abdominal periaortic lymph nodes were removed, cell suspensions were prepared, and cells were cultured at 2.5 × 106/mL in 96-well, flat-bottom microtiter plates with increasing concentration of ovalbumin (50, 170, and 500 μg/mL).
Reverse transcription-polymerase chain reaction (RT-PCR).
Total RNA was extracted from spleen cells as previously described.16 One microgram of RNA was reverse transcribed using random hexamer primers (Perkin Elmer, Norwalk, CT) in a thermocycler (42°C for 30 minutes and 99°C for 5 minutes) and the cDNA amplification was performed as previously decribed for 30 cycles for cytokine expression and 24 cycles for GAPDH.16Oligonucleotide primers were as follows: p40 forward, 5′-CGTGCTATGGCTGGTGCAAAG-3′; p40 reverse, 5′-GAACACATGCCCACTTGCTG-3′; p35 forward, 5′-ACCAGCACATTGAAGACCTG-3′; p35 reverse, 5′-GACTGCATCAGCTCATCGAT-3′; GAPDH forward, 5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′; and GAPDH reverse, 5′-CATGTGGGCCATGAGGTCCACCAC-3′. The annealing temperature was 60°C. Products of PCR amplifications were separated in a 2.0% agarose gel (Sigma) containing ethidium bromide (0.5 μg/mL) and 0.5× Tris-Borate-EDTA buffer (Fisher Scientific, Pittsburgh, PA). PCR amplification products were illuminated with UV light and a negative image photograph taken (Polaroid type 55 film; Polaroid, Cambridge, MA). The photographs were scanned on a densitometer using ImageQuant software (Molecular Dynamics, Sunnyvale, CA). Data are presented as the ratio of cytokine densitometric units to the GAPDH units for each condition.
Cytokine measurement.
IFNγ was measured with an enzyme-linked immunosorbent assay (ELISA) kit, kindly provided by Endogen, Inc. Total and p70 IL-12 were measured with ELISA kits kindly provided by Genzyme Corp (Cambridge, MA).
RESULTS
Inhibition of ICE activity reduces LPS-induced IFNγ production.
Splenocytes from WT mice were cultured for 24 hours with LPS (1μg/mL) in the presence or absence of an ICE inhibitor (20 μmol/L) or of IL-1Ra (10 μg/mL). As shown in Fig 1, the ICE inhibitor reduced IFNγ production by 70% (P < .01), whereas IL-1Ra had no significant effect.
An ICE inhibitor, but not IL-1Ra, reduces LPS-induced IFNγ production. Splenocytes from WT mice were incubated with LPS alone (1 μg/mL) or with LPS the presence of either IL-1Ra (10 μg/mL) or of an ICE inhibitor (20 μmol/L). IFNγ levels were measured 24 hours later. Data are the mean ± SEM of 6 mice per group. **P < .01 versus LPS alone by ANOVA for repeated measures.
An ICE inhibitor, but not IL-1Ra, reduces LPS-induced IFNγ production. Splenocytes from WT mice were incubated with LPS alone (1 μg/mL) or with LPS the presence of either IL-1Ra (10 μg/mL) or of an ICE inhibitor (20 μmol/L). IFNγ levels were measured 24 hours later. Data are the mean ± SEM of 6 mice per group. **P < .01 versus LPS alone by ANOVA for repeated measures.
Reduced production of IFNγ in ICE-deficient mice.
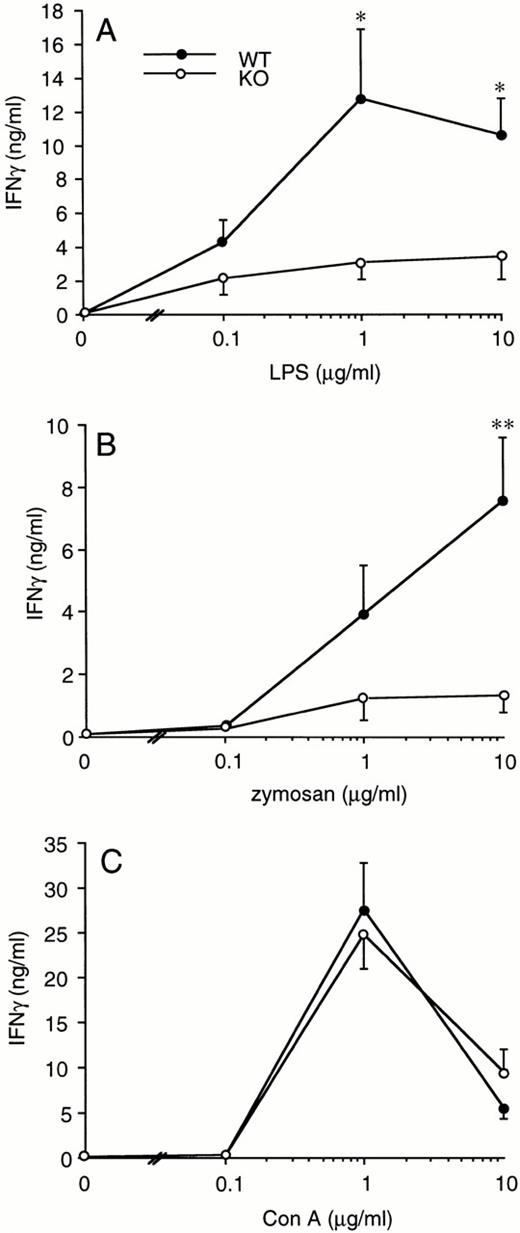
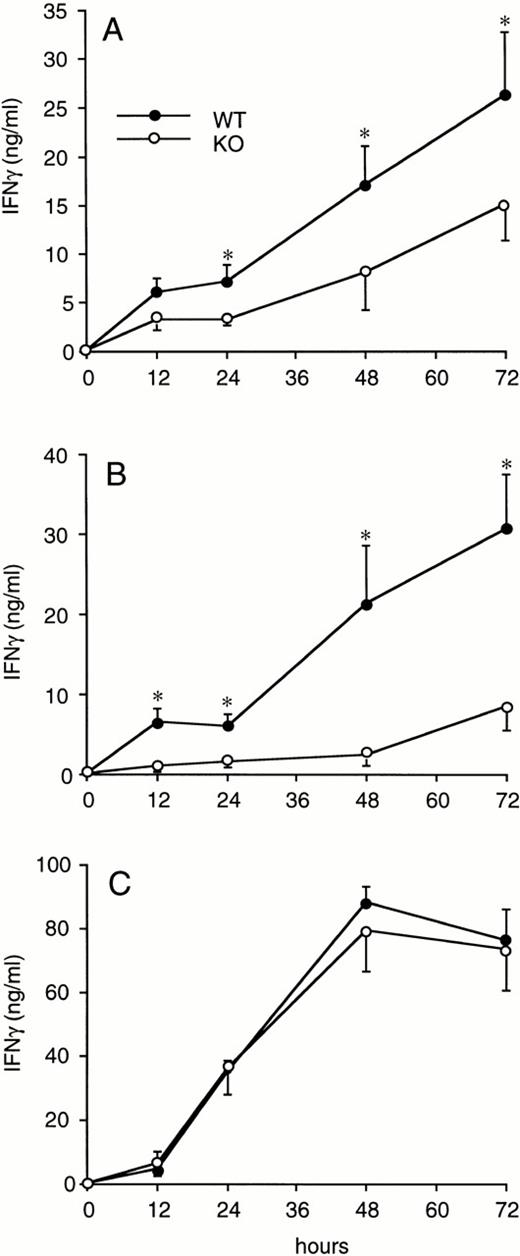
Splenocytes obtained from WT or ICE-deficient mice were cultured in vitro for 24 hours in the presence of various concentrations of LPS, zymosan, or Con A. Significantly lower levels of IFNγ were produced in cultures from ICE-deficient splenocytes stimulated with either LPS (Fig 2A) or zymosan (Fig 2B). However, when Con A was used as a stimulus, the production of IFNγ in ICE-deficient mice did not differ from that observed in WT mice (Fig 2C). These differences between WT and ICE-deficient mice were observed at each time point over 72 hours of culture. As shown in Fig 3A, significantly lower levels of IFNγ were found in cultures of ICE-deficient splenocytes stimulated with LPS for 24, 48, or 72 hours. Markedly reduced levels of IFNγ were also measured from ICE-deficient splenocytes stimulated with zymosan for 12, 24, 48, or 72 hours (Fig 3B). In contrast, no differences between WT and ICE-deficient mice were observed at any time point when the cells were stimulated with Con A (Fig 3C).
IFNγ production in splenocytes from WT and ICE-deficient mice. Splenocytes from WT (•) or ICE-deficient (○) mice were incubated with the indicated concentration of LPS (A), zymosan (B), or Con A (C) for 24 hours and levels of IFNγ measured. Data are the mean ± SEM of 9 mice per group. *P < .05; **P < .01 versus WT by factorial ANOVA.
IFNγ production in splenocytes from WT and ICE-deficient mice. Splenocytes from WT (•) or ICE-deficient (○) mice were incubated with the indicated concentration of LPS (A), zymosan (B), or Con A (C) for 24 hours and levels of IFNγ measured. Data are the mean ± SEM of 9 mice per group. *P < .05; **P < .01 versus WT by factorial ANOVA.
Time course of IFNγ production in splenocytes from WT and ICE-deficient mice. Splenocytes from WT (•) or ICE-deficient (○) mice were incubated for the indicated times with 1 μg/mL of LPS (A), 10 μg/mL of zymosan (B), or 1 μg/mL of ConA (C) and levels of IFNγ were measured. Data are the mean ± SEM of 6 mice per group. *P < .05 versus WT by factorial ANOVA.
Time course of IFNγ production in splenocytes from WT and ICE-deficient mice. Splenocytes from WT (•) or ICE-deficient (○) mice were incubated for the indicated times with 1 μg/mL of LPS (A), 10 μg/mL of zymosan (B), or 1 μg/mL of ConA (C) and levels of IFNγ were measured. Data are the mean ± SEM of 6 mice per group. *P < .05 versus WT by factorial ANOVA.
Because the most striking differences between WT and ICE-deficient mice for IFNγ production were observed after zymosan, this stimulus was chosen for further investigation.
Effect of blockade of endogenous IL-18 on IFNγ production.
Splenocytes from WT mice were incubated for 24 or 72 hours with LPS (1 μg/mL), zymosan (10 μg/mL), or Con A (1 μg/mL) in the presence of different dilutions of anti–IL-18 antiserum. As shown in Table 1, blockade of endogenous IL-18 significantly reduced LPS- and zymosan-induced IFNγ at both 24 and 72 hours, but did not significantly alter IFNγ production when Con A was the stimulus. At a 1:100 dilution of anti–IL-18 antiserum, 24-hour LPS-induced IFNγ was inhibited by 83%, whereas zymosan-induced IFNγ was inhibited by 66% compared with controls. The effect of the anti–IL-18 antiserum was even more evident in the 72-hour culture. At this time point, a 1:100 dilution of antiserum reduced LPS-induced IFNγ production by 90% and zymosan-induced IFNγ levels by 75%. Addition of normal rabbit serum did not significanlty alter IFNγ production after any of the three stimuli used, thus ruling out a possible nonspecific effect of rabbit serum on IFNγ levels (data not shown). These data confirm the results obtained in ICE-deficient mice and show that endogenous IL-18 actually plays a critical role in the induction of IFNγ after LPS or zymosan.
Effect of IL-18 Blockade on IFNγ Production After Stimulation With LPS, Zymosan, or Con A
| . | No Antiserum . | Anti–IL-18 1:400 . | Anti–IL-18 1:200 . | Anti–IL-18 1:100 . |
|---|---|---|---|---|
| 24 hours | ||||
| LPS | 12.99 ± 1.40 | 6.52 ± 3.55-150 | 3.84 ± 2.36-151 | 2.18 ± 1.22-151 |
| Zymosan | 10.89 ± 1.71 | 9.82 ± 2.74 | 5.11 ± 1.86-150 | 3.74 ± 1.53-150 |
| Con A | 48.30 ± 6.03 | 45.85 ± 7.23 | 47.26 ± 7.57 | 42.65 ± 6.81 |
| 72 hours | ||||
| LPS | 22.60 ± 7.50 | 5.00 ± 1.95-151 | 3.40 ± 1.85-151 | 2.35 ± 1.45-151 |
| Zymosan | 17.58 ± 2.84 | 5.27 ± 0.61-151 | 6.50 ± 3.17-151 | 4.34 ± 1.60-151 |
| . | No Antiserum . | Anti–IL-18 1:400 . | Anti–IL-18 1:200 . | Anti–IL-18 1:100 . |
|---|---|---|---|---|
| 24 hours | ||||
| LPS | 12.99 ± 1.40 | 6.52 ± 3.55-150 | 3.84 ± 2.36-151 | 2.18 ± 1.22-151 |
| Zymosan | 10.89 ± 1.71 | 9.82 ± 2.74 | 5.11 ± 1.86-150 | 3.74 ± 1.53-150 |
| Con A | 48.30 ± 6.03 | 45.85 ± 7.23 | 47.26 ± 7.57 | 42.65 ± 6.81 |
| 72 hours | ||||
| LPS | 22.60 ± 7.50 | 5.00 ± 1.95-151 | 3.40 ± 1.85-151 | 2.35 ± 1.45-151 |
| Zymosan | 17.58 ± 2.84 | 5.27 ± 0.61-151 | 6.50 ± 3.17-151 | 4.34 ± 1.60-151 |
Splenocytes from WT mice were incubated with LPS (1 μg/mL), zymosan (10 μg/mL), or Con A (1 μg/mL) for 24 or 72 hours in the presence or absence of rabbit antimurine IL-18 antiserum at the indicated dilutions. Addition to the cultures of normal rabbit serum at the same concentrations did not significantly alter IFNγ production (data not shown). IFNγ levels were measured by ELISA. Data are the mean ± SEM of 3 mice per group.
P < .05.
P < .01 versus respective “No Antiserum” by ANOVA for repeated measures.
Induction of IL-12 in ICE-deficient mice.
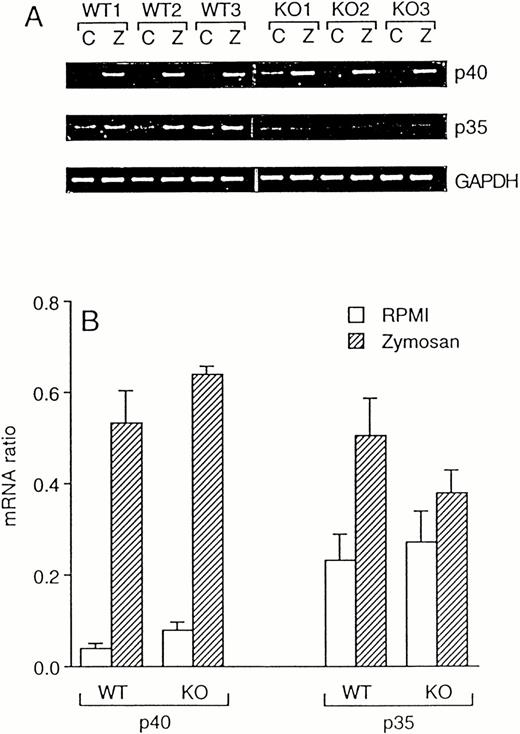
Because IL-12 is a potent inducer of IFNγ,17 we investigated whether the reduced levels of IFNγ in ICE-deficient mice are due to a deficient production of this cytokine. However, despite an 80% reduced IFNγ production in zymosan-stimulated ICE-deficient spleen cells, steady-state mRNA expression for both the p40 and the p35 subunits of IL-12 was induced equally in WT and in ICE-deficient mice (Fig 4). At the protein level, no differences were observed between WT and ICE-deficient mice for production of the active p70 heterodimer 24 hours after stimulation with zymosan (9.68 ± 1.39 and 10.22 ± 1.70 pg/mL in WT and ICE-deficient mice, respectively). However, levels of zymosan-stimulated total IL-12 (as assessed by an ELISA kit that measures p40 monomer, p40 homodimer, and p70 heterodimer) were higher in ICE-deficient mice compared with WT (496.72 ± 42.58 and 879.09 ± 88.46 pg/mL in WT and ICE-deficient mice, respectively; P< .01 by Student's t-test).
Zymosan-induced IL-12 mRNA in WT and ICE-deficient mice. Splenocytes from WT and ICE-deficient mice were incubated for 4 hours with RPMI or 10 μg/mL of zymosan. RT-PCR was performed for the p40 and p35 subunits of IL-12. Minor nonspecific amplicons were noted when p40 was amplified. GAPDH was used as an internal control. Amplification products for p40, p35, and GAPDH for 3 WT and 3 KO mice are shown in (A) (C, RPMI; Z, Zymosan). (B) shows mRNA ratios (mean ± SEM, n = 3).
Zymosan-induced IL-12 mRNA in WT and ICE-deficient mice. Splenocytes from WT and ICE-deficient mice were incubated for 4 hours with RPMI or 10 μg/mL of zymosan. RT-PCR was performed for the p40 and p35 subunits of IL-12. Minor nonspecific amplicons were noted when p40 was amplified. GAPDH was used as an internal control. Amplification products for p40, p35, and GAPDH for 3 WT and 3 KO mice are shown in (A) (C, RPMI; Z, Zymosan). (B) shows mRNA ratios (mean ± SEM, n = 3).
Effect of IL-18 on IFNγ production.
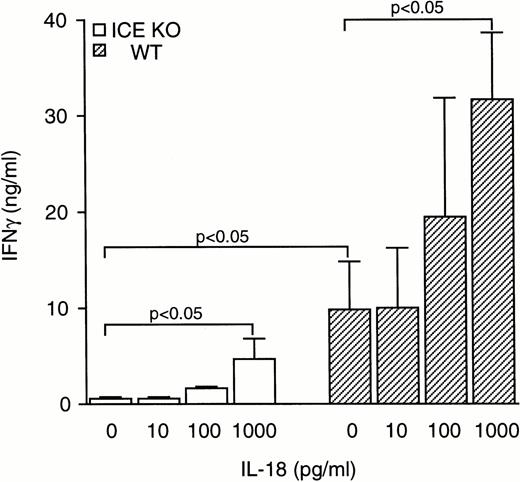
To assess whether the reduced levels of IFNγ observed in ICE-deficient mice could be normalized by addition of exogenous IL-18, splenocytes were stimulated with zymosan in the presence of increasing concentrations of IL-18. As shown in Fig 5, IL-18 increased zymosan-induced IFNγ production both in WT and in ICE-deficient mice. At 1 ng/mL, IL-18 added to cultures of ICE-deficient splenocytes restored IFNγ production to levels no longer significantly different from those observed in WT cells stimulated with zymosan alone. However, this concentration of IL-18 markedly increased IFNγ production in WT mice. At 1 ng/mL IL-18, a ninefold and a threefold increase in IFNγ production was observed in ICE-deficient and in WT mice, respectively. The difference in IFNγ production between WT and ICE-deficient mice could not be narrowed even when higher concentrations of IL-18 were used. At 100 ng/mL of IL-18, splenocytes from ICE-deficient mice produced 29.05 ± 10.06 ng/mL of IFNγ, whereas cells from WT mice produced 59.86 ± 9.85 ng/mL of IFNγ. Although the augmentation in IFNγ production using 100 ng/mL of IL-18 in ICE-deficient splenocytes represents a 56-fold increase over zymosan alone, the total IFNγ production was still 50% lower than that observed in WT splenocytes.
Effect of IL-18 on IFNγ production. Splenocytes from WT (▨) or ICE-deficient (□) mice were incubated for 24 hours with 10 μg/mL of zymosan in the presence of increasing concentrations of IL-18 and levels of IFNγ were measured. Data are the mean ± SEM of 5 mice per group. The unpaired Student's t-test was used for comparison between WT and ICE-deficient mice, whereas for comparisons within a group, ANOVA for repeated measures was used.
Effect of IL-18 on IFNγ production. Splenocytes from WT (▨) or ICE-deficient (□) mice were incubated for 24 hours with 10 μg/mL of zymosan in the presence of increasing concentrations of IL-18 and levels of IFNγ were measured. Data are the mean ± SEM of 5 mice per group. The unpaired Student's t-test was used for comparison between WT and ICE-deficient mice, whereas for comparisons within a group, ANOVA for repeated measures was used.
These findings may reflect the lack of an additional factor involved in IFNγ production in ICE-deficient mice. However, zymosan-induced IFNγ production was not suppressed in splenocytes obtained from IL-1β–deficient mice (4.71 ± 2.51 ng/mL and 5.93 ± 1.93 ng/mL in WT and IL-1β–deficient mice, respectively, with 1 μg/mL of zymosan). Therefore, a lack of IL-1β release in splenocytes from ICE-deficient mice does not account for the reduced IFNγ production after zymosan stimulation.
Mitogenic responses in ICE-deficient mice.
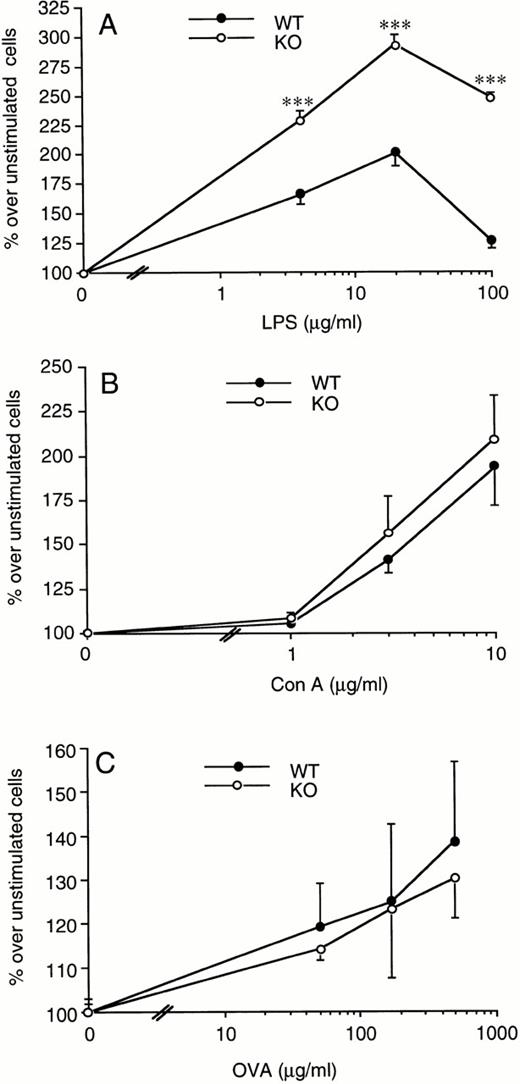
Splenocytes from WT or ICE-deficient mice were incubated with increasing concentrations of LPS and proliferation assessed after 72 hours. We consistently observed a greater proliferation rate in cells obtained from ICE-deficient compared with WT mice at each of the LPS concentrations tested (Fig 6A). The increase in spleen cell proliferation in ICE-deficient mice ranged from 10% to 60%, depending on the experiment and the dose of LPS used. On the other hand, when Con A was used as a mitogen, no significant difference was observed between WT and ICE-deficient mice (Fig 6B). Comparable results were obtained when LPS- or Con A-induced proliferation was measured at either 48 or 96 hours (data not shown).
Increased LPS-induced spleen cell proliferation in ICE-deficient mice. Splenocytes from WT (•) or ICE-deficient (○) mice were incubated for 72 hours with the indicated concentrations of LPS (A) or Con A (B) and proliferation was assessed. Lymph node cells from WT and ICE-deficient mice immunized 14 days before with ovalbumin were incubated for 72 hours with the indicated concentrations of ovalbumin and proliferation was assessed (C). Data are the mean ± SEM of 6 mice per group and are expressed as the percentage of change in MTS absorbance compared with unstimulated cells (100%). ***P< .001 versus WT by factorial ANOVA.
Increased LPS-induced spleen cell proliferation in ICE-deficient mice. Splenocytes from WT (•) or ICE-deficient (○) mice were incubated for 72 hours with the indicated concentrations of LPS (A) or Con A (B) and proliferation was assessed. Lymph node cells from WT and ICE-deficient mice immunized 14 days before with ovalbumin were incubated for 72 hours with the indicated concentrations of ovalbumin and proliferation was assessed (C). Data are the mean ± SEM of 6 mice per group and are expressed as the percentage of change in MTS absorbance compared with unstimulated cells (100%). ***P< .001 versus WT by factorial ANOVA.
In addition, no significant differences between WT and ICE-deficient mice were observed in the antigen-induced proliferation of draining lymph node cells obtained from ovalbumin-immunized mice (Fig 6C).
Effect of IL-18 and IFNγ on LPS-induced spleen cell proliferation.
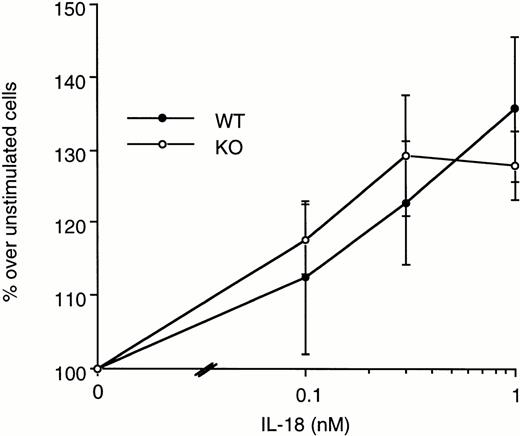
To investigate the augmented LPS-induced spleen cell proliferation observed in ICE-deficient mice, increasing concentrations of exogenous IL-18 were added to spleen cells of WT and ICE-deficient mice in the presence of LPS, and proliferation was assessed. IL-18 (1 to 100 ng/mL) did not significantly alter LPS-induced spleen cell proliferation in neither WT nor ICE-deficient mice (data not shown). When IL-18 was added alone to spleen cells, in the absence of other stimuli, a significant induction of cell proliferation was observed both in WT and in ICE-deficient mice (Fig 7).
Effect of IL-18 on spleen cell proliferation. Splenocytes from WT (•) or ICE-deficient (○) mice were incubated with the indicated concentrations of IL-18. Proliferation was assessed after 72 hours of incubation. Data are the mean ± SEM of 3 mice per group and are expressed as the percentage of change in MTS absorbance over unstimulated cells (100%).
Effect of IL-18 on spleen cell proliferation. Splenocytes from WT (•) or ICE-deficient (○) mice were incubated with the indicated concentrations of IL-18. Proliferation was assessed after 72 hours of incubation. Data are the mean ± SEM of 3 mice per group and are expressed as the percentage of change in MTS absorbance over unstimulated cells (100%).
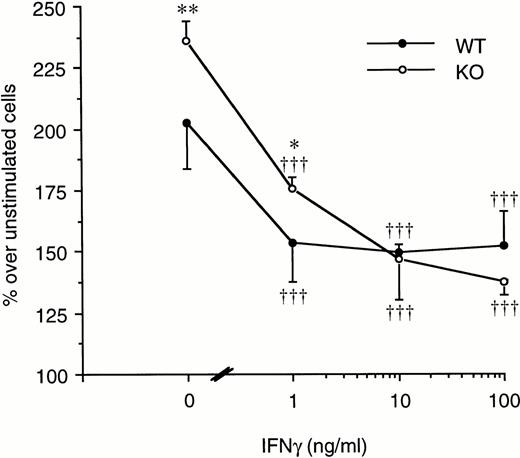
On the other hand, LPS-induced splenocyte proliferation was significantly inhibited both in WT and in ICE-deficient mice when the cells were cultured in the presence of increasing concentrations of IFNγ. As shown in Fig 8, at 10 ng/mL of IFNγ, the proliferation of ICE-deficient splenocytes was no longer different from that of WT cells.
Effect of IFNγ on LPS-induced spleen cell proliferation. Splenocytes from WT (•) or ICE-deficient (○) mice were incubated with 20 μg/mL of LPS in the presence of increasing concentrations of IFNγ. Proliferation was assessed after 72 hours of incubation. Data are the mean ± SEM of 3 mice per group and are expressed as the percentage of change in MTS absorbance over unstimulated cells (100%). *P < .05; **P < .01 versus WT by factorial ANOVA. †††P < .001 versus LPS alone by ANOVA for repeated measures.
Effect of IFNγ on LPS-induced spleen cell proliferation. Splenocytes from WT (•) or ICE-deficient (○) mice were incubated with 20 μg/mL of LPS in the presence of increasing concentrations of IFNγ. Proliferation was assessed after 72 hours of incubation. Data are the mean ± SEM of 3 mice per group and are expressed as the percentage of change in MTS absorbance over unstimulated cells (100%). *P < .05; **P < .01 versus WT by factorial ANOVA. †††P < .001 versus LPS alone by ANOVA for repeated measures.
The effect of neutralization of IL-18 or IFNγ on LPS-induced spleen cell proliferation is shown in Table 2. When the proliferation was measured 48 hours after LPS stimulation, neutralization of either IL-18 or IFNγ significantly increased spleen cell proliferation. However, when spleen cell proliferation was measured 72 hours after LPS, only neutralization of IL-18, but not of IFNγ, was effective.
Effect of Neutralization of IL-18 or IFNγ on LPS-Induced Spleen Cell Proliferation
| . | LPS Alone . | Anti–IL-18 1:400 . | Anti–IL-18 1:200 . | Anti–IL-18 1:100 . | Anti-IFNγ (10 μg/mL) . | Anti-IFNγ (30 μg/mL) . |
|---|---|---|---|---|---|---|
| 48 h | 163.6 ± 7.4 | 176.0 ± 16.2 | 173.9 ± 12.8 | 190.0 ± 13.6* | 179.8 ± 11.6† | 180.3 ± 11.3† |
| 72 h | 164.6 ± 3.8 | 185.8 ± 11.0 | 202.9 ± 19.5† | 207.3 ± 19.7† | 172.4 ± 5.8 | 168.2 ± 5.8 |
| . | LPS Alone . | Anti–IL-18 1:400 . | Anti–IL-18 1:200 . | Anti–IL-18 1:100 . | Anti-IFNγ (10 μg/mL) . | Anti-IFNγ (30 μg/mL) . |
|---|---|---|---|---|---|---|
| 48 h | 163.6 ± 7.4 | 176.0 ± 16.2 | 173.9 ± 12.8 | 190.0 ± 13.6* | 179.8 ± 11.6† | 180.3 ± 11.3† |
| 72 h | 164.6 ± 3.8 | 185.8 ± 11.0 | 202.9 ± 19.5† | 207.3 ± 19.7† | 172.4 ± 5.8 | 168.2 ± 5.8 |
Data are expressed as the percentage of change in MTS absorbance compared with unstimulated cells (100%). Splenocytes from WT mice were incubated with LPS (20 μg/mL) for 48 or 72 hours in the presence or absence of rabbit antimurine IL-18 antiserum at the indicated dilutions or rat antimouse IFNγ at the indicated concentrations. Addition to the cultures of normal rabbit serum at the same concentrations did not significantly alter cell proliferation (data not shown). Data are the mean ± SEM of 3 mice per group.
P < .05.
P < .01 versus respective LPS alone by ANOVA for repeated measures.
Because splenocytes from ICE-deficient mice have decreased IL-1 release after LPS stimulation, we questioned whether the lack of IL-1 in ICE-deficient mice could account for the difference in spleen cells proliferation. To assess a role for endogenous IL-1 activity, we blocked IL-1 receptors with IL-1Ra (10 μg/mL). The addition of IL-1Ra had no effect on LPS-induced splenocyte proliferation (data not shown). Therefore, a lack of IL-1 in spleen cell cultures in ICE-deficient mice does not account for the increased proliferation of ICE-deficient splenocytes to LPS.
DISCUSSION
Because of the inability to process pro–IL-18, ICE-deficient mice injected with LPS, with or without preconditioning with P acnes, exhibit defective IFNγ production.8,9 In the present report, we investigated whether endogenous IL-18 plays a role in the induction of IFNγ after various stimuli. Markedly reduced levels of IFNγ were present in splenocytes obtained from ICE-deficient mice after stimulation with two inflammatory stimuli, LPS and zymosan. In contrast, using a direct T-cell mitogen, such as Con A, no differences in IFNγ production were observed between WT and ICE-deficient mice. Two explanations are possible for this phenomenon: (1) IL-18, similar to IL-12, is not required for IFNγ production after Con A stimulation18; and/or (2) ICE is not necessary for the cleavage of pro–IL-18 when Con A is the stimulus. We have previously shown that ICE is not always indispensable for release of active IL-1β and that the requirement for ICE in IL-1β processing is stimulus-dependent.19 It is therefore possible that, similar to pro–IL-1β , ICE-independent pathways might also exist for the cleavage of pro–IL-18. However, the observation that inhibition of endogenous IL-18 reduces IFNγ production after LPS or zymosan, but not after Con A, suggests that the first hypothesis is probably correct.
In addition, it should be noted that T lymphocytes as well as B cells, NK cells, and monocytes/macrophages can produce IFNγ when an inflammatory stimulus such as LPS is used.20-22 When LPS is the stimulus, the presence of monocytes/macrophages is also critical, because these cells provide the necessary cytokines (ie, IL-12 and IL-18) that induce IFNγ production from lymphocytes and NK cells.23 By contrast, a mitogen such as Con A can react directly with T cells to induce IFNγ production without the need of accessory cells, although cytokines produced by antigen-presenting cells or by bystander lymphocytes (eg, IL-2) play an important regulatory role.24
The results obtained in ICE-deficient mice were confirmed by experiments in which endogenous IL-18 was blocked with a neutralizing antiserum. This resulted in a reduction in LPS- and zymosan-induced IFNγ levels, but had no significant effect on Con A-induced IFNγ production. These results clearly show the critical role played by endogenous IL-18 in the production of IFNγ after an inflammatory stimulus and further strengthen the observation that a decreased processing of IL-18 is responsible for the reduced IFNγ levels observed in ICE-deficient mice. Furthermore, inhibition of ICE activity reduced LPS-induced IFNγ production. However, blockade of IL-1 with IL-1Ra had no inhibitory effect on IFNγ levels. These data show that the reduced IFNγ levels observed in ICE-deficient mice are not due to the lack of bioactive IL-1.
Expectedly, exogenous IL-18 increased zymosan-induced IFNγ production in both WT and ICE-deficient mice. Although the relative increase in IFNγ production was higher in ICE-deficient than in WT mice, the absolute amount of IFNγ was consistently lower (50%) in cultures from ICE-deficient mice, even when concentrations of exogenous IL-18 up to 100 ng/mL were added. These findings may reflect the lack of an additional factor involved in IFNγ production in ICE-deficient mice. Because zymosan-induced IFNγ production was not suppressed in splenocytes obtained from IL-1β–deficient mice, it is unlikely that the missing factor is IL-1β. In addition, mRNA expression for both subunits of IL-12, as well as protein levels for the active p70 heterodimer, was not reduced in ICE-deficient mice, thus ruling out the possibility that defective IL-12 production might be responsible for the reduced IFNγ levels in ICE-deficient mice. However, homodimers of the p40 subunit of IL-12 can act as antagonists of the p70 heterodimer and hence suppress IFNγ induction.25 Levels of total IL-12 were increased in ICE-deficient splenocytes after stimulation with zymosan, possibly reflecting enhanced production of the p40 homodimer. Therefore, the suppressive activity of p40 homodimers on IFNγ production might be enhanced in ICE-deficient mice, thus contributing to the low IFNγ levels observed in these mice.
In general, IFNγ inhibits proliferation of various cells.10,11 Accordingly, IFNγ-deficient mice have augmented spleen cell proliferation in response to Con A, which is suppressed by exogenous IFNγ.26 Because IFNγ production after Con A stimulation is normal in ICE-deficient mice, we did not observe increased splenocyte proliferation with this stimulus. In addition, antigen-induced proliferation of lymph node cells after immunization with ovalbumin was not altered in ICE-deficient mice.
However, consistent with the reduced levels of IFNγ observed in ICE-deficient mice after LPS stimulation, spleen cells from ICE-deficient mice underwent increased proliferation when incubated with LPS. Reconstitution with exogenous IFNγ inhibited LPS-induced spleen cell proliferation, and the difference between WT and ICE-deficient mice was no longer observed. Accordingly, blockade of endogenous IL-18 or IFNγ enhanced LPS-induced spleen cell proliferation. Neutralization of IFNγ was effective only when proliferation was measured at 48 hours, but not at 72 hours, after LPS stimulation. These data suggest the role for IL-18 in regulating cell proliferation is only partly dependent on induction of IFNγ. When specifically examined, exogenous IL-18 did not influence LPS-induced spleen cell proliferation in WT or in ICE-deficient mice. These results may be possibly confounded by the fact that IL-18, by itself, as previously shown, can provide a proliferative signal.2 5The lack of reduction in cell proliferation observed after coincubation with LPS and IL-18 is thus probably a result of two opposite effects of this cytokine: induction of IFNγ, which inhibits cell proliferation, and a direct proliferative signal for T cells by IL-18 itself.
Inhibitors of ICE activity are effective in experimental models of inflammatory diseases, such as pancreatitis and collagen-induced arthritis.27,28 Inhibition of ICE activity is also effective in reducing ischemic and excitotoxic neuronal damage29 and in delaying lethality in a model of amyotrophic lateral sclerosis.30 In addition, ICE-deficient mice are protected against LPS-induced toxicity.31 The reduction in IL-1β and IL-18 release, and consequently of IFNγ production, in these models should be considered to account for the ameliorative effects of ICE inhibitors. In view of the possible use of ICE inhibitors as therapeutic agents, it is important to identify those disease conditions in which ICE-cleaved IL-18 plays a critical role.
From the present studies, it appears that T-cell stimuli such as mitogens or specific antigens drive IFNγ production without the need for IL-18 processing by ICE. On the other hand, microbial and inflammatory IFNγ production is clearly dependent on ICE-mediated cleavage of pro–IL-18. Thus, differential IFNγ production reflects the stimulus and inhibiting ICE appears to spare IFNγ production induced by T-cell stimuli.
ACKNOWLEDGMENT
The authors thank Drs Richard A. Flavell and K. Kuida for providing the ICE-deficient mice and Dr H. Zheng for the IL-1β–deficient mice. We also thank J. Keutzer at Genzyme Corp for kindly providing kits for IL-12 measurement.
Supported by National Institutes of Health Grant No. AI-15614.
Address reprint requests to Charles A. Dinarello, MD, Division of Infectious Diseases, University of Colorado Health Sciences Center, 4200 E Ninth Ave, B168, Denver, CO 80262.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal