Abstract
Ligneous conjunctivitis is a rare form of chronic pseudomembranous conjunctivitis that is associated with systemic membranous pathological changes. A probable link between plasminogen and ligneous conjunctivitis has been indicated by the recent diagnoses of plasminogen deficiency in five patients suffering from ligneous conjunctivitis. The current study reports that plasminogen-deficient mice develop conjunctival lesions indistinguishable from human ligneous conjunctivitis in both appearance and histology. Both human and mouse lesions contain acellular material rich in fibrin, and aberrant or disrupted epithelium. The incidence of lesion development in mice increases with age and is strongly influenced by genetic background. Interestingly, ligneous conjunctivitis was not observed in plasminogen-deficient mice simultaneously lacking fibrinogen. This study provides direct evidence that plasminogen deficiency is one cause of ligneous conjunctivitis and suggests that plasminogen-deficient mice may be an excellent model for the development of therapeutic strategies for the treatment of this debilitating disease.
LIGNEOUS CONJUNCTIVITIS is a rare form of chronic membranous conjunctivitis of unknown origin. Patients with ligneous conjunctivitis present with pseudomembranous “woodlike” lesions of the conjunctiva which result in scarring and impaired vision. White, yellowish, or red nodules form on the tarsal and bulbar conjunctivae, which can result in eversion of the eyelid.1Secondary corneal involvement including corneal melting, scarring, and development of extensive sessile lesions has also been reported.2 3
This disorder occurs most frequently in young children, with a slight predominance in females, and an autosomal recessive inheritance pattern is apparent in some cases.1,3,4 Increased childhood mortality rates accompany ligneous conjunctivitis due to associated systemic pathological changes,5-7 although elderly patients have been described.3 The chronic phase of this condition may persist for years, or spontaneously remit and recur. Treatment with antibiotics, antifungals, or immunosuppressants has been largely ineffective6,8,9 and attempts to identify a microbial causal agent have had little success.6,10 Surgical excision of conjunctival lesions is invariably followed by rapid reformation and often worsens the condition.1,2,7,11 Furthermore, several reports indicate that in rare cases, conjunctival surgery can initiate the formation of ligneous lesions.12-14 However, De Cock et al15 have reported remission of ligneous conjunctivitis in some patients after excision of the membranes followed by cauterization and application of topical heparin. The pathological changes associated with this condition are not restricted to the eye. Rather, lesions in other mucous membranes such as the nasopharynx, larynx, tongue, trachea, middle ear, gingiva, peritoneum, vagina, and cervix may occur in association with, or in place of, ocular manifestations.5,11,16-22 Occlusive hydrocephalus has also been noted in some patients with ligneous conjunctivitis.3,7 10
Histological examination of the membranous conjunctival lesions shows extensive epithelial ulceration with hyperplasia and extension of the epithelial layer into the substantia propria in the form of cysts and glandlike structures.2,7 Mucopolysaccharide exudate frequently overlies large areas of ulceration.3,11 Lesions contain large, sparsely cellular deposits of eosinophilic, periodic acid—Schiff-positive (PAS+) amorphous material with adjacent acute and/or chronic inflammatory cell infiltrates composed of neutrophils, T cells, macrophages, B cells, and mast cells.3,11-13,17,23 Neovascularization and deposition of plasma proteins such as immunoglobulin and albumin are frequently present, whereas lipid, amyloid, and keratin are generally not detectable.2,17 The amorphous deposits contain fibrillar material, consistent with fibrin, and stain intensely for fibrin by immunohistochemistry.3,23 24
Ligneous conjunctivitis has been associated with plasminogen (Plg) in recent reports describing five unrelated patients with severe Plg deficiency.25-27 Plg is an abundant plasma protein which is the zymogen precursor of the serine protease, plasmin, the key fibrinolytic enzyme.28 Plg-deficient (Plg−/−) mice were recently generated to define in greater detail the physiological roles of plasmin(ogen) in vivo.29,30 These mice develop to term and generally survive to adulthood, but the phenotypic consequences of Plg deficiency are severe and life expectancy is short. Plg−/− mice experience widespread thrombotic occlusions within terminal vessels, organ damage, and wasting. Fibrin-rich ulcerative lesions develop throughout the gastrointestinal, respiratory, and female genital tracts.29 31 The current study reports that Plg deficiency in mice results in the development of fibrin-rich conjunctival lesions that are indistinguishable from human ligneous conjunctivitis.
MATERIALS AND METHODS
Generation of cohorts of inbred and outbred mice.
Gene-deficient mice were generated in accordance with National Institutes of Health recombinant DNA guidelines, and study protocols were approved by the Children's Hospital Research Foundation Institutional Animal Care and Use Committee. All mice used in these studies were maintained in parallel by the same caregivers. Mice with single and combined deficits in Plg and fibrinogen (Fib) were genotyped as described previously.31 The genotypes of mice with combined tissue-type plasminogen activator (tPA) and urokinase (uPA) deficiencies32 were established by polymerase chain reaction (PCR), using ear biopsy DNA as a template. The wild-type tPA allele was detected with primers complementary to exon 10 (tPAEx10-131) and exon 11 (tPAEx11-1, 5′-TCTGCCCAAGACCACTTTAAGATGATT-3′) that together yield a 350-bp PCR product. The targeted tPA allele was detected by using tPAEx11-1 (see above) and a primer complementary to the PGK-Neo cassette inserted into the disrupted tPA gene (PGK-Neo, 5′-GTGCGAGGCCAGAGGCCACTTGTGTAGCG-3′) that together yield a 300-bp PCR product. The wild-type uPA allele was detected with primers complementary to exon 11 (uPAEx11-1, 5′-GCGATTCTGGAGGACCGCTTATCT-3′ and uPAEx11-3, 5′-ATTGAATCCAGTCCAGGAAGTGTGAGACCC-3′) that together yield a 141-bp PCR product. The targeted uPA allele was detected by using primers uPAEx11-3 (see above) and PGK-Neo 5′ that together yield a 160-bp PCR product.
The incidence of ligneous conjunctivitis was assessed in Plg−/− mice in a mixed 129/Black Swiss background29 and in mice backcrossed for six generations to C57B1/6J mice (Jackson Laboratories, Bar Harbor, ME). Groups of older Plg−/− mice of both backgrounds (C57B1/6J, n = 13; 129/Black Swiss, n = 18) and Plg+/− and Plg+/+ littermate controls (n = 24) were anesthetized with ketamine/xylazine/acepromazine (4:1:1), and eyelids were everted with forceps and examined with a dissecting microscope.
In later detailed studies, a prospective cohort of 17 C57B1/6J Plg−/− mice and 27 Plg+/− and Plg+/+ littermate control mice, aged between 51 to 70 days at the beginning of the observational period, were observed until 133 to 152 days of age. Mice were inspected weekly by an investigator unaware of the genotypes of the mice. Conjunctival lesion development was recorded along with general health parameters, including weight and development of rectal prolapse.
In a separate study, the development of ligneous conjunctivitis was investigated in Plg−/− (n = 17) and Plg−/−/Fib−/− (n = 11) mice. These mice were generated from a cross between inbred Plg−/−C57B1/6J33 and inbred Fib−/− C57B1/6J mice.34 Mice were age-matched with an age range of 60 to 172 days and a median age of 109 days. Lesions were detected in anesthetized mice by eversion of eyelids and viewed with a dissecting microscope. The presence of conjunctival lesions was explored in mice lacking both uPA and tPA in a retrospective evaluation of arbitrarily selected mice that had been sacrificed, formalin-fixed, and stored (n = 6).
Histopathology.
Eyes, eyelids, and surrounding skin were excised and fixed en bloc in neutral-buffered formalin (Sigma, St Louis, MO). Tissues were paraffin-embedded and sectioned at a thickness of 4 μm. Longitudinal, sagittal sections were taken at 180-μm intervals for sequential analysis. Tissue sections were routinely stained with hematoxylin and eosin, PAS stain, or Leder stain.35
Immunohistology.
Fibrin(ogen) was detected with a polyclonal rabbit anti-mouse fibrinogen serum (diluted 1:1,000) by using the Vectastain Elite ABC Kit (Vector Laboratories, Burlingame, CA) and diaminobenzidine (DAB; Sigma) substrate. Negative controls for staining consisted of parallel staining of conjunctival tissue from Fib−/−mice34 and application of nonimmune rabbit serum in place of the primary antibody in Plg−/− mice. Mouse immunoglobulin was detected with biotinylated anti-mouse IgG (Vector) and mouse CD4 and CD8 were detected with GK1.5 (monoclonal anti-mouse CD4, ATCC) and 2.43 (monoclonal anti-mouse CD8, ATCC) and the Vectastain Elite Kit.
Detection of Plg protein in plasma.
Plasma Plg was purified by lysine-Sepharose chromatography. Citrated plasma samples (60 μL) were diluted in an equal volume of buffer B (1.5 mmol/L potassium phosphate; 8 mmol/L sodium phosphate; 14 mmol/L sodium chloride; 3 mmol/L potassium chloride, pH 7.3) and combined with 60 μL (settled volume) of lysine-Sepharose 4B (Pharmacia Biotech, Piscataway, NJ). The suspensions were incubated at room temperature for 30 minutes with continuous mixing. Sepharose beads were collected by centrifugation at 3,000g for 30 seconds and resuspended in 1 mL of buffer B. This wash step was repeated eight times with the OD280 of the third wash being less than 0.02. Bound material was eluted from the lysine-Sepharose by a 30-minute incubation (with continuous mixing) with an equal volume of buffer B containing 0.4 mol/L ε-amino caproic acid. The eluates were diluted in sample buffer containing 2% sodium dodecyl sulfate (SDS) for gel fractionation and subsequent Plg detection by Western blotting. Proteins were fractionated by SDS-polyacrylamide gel electrophoresis (12% acrylamide), transferred to Immobilon P membranes (Millipore, Bedford, MA), and Plg was detected with sheep anti-rat Plg antiserum (kindly provided by Dr E. Reich, SUNY, Stony Brook, NY) that cross-reacts with mouse plasminogen as described previously.29 Bound primary antibody was detected by using the Vectastain ABC (peroxidase) kit (Vector) and the ECL chemiluminescence system (Amersham, Arlington Heights, IL).
Fib enzyme-linked immunosorbent assay (ELISA).
Plasma Fib levels of 129/Black Swiss Plg−/− and C57B1/6J Plg−/− mice were determined with an Fib-specific ELISA (Asserachrom-Fibrinogen; Diagnostica Stago, France) by using purified mouse Fib as a standard. Purified mouse Fib was a gift from Dr D.I. Simon (Brigham and Women's Hospital, Boston, MA).
RESULTS
Development of conjunctival and corneal lesions.
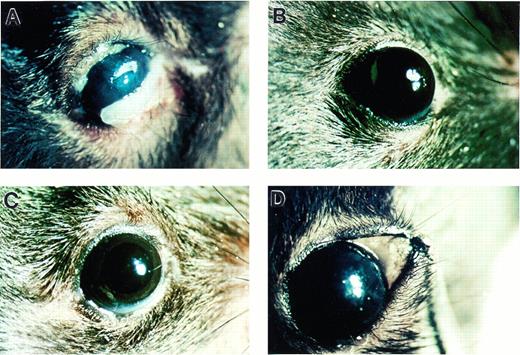
Conjunctival lesions were grossly apparent in adult C57B1/6J Plg−/− mice (Fig 1A),whereas no lesions resembling ligneous conjunctivitis were observed in either Plg+/− or Plg+/+ littermate control mice (Fig 1B through D). The earliest lesions appeared as subtle thickenings of the lower or upper eyelid. These lesions were accompanied by a whitish, irregular surface of the palpebral conjunctiva, an increase in vascularization of the lid, and external accumulations of mucus (Fig 2A). Corneal defects such as stromal haze and epithelial irregularities were occasionally present in mice with early conjunctival lesions (Fig 2B), but lesions always occurred in the palpebral conjunctiva prior to corneal involvement.
Photomicrographs indicating development of conjunctival lesions in Plg−/− (A) but not in Plg+/−(B) and Plg+/+ (C) mice. (D) Exposed palpebral conjunctiva from a Plg+/− mouse shows normal conjunctival tissue.
Photomicrographs indicating development of conjunctival lesions in Plg−/− (A) but not in Plg+/−(B) and Plg+/+ (C) mice. (D) Exposed palpebral conjunctiva from a Plg+/− mouse shows normal conjunctival tissue.
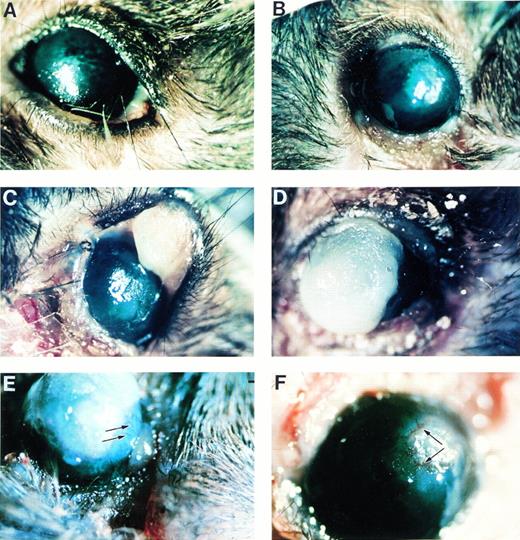
Photomicrographs of gross pathology of ligneous lesions in Plg−/− mice, showing mild and severe manifestations. (A) Thickened eyelid representing an early or mild lesion. Note that the observed cataract formed postmortem. (B) Stromal haze and epithelial defect; (C) pedunculated lesion in the upper lid, with ulceration and hemorrhage in the lower lid; (D) plaque extending above the corneal surface; (E) neovascularization of the cornea extending from the limbus into the plaque (arrows); and (F) corneal neovascularization around the circumference of a plaque (arrows).
Photomicrographs of gross pathology of ligneous lesions in Plg−/− mice, showing mild and severe manifestations. (A) Thickened eyelid representing an early or mild lesion. Note that the observed cataract formed postmortem. (B) Stromal haze and epithelial defect; (C) pedunculated lesion in the upper lid, with ulceration and hemorrhage in the lower lid; (D) plaque extending above the corneal surface; (E) neovascularization of the cornea extending from the limbus into the plaque (arrows); and (F) corneal neovascularization around the circumference of a plaque (arrows).
Lesions were progressive in nature, developing over a period of several weeks into extensive, pedunculated plaques (Fig 2C). Advanced lesions occurred in the lid margin, along the entire palpebral and bulbar conjunctivae, and on the cornea. Palpebral conjunctival alterations resulted in hypertrophied, everted eyelids with ulceration and hemorrhage. Scarring and hair loss were also commonly associated with advanced lesions. Ulceration and necrosis were observed in the cornea, and large lesions that extended 1 to 2 mm above the corneal surface developed in some mice (Fig 2D). Stromal opacification and neovascularization extending from the limbus were apparent throughout the lesion (Fig 2E and F). Occasionally, foreign bodies such as cage-bedding material were seen embedded in lesions.
Frequency of lesion development.
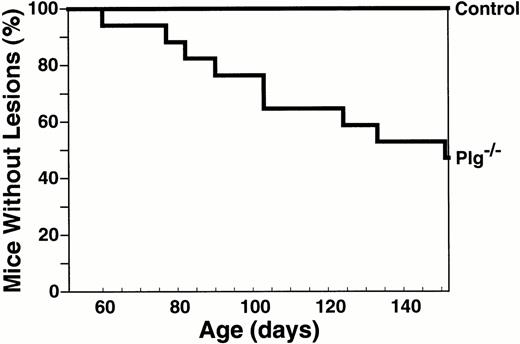
To define the age- and gender-dependence of ligneous lesion formation, a prospective cohort of highly inbred C57B1/6J Plg−/−mice was monitored for ocular lesion development over a period of several months. Changes in body weight and development of rectal lesions (a common feature of adult Plg−/− mice) were also recorded. Nine of 17 mice (53%) developed conjunctival lesions during the observational period (Fig 3).The median age of onset of lesions was in young adulthood, at 151 days. Gender was not a significant factor in the development of conjunctival lesions; lesions developed in four of eight female mice (50%; median age, 151 days) and five of nine male mice (56%; median age, 124 days) during the observational period. Development of lesions was predominantly bilateral. Thus, occurrence of a lesion in either eye was associated with a high incidence of involvement of the other eye. All conjunctival lesions developed after 60 days of age. At this age, the mean weight of inbred C57B1/6J Plg−/− mice was already significantly lower than that of littermate control mice [weight of 60-day-old Plg−/− and Plg+/+ mice was 18.5 ± 0.8 g (n = 16) and 23.5 ± 0.6 g (n = 14), respectively (mean ± SEM, P = .0001, Student's t-test)]. Rectal lesions developed in 12 of 17 mice (71%) with a median age of 126 days. Of the nine mice in the cohort that developed conjunctival lesions, eight also developed rectal lesions during the same period. A significant correlation was found between the age of onset of conjunctival lesions (median age, 151 days) and rectal lesions (median age, 126 days; P = .002; Kendall Rank Correlation Coefficient).
Development of ligneous conjunctivitis in Plg−/− mice, as a function of age. The development of ligneous lesions of the conjunctiva was followed in a prospective cohort of mice consisting of 17 Plg−/− mice and 27 littermate control mice of an inbred C57Bl/6J background until 151 days of age.
Development of ligneous conjunctivitis in Plg−/− mice, as a function of age. The development of ligneous lesions of the conjunctiva was followed in a prospective cohort of mice consisting of 17 Plg−/− mice and 27 littermate control mice of an inbred C57Bl/6J background until 151 days of age.
The genetic background of mice had a marked effect on both the frequency of lesion development and the severity of the lesions. In a separate study, a random selection of older mice from two genetic backgrounds of Plg−/− mice were examined for ocular lesion formation, including inbred C57B1/6J mice (median age, 190 days; range, 132 to 324 days) and outbred 129/Black Swiss mice29(median age, 235 days; range, 201 to 397 days). Thirteen of thirteen (100%) C57B1/6J mice included in this selection had conjunctival lesions at the time of examination. A significantly lower incidence of ligneous lesions, 3 of 18 mice (17%), and milder lesions were observed in the 129/Black Swiss background (P = .0001, Chi-square analysis).
To investigate the difference in susceptibility to ligneous lesion development between the two genetic backgrounds, hematologic parameters, including plasma Fib and Plg antigen levels, were investigated. Consistent with earlier results in 129/Black Swiss Plg−/− mice documenting that hepatic Plg mRNA, plasma Plg protein, and Plg activity were undetectable in assays sensitive to at least three orders-of-magnitude below normal,29 parallel analyses of plasma collected from adult Plg−/− mice of both C57B1/6J and 129/Black Swiss genetic backgrounds (median age, 80 days) showed a complete absence of Plg antigen, regardless of genetic background (data not shown). In contrast, Plg antigen was easily detected and was at similar levels in the plasma of Plg+/− mice of both genetic backgrounds (data not shown). Through use of a specific ELISA, parallel assays of plasma Fib-related antigen levels indicated that circulating Fib antigen was not appreciably different in Plg−/− mice of each genetic background (129/Black Swiss, 2.4 ± 0.5 mg/mL; C57B1/6J, 2.8 ± 0.7 mg/mL).
To investigate if Fib deficiency protects Plg mice from the development of ligneous conjunctivitis, a group of 11 Plg−/−/Fib−/− mice in a C57BL/6J background and 17 Plg−/− littermates were inspected. The median age in both groups was 109 days and the range was 60 to 172 days. Conjunctival lesions were detected in none of eleven Plg−/−/Fib−/− mice. In contrast, 7 of 17 Plg−/− littermates showed ligneous lesions (P = .014, Chi-square test). Additionally, formalin-fixed cadaveric mice in a hybrid 129/C57BL/6J genetic background and with combined uPA and tPA deficiency were retrospectively investigated for conjunctival lesion development. Of six mice examined, ligneous lesions were observed in two mice (median age, 139 days; range, 134 to 139 days). These observations were confirmed histologically. Thus, ligneous conjunctivitis seems to be a consequence of failed Plg activation resulting in impaired fibrin clearance.
Microscopic analyses of conjunctival and corneal lesions.
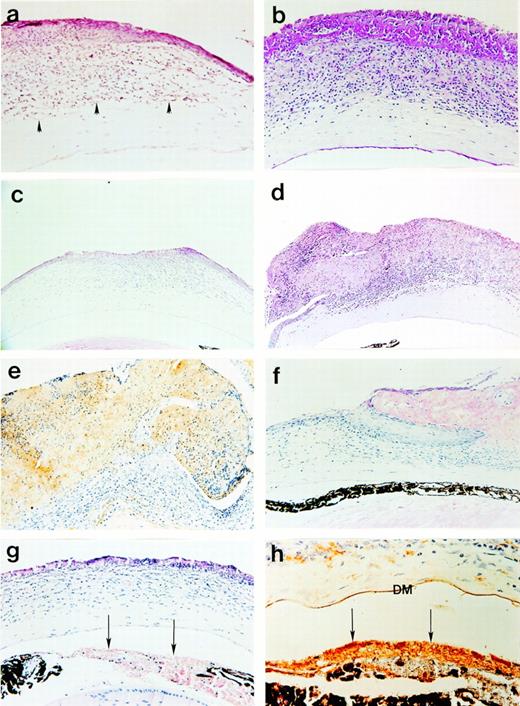
Consistent with the conjunctival and corneal defects observed grossly in Plg−/− mice, microscopic analyses of sectioned tissues revealed abnormal tissue organization. Extensive disruption of the conjunctival epithelium was evident in all lesions, with associated hypertrophy, disorganization, and reduplication consistent with chronic, recurrent ulceration and attempted re-epithelialization (Fig4). Amorphous, eosinophilic deposits were associated with areas of disrupted epithelium. These deposits were largely acellular and PAS+, indicating mucopolysaccharide (Fig 4C and D). These areas were shown to contain appreciable amounts of fibrin(ogen) (Fig 4E) and immunoglobulin (data not shown) by immunohistochemistry. No staining was observed with normal rabbit serum in Plg−/− mice (bottom, Fig 4E) or with Fib-specific antisera in the conjunctivae of Fib-deficient mice (data not shown). Acute inflammatory infiltrates with a predominance of neutrophils frequently accompanied conjunctival lesions (Fig 4F). Occasionally, CD4+ and CD8+lymphocytes were identified in the infiltrates by immunohistochemistry (data not shown). Tissue disorganization was highly evident in lesions containing “islands” of epithelial cells (including goblet cells) surrounded by PAS+ material (Fig 4G) or in lesions exhibiting epithelial reduplication around similar amorphous deposits (Fig 4H).
Photomicrographs of (a) normal palpebral conjunctiva from a Plg+/− mouse in contrast with (b through h) Plg−/− mice. (b) Large eosinophilic deposits with low cellular density constitute the majority of the area occupied by lesions. (c) Disrupted epithelium was associated with (d) amorphous PAS+ material (in the same lesion). (e) The amorphous material stained with an anti-mouse Fib antiserum (top) but not with normal rabbit serum (bottom; arrowheads indicate lesion area). (f) An acute inflammatory infiltrate with prominent neutrophils (arrows) is adjacent to amorphous, eosinophilic material. (g) Epithelial cells, including PAS+ goblet cells, existing as a discreet cluster (*) inside the eyelid show the degree of disorganization of the epithelium. (h) Reduplication of the epithelium (denoted by “e”) occurred adjacent to eosinophilic deposits. Panels b, c, and h are hematoxylin and eosin preparations; panels a, d, and g are PAS preparations; panel f is a Leder stain. Original magnifications are 100× (panels b and e), 200× (panels a, c, d, g, and h) and 400× (panel f).
Photomicrographs of (a) normal palpebral conjunctiva from a Plg+/− mouse in contrast with (b through h) Plg−/− mice. (b) Large eosinophilic deposits with low cellular density constitute the majority of the area occupied by lesions. (c) Disrupted epithelium was associated with (d) amorphous PAS+ material (in the same lesion). (e) The amorphous material stained with an anti-mouse Fib antiserum (top) but not with normal rabbit serum (bottom; arrowheads indicate lesion area). (f) An acute inflammatory infiltrate with prominent neutrophils (arrows) is adjacent to amorphous, eosinophilic material. (g) Epithelial cells, including PAS+ goblet cells, existing as a discreet cluster (*) inside the eyelid show the degree of disorganization of the epithelium. (h) Reduplication of the epithelium (denoted by “e”) occurred adjacent to eosinophilic deposits. Panels b, c, and h are hematoxylin and eosin preparations; panels a, d, and g are PAS preparations; panel f is a Leder stain. Original magnifications are 100× (panels b and e), 200× (panels a, c, d, g, and h) and 400× (panel f).
Marked abnormalities were also evident in the microscopic analyses of the cornea. Stromal vascularization, increased cellularity, and deposition of PAS+ material were frequently associated with corneal lesions (Fig 5A and B). Ulceration or destruction of the anterior epithelium and stroma were noted (Fig 5C) with extensive, protruding plaques overlying the cornea (Fig 5D). Fibrin(ogen) was found to be a significant component of such corneal plaques (Fig 5E). Corneal epithelium was frequently absent, necrotic, or hypertrophied. Epithelium accompanying severe corneal lesions was occasionally observed to form aberrant wedge-shaped projections, adjacent to amorphous fibrin-containing deposits, indicating that impaired re-epithelialization is a feature of these diseased tissues (Fig 5F). Formation of a pupillary or a retrocorneal membrane was observed in several mice, consistent with chronic intraocular inflammation (Fig 5G). This aberrant membrane stained intensely for fibrin(ogen) (Fig 5H). The formation of extensive fibrin(ogen)-rich plaques overlying the cornea, with associated epithelial ulceration, hypertrophy, and disorganization are consistent with an ongoing process of tissue injury, repair, and partial epithelialization.
Photomicrographs of corneal lesions in Plg−/− mice. (a) Stromal vascularization (arrowheads) in regions underlying extended plaques; (b) Extensive cellular infiltration and anterior deposition of PAS+ material, yet relatively normal posterior stroma with only mild inflammatory infiltration; (c) corneal ulceration; (d) An extensive corneal plaque which contained (e) abundant fibrin(ogen) matrix; (f) Formation of a wedge-like projection of epithelial cells was identified underlying but not penetrating the shoulder region of a plaque. The acellular, eosinophilic material above the “wedge” appeared to bisect the epithelial layer, leaving a thin layer of necrotic squamous cells exposed. (g) A pupillary membrane (arrows) formed in mice with severe corneal lesions. (h) This aberrant membrane stained intensely for fibrin(ogen) (arrows) by immunohistochemistry (DM denotes Descemet's membrane). Panels a, c, d, f, and g are hematoxylin- and eosin-stained, panel b is PAS-stained, and panels e and h are fibrin(ogen) immunohistochemistry. Original magnifications are 100× (panels c and d), 200× (panels a, b, e, f, and g) and 400× (panel h).
Photomicrographs of corneal lesions in Plg−/− mice. (a) Stromal vascularization (arrowheads) in regions underlying extended plaques; (b) Extensive cellular infiltration and anterior deposition of PAS+ material, yet relatively normal posterior stroma with only mild inflammatory infiltration; (c) corneal ulceration; (d) An extensive corneal plaque which contained (e) abundant fibrin(ogen) matrix; (f) Formation of a wedge-like projection of epithelial cells was identified underlying but not penetrating the shoulder region of a plaque. The acellular, eosinophilic material above the “wedge” appeared to bisect the epithelial layer, leaving a thin layer of necrotic squamous cells exposed. (g) A pupillary membrane (arrows) formed in mice with severe corneal lesions. (h) This aberrant membrane stained intensely for fibrin(ogen) (arrows) by immunohistochemistry (DM denotes Descemet's membrane). Panels a, c, d, f, and g are hematoxylin- and eosin-stained, panel b is PAS-stained, and panels e and h are fibrin(ogen) immunohistochemistry. Original magnifications are 100× (panels c and d), 200× (panels a, b, e, f, and g) and 400× (panel h).
DISCUSSION
Plg−/− mice spontaneously develop palpebral and bulbar conjunctivitis. Conjunctival lesions occurred with a high penetrance, often with bilateral involvement and with no evidence of a gender bias. The development of conjunctival lesions increased as a function of age and was associated with a general decline in health (indicated by a significant deviation from normal body weight and the development of rectal prolapse)29 in Plg−/− mice. The conjunctival lesions described closely resembled human ligneous conjunctivitis in gross appearance, distribution, and histologic features, including lesion composition and organization.2,3The current studies show that Plg deficiency alone is sufficient for the development of ligneous conjunctivitis in mice and that lesion development may be another manifestation of the many progressive disorders which befall Plg−/− mice with increasing age.29 30
Conjunctival lesions were a feature of adult Plg−/−mice in each of two genetic backgrounds evaluated, although mice with a mixed 129/Black Swiss background were significantly less susceptible to conjunctival lesion formation than the C57B1/6J inbred cohort followed in this study. Thus, additional genetic factors seem to contribute to lesion incidence and severity in Plg−/− mice. The secondary genetic factors that contribute, in combination with Plg deficiency, to the risk for developing conjunctival lesions are presently unknown, but differences in plasma Plg and/or Fib levels in Plg−/− mice were excluded. However, this does not preclude background-specific differences in the regulation of the coagulation system, or differences in the availability and efficiency of Plg-independent pathways for fibrin clearance. Whatever the factors are in Plg−/− mice that contribute to the risk of conjunctival and corneal lesions, it is clear that the loss of Fib prevents the development of these lesions. This finding strongly implies that diminished fibrinolysis and fibrin clearance in Plg−/− mice is mechanistically related to conjunctival lesion development.
Mice deficient in both Plg activators (uPA and tPA) have previously been reported to suffer from similar pathological manifestations as Plg−/− mice.29,32 Although no data have been collected regarding the frequency or timing of lesion development in mice with combined uPA/tPA deficiency, we have observed conjunctival and corneal lesions in these mice that histologically resemble those of Plg−/− mice. This indicates that ligneous conjunctivitis can be caused by more than one genetic disorder, and disorders other than Plg and Plg activator deficiency may also be encountered in ligneous conjunctivitis patients. It should also be noted that patients may develop ligneous conjunctivitis without any major underlying genetic deficit; a case report of a patient being treated for menorrhagia with tranexamic acid, a plasmin inhibitor, describes that systemic inhibition of plasmin was associated with the development of ligneous lesions in the conjunctiva, which regressed on cessation of treatment.19 Taken together, these data suggest that similar pathological processes may lead to the formation of ligneous lesions when plasmin-dependent events are interrupted in both humans and mice.
Conjunctival lesions of Plg−/− mice are invariably associated with epithelial disruptions. Minor wounding or disruption of the epithelium may be the initiating event in the formation of ligneous lesions. In normal human and mouse skin, injury results in the rapid deposition of fibrin to control blood loss and to provide a provisional extracellular matrix to support the formation of granulation tissue and re-epithelialization.36 The cellular organization of wound fields requires migration of keratinocytes, endothelial cells, fibroblasts, and other cell types through the fibrin-rich matrix and hence, pericellular proteolysis. In Plg−/− mice, a lack of plasmin-mediated proteolysis is a major impediment to healing in skin wound fields,37 characterized by impaired keratinocyte migration from the wound edges. Remarkably, these defects can be ameliorated by simultaneous Fib deficiency.31 Based on the presence of local epithelial defects and persistent fibrin deposits within conjunctival lesions of Plg−/− mice, together with the findings that defective wound repair and the deleterious formation of ocular lesions are corrected, in parallel, by simultaneous Fib deficiency, it seems likely that compromised tissue repair contributes to conjunctival lesion formation in these mice. The presence of persistent fibrin deposits adjacent to regions of epithelial disruption in the conjunctivae of Plg−/−mice may both stimulate inflammatory responses and impede timely re-epithelialization. Indeed, as reported previously for incisional skin wounds in Plg−/− mice,37 epithelial cells within conjunctival lesions were frequently observed at the periphery of the fibrinous deposits, seemingly unable to penetrate the fibrin-rich matrix in the absence of plasmin-mediated proteolysis. Thus, conjunctival lesions formed in Plg−/− mice may result from minor epithelial disruption, chronic inflammation, and a failure to resolve local tissue damage.
Clinical case studies in the literature provide additional support for the hypothesis that tissue injury is an initiating event in the formation of ligneous lesions in the conjunctiva. Several reports document induction of lesions shortly after surgical procedures12-14 and one case identifies a dog bite to the eyelid one month before ligneous lesion development.3Foreign bodies have been identified within human ligneous lesions and the aggravating effect of this material may have caused injury and inflammation sufficient to initiate lesion formation in these individuals.3 Although the mice in the current study were not subjected to invasive procedures, foreign bodies consisting of cage-bedding material were occasionally identified embedded in plaques. Scratching, or abrasion of foreign bodies against the conjunctival epithelium, may initiate development of ligneous lesions by disrupting the epithelium and underlying tissue, resulting in the accumulation of fibrin which persists in the absence of plasmin. Repetitive minor trauma and the progressive distortion of normal structure may set up a cycle of repetitive injury and abortive healing, resulting in the progression of these lesions.
Tissue injury may also result from microvascular thrombotic occlusion. As a de novo event, this seems unlikely because tissues in many anatomic locations are unaffected. However, lesion distribution in Plg−/− mice tends to occur in areas where microtrauma would be suspected. It is possible that microtrauma, secondary to mechanical tissue stress or stretching in such areas as the rectum or conjunctiva, may induce microthrombosis that results in localized tissue ischemia as an early event in the evolution of such lesions. Whatever the nature of the initiating event, concurrent diminution of plasmin-mediated proteolysis appears to be essential for ligneous lesion development.
Occurrence of lesions in the palpebral conjunctiva was always observed before corneal involvement. It is likely that involvement of the cornea is secondary to conjunctival lesion development, and may result from abrasion of ulcerated palpebral lesions against the corneal epithelium during eye closure and/or drying caused by impaired eye closure. Corneal injury induced by epithelial scraping with a blade in Plg−/− mice results in impaired wound healing, persistent fibrin-containing matrix, and corneal opacification.38 Corneal caps of the type seen in human ligneous conjunctivitis may develop in Plg−/− mice after repetitive surgical removal of the epithelium, but this has not yet been explored.
Recent reports in the literature have identified several patients with severe Plg deficiency.25-27 In addition to developing ligneous conjunctivitis, these patients sustained other clinically recognized disorders, including hyperviscosity of tracheobronchial and nasopharyngeal secretions, bronchopneumonia, gingival hyperplasia, and hydrocephalus. Given the obvious differences between humans and mice in anatomy, physiology, diet, environmental challenges (eg, pathogen exposure), and life expectancy, as well as potentially significant differences in Plg-independent proteolytic pathways, there is no reason to expect the Plg−/− humans and Plg−/−laboratory mice to be phenotypically identical. Nevertheless, there are many notable similarities between Plg−/− mice and humans, and more may be recognized as additional detailed studies are performed in each species. For example, pathological disturbances involving mucous membranes have been previously reported in both human ligneous conjunctivitis patients and in Plg−/−mice.5,17,18,26,29,30,39 Two recent reports describe impaired wound healing in two Plg−/−patients25,26 and retarded postnatal growth in one patient,25 both of which have been well documented in Plg−/− mice.29,30,40 Also, as noted in two patient case studies, hydrocephalus has been observed in Plg−/− mice, but the phenotype seems to be very rare (unpublished results, October 1997). One striking phenotypic feature of Plg−/− mice that has not yet been explored in patients with confirmed Plg deficiency is the development of thrombotic occlusions in terminal microvasculature, particularly in the gastrointestinal tract. Detailed histological evaluation of patient tissues collected at biopsy or autopsy will be useful in establishing whether this feature is shared between species. However, with regard to the hemostatic consequences of Plg deficiency, it is notable that presently there is no evidence of occlusive thrombotic events in large vessels of either Plg−/− mice or humans.25,26,29 30
Complete remission of ligneous conjunctivitis, including dissolution of fibrin and resolution of pseudomembranes, has been reported in a male infant after replacement therapy with lys-Plg.26 Increases in plasma D-dimer formation after addition of Plg indicated an otherwise intact capacity for fibrinolysis in this patient. Fibrin-containing deposits reappeared after cessation of treatment. Whether the therapeutic benefit of Plg administration will be equally apparent in other patients remains to be established. Rapid Plg clearance may be a sufficient limitation of this approach.25 Systemic antithrombotic drugs may prove to be an effective alternative but this has not yet been explored.
The current studies show that Plg deficiency results in the development of ligneous conjunctivitis in mice. These studies identify the Plg−/− mouse as an appropriate model for human ligneous conjunctivitis. Furthermore, Plg−/− mice may be useful in the development of clinical strategies to treat or prevent the life-threatening pathological conditions associated with ligneous conjunctivitis.
ACKNOWLEDGMENT
We are grateful to Dr Peter Gartside for assistance with statistical analyses, and Heidi Schiman and Chonnettia Jones for assistance with histological analyses and the Fib ELISA, respectively.
Supported by a Career Development Award from Research to Prevent Blindness, New York, NY (A.H.K.) and Grant No. HL47826 from the National Institutes of Health (J.L.D.). This study was performed during the tenure of an Established Investigatorship (J.L.D.) from the American Heart Association (93002570).
Address reprint requests to T.H. Bugge, PhD, Division of Developmental Biology, Children's Hospital Research Foundation, 3333 Burnet Ave NRB 2018A, Cincinnati, OH 45229.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal