Abstract
Interleukin-7 (IL-7) is a B-cell growth factor produced by both bone marrow stroma cells and follicular dendritic cells (FDCs) located in primary lymphoid follicles and germinal centers. In this study, we have evaluated the role of IL-7 on human Ig class switching. IL-7 was added to peripheral blood mononuclear cells (PBMCs) or tonsillar B cells in the absence or presence of IL-4 and/or anti-CD40 monoclonal antibody (MoAb). Alone, IL-7 did not affect Ig production by PBMCs or by anti-CD40 MoAb-stimulated B cells. Rather, IL-7 potentiated IL-4–induced IgE and IgG4 production by PBMCs. In parallel, IgG3 production was also enhanced but to a lesser extent, whereas the production of the other isotypes was unaltered. The activity of IL-2, IL-9, or IL-15, which share usage of the common γ chain for signaling, was also assessed. IL-9, like IL-7, potentiated mainly IgE and IgG4 production by IL-4–stimulated PBMCs. IL-15, in contrast, was ineffective, whereas IL-2 enhanced the production of all isotypes. More precisely, IL-7 potentiation of IgE and IgG4 production required the presence of T cells and was accompanied by an increase of the expression of two soluble molecules favoring preferentially IgE and IgG4 synthesis: CD23 (sCD23) and IL-9. Moreover, neutralizing anti-CD23 and anti–IL-9 antibodies partly inhibited the increase of IgE synthesis induced by IL-7. Thus, IL-7 produced locally in the germinal centers by FDCs may interact with T cells and potentiate human IgE and IgG4 switching by favoring IL-9 and sCD23 production.
INTERLEUKIN-7 (IL-7), produced by stroma cells and follicular dendritic cells (FDCs), exerts its effects through a receptor (IL7R) composed of an α and a γ chain.1-4The γ chain (γc) is a common component of the receptors for IL-2, IL-4, IL-9, and IL-15.5-7 IL7R controls T-cell lymphopoiesis and potentiates the proliferation and the production of lymphokines by mature T cells.8-10 IL-7 also has a critical role in B-cell lymphopoiesis because it favors the commitment of murine progenitor cells towards pro-B cells and promotes the growth and differentiation of pro-B and early pre-B cells.2,11Furthermore, IL-7 enhances Ig recombinase gene activity in human progenitor B cells and consequently may affect VDJ recombination.12 However, the effect of IL-7 on isotype switching remains undefined.
After activation, surface IgM+IgD+ human B cells switch to express different isotypes of antibody (Ab; IgG1-4, IgA1-2, or IgE). This process, which occurs mainly in the T-cell–rich apical zone of germinal centers is highly controlled by soluble cytokines and by B-T–cell interactions involving the CD40 molecule.13-15 The regulation of IgE switching, which has been extensively studied, requires two primary signals: the first, furnished by IL-4 or IL-13, induces the expression of the sterile ɛ transcript; the second, provided by the B-T–cell interaction and mimicked in vitro by CD40 triggering (using either CD40 ligand [CD40L] or an anti-CD40 monoclonal antibody [MoAb]), induces the expression of the mature ɛ transcript.16,17 IgE production is tightly controlled by costimulatory signals mediated by cell-cell contact and soluble mediators: The triggering of CD21 with CD23 and the cytokines IL-2, IL-6, and IL-9 upregulate IgE production,18-21 whereas interferon-α (IFN-α), IFN-γ, transforming growth factor-β (TGF-β), IL-10, IL-12, and PGE2 induce downregulation.22-25
Based on the observation that IL-7 is produced in the germinal centers where the switch process takes place,4 14 we have evaluated whether or not it may affect isotype switching. We report that IL-7 has no direct effect on human B cells but potentiates, in the presence of T cells, IL-4–induced IgE and IgG4 switching.
MATERIALS AND METHODS
Cell preparations.
Peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation on Ficoll/Paque (Pharmacia, Uppsala, Sweden). In some experiments, monocytes were removed by a two-step adherence assay on Sephadex G10 columns (Pharmacia), as described.26 In others, T-cell depletion was performed by sheep red blood cells rosetting. Residual T cells were labeled with anti-CD3 MoAb (Immunotech, Marseille, France) and removed with magnetic beads coated with anti-mouse Ig Ab (Dynal, Oslo, Norway). The percentage of residual monocytes or T cells determined by flow cytometry on a FACStar Plus cytofluorometer (Becton Dickinson, Erembodegem, Belgium) after labeling with fluorescein isothiocyanate (FITC)-labeled anti-CD14 or anti-CD2 MoAbs (both from Becton Dickinson), respectively, were <1%. Human B cells were purified from tonsils as described.27 Cells were cultured in enriched Iscove's medium.27
Ig assays.
Cells were added at 2 × 105/200 μL/well in 96-well plates (Nunc, Roskilde, Denmark) and stimulated, in replicates of five, with 0.5 to 50 ng/mL of human recombinant IL-7 (R&D Systems, Minneapolis, MN), 10 ng/mL of either IL-2 (Geneva Biomedical Research Institute [GBRI], Geneva, Switzerland), IL-9 (R&D Systems), or IL-15 (Genzyme, Cambridge, MA) in the absence or presence of 200 U/mL of IL-4 (GBRI), 0.1 μg/mL anti-CD40 MoAb (Serotec, Oxford, UK), or combinations of IL-4 plus anti-CD40 MoAb. In some experiments, PBMCs were stimulated with IL-4 in the presence of 10 ng/mL of IL-1α, IL-3, IL-5 (all from GBRI), IL-8, IL-9, IL-11, IL-12, IFN-γ (all from R&D Systems), IL-6, tumor necrosis factor-α (both from AMS Biotechnology, Lugano, Switzerland), or TGFβ1 (Sigma, St Louis, MO). In other experiments, PBMCs were stimulated with IL-4 in the presence or absence of 10 ng/mL of IL-7 with or without 20 μg/mL of neutralizing mouse IgG1 anti-CD23 MoAb (clone Mab 25, Serotec),28 neutralizing goat IgG anti–IL-9 Ab (R&D Systems), control mouse IgG1 MoAb, or control goat IgG Ab (both from Sigma). Supernatants were collected at day 12 to quantify Ig.
Ig quantification.
IgA1-2, IgE, IgG1-4, and IgM were quantified by enzyme-linked immunosorbent assay (ELISA) as described.27 29 Results are expressed in ng/mL or as a percentage of increase of Ig production defined as followed: (A−O/O) × 100, where A and O were the concentrations of Ig produced in the presence or absence of IL-7, respectively.
Proliferation assays.
Cells (2 × 105/200 μL/well) were stimulated in quintuplicate as described and pulsed at day 2 with 0.25 μCi/well3H-thymidine (Amersham International, Amersham, UK) for 6 hours. Radioactive incorporation was measured by standard liquid scintillation counting. Results are given in counts per minute (cpm) or as a stimulation index (SI) calculated as follows: A/O, where A and O were the cpm values obtained when cells were or were not cultured in the presence of the cytokine tested, respectively.
Immunostaining.
PBMCs were stimulated with 200 U/mL of IL-4 in the absence or presence of 10 ng/mL of IL-7. The expression of different cell surface antigens was evaluated at different time points by flow cytometry on B and T cells derived from PBMCs. PBMCs were incubated with FITC-labeled anti-CD21, -CD23, -CD40, control MoAbs (all from Becton Dickinson), CD23-, or glycophorin-A–fluorescent liposomes (used as a negative control) or with biotinylated anti-CD40L MoAb (all from GBRI). The binding of biotin–anti-CD40L was shown by FITC-labeled streptavidin (Sigma). B and T cells were identified using phycoerythrin (PE)-labeled anti-CD19 or anti-CD3 MoAbs, respectively (both from Becton Dickinson). Results are given as the expression of surface markers (frequency and mean fluorescence intensity [MFI] of positive cells) among B and T cells.
Quantification of soluble mediators.
IL-4 and IFN-γ were quantified by ELISA using specific capture and detection MoAbs from Pharmingen (San Diego, CA) and Genzyme, respectively. IL-6 and soluble CD23 (sCD23) were measured with ELISA kits from R&D Systems and Innogenetics (Zwijndrecht, Belgium), respectively.
Analysis of IL-9 mRNA expression by polymerase chain reaction (PCR).
PBMCs were either unstimulated or stimulated with 200 U/mL of IL-4 and/or 10 ng/mL of IL-7 for 24 hours. IL-9 mRNA expression was analyzed by PCR as previously described.30 Briefly, pelleted cells were resuspended in 1 mL of Trizol reagent (Life technologies, Basel, Switzerland). After addition of 0.2 mL chloroform, total RNA was precipitated by isopropylic alcohol. The single-strand cDNA was synthesized using 1 μg of total RNA by reverse transcription using an oligo-dT primer (Pharmacia). cDNA corresponding to 20 ng of total RNA was used for the amplification reactions. Each PCR reaction tube contained 10 mmol/L Tris-HCl (pH 8.3), 50 mmol/L KCl, 1 mmol/L MgCl2, 0.01% gelatin, 2 U AmpliTaq DNA polymerase (Perkin-Elmer Cetus, Norwalk, CT), 0.2 mmol/L of each deoxynucleotide (Pharmacia) and 1 μmol/L of each primer. Sequences of the oligonucleotides for the IL-9 transcript are as follows: 5′-ATGCTTCTGGCCATGGTCCT-3′ and 5′-TATCTTGCCTCTCATCCCTC-3′. Control consisted of amplifying β-actin transcripts using the following primers: 5′-CGATTTCCCGCTCGGCCGTGGTGGTGAAGC-3′ and 5′-GGCGACGAGGCCCAGAGCAAGAGAGGCATC-3′. The amplification consisted of one cycle for 4 minutes at 94°C followed by 35 cycles for 30 seconds at 94°C, 60°C for 1 minute, and 72°C for 1 minute. The final extension cycle was for 5 minutes at 72°C. PCR products were size-separated on a 1% agarose gel and visualized by ethidium bromide staining.
Analysis of IgE mRNA transcription by Northern blotting.
PBMCs were either unstimulated or stimulated for 10 days with 200 U/mL of IL-4 and/or 10 ng/mL of IL-7. Total RNA extraction and hybridizations were performed with cRNA probes complementary to Cɛ and β-actin mRNA.17 IgE protein was quantified in the supernatants by ELISA.
Statistical analysis.
Statistical significance was determined using the Student'st-test.
RESULTS
IL-7 potentiates IL-4–induced IgE and IgG4 production by PBMCs.
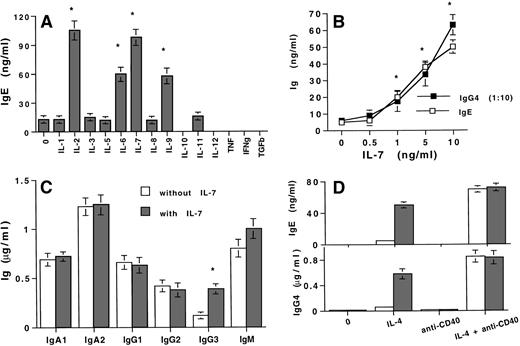
The production of IgE, which results from the stimulation of PBMCs with IL-4, is upregulated by different cytokines such as IL-2, IL-6, and IL-919-25 (Fig 1A). We report that IL-7 also potentiates IL-4–induced IgE and IgG4 synthesis; this effect is significant with 1 (P < .01) and maximal with 10 ng/mL of IL-7 (increase in IgE and IgG4 of 850% ± 120% and 990% ± 125%, respectively, mean percent ± standard deviation [SD], n = 6; Fig 1B). In parallel, IgG3 production is weakly increased (increase of 264% ± 90%), whereas IgA1-2, IgG1-2, and IgM production are unaffected (Fig 1C). As expected,8 31 IL-7 also increases the proliferation of PBMCs either unstimulated (SI, 5 ± 1.2, mean ± SD, n = 3) or stimulated with IL-4 (SI, 4 ± 0.9; Table 1).
Effect of IL-7 on Ig production by PBMCs from healthy subject. (A) IL-7 increases IgE production by IL-4–stimulated PBMCs. IL-4–stimulated PBMCs were incubated with 10 ng/mL of the different cytokines and IgE was quantified by ELISA. (B) IL-7 increases IgE and IgG4 production in a dose-dependent manner. PBMCs were stimulated with IL-4 with or without 0.5 to 50 ng/mL of IL-7. IgE (□) and IgG4 (1:10) (▪) were quantified by ELISA. (C) Effect of IL-7 on the production of other isotypes by 200 U/mL of IL-4–stimulated PBMCs. PBMCs were stimulated with 200 U/mL of IL-4 without (□) or with (▪) 10 ng/mL of IL-7 before quantification of IgA1, IgA2, IgG1-3, and IgM by ELISA. (D) IL-7 potentiates IL-4–induced IgE and IgG4 production in the absence of anti-CD40 MoAb. IgE and IgG4 were quantified in the supernatants of PBMCs either unstimulated or stimulated with IL-4, anti-CD40 MoAb, or combination of IL-4 plus anti-CD40 MoAb in the absence (□) or presence (▪) of 10 ng/mL IL-7. (A-D) Ig isotypes were quantified in the day-12 supernatants. Results are expressed in ng or μg/mL (as mean ± SD of quintuplicate values) and are representative of one of four (A and C) or six (B and D) separate experiments. * indicates P < .01.
Effect of IL-7 on Ig production by PBMCs from healthy subject. (A) IL-7 increases IgE production by IL-4–stimulated PBMCs. IL-4–stimulated PBMCs were incubated with 10 ng/mL of the different cytokines and IgE was quantified by ELISA. (B) IL-7 increases IgE and IgG4 production in a dose-dependent manner. PBMCs were stimulated with IL-4 with or without 0.5 to 50 ng/mL of IL-7. IgE (□) and IgG4 (1:10) (▪) were quantified by ELISA. (C) Effect of IL-7 on the production of other isotypes by 200 U/mL of IL-4–stimulated PBMCs. PBMCs were stimulated with 200 U/mL of IL-4 without (□) or with (▪) 10 ng/mL of IL-7 before quantification of IgA1, IgA2, IgG1-3, and IgM by ELISA. (D) IL-7 potentiates IL-4–induced IgE and IgG4 production in the absence of anti-CD40 MoAb. IgE and IgG4 were quantified in the supernatants of PBMCs either unstimulated or stimulated with IL-4, anti-CD40 MoAb, or combination of IL-4 plus anti-CD40 MoAb in the absence (□) or presence (▪) of 10 ng/mL IL-7. (A-D) Ig isotypes were quantified in the day-12 supernatants. Results are expressed in ng or μg/mL (as mean ± SD of quintuplicate values) and are representative of one of four (A and C) or six (B and D) separate experiments. * indicates P < .01.
Comparison Between the Effect of IL-7 and Either IL-2, IL-9, or IL-15 on Proliferation and IgE Production by PBMCs or Purified B cells
| . | PBMCs . | Purified Tonsillar B Cells . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Proliferation (cpm × 103) . | IgE (ng/mL) . | Proliferation (cpm × 103) . | IgE (ng/mL) . | ||||||
| None . | IL-4 . | None . | IL-4 . | None . | α-CD40 . | IL-4 . | α-CD40 . | IL-4 + α-CD40 . | |
| None | 3 ± 0.6 | 11 ± 2 | ND | 6 ± 1 | 2.5 ± 0.2 | 4.8 ± 1 | ND | ND | 48 ± 5 |
| IL-2 | 55 ± 6 | 117 ± 26 | ND | 63 ± 12 | 36 ± 6 | 28 ± 2 | ND | ND | 52 ± 11 |
| IL-7 | 16 ± 1 | 42 ± 5 | ND | 48 ± 7 | 2.8 ± 0.3 | 4.6 ± 0.8 | ND | ND | 47 ± 8 |
| IL-9 | 3 ± 0.4 | 13 ± 2 | ND | 24 ± 5 | 7.2 ± 0.6 | 13 ± 2 | ND | ND | 50 ± 5 |
| IL-15 | 56 ± 8 | 130 ± 15 | ND | 5 ± 1 | 23 ± 4 | 25 ± 3 | ND | ND | 45 ± 6 |
| . | PBMCs . | Purified Tonsillar B Cells . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Proliferation (cpm × 103) . | IgE (ng/mL) . | Proliferation (cpm × 103) . | IgE (ng/mL) . | ||||||
| None . | IL-4 . | None . | IL-4 . | None . | α-CD40 . | IL-4 . | α-CD40 . | IL-4 + α-CD40 . | |
| None | 3 ± 0.6 | 11 ± 2 | ND | 6 ± 1 | 2.5 ± 0.2 | 4.8 ± 1 | ND | ND | 48 ± 5 |
| IL-2 | 55 ± 6 | 117 ± 26 | ND | 63 ± 12 | 36 ± 6 | 28 ± 2 | ND | ND | 52 ± 11 |
| IL-7 | 16 ± 1 | 42 ± 5 | ND | 48 ± 7 | 2.8 ± 0.3 | 4.6 ± 0.8 | ND | ND | 47 ± 8 |
| IL-9 | 3 ± 0.4 | 13 ± 2 | ND | 24 ± 5 | 7.2 ± 0.6 | 13 ± 2 | ND | ND | 50 ± 5 |
| IL-15 | 56 ± 8 | 130 ± 15 | ND | 5 ± 1 | 23 ± 4 | 25 ± 3 | ND | ND | 45 ± 6 |
PBMCs and tonsillar B cells were either unstimulated or stimulated with IL-4 and/or anti-CD40 MAb in the absence or presence of 10 ng/mL of IL-2, IL-7, IL-9, or IL-15. Profiferation was measured at day 3 and IgE were quantified in the supernatants at day 12. Results are expressed in cpm × 103 or in ng/mL, respectively, as mean ± SD of quintuplicate values. Data are representative of one of three experiments.
Abbreviation: ND, not detectable.
PBMCs either unstimulated or stimulated with anti-CD40 MoAb produce low levels of IgG4 (<25 ng/mL, n = 6) and no IgE. In both cases, addition of IL-7 does not induce IgE nor affect the production of IgG4 (Fig 1D) or other Ig isotypes (data not shown). Moreover, the stimulation of PBMCs with IL-4 plus anti-CD40 MoAb results in a large production of IgE and IgG4, which is unaffected by IL-7 (Fig 1D). Thus, IL-7 synergizes with IL-4 to induce IgE and IgG4 production by PBMCs.
IL-7 increases IL-4–induced ɛ transcript expression by PBMCs.
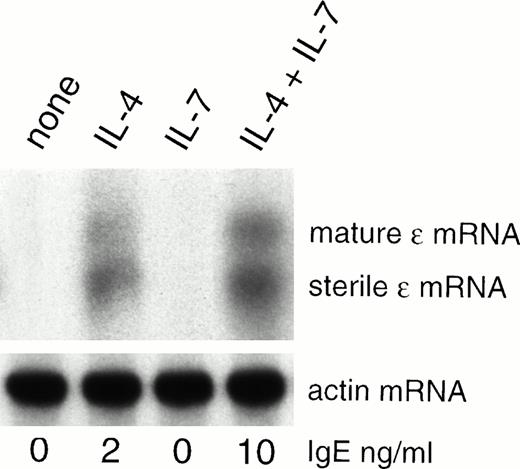
In view of the preceding results, we have evaluated whether or not IL-7 affects the expression of the ɛ transcripts. PBMCs either unstimulated or stimulated with IL-7 do not express the ɛ transcripts. A stimulation with IL-4 is required to induce their expression resulting in IgE production (Fig2).16 17 Addition of IL-7 enhances the expression of both the sterile and mature ɛ transcripts induced by IL-4 (Fig 2). Thus, IL-7 potentiates IL-4–induced IgE synthesis by enhancing the ɛ transcript expression.
IL-7 enhances ɛ transcripts expression. PBMCs were either unstimulated or stimulated with IL-7 and/or IL-4 for 10 days. RNA was isolated and used for Northern blot analysis using probes complementary to Cɛ (upper panel). As control, probes specific for actin were used (lower panel). In parallel, IgE was quantified in the day-10 supernatants.
IL-7 enhances ɛ transcripts expression. PBMCs were either unstimulated or stimulated with IL-7 and/or IL-4 for 10 days. RNA was isolated and used for Northern blot analysis using probes complementary to Cɛ (upper panel). As control, probes specific for actin were used (lower panel). In parallel, IgE was quantified in the day-10 supernatants.
Comparison between the effect of IL-7 and other cytokines in which receptors use the γc.
Like IL-7, the cytokines IL-2, IL-4, IL-9, and IL-15 have specific receptors that use the γc and modulate B-cell functions.5-7,16,19-21,32 Moreover, IL-2, IL-4, and IL-9 control IgE synthesis.16 19-21 As such, we have compared the effect of these cytokines on IgE synthesis with those of IL-7.
IL-2, IL-7, IL-9, and IL-15 are unable to replace IL-4 or anti-CD40 MoAb in inducing IgE production by purified B cells nor do they modulate IgE production induced by IL-4 plus anti-CD40 MoAb (Table 1). Nevertheless, IL-2, IL-15,32 and to a lesser extent IL-9 have a direct effect on B cells because they increase the proliferation of B cells either unstimulated or stimulated with an anti-CD40 MoAb, whereas IL-7 is ineffective (SI <2; Table 1).
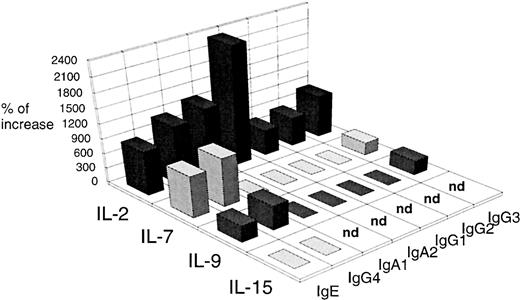
Except for IL-4, none of these cytokines induce IgE (Table 1) nor IgG4 production (data not shown) by PBMCs. However, IL-2, IL-7, and IL-9 potentiate IL-4–induced IgE and IgG4 synthesis by PBMCs whereas IL-15 is ineffective. IL-2 and IL-7 are more efficient than IL-9 (increase of IgE production of 900% ± 100%, 850% ± 120%, and 336% ± 66%, respectively; mean% ± SD, n = 3; Fig 3). As observed with IL-7, IL-9 does not significantly affect IgA1, IgA2, IgG1, and IgG2 production and only increases IgG3 production to a small extent (increase of 220% ± 79%; Fig 3). In contrast, IL-2 potentiates the production of all the isotypes, mainly IgA1 and IgA2 (increase of 1,460% ± 150% and 2,300% ± 350%, respectively; Fig 3). As previously reported,33 in contrast to the T-cell growth factors IL-2, IL-7, and IL-15,8,31 34 IL-9 does not enhance the proliferation of PBMCs either unstimulated or stimulated with IL-4 (SI <2; Table 1). Thus, IL-7 appears more potent than IL-9 and more selective than IL-2 in potentiating IL-4–induced IgE and IgG4 synthesis by PBMCs.
Comparison of the effect that IL-2, IL-7, IL-9, and IL-15 have on Ig production by IL-4–stimulated PBMCs. PBMCs were stimulated with 200 U/mL of IL-4 without or with 10 ng/mL IL-2, IL-7, IL-9, or IL-15. IgA1, IgA2, IgE, and IgG1-4 were quantified using supernatants harvested after 12 days of culture. Results are expressed in percent of Ig increase as described in the Material and Methods and are representative of one of three experiments.
Comparison of the effect that IL-2, IL-7, IL-9, and IL-15 have on Ig production by IL-4–stimulated PBMCs. PBMCs were stimulated with 200 U/mL of IL-4 without or with 10 ng/mL IL-2, IL-7, IL-9, or IL-15. IgA1, IgA2, IgE, and IgG1-4 were quantified using supernatants harvested after 12 days of culture. Results are expressed in percent of Ig increase as described in the Material and Methods and are representative of one of three experiments.
IL-7 requires the presence of T cells to potentiate IgE and IgG4 production.
To test whether IL-7 enhances IgE synthesis by acting directly on B cells, we have compared its ability to modulate IgE production by purified B cells and by PBMCs depleted either in monocytes or in T cells.
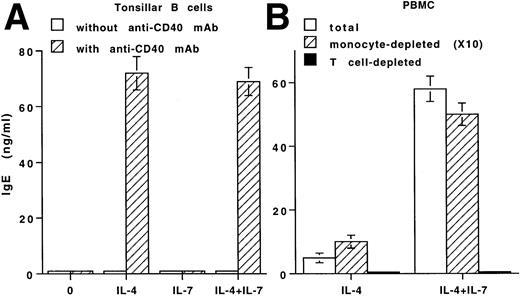
B cells require a stimulation via both the IL-4 receptor and CD40 to produce IgE.16 IL-7 does not induce IgE production by tonsillar B cells, either unstimulated or stimulated, with IL-4 or anti-CD40 MoAb nor does it modulate IgE production induced by IL-4 plus anti-CD40 MoAb (Fig 4A). Such lack of activity of IL-7 was also observed with purified peripheral blood B cells (data not shown). In parallel, IL-7 does not affect other isotypes' production by anti-CD40 MoAb-stimulated B cells (data not shown) nor the proliferation of B cells, either unstimulated or stimulated, with anti-CD40 MoAb (SI <2; Table 1).
Evaluation of the effect of IL-7 on IgE production by different cell populations. (A) IL-7 does not affect IgE production by purified B cells. Tonsillar B cells were either unstimulated or stimulated with IL-7 and/or IL-4 in the absence (□) or presence (▨) of anti-CD40 MoAb. IgE were quantified in the day-12 supernatants. (B) The effect of IL-7 on IgE production requires the presence of T cells. PBMCs depleted or not (□) of monocytes (×10) (▨) or of T cells (▪) were either unstimulated or stimulated with IL-7 and/or IL-4. IgE was quantified in the supernatant of 12 days. (A and B) Results are expressed in ng/mL (as mean ± SD of quintuplicate values) and are representative of one of three separate experiments.
Evaluation of the effect of IL-7 on IgE production by different cell populations. (A) IL-7 does not affect IgE production by purified B cells. Tonsillar B cells were either unstimulated or stimulated with IL-7 and/or IL-4 in the absence (□) or presence (▨) of anti-CD40 MoAb. IgE were quantified in the day-12 supernatants. (B) The effect of IL-7 on IgE production requires the presence of T cells. PBMCs depleted or not (□) of monocytes (×10) (▨) or of T cells (▪) were either unstimulated or stimulated with IL-7 and/or IL-4. IgE was quantified in the supernatant of 12 days. (A and B) Results are expressed in ng/mL (as mean ± SD of quintuplicate values) and are representative of one of three separate experiments.
Monocyte-depleted PBMCs stimulated with IL-4 produce very low levels of IgE (0.8 ng/mL ± 0.3, mean ± SD, n = 3),19 24 and the addition of IL-7 potentiates this production (increase of 501% ± 120%, n = 3; Fig 4B).
As expected, IL-4 or anti-CD40 MoAb does not induce IgE production by PBMCs in which T cells are depleted (Fig 4B) or cultured in an insert (data not shown).15,16 19 In both conditions, addition of IL-7 is unable to induce it. Thus, IL-7 does not modulate IgE production by purified B cells but potentiates IL-4–induced IgE synthesis via T-cell–mediated events.
Effect of IL-7 on IgE-regulating pathways.
To understand the mechanism responsible for the effect of IL-7 on IgE and IgG4 synthesis by PBMCs, we have evaluated whether or not IL-7 modulates the expression of cell-associated (CD21, CD23, CD40, and CD40L) or soluble molecules (IL-4, IL-6, IL-9, IFN-γ, and sCD23) that control IgE and IgG4 production.16-21 23
Stimulation of PBMCs with IL-4 results in an increase of membrane CD23 expression and in the binding of CD23-liposomes to B cells16,18; in contrast, CD21 and CD40 expression is unaffected and CD40L expression remains undetectable. Addition of IL-7 to IL-4–stimulated PBMCs for 1, 3, or 5 days does not modulate the expression of CD21, CD23, and CD40 nor affect the binding of CD23 liposomes and does not induce CD40L expression on B cells (Table 2A). In parallel, the expression of both CD23 and CD40L is undetectable on T cells derived from PBMCs either unstimulated or stimulated with IL-4 and/or IL-7, whatever the time point analyzed (data not shown).
Effect of IL-7 on Molecules Controlling IgE Synthesis
| AStimulus . | CD21 . | CD23-1 . | CD23 . | CD40 . | CD40L . |
|---|---|---|---|---|---|
| None | 93% 54 | 42% 55 | 2% 53 | 99% 138 | <1% |
| IL-7 | 90% 53 | 46% 63 | 2% 98 | 99% 138 | <1% |
| IL-4 | 90% 54 | 71% 159 | 38% 110 | 99% 220 | <1% |
| IL-4 + IL-7 | 88% 53 | 74% 151 | 38% 107 | 99% 219 | <1% |
| AStimulus . | CD21 . | CD23-1 . | CD23 . | CD40 . | CD40L . |
|---|---|---|---|---|---|
| None | 93% 54 | 42% 55 | 2% 53 | 99% 138 | <1% |
| IL-7 | 90% 53 | 46% 63 | 2% 98 | 99% 138 | <1% |
| IL-4 | 90% 54 | 71% 159 | 38% 110 | 99% 220 | <1% |
| IL-4 + IL-7 | 88% 53 | 74% 151 | 38% 107 | 99% 219 | <1% |
| BStimulus . | IL-4 . | IL-6 . | IFNγ . | s. CD23 . |
|---|---|---|---|---|
| None | <0.1 | 76 ± 7 | 3.1 ± 0.2 | 2.4 ± 0.2 |
| IL-7 | <0.1 | 112 ± 15 | 7.7 ± 1.2 | 3.8 ± 0.5 |
| IL-4 | ND | 13 ± 3 | 1.7 ± 0.4 | 8.9 ± 1.1 |
| IL-4 + IL-7 | ND | 26 ± 5 | 1.8 ± 0.3 | 23.6 ± 4.2 |
| BStimulus . | IL-4 . | IL-6 . | IFNγ . | s. CD23 . |
|---|---|---|---|---|
| None | <0.1 | 76 ± 7 | 3.1 ± 0.2 | 2.4 ± 0.2 |
| IL-7 | <0.1 | 112 ± 15 | 7.7 ± 1.2 | 3.8 ± 0.5 |
| IL-4 | ND | 13 ± 3 | 1.7 ± 0.4 | 8.9 ± 1.1 |
| IL-4 + IL-7 | ND | 26 ± 5 | 1.8 ± 0.3 | 23.6 ± 4.2 |
PBMCs were either unstimulated or stimulated with IL-4 and/or 10 ng/mL IL-7. (A) The B-cell surface expression of CD21, CD23, CD40, CD40L, and the binding of CD23-liposomes (CD23-I) was evaluated by FACS analysis at day 3. (B) Cytokines and sCD23 were quantified in 3-day supernatants. Results are expressed in ng/mL (mean ± SD of triplicate values). The percentage and the MFI of the positive B cells is reported. Data are representative of one of four experiments.
Abbreviations: ND, not done; MFI, mean fluorescence intensity.
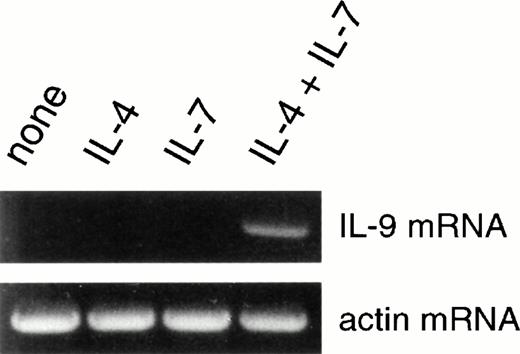
IL-7 increases the spontaneous production of IL-6, IFN-γ, and sCD23 by PBMCs (increase of 51% ± 9%, 150% ± 21%, and 63% ± 12%, n = 4) but does not induce IL-4 production (Table 2B). When added to IL-4–stimulated PBMCs, IL-7 does not affect IFN-γ production but upregulates IL-6 and sCD23 production (increase of 102% ± 18% and 190% ± 27%, respectively; Table 2B). Interestingly, the expression of mRNA encoding for the T-cell–derived cytokine, IL-9, is undetectable in PBMCs stimulated for 24 hours with IL-4 or IL-7 but is induced by stimulation with both IL-4 plus IL-7 (Fig 5). Thus, IL-7 increases sCD23 and to a lesser extent IL-6 production and upregulates IL-9 mRNA transcription by IL-4–stimulated PBMCs.
IL-7 synergizes with IL-4 to upregulate IL-9 mRNA expression. PBMCs were either unstimulated or stimulated with IL-4 and/or IL-7. After 24 hours, IL-9 mRNA expression was evaluated by PCR.
IL-7 synergizes with IL-4 to upregulate IL-9 mRNA expression. PBMCs were either unstimulated or stimulated with IL-4 and/or IL-7. After 24 hours, IL-9 mRNA expression was evaluated by PCR.
IL-7 potentiation of IgE synthesis is mediated at least partly through an enhancement of both sCD23 and IL-9 expression.
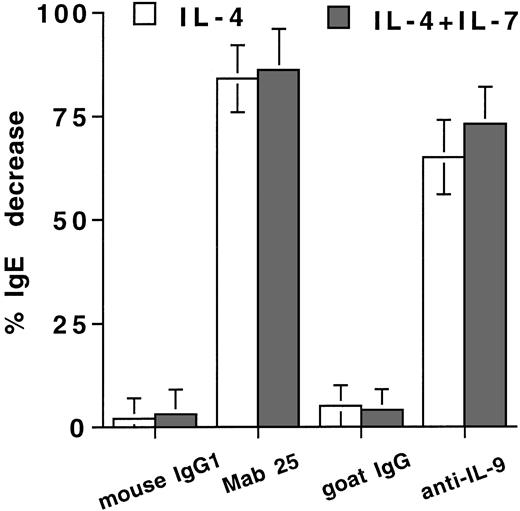
As IL-9 and sCD23 costimulate preferentially IgE and IgG4 synthesis by IL-4–stimulated PBMCs,18,20,21 we have evaluated whether IL-7 enhances IgE synthesis by increasing their production. As expected, the neutralizing anti-CD23 MoAb, Mab 25, decreases IgE production by IL-4–stimulated PBMCs (decrease of 84% ± 8%, n = 4)18 28 (Fig 6). A neutralizing anti–IL-9 Ab also decreases IgE synthesis by IL-4–stimulated PBMCs (decrease of 65% ± 11%; Fig 6). When PBMCs are stimulated with IL-4 plus IL-7, addition of the anti-CD23 or anti–IL-9 Ab decreases IgE production to a similar extent as observed in the absence of IL-7 (decrease of 86% ± 10% and 73% ± 9%, respectively; Fig 6).
IL-7 potentiation of IL-4–induced IgE synthesis is at least partly through sCD23 and IL-9. PBMCs were stimulated with IL-4 in the absence (□) or presence of 10 ng/mL IL-7 (▪) with or without neutralizing anti-CD23 MoAb (Mab 25), neutralizing goat anti–IL-9 IgG, control mouse IgG1, or control goat IgG. IgE was quantified in the day-12 supernatants. Results are expressed in percent of decrease as mean ± SD of four experiments.
IL-7 potentiation of IL-4–induced IgE synthesis is at least partly through sCD23 and IL-9. PBMCs were stimulated with IL-4 in the absence (□) or presence of 10 ng/mL IL-7 (▪) with or without neutralizing anti-CD23 MoAb (Mab 25), neutralizing goat anti–IL-9 IgG, control mouse IgG1, or control goat IgG. IgE was quantified in the day-12 supernatants. Results are expressed in percent of decrease as mean ± SD of four experiments.
Thus, IL-7 may potentiate IgE synthesis, at least in part, by enhancing both sCD23 and IL-9 production.
DISCUSSION
We have shown in this study that IL-7 synergizes with IL-4 to induce human IgE and IgG4 production in a T-cell–dependent manner. This effect is mediated at least in part through an increase of both sCD23 and IL-9 production.
The lymphokines that use the γc for signal transduction, IL-2, IL-4, IL-7, IL-9, and IL-15, affect B-cell functions.19-21,32Thus, we have compared their effect on IgE synthesis. Firstly, in contrast to IL-4, we confirm that IL-2, IL-9, and IL-15 are not switch factors for IgE and extend this observation to IL-7. Secondly, although IL-2 and IL-15 have comparable activities on purified B cells (both increase proliferation and IgA, IgG, and IgM production induced by anti-CD40 MoAb),32 we found that they differentially modulate IgE and IgG4 production by IL-4–stimulated PBMCs. IL-2 induces a potent increase of the production of IgE, IgG4,19and other isotypes by IL-4–stimulated PBMCs. In contrast, IL-15 does not increase IgE and IgG4 production. These data suggest that the effects of IL-2 and IL-15 on T cells or monocytes probably differ. Thirdly, we have shown that IL-7 and IL-9 have a similar effect on IgE and IgG4 production by PBMCs because they both potentiate IL-4–induced IgE and IgG4 and, in a lower extent, IgG3 production. Although it has been proposed that IL-9 may potentiate IL-4–induced IgE synthesis by acting directly on B cells,20 21 we reported no direct effect of IL-9 on IgE production by IL-4 plus anti-CD40 MoAb-stimulated B cells. Finally, although IL-2, IL-4, IL-7, and IL-9 positively control IgE synthesis, each lymphokine presents an individual pattern of effects based on differences in intensity and selectivity of the response and on the nature and the degree of maturation of the target cells. These differences could be related to an effect on distinct cell subpopulations or to binding to receptors associated with a specific intracellular signaling pathway. Nevertheless, the main point is that, in contrast to IL-2, both IL-7 and IL-9 costimulate preferentially IgE and IgG4 synthesis.
IL-7 is crucial for murine B lymphopoiesis2 but poorly affects human precursor B-cell proliferation and development.12,35 Furthermore, IL7R expression disappears during the B-cell maturation process.2,36 Both these observations may explain the absence of effect of IL-7 on B-cell proliferation reported in this study. Interestingly, results suggest that IL-7 acts through T cells to potentiate IgE and IgG4 production. Thus, IL-7 may affect the production/expression by T cells of factors that control IgE synthesis, such as IL-4, IL-6, IL-9, IL-13, IFN-γ, sCD23, or the cell surface molecules CD23, CD21, or CD40L.16,18,19-21,23,30 Based on different data, it seems unlikely that IL-7 increases IgE production by enhancing the expression of the IgE switch factors, IL-4 and CD40L. Supraoptimal concentrations of exogenous IL-4 are present in these experiments. In accordance with data showing that IL-7 potentiates IL-4 production by T cells but does not induce it,9,10 we found that PBMCs stimulated with IL-7 do not produce IL-4. Moreover, membrane CD40L expression is undetectable on T cells derived from IL-437 or IL-4 plus IL-7–stimulated PBMCs. Finally, IL-7 does not replace IL-4 or CD40 triggering and, as such, is not a switch factor for IgE.
We have then evaluated whether IL-7 modulated the production of lymphokines such as IL-619 or IFN-γ,23 which potentiate or inhibit IgE synthesis, respectively. IL-7 induces a weak increase of IL-6 production by IL-4–stimulated PBMCs. This effect cannot explain the selective increase of IgE and IgG4 production induced by IL-7, because IL-6 also favors the other isotypes' production.38 As IFN-γ decreases the synergistic effect of IL-4 and IL-7 on IgE production (data not shown), we could have expected IL-7 to decrease IFN-γ production by IL-4–stimulated PBMCs. However, IL-7 does not affect IFN-γ production by IL-4–stimulated PBMCs but, in contrast, increases IFN-γ production by unstimulated PBMCs.10
Finally, as signaling through CD2118 and IL-9R preferentially costimulate IgE/IgG4 production, we have tested whether or not IL-7 affects IL-9 production and/or CD23-CD21 interaction. Although IL-7 increases membrane CD23 expression on phytohemagglutinin-activated T cells,39 it does not induce CD23 expression on T cells derived from IL-4–stimulated PBMCs or increase CD21 and CD23 expression on B cells or CD23 binding to CD21. IL-7 has been shown to enhance sCD23 production by human T cells.40 In accordance with this observation, we report that IL-7 increases sCD23 production by IL-4–stimulated PBMCs and that this increase may explain its costimulatory role on IgE production.18 Then, based on the observation that IL-7 synergizes with IL-4 in upregulating the expression of the T-cell–derived cytokine IL-9,30 we have analyzed the effects of a neutralizing anti–IL-9 Ab on IgE synthesis. This Ab decreased IgE synthesis by PBMCs stimulated by IL-4 in the presence or absence of IL-7, thereby reinforcing the costimulatory role of IL-9 in the regulation of IgE synthesis.20 21 In conclusion, although we cannot exclude the involvement of other mechanisms, these data suggest that IL-7 potentiates IL-4–induced IgE synthesis by enhancing sCD23 and IL-9 production.
In conclusion, these data provide evidence for a role of IL-7 in the regulation of human isotype switching. IL-7 synergizes with IL-4 to induce IgE and IgG4 synthesis. This effect requires the presence of T cells and is, at least in part, caused by an enhancement of sCD23 and IL-9 production. Isotype switching, which has been recently shown to occur in T-cell–rich zones of the secondary follicles from human tonsils, is directed by multiple signals provided by T cells.13-15 Thus, IL-7 produced locally by the FDCs4 may act on T cells to potentiate IL-4–induced IgE switching. Allergic disorders are associated with a production of IgE and with a predominant Th2 T-cell response (characterized by the production of IL-4, IL-5, and IL-941,42). The recent evidence of IL-7 together with IL-4 and IL-5 in the skin from atopic patients after allergenic challenge reinforces a potential role of IL-7 in IgE-associated diseases.43
ACKNOWLEDGMENT
We thank Dr M.H. Kosco-Vilbois for critical reading of the manuscript.
Address reprint requests to Pascale Jeannin, PhD, Centre d'Immunologie Pierre Fabre, 5, Av Napoleon III, BP 97, F-74164 Saint-Julien en Genevois, Switzerland.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal