Abstract
Antitissue factor antibody attenuated the coagulopathic and lethal responses to LD100Escherichia coli, whereas active site inhibited factor Xa inhibited only the coagulopathic response. In this study, we wished to determine: (1) whether active site inhibited factor VIIa blocks the coagulopathic and/or attenuates the lethal effects of LD100E coli and (2) whether these effects are accompanied by attenuation of the inflammatory cytokine response to LD100E coli. Eight baboons infused for 2 hours with LD100E coli also were given five bolus infusions of DEGR VIIa of 280 μg/kg at T = −10 minutes, +2, 4, 6, and 8 hours and observed for changes in vital signs, and the concentrations of hemostatic components (fibrinogen, platelets, fibrin degradation products) and inflammatory mediators (tumor necrosis factor [TNF], interleukin-6 [IL-6], IL-8) at T = 0, 1, 2, 4, 6, and 8 hours. Eight control baboons were also infused with LD100E coli alone and followed as described above. Four of the eight baboons treated with DEGR VIIa were permanent 7-day survivors versus none in the control group. The mean survival times for the treated and control groups were 116 ± 22 and 26 ± 8 hours, respectively. These values differed significantly from each other, (P = .0008). The decrease in platelet and fibrinogen concentrations and the increase in fibrin degradation products observed in the control group were significantly attenuated in the treated group, as was thrombosis of renal glomerular capillaries. Treatment with DEGR VIIa showed no effect on the peak TNF response to LD100E coli at T = 2 hours (170 ± 32 v120 ± 35 ng/mL). DEGR VIIa, however, did attenuate the IL-6 and IL-8 responses at T = 8 hours (ie, the IL-6 concentrations were 81 ± 10 for treated and 1,256 ± 236 for the control groups and the IL-8 concentrations were 28 ± 3.9 for the treated and 60 ± 8.2 for the control group). These values for IL-6 and IL-8 differed significantly from each other between the treated and control groups (P = .0001 and .0074, respectively). It should be noted that the initial responses of IL-6 and IL-8 up to T = 4 hours were not attenuated. We concluded that DEGR VIIa treatment attenuates inflammatory, as well as hemostatic system responses to LD100E coli. We hypothesize that this occurs through interference with the assembly and/or interactions of tissue factor/VIIa complexes.
IN PREVIOUS STUDIES, we have shown that tissue factor plays an important role in mediation of the disseminated intravascular coagulopathic (DIC) response to LD100Escherichia coli in the baboon.1We found that of several monoclonal antibodies against tissue factor, only one antibody inhibited both tissue factor coagulant activity in vitro and attenuated the coagulopathic response to and protected the baboon from the lethal effects of LD100E coli. While this observation was important in describing the role of tissue factor in producing DIC in this model, we wished to intervene in this model using factor VIIa, the active site of which was inhibited with Dansyl-Glu-Gly-Arg chloromethylketone (DEGR VIIa) to further understand the role and importance of additional factors mediating the DIC and the lethal responses to E coli. In these studies, we addressed the following three questions: First, does factor VIIa also participate in mediating the DIC response and does active site inhibited fVIIa also protect against the lethal effects of LD100E coli? If DEGR VIIa attenuated these responses, this would suggest that the tissue factor/fVIIa complex contributes to them and strengthens evidence supporting the role already described for tissue factor alone. Second, how does intervention with DEGR VIIa compare with intervention with DEGR Xa? Previously, we showed that DEGR Xa inhibited only the coagulopathic response to LD100E coli.2 If DEGR VIIa, in contrast to DEGR Xa, inhibited both the coagulopathic and both the inflammatory and lethal responses to LD100E coli, this would suggest that those factors tightly associated with tissue factor (ie, fVIIa) might play a role different from those less tightly associated with tissue factor (ie, fXa). Third, to what extent and at what point during these responses to LD100E coli does DEGR VIIa attenuate elements of the inflammatory response (tumor necrosis factor [TNF], interleukin-6 [IL-6], and IL-8)? If DEGR VIIa exhibits distinct antiinflammatory, as well as anticoagulant effects, this would support current evidence linking the TF/fVII complex to the inflammatory, as well as to coagulopathic responses to LD100E coli1,3-5 and support the concept that control of coagulant events involving proximal (upstream) coagulant factors might also influence cellular signaling and inflammatory events.
MATERIALS AND METHODS
Materials
E coli: Escherichia coli 086:K61H of 33985 American Type Culture Collection, Rockville, MD. These bacteria were previously isolated from a stool specimen at Children's Memorial Hospital, Oklahoma City, OK. They were stored in the lyophilized state at 4°C after growth in tryptic soybean agar and were reconstituted and characterized as described by Hinshaw et al.6
Preexperimentation and Experimentation Procedures
Papio c cynocephalus orPapio c anubis baboons were purchased from a breeding colony maintained at the University of Oklahoma Health Sciences Center or from Biomedical Research Foundation, Inc, Houston, TX. Animals weighed 4 to 14 kg, had leukocyte concentrations not exceeding 10,000/μL, and hematocrits above 36%. They were screened for tuberculosis. These animals were held for 30 days at the OUHSC animal facility on campus where the infusion studies were performed. The animals were observed continuously during the first 8 hours postinfusion. All animals were followed for up to 7 days, at which time they were killed and tissues examined at postmortem.
Infusion Procedures
Experiments were performed on 16 baboons. Animals were fasted overnight before the study, administered water ad libitum, and immobilized on the morning of the experiment with Ketamine (14 ± 0.5 mg/kg intramuscularly). Animals then were administered sodium pentobarbital (2 mg/kg) through a percutaneous catheter in the cephalic vein of the forearm and maintained at a light level of surgical anesthesia with supplemental bolus infusions, approximately every 20 to 40 minutes for 8 hours. The animals were intubated orally and allowed to breath spontaneously.
A superficial femoral vein was exposed aseptically and cannulated for sampling blood. This cannulization involved insertion of the catheter into the superficial femoral vein and advanced into the inferior vena cava. Each baboon was placed on its side in contact with a controlled temperature heating pad. The blood pressure and rectal temperature were monitored using a Dinamap Research monitor, model no. 1255 (Critikon, Inc, Tampa, FL) and a telethermometer (Yellow Springs Instrument Co, Yellow Springs, OH), respectively. The percutaneous catheter in the cephalic vein was used to infuse DEGR VIIa and E coliorganisms. At the conclusion of the initial 8-hour observation period, both the cephalic and superficial femoral vein catheters were removed, the superficial femoral vein was ligated, and the animals were returned to their cages.
Sampling
The vital signs and cardiovascular responses were monitored, and blood samples were collected at 0, 1, 2, 4, 6, and 8 hours. T = 0 designated the point at which the infusion of E coli was started. The whole blood samples at each drawing included: 1.0 mL anticoagulated with EDTA for complete blood count (CBC), hematocrit, platelet count, and differential; 1.8 mL anticoagulated with 3.8% sodium citrate for fibrinogen,9 TNF, IL-6, IL-8, and DEGR VIIa; 1.0 mL in trasylol/thrombin for fibrin degradation products10; 1.0 mL of clotted blood for blood urea nitrogen,11 creatinine,12 and serum glutamic-pyruvic transaminase13; and 0.5 mL whole blood collected at T-0 and T = 2 hours for E coli colony counts.6 This totaled approximately 5.3 mL of blood at each sampling period. Not more than 10% of the baboon's total blood volume was withdrawn over the 8-hour monitoring period. This study protocol received prior approval by the Institutional Animal Care and Use Committees of the Oklahoma Medical Research Foundation and the University of Oklahoma Health Sciences Center.
Experimental Groups and Plasma Concentration of DEGR VIIa
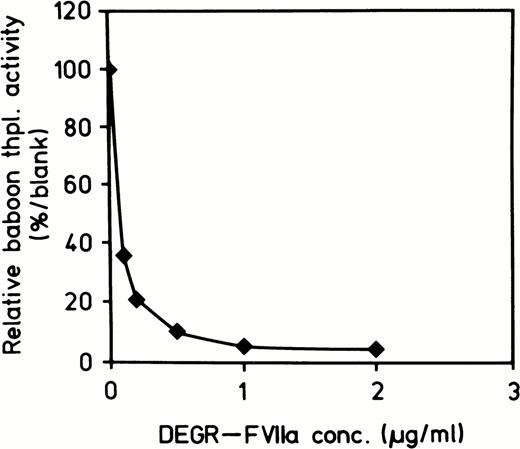
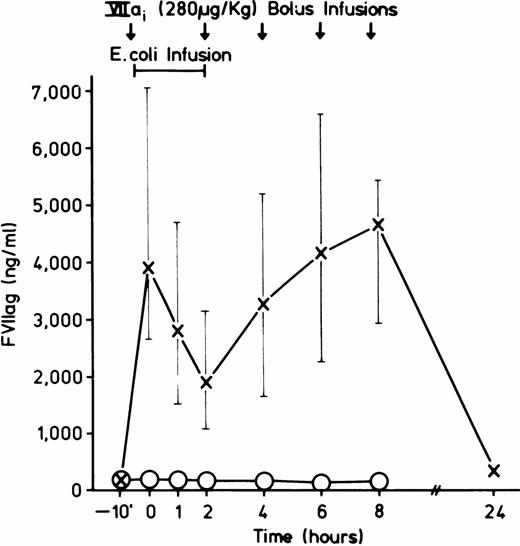
Table 1 lists the animals, their sex and weight, the concentrations of E coli organisms, and DEGR VIIa used in the experiments, and the duration of their survival. E coli (approximately 2 to 3 × 1010 colony-forming unit [CFU]/kg) infusion was begun at T-0 and given as a continuous infusion until T = 2 hours. DEGR VIIa (280 μg/kg) had a T½ of approximately 20 minutes. Therefore, it was given as a bolus at T-10 minutes and again at T = 2, 4, 6, and 8 hours. Figure 1 shows an in vitro assay demonstrating a dose-dependent inhibition of thromboplastin coagulant activity by DEGR VIIa. Figure 2shows the concentrations of DEGR VIIa antigen in the treated animals versus untreated animals.
Summary of Sex, Weight, E coli Dose, DEGR VIIa Dose, and Survival of the DEGR VIIa-Treated and Control Groups
| Experiment No. . | Sex . | Weight (kg) . | E coliStock (CFU × 1010/mL) . | E coli at T + 120′ (CFU × 106/ mL blood) . | VIIa1 (280 μg/kg) × 5 . | Survival (h) . |
|---|---|---|---|---|---|---|
| Treated | ||||||
| VIIa1 1 | Female | 5.9 | 1.59 | 3.85 | Yes | 43 |
| VIIa1 2 | Female | 3.6 | 1.65 | 0.09 | Yes | 119 |
| VIIa1 3 | Female | 4.1 | 1.54 | 22.60 | Yes | 168 |
| VIIa1 6 | Male | 4.2 | 1.52 | 13.00 | Yes | 168 |
| VIIa1 8 | Female | 5.4 | 8.40 | 27.00 | Yes | 31 |
| VIIa1 9 | Male | 5.7 | 1.30 | 6.05 | Yes | 65 |
| VIIa1 10 | Male | 5.7 | 1.44 | 29.00 | Yes | 168 |
| VIIa1 12 | Male | 6.6 | 1.36 | 2.32 | Yes | 168 |
| Average SE | 5.2 ± 0.4 | 2.35 ± 0.86 | 12.98 ± 4.13 | 116 ± 22* | ||
| Control | ||||||
| 4 | Female | 4.3 | 1.66 | 13.90 | No | 86 |
| 5 | Female | 5.2 | 1.50 | 27.10 | No | 15 |
| 7 | Male | 6.4 | 7.25 | 4.32 | No | 26 |
| 13 | Female | 4.8 | 1.37 | 16.4 | No | 26 |
| 14 | Female | 7.5 | 1.36 | 10.8 | No | 24 |
| 15 | Male | 14.8 | 1.46 | 9.90 | No | 14 |
| 16 | Female | 11.7 | 8.23 | 2.43 | No | 15 |
| 17 | Female | 7.3 | 5.03 | 12.1 | No | 14 |
| Average SE | 7.7 ± 1.9 | 3.48 ± 1.21 | 12.11 ± 4.82 | 26 ± 8 |
| Experiment No. . | Sex . | Weight (kg) . | E coliStock (CFU × 1010/mL) . | E coli at T + 120′ (CFU × 106/ mL blood) . | VIIa1 (280 μg/kg) × 5 . | Survival (h) . |
|---|---|---|---|---|---|---|
| Treated | ||||||
| VIIa1 1 | Female | 5.9 | 1.59 | 3.85 | Yes | 43 |
| VIIa1 2 | Female | 3.6 | 1.65 | 0.09 | Yes | 119 |
| VIIa1 3 | Female | 4.1 | 1.54 | 22.60 | Yes | 168 |
| VIIa1 6 | Male | 4.2 | 1.52 | 13.00 | Yes | 168 |
| VIIa1 8 | Female | 5.4 | 8.40 | 27.00 | Yes | 31 |
| VIIa1 9 | Male | 5.7 | 1.30 | 6.05 | Yes | 65 |
| VIIa1 10 | Male | 5.7 | 1.44 | 29.00 | Yes | 168 |
| VIIa1 12 | Male | 6.6 | 1.36 | 2.32 | Yes | 168 |
| Average SE | 5.2 ± 0.4 | 2.35 ± 0.86 | 12.98 ± 4.13 | 116 ± 22* | ||
| Control | ||||||
| 4 | Female | 4.3 | 1.66 | 13.90 | No | 86 |
| 5 | Female | 5.2 | 1.50 | 27.10 | No | 15 |
| 7 | Male | 6.4 | 7.25 | 4.32 | No | 26 |
| 13 | Female | 4.8 | 1.37 | 16.4 | No | 26 |
| 14 | Female | 7.5 | 1.36 | 10.8 | No | 24 |
| 15 | Male | 14.8 | 1.46 | 9.90 | No | 14 |
| 16 | Female | 11.7 | 8.23 | 2.43 | No | 15 |
| 17 | Female | 7.3 | 5.03 | 12.1 | No | 14 |
| Average SE | 7.7 ± 1.9 | 3.48 ± 1.21 | 12.11 ± 4.82 | 26 ± 8 |
*Denotes that value of the treated group differs significantly from that of the control group, (P value = .0008).
Dose-response curve of inhibition of thromboplastin-induced coagulant activity of baboon plasma by increasing concentrations of DEGR VIIa. A baboon thromboplastin standard curve was constructed using dilutions of baboon thromboplastin in normal baboon plasma. Active site inhibited fVIIa was added to 1:5 dilution of thromboplastin (100%) and the clotting times obtained were converted to thromboplastin activity using the standard curve. The plot was then obtained by plotting DEGR VII concentration versus thromboplastin activity.
Dose-response curve of inhibition of thromboplastin-induced coagulant activity of baboon plasma by increasing concentrations of DEGR VIIa. A baboon thromboplastin standard curve was constructed using dilutions of baboon thromboplastin in normal baboon plasma. Active site inhibited fVIIa was added to 1:5 dilution of thromboplastin (100%) and the clotting times obtained were converted to thromboplastin activity using the standard curve. The plot was then obtained by plotting DEGR VII concentration versus thromboplastin activity.
Average concentration of DEGR VIIa observed in the DEGR VIIa treated (X-X, N = 8) and control group (○-○, N = 8). Samples were drawn just before each bolus infusion.
Average concentration of DEGR VIIa observed in the DEGR VIIa treated (X-X, N = 8) and control group (○-○, N = 8). Samples were drawn just before each bolus infusion.
Assays
TNF enzyme-linked immunosorbent assay (ELISA).
Microtiter plates (Dynatech, Chantilly, VA) were coated overnight at 4°C with a polyclonal antibody against human (r)TNF (R & D Systems no. AB-210-NA, Minneapolis, MN), 5 μg/mL in 50 mmol/L carbonate buffer, pH 9.6, 50 μL/well. The wells were washed three times with 20 mmol/L Tris-HCl, 0.15 mol/L NaCl, 0.1% wt/vol Tween 20, pH 7.5 (wash buffer), and then incubated at room temperature for 1 hour with 20 mmol/L Tris-HCl, 0.15 mol/L NaCl, 1% wt/vol bovine serum albumin (BSA), pH 7.5, 50 μL/well. After washing three times again, citrated plasma samples, which had been diluted in 20 mmol/L Tris-HCl, 0.15 mol/L NaCl, 0.1% wt/vol BSA, pH 7.5 (diluting buffer), were added in duplicate to the wells, 50 μL/well. A standard curve of normal baboon plasma, diluted 1:10, to which human (r)TNF had been added was run from 0.5 to 7.0 ng/mL. The plate was incubated for 1 hour at 37°C and washed. To detect bound TNF, antibody against human (r)TNF (R & D Systems no. AB-210-NA) was biotinylated by the method of Gretch et al14 and 50 μL of 1 μg/mL biotinylated antibody was added to the plate and incubated for 1 hour at 37°C. After washing, 50 μL of 0.25 μg/mL streptavidin-alkaline phosphatase conjugate (BRL no. 9589SA; GIBCO-BRL, Gaithersburg, MD) was added, and the plate was incubated for 1 hour at 37°C. After thorough washing, nicotinamide adenine dinucleotide phosphate (NADPH) substrate stock solution from the ELISA amplification kit (BRL no. 9589SA) was added, 50 μL/well, and the plate incubated 10 minutes at room temperature. Amplifier solution was then added, 50 μL/well, and incubation continued at room temperature for ≈20 minutes, until color development. The A490 was measured with the microplate reader. The data was analyzed using a 4-parameter curve fitting Softmax program (Molecular Devices, Menlo Park, CA).
IL-6 ELISA.
The IL-6 ELISA assay was performed in a manner similar to that described for TNF. Microtiter plates (Nunc, Roskilde, Denmark) were coated overnight with 100 μL of 0.5 μg/mL MoAb α IL-6 under the same conditions and with the same buffers as described for TNF. The MoAb α IL-6 clone 8 at 5.0 mg/mL stock was a gift of Dr L. Arden (BCL, Amsterdam, Holland). The succeeding wash steps, blocking with BSA, and incubation of plasma samples were as described above. Biotinylated goat anti–IL-6 (1 μg/mL), (R&D Systems, no. AB-206-NA) plus streptavidin-horseradish peroxidase congugate and trimethyl benzamodine (TMB) substrate also were used in quantitating the antibody reaction as described above.
IL-8 ELISA.
The IL-8 ELISA assay was also performed in a manner similar to that described for TNF. Microtiter plates (Costar no. 2596; Costar, Cambridge, MA) were coated overnight with 50 μL of 0.5 μg/mL MoAb α IL-8 (R&D Systems, no. MAB208) under the same conditions and with the same buffers as described above for TNF. The succeeding wash steps, blocking with BSA, and incubation of plasma samples were as described above. Biotinylated goat anti–IL-8 (1 μg/mL) (R&D Systems, no. AB-208-NA) plus strepovidin-horseradish peroxidase congugate and TMB substrate in quantitating the antibody reactions also were used as described above.
DEGR VIIa ELISA.
Total VIIa antigen levels were determined using a solid-phase double antibody enzyme-linked immunoassay.15
Statistical Analysis and Scoring of Tissue Histopathology
Statistical analysis.
All data are presented as mean ± standard error of mean (SEM). The survival data in Table 1 was analyzed using the Kaplan-Meier Product-Limit Estimate of Survival Function to compare the survival times of treated versus control groups, as the data included censored observations (ie, times of survival were measured only up to 168 hours, “permanent survivors”). The clinical laboratory data in Table 2were analyzed using an analysis of variance with Duncan's multicomparison test to determine significant differences (P< .05) between groups at given times and within certain variables. An analysis of variance with Dunnett's multicomparison test was also used to determine significant differences (P < .05) between time 0 (T-0) or baseline and subsequent times for a given variable and a given group. The cytokine data in Table 4 were analyzed using the Wilcoxon-Rank Sum test to compare the IL-6 and IL-8 assay results at T = 8 hours of the treated versus the control groups. The pathologic lesions of adrenal glands, kidneys, and lungs were analyzed by dividing their description into five categories: thrombosis, hemorrhage, congestion, white cell influx, and necrosis. The tissues were rated according to the severity of the histopathologic lesions. The scale ranged from 1 to +4, with 4 being the most severe. All microscopic sections were read by Dr Kosanke (Oklahoma Health Sciences Center, Oklahoma City), a veterinary pathologist, who was blinded as to which study was being analyzed. The Kruskal-Wallis test, a nonparametric test, was used to determine significant differences (P < .05) between groups for a given pathologic lesion.
Summary of Clinical Laboratory Parameters of DEGR VIIa-Treated and Control Groups
| Observations . | T-0 . | T + 1 h . | T + 2 h . | T + 4 h . | T + 6 h . | T + 8 h . |
|---|---|---|---|---|---|---|
| LD100E coli plus DEGR VIIa | ||||||
| MSAP (mm Hg) | 110 ± 3 | 102 ± 5 | 63 ± 6 | 58 ± 4* | 73 ± 4* | 74 ± 3* |
| Inflammatory | ||||||
| WBC (×103/μL) | 5.0 ± 0.6 | 1.4 ± 0.1 | 1.1 ± 0.1 | 1.3 ± 0.2 | 1.5 ± 0.2 | 2.0 ± 0.4 |
| Hemostasis | ||||||
| Hematocrit (mm) | 39 ± 0.4 | 38 ± 1.0 | 37 ± 1.0 | 38 ± 1.1 | 40 ± 1.1 | 40 ± 1.0 |
| Platelets (%) | 100 | 76 ± 5.1 | 65 ± 8.3 | 70 ± 10.1 | 66 ± 6.8* | 60 ± 6.1* |
| Fibrinogen (%) | 100 | 92 ± 3.7 | 83 ± 2.8 | 80 ± 2.7* | 76 ± 3.4* | 77 ± 4.0* |
| FDP (μg/dL) | <10 | — | — | — | — | 34 ± 21* |
| Cell injury | ||||||
| BUN (mg/dL) | 17.8 ± 1.4 | — | — | — | — | 27.4 ± 1.0 |
| CR (mg/dL) | 0.53 ± 0.03 | — | — | — | — | 1.2 ± 0.2 |
| SGPT (U/L) | 37 ± 3 | — | — | — | — | 190 ± 97 |
| Renal path (0-4+) | — | — | — | — | — | 0.05 ± 0.1* |
| LD100E coli alone | ||||||
| MSAP (mm Hg) | 116 ± 9 | 118 ± 7 | 69 ± 8 | 74 ± 4 | 83 ± 4 | 84 ± 4 |
| Inflammatory | ||||||
| WBC (×103/μL) | 6.3 ± 1.1 | 1.8 ± 0.3 | 1.1 ± 0.2 | 1.2 ± 0.2 | 1.6 ± 0.3 | 2.4 ± 0.5 |
| Hemostasis | ||||||
| Hematocrit (mm) | 40 ± 0.1 | 42 ± 1.0 | 39 ± 1.1 | 40 ± 1.9 | 42 ± 1.5 | 42 ± 1.4 |
| Platelets (%) | 100 | 72 ± 7.2 | 60 ± 9.1 | 51 ± 6.7 | 43 ± 5.5 | 34 ± 4.5 |
| Fibrinogen (%) | 100 | 89 ± 5.1 | 63 ± 10 | 13 ± 7.9 | 6.2 ± 5.8 | 7 ± 5.7 |
| FDP (μg/dL) | <10 | — | — | — | — | 311 ± 84 |
| Cell injury | ||||||
| BUN (mg/dL) | 18 ± 3.1 | — | — | — | — | 25 ± 2.4 |
| CR (mg/dL) | 0.65 ± 0.02 | — | — | — | — | 2.0 ± 0.3 |
| SGPT (U/L) | 36 ± 6 | — | — | — | — | 104 ± 32 |
| Renal Path (0-4+) | — | — | — | — | — | 3.2 ± 0.4 |
| Observations . | T-0 . | T + 1 h . | T + 2 h . | T + 4 h . | T + 6 h . | T + 8 h . |
|---|---|---|---|---|---|---|
| LD100E coli plus DEGR VIIa | ||||||
| MSAP (mm Hg) | 110 ± 3 | 102 ± 5 | 63 ± 6 | 58 ± 4* | 73 ± 4* | 74 ± 3* |
| Inflammatory | ||||||
| WBC (×103/μL) | 5.0 ± 0.6 | 1.4 ± 0.1 | 1.1 ± 0.1 | 1.3 ± 0.2 | 1.5 ± 0.2 | 2.0 ± 0.4 |
| Hemostasis | ||||||
| Hematocrit (mm) | 39 ± 0.4 | 38 ± 1.0 | 37 ± 1.0 | 38 ± 1.1 | 40 ± 1.1 | 40 ± 1.0 |
| Platelets (%) | 100 | 76 ± 5.1 | 65 ± 8.3 | 70 ± 10.1 | 66 ± 6.8* | 60 ± 6.1* |
| Fibrinogen (%) | 100 | 92 ± 3.7 | 83 ± 2.8 | 80 ± 2.7* | 76 ± 3.4* | 77 ± 4.0* |
| FDP (μg/dL) | <10 | — | — | — | — | 34 ± 21* |
| Cell injury | ||||||
| BUN (mg/dL) | 17.8 ± 1.4 | — | — | — | — | 27.4 ± 1.0 |
| CR (mg/dL) | 0.53 ± 0.03 | — | — | — | — | 1.2 ± 0.2 |
| SGPT (U/L) | 37 ± 3 | — | — | — | — | 190 ± 97 |
| Renal path (0-4+) | — | — | — | — | — | 0.05 ± 0.1* |
| LD100E coli alone | ||||||
| MSAP (mm Hg) | 116 ± 9 | 118 ± 7 | 69 ± 8 | 74 ± 4 | 83 ± 4 | 84 ± 4 |
| Inflammatory | ||||||
| WBC (×103/μL) | 6.3 ± 1.1 | 1.8 ± 0.3 | 1.1 ± 0.2 | 1.2 ± 0.2 | 1.6 ± 0.3 | 2.4 ± 0.5 |
| Hemostasis | ||||||
| Hematocrit (mm) | 40 ± 0.1 | 42 ± 1.0 | 39 ± 1.1 | 40 ± 1.9 | 42 ± 1.5 | 42 ± 1.4 |
| Platelets (%) | 100 | 72 ± 7.2 | 60 ± 9.1 | 51 ± 6.7 | 43 ± 5.5 | 34 ± 4.5 |
| Fibrinogen (%) | 100 | 89 ± 5.1 | 63 ± 10 | 13 ± 7.9 | 6.2 ± 5.8 | 7 ± 5.7 |
| FDP (μg/dL) | <10 | — | — | — | — | 311 ± 84 |
| Cell injury | ||||||
| BUN (mg/dL) | 18 ± 3.1 | — | — | — | — | 25 ± 2.4 |
| CR (mg/dL) | 0.65 ± 0.02 | — | — | — | — | 2.0 ± 0.3 |
| SGPT (U/L) | 36 ± 6 | — | — | — | — | 104 ± 32 |
| Renal Path (0-4+) | — | — | — | — | — | 3.2 ± 0.4 |
*Denotes those values of the treated group that differ significantly from those of the control group (Pvalue ≥ .05).
IL-6 and IL-8 Concentrations at T = 8 Hours of the DEGR VIIa-Treated and Control Groups
| Experiment No. . | IL-6 (ng/mL) . | IL-8 (ng/mL) . |
|---|---|---|
| Treated | ||
| VIIa1 1 | 65 | 44 |
| VIIa1 2 | 62 | 26 |
| VIIa1 3 | 69 | 33 |
| VIIa1 6 | 106 | 35 |
| VIIa1 8 | 89 | 15 |
| VIIa1 9 | 134 | 38 |
| VIIa1 10 | 44 | 20 |
| VIIa1 12 | 82 | 15 |
| Average SE | 81 ± 103-150 | 28 ± 3.93-150 |
| Control | ||
| 4 | 723 | 31 |
| 5 | 1,336 | 64 |
| 7 | 1,244 | 69 |
| 13 | 285 | 24 |
| 14 | 936 | 53 |
| 15 | 2,413 | 93 |
| 16 | 1,200 | 78 |
| 17 | 1,908 | 69 |
| Average SE | 1,256 ± 236 | 60 ± 8.2 |
| Experiment No. . | IL-6 (ng/mL) . | IL-8 (ng/mL) . |
|---|---|---|
| Treated | ||
| VIIa1 1 | 65 | 44 |
| VIIa1 2 | 62 | 26 |
| VIIa1 3 | 69 | 33 |
| VIIa1 6 | 106 | 35 |
| VIIa1 8 | 89 | 15 |
| VIIa1 9 | 134 | 38 |
| VIIa1 10 | 44 | 20 |
| VIIa1 12 | 82 | 15 |
| Average SE | 81 ± 103-150 | 28 ± 3.93-150 |
| Control | ||
| 4 | 723 | 31 |
| 5 | 1,336 | 64 |
| 7 | 1,244 | 69 |
| 13 | 285 | 24 |
| 14 | 936 | 53 |
| 15 | 2,413 | 93 |
| 16 | 1,200 | 78 |
| 17 | 1,908 | 69 |
| Average SE | 1,256 ± 236 | 60 ± 8.2 |
The concentration of IL-6 at T = 4 hours was 187 ± 30 ng/mL and 383 ± 144 ng/mL for the treated and control groups, respectively. The concentration of IL-8 at T = 4 hours was 51 ± 6 ng/mL and 60 ± 15 ng/mL for the treated and control groups, respectively. The limit of detection for IL-6 and IL-8 was 2.5 and 0.2 ng/mL, respectively.
Denotes that those values of the treated group differ significantly from those of the control group. The P values for IL-6– and IL-8–treated versus control groups were P = .0001 andP = .0074, respectively.
RESULTS
Table 1 shows that coinfusion of DEGR VIIa with LD100E coli was associated with an average survival time of 116 hours versus 26 hours for the control group. There were four permanent (168 hours) survivors in the treated versus none in the control group. Table 2 summarizes the clinical laboratory studies. The decrease in platelet, fibrinogen concentrations and increase in fibrin degradation product (FDP) concentration observed in the control group at T = 6 to 8 hours were attenuated significantly in the treated group. The mean systemic arterial blood pressure (MSAP) of the treated group was significantly lower than that of the control group at T = 6 to 8 hours. Coinfusion of DEGR VIIa with LD100E coli had no effect, however, on the responses of other clinical laboratory parameters including the white cells and markers of renal (blood urea nitrogen [BUN], creatinine [CR]) and liver (serum glutamic-pyruvic transaminase [SGPT]) function. Table 3shows that renal glomerular capillary thrombosis was almost completely inhibited in the treated versus that observed in the control group. The presence or absence of thrombotic lesions appear to be independent of the length of survival.
Renal Glomerular Capillary Thrombosis Scores and Survival Times of the DEGR VIIa-Treated and Control Groups
| Experiment No. . | Thrombosis Score (0-4+) . | Survival (h) . |
|---|---|---|
| Treated | ||
| VIIa1 1 | 0.0 | 43 |
| VIIa1 2 | 0.0 | 119 |
| VIIa1 3 | 0.0 | 168 |
| VIIa1 6 | 0.0 | 168 |
| VIIa1 8 | 0.0 | 31 |
| VIIa1 9 | 0.4 | 65 |
| VIIa1 10 | 0.0 | 168 |
| VIIa1 12 | 0.0 | 168 |
| Average SE | 0.05 ± 0.01* | 116 ± 22* |
| Control | ||
| 4 | 3.8 | 86 |
| 5 | 2.7 | 15 |
| 7 | 4.0 | 26 |
| 13 | 2.3 | 26 |
| 14 | 2.5 | 24 |
| 15 | 3.3 | 14 |
| 16 | 3.0 | 15 |
| 17 | 4.0 | 14 |
| Average SE | 3.2 ± 0.4 | 26 ± 8 |
| Experiment No. . | Thrombosis Score (0-4+) . | Survival (h) . |
|---|---|---|
| Treated | ||
| VIIa1 1 | 0.0 | 43 |
| VIIa1 2 | 0.0 | 119 |
| VIIa1 3 | 0.0 | 168 |
| VIIa1 6 | 0.0 | 168 |
| VIIa1 8 | 0.0 | 31 |
| VIIa1 9 | 0.4 | 65 |
| VIIa1 10 | 0.0 | 168 |
| VIIa1 12 | 0.0 | 168 |
| Average SE | 0.05 ± 0.01* | 116 ± 22* |
| Control | ||
| 4 | 3.8 | 86 |
| 5 | 2.7 | 15 |
| 7 | 4.0 | 26 |
| 13 | 2.3 | 26 |
| 14 | 2.5 | 24 |
| 15 | 3.3 | 14 |
| 16 | 3.0 | 15 |
| 17 | 4.0 | 14 |
| Average SE | 3.2 ± 0.4 | 26 ± 8 |
*Denotes those values of the treated group that differ significantly from those of the control group (the P values for thrombosis score and survival were P = <.0001 and .0008, respectively).
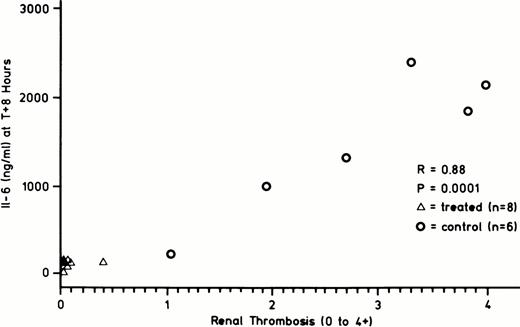
Table 4 shows that the IL-6 and IL-8 concentrations at T = 8 hours were significantly lower in the treated than in the control group. This table illustrates that while the IL-6 and IL-8 concentrations of the treated and control groups were comparable at T = 4 hours, that of the treated group decreased after T = 4 hours, while that of the control group remained elevated out to T + 8 hours. In contrast to IL-6 and IL-8, there was no difference in the TNF response between the treated and control groups. Peak TNF concentrations at T = 2 hours were 170 ± 32 ng/mL and 120 ± 35 ng/mL in the treated and control groups, respectively. Given the reports associating IL-6 cytokine with procoagulant activity,16-18 the association between IL-6 concentration and extent of renal glomerular capillary thrombosis was examined. Figure 3 shows that there is a significant correlation between the extent of renal glomerular capillary thrombosis and the plasma concentration of IL-6.
Correlation between IL-6 concentration at T + 8 hours (ng/mL) and degree of renal glomerular capillary thrombosis (0 to 4+) observed at postmortem. DEGR VIIa treated group (▵-▵, N = 8), control group (○-○, N = 6). R = .88, P = .0001.
Correlation between IL-6 concentration at T + 8 hours (ng/mL) and degree of renal glomerular capillary thrombosis (0 to 4+) observed at postmortem. DEGR VIIa treated group (▵-▵, N = 8), control group (○-○, N = 6). R = .88, P = .0001.
DISCUSSION
The first question raised in the introduction was does DEGR VIIa attenuate DIC and prolong survival? DEGR VIIa clearly inhibited fibrinogen consumption and the generation of fibrin degradation products. It also attenuated to a lesser degree the consumption of platelets, while dramatically inhibiting renal glomerular capillary thrombosis. The renal pathology observed did not appear to be affected by differences in times of survival. The extent of thrombosis in tissues recovered from animals in the control group that died in 14 to 26 hours, did not differ greatly from those that died in 86 hours. Likewise, little or no thrombosis was observed in tissues recovered from animals in the treated group regardless of whether they died in 31 to 119 hours or were permanent survivors (168 hours). Thus, we concluded that the extent of lesions appeared to be more dependent on whether the animals were infused with DEGR VIIa and not on duration of survival.
Although the difference in the survival times of the treated and the untreated groups did reach significance (P = .0008), the attenuation of the response to LD100E coli by DEGR VIIa did not appear to be uniform, as the treated group was split between four permanent survivors and four nonsurvivors. This raised the question of a subgroup within the treated group, which is more responsive to treatment. We know from experience over the last 10 years that the response to infusion of LD100E coli alone can vary from a capillary leak, shock, and death within 12 hours at one extreme to microvascular thrombosis, renal failure, and death in 3 to 4 days.19 The incidence of these two extremes was 10% and 8%, respectively. The majority of animals (approximately 75%) exhibited a classical consumptive coagulopathy and succumbed between 12 and 30 hours.19 Although the small number of animals studied preclude drawing a conclusion, these results raise the possibility that the differences in the effectiveness of DEGR VIIa in mitigating the response to LD100E coli may be related to these variations of the host response to E coli.
The second question raised in the introduction was how does intervention with DEGR VIIa compare with intervention with DEGR Xa, as both are efficient inhibitors of the DIC response to E coli? We concluded that DEGR VIIa could mitigate the lethal effects of E coli, whereas DEGR Xa,2 heparin, and hirudin20 do not. This again suggests that like tissue factor pathway inhibitor (TFPI),3 DEGR VIIa interacts with tissue factor or tissue factor/fVIIa complex in such a manner as to attenuate lethal inflammatory, as well as procoagulant responses.
This observation raised the third question with respect to the effect of DEGR VIIa intervention on cytokine release and what, if any, correlation might exist between the alteration of cytokine release and development of renal glomerular capillary thrombosis. We concluded that in addition to inhibiting the DIC response and mitigating the lethal effects of LD100E coli, DEGR VIIa also inhibited the elevation of IL-6 and to a lesser degree that of IL-8 observed beyond T = 4 hours after the infusion of E coli. This broadens the effects of DEGR VIIa to include antiinflammatory effects, as postulated by Taylor.21 Evidence supporting this concept is offered by Rottingen et al.4 They observed that addition of factor VIIa caused Ca2+ transients in 100% of cells from the kidney cell line MDCK, a constitutive tissue factor producer. They concluded that tissue factor mediates a cytosolic Ca2+signal on interaction with its ligand, factor VIIa. This is consistent with the characterization of tissue factor as being related to the class 2 cytokine receptor superfamily. These investigators later showed that DEGR VIIa did not illicit this calcium flux.5 Both of these observations lend support to the hypothesis that DEGR VIIa by blocking tissue factor interaction with native factor VII not only blocks the coagulant response, but also may attenuate the inflammatory response by inhibiting this calcium flux. This also may explain why DEGR Xa fails to protect while inhibiting the coagulant response, as it does not interfere with the tissue factor-factor VIIa interaction.
These data also raised the question of where this action of DEGR VIIa is taking place in the vasculature. The fact that the TNF response is not inhibited at all and that the IL-6 and IL-8 responses are attenuated only after T = 3 to 4 hours raises the question of the target of DEGR VIIa action within the vasculature. Monocytes, fixed macrophages, and endothelium are all in contact with the blood and contribute to varying degrees to the cytokine response to LD100E coli. Because DEGR VIIa and tissue factor pathway inhibitor (TFPI) both exhibit this selective attenuation, we concluded that the phenomenon is real. This raises the question of whether these selective effects reflect action on either different tissues (endothelium v monoctye/macrophage) or at different stages (early v late) of the response of the same tissue.
Finally, we concluded that there was a correlation between the plasma concentration of IL-6 and the degree of renal glomerular capillary thrombosis. This supports previous studies associating IL-6 with increased procoagulant activity.16-18 IL-6 levels also returned to normal in animals infused with sublethal concentrations ofE coli (data not reported). Thus, the treated animals receiving LD100E coli and control animals receiving sublethal E coli showed similar patterns of IL-6 release, peaking at T = 3 to 4 hours followed by a return toward normal by T = 6 to 8 hours. In contrast, those control animals receiving only LD100E coli showed a similar release of IL-6 for the first 3 to 4 hours which, however, remained elevated out to T + 8 hours. It is interesting to note, however, that while a return of IL-6 toward normal was associated with survival, this pattern did not differentiate between the four of eight treated animals that survived from the four of eight treated animals that died. IL-6 returned toward normal in all eight cases of the treated animals regardless of whether they survived. This raises the question of whether in those four animals other overriding factors, which determine survival, came into play (eg, target cell sensitivity to TNF, etc).
ACKNOWLEDGMENT
We acknowledge the excellent assistance in preparing the manuscript performed by Joy Albert-Gorr and Penny Antkowiak and figures by Richard Irish.
Supported in part by National Institutes of Health (Bethesda, MD) Grant No. 2R01 GM37704 (to F.B.T.).
Address reprint requests to F.B. Taylor, Jr, MD, Cardiovascular Biology Research, Oklahoma Medical Research Foundation, 825 NE 13th St, Oklahoma City, OK 73l04.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal