Abstract
T-cell activation in response to interleukin-12 (IL-12) is mediated through signaling events that include the tyrosine phosphorylation of STAT4. IL-12 responsiveness and the ability of IL-12 to activate STAT4 is different in T cells induced to differentiate into a Th1 or Th2 phenotype. In this report, we show that STAT5, STAT1α, and STAT1β, in addition to STAT4, are tyrosine phosphorylated in response to IL-12 in phytohemagglutinin (PHA)-activated human T cells. To understand how the activation of these STATs contributes to T-cell IL-12 responsiveness, we analyzed the IL-12–induced activation of STAT5 and STAT1 in T cells stimulated to undergo Th1 or Th2 differentiation. The IL-12–induced tyrosine phosphorylation of STAT5 and STAT1, but not STAT4, is augmented in T cells activated into Th1 cells with PHA + interferon-γ (IFN-γ) compared with T cells activated with PHA alone. STAT5 DNA binding induced by IL-12 is also augmented in T cells activated with PHA + IFN-γ compared with T cells activated with PHA alone, whereas STAT4 DNA binding is not increased. In contrast, the IL-12–induced activation of these STATs is inhibited in T cells activated into Th2 cells with PHA + IL-4. The enhancement of IL-12 signaling by IFN-γ is not a direct effect of IFN-γ on T cells, but rather is mediated by IL-12 that is produced by antigen-presenting cells in response to IFN-γ. This positive autoregulatory effect of IL-12 on the activation of select STATs correlates with an increase in T-cell IFN-γ production in response to IL-12. These findings suggest that the activation of STAT5 and STAT1 may augment select STAT4-dependent functional responses to IL-12 in Th1 cells.
INTERLEUKIN-12 (IL-12) is a heterodimeric cytokine produced by antigen-presenting cells (APCs)1-3that stimulates proliferation,4 cytolytic activity,5,6 and interferon-γ (IFN-γ) production7 by T and natural killer (NK) cells. IL-12 also promotes the development of T helper type 1 (Th1) cells that produce IFN-γ and IL-2 and augment cellular immune responses.8,9Lymphocyte responsiveness to IL-12 is dependent to a considerable extent on the expression of high-affinity IL-12 receptors.10-12 Whereas the low-affinity binding sites detected on activated T and NK cells are composed of the low-affinity IL-12Rβ1 subunit alone,12,13 high-affinity binding is mediated through the combination of IL-12Rβ1 and at least one additional low-affinity subunit.12 A second low-affinity IL-12R subunit, designated IL-12Rβ2, was recently cloned and appears to be vital to IL-12 signaling in that it forms a high-affinity receptor in combination with IL-12Rβ1 in cotransfected Cos cells and has a cytoplasmic domain that contains three tyrosine residues.14 Furthermore, the expression of IL-12Rβ2 appears to be necessary for the activation of STAT4 by IL-12.15 16
Upon binding to its receptor, IL-12 stimulates the tyrosine phosphorylation of the Jak2 and Tyk2 protein tyrosine kinases in T and NK cells.17 This is in distinction to IL-2, which instead activates Jak1 and Jak3.18 IL-12 also induces the tyrosine phosphorylation of STAT4 in T and NK cells,19 an event that so far appears to be unique to IL-12 and IFN-α stimulation.20 However, like IL-2, IL-12 also activates STAT319 and perhaps STAT121 as well. IL-2 activates STAT5,22 but it is not known whether IL-12 is also capable of activating STAT5. Although IL-12 activates multiple STATs, the main functional effects of IL-12 on T and NK cells are lost in mice lacking the STAT4 gene.23 This suggests that STAT4 is necessary for IL-12 signaling. However, because the response to IL-12 in STAT1 and/or STAT3 knockout mice has not yet been analyzed, it is not clear how and to what extent these other STATs contribute to lymphocyte IL-12 responsiveness.
Apart from experiments using STAT4 knockout mice, information regarding both the role of Jaks and STATs and the role of IL-12 receptors in mediating the functional effects of IL-12 has been gained through observing the alterations in T-cell IL-12 responsiveness that occur as part of Th1 and Th2 development. For example, murine CD4+T-cell clones stimulated to undergo Th2 differentiation in the presence of antigen plus IL-4 lose the ability to produce IFN-γ in response to IL-12.21 This impairment of IL-12 function is accompanied by the loss of IL-12–induced Jak2 and STAT4 tyrosine phosphorylation, suggesting that these are both integral components of IL-12 signaling required for the induction of T-cell IFN-γ production. Human T cells activated with the mitogen phytohemagglutinin (PHA) upregulate the expression of high- and low-affinity IL-12 receptors and respond to IL-12. However, when IL-4 is present during activation with PHA, T cells are unable to upregulate the expression of high-affinity IL-12 receptors and subsequently respond poorly to IL-12.12 In contrast, when IFN-γ, a cytokine that promotes Th1 development, is present during PHA activation, the expression of high-affinity IL-12 receptors is augmented.12 This high-affinity receptor upregulation is accompanied by a heightened production of IFN-γ in response to IL-12. Because neither IL-4 nor IFN-γ affect the expression of IL-12Rβ1 on T cells activated with PHA,12their opposing effects on high-affinity IL-12 receptor expression are likely due to modulation of expression of either the IL-12Rβ2 subunit and/or a third as yet undiscovered IL-12R subunit.
Support for the hypothesis that IL-4 and IFN-γ have opposing effects on IL-12Rβ2 expression comes from a recent report using Th clones from transgenic mice that showed that the stimulation of Th cells through the TCR in the presence of IL-4, leading to Th2 development, prevented the transcription of the IL-12Rβ2 subunit gene while leaving IL-12Rβ1 gene transcription unaffected.15 This loss of IL-12Rβ2 gene transcription was accompanied by the inability of IL-12 to activate STAT4. In contrast, IL-12Rβ2 gene transcription could be restored to Th2 clones developing in the presence of IL-4 through the simultaneous stimulation with IFN-γ. However, in another recent study using human Th clones,16 IL-4 was also able to inhibit IL-12Rβ2 mRNA production, but this effect could not be overcome with IFN-γ. Interestingly, IL-12 and IFN-α were able to override the inhibition of IL-12Rβ2 gene transcription in IL-4–stimulated human Th clones. Although neither of these studies explored whether IFN-γ alone, in the absence of IL-12 or IL-4, can upregulate IL-12Rβ2 expression during the activation of naive T cells through the TCR, they suggested that there might be important differences between the human and murine systems regarding the interactions between regulatory cytokines involved in the control of high-affinity IL-12 receptor expression on T cells.
Because IL-12 binding studies have suggested that IL-4 and IFN-γ both appear to be capable of altering T-cell IL-12 responsiveness by differentially modulating the expression of one or more IL-12 receptor subunits that are critical for high-affinity IL-12 binding on human T cells, we examined the impact of such changes in receptor expression on IL-12 signaling to provide insight into which Jaks and STATs are used by T cells to maximize the functional effects of IL-12. In this report, we show that STAT5, STAT1α, and STAT1β, in addition to STAT4, are tyrosine phosphorylated in response to IL-12 in T cells derived from the activation of peripheral blood mononuclear cells (PBMC) with PHA. The IL-12–induced activation of STAT1 and STAT5, but not STAT4, is greatly augmented in T cells activated with PHA + IFN-γ, as is the activation of Jak2 and Tyk2. In contrast, the activation of these components of IL-12 signaling is inhibited in T cells activated with PHA + IL-4. The observed effect of IFN-γ on T-cell IL-12 signaling is mediated by IL-12 that is produced by PHA-activated PBMC in response to IFN-γ. This autoregulatory effect of IL-12 on IL-12 signaling correlates with an enhancement of both high-affinity IL-12 receptor expression and IL-12–induced IFN-γ production, but not with any significant enhancement of IL-12–induced proliferation. These findings suggest that the activation of STAT5 and STAT1 may be a mechanism through which Th1 cells can augment the magnitude of select functional responses to IL-12.
MATERIALS AND METHODS
Cytokines and Antibodies
Recombinant human IL-4 (specific activity, 1 × 107U/mg) and IFN-γ (specific activity, 4.75 × 107U/mg) were purchased from Genzyme (Cambridge, MA). IL-2 (specific activity, 3.9 × 106 U/mL) was generously provided by Amgen (Thousand Oaks, CA). Recombinant human IL-12 (specific activity, 1.7 × 107 U/mg) was generously provided by Dr Steven Herrmann at Genetics Institute (Cambridge, MA). Neutralizing antibodies to IL-12 (C8.6) and IFN-γ (25718.11) were purchased from Endogen (Cambridge, MA) and R&D Systems (Minneapolis, MN), respectively. Antibodies to Jak2, Jak3, and Tyk2 were purchased from Upstate Biotechnology (Lake Placid, NY), and the antibodies to STAT4 and STAT5 were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). The antibody to STAT1 was purchased from Transduction Laboratories (Lexington, KY) and horseradish peroxidase (HRP)-conjugated rabbit antimouse and goat antirabbit antibodies were purchased from Calbiochem (San Diego, CA). The antiphosphotyrosine antibody (4G10) was a gift from Dr Thomas Roberts (Dana-Farber Cancer Institute, Boston, MA). The phospho-STAT1 antibody recognizes the tyrosine-phosphorylated forms of STAT1α and STAT1β and cross-reacts with the tyrosine-phosphorylated form of STAT5.24-26 The phospho-STAT5 antibody was raised in rabbits to a phosphopeptide containing amino acids 687-698 of ovine STAT5, with phosphotyrosine in position 694, and specifically recognizes the tyrosine phosphorylated form of STAT5.
Isolation and Activation of T Cells
Whole blood was obtained through venipuncture from volunteer donors ranging in age from 24 to 50 years. Experiments were repeated using blood from different donors. PBMC were isolated from whole blood through density gradient centifugation using Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden). PBMC were cultured at a starting concentration of 1 × 106 cells/mL in RPMI 1640 medium (Sigma Chemical Co, St Louis, MO) containing 15% fetal calf serum (PAA Laboratories, Newport Beach, CA), 2% L-glutamine, 1% sodium pyruvate, 1% gentamicin, and 1% penicillin-streptomycin. For activation, PHA (Murex, Dartford, UK) was added to the culture medium at day 0 at a concentration of 2.5 μg/mL. Where indicated, cytokines were added to the cultures at the same time as the PHA, using either IFN-γ at 1,000 U/mL, IL-4 at 20 ng/mL, or IL-12 at a concentration of 1 pmol/L. Neutralizing IFN-γ or IL-12 antibodies were also added at day 0 where indicated, at concentrations of 5 μg/mL and 10 μg/mL, respectively. Cells were cultured at 37°C in 5% CO2. Seventy-two to 96 hours after the start of activation, cells were routinely greater than 95% CD3+CD56−, 0% CD3−CD56+.
T-Cell Stimulation and Preparation of Whole Cell Lysates for Immunoprecipitations and Western Blots
After activating T cells with PHA with or without cytokines and antibodies, cells were acid-treated to remove any bound PHA and/or cytokine in a solution containing 10 mmol/L citrate, 140 mmol/L NaCl, and 50 μg/mL bovine serum albumin (BSA), pH 4.0, for 1 minute, washed with RPMI 1640 medium, and recultured for an additional 18 hours in starvation medium consisting of RPMI 1640 with 2.5% fetal calf serum before use in stimulation experiments. Anti–IL-12 or anti–IFN-γ antibodies were added again to the starvation medium where indicated. On day 4 after the start of activation, cells were washed and stimulated for 20 minutes at 37°C in a total volume of 800 μL using either medium alone (RPMI 1640 + 2.5% fetal calf serum) or medium plus either 100 pmol/L IL-12 or 100 pmol/L IL-2. After stimulation, cells were washed once with ice-cold phosphate-buffered saline (PBS) and then lysed on ice for 20 minutes in lysis buffer containing 1% NP-40, 50 mmol/L Tris, pH 8.0, 150 mmol/L NaCl, 2 mmol/L EDTA, 2 μg/mL aprotinin, 100 μg/mL phenylmethylsulfonyl fluoride (PMSF), 1 mmol/L sodium orthovanadate, and 1 mmol/L NaF. Aliquots of whole cell lysates were mixed with an equal volume of 2× reducing sample buffer and boiled, and proteins were resolved on a 7.5% polyacrylamide gel. For immunoprecipitations, antibodies to Jak2, Jak3, Tyk2, STAT4, or STAT5 were added to the lysates and incubated overnight at 4°C. Antibody-protein complexes were then immunoprecipitated from the lysates by adding protein A beads (Pharmacia Biotech) and incubating with rotation at 4°C for 4 hours. The beads were washed twice with ice-cold PBS and boiled in reducing sample buffer, and precipitated proteins were resolved on a 7.5% polyacrylamide gel.
For Western blots, proteins were transferred to a nitrocellulose membrane (Schleicher & Schuell, Keene, NH) by electroblotting, and membranes were then blocked for 30 minutes in Tris-buffered saline (TBS) containing 0.1% Tween-20 (BioRad, Hercules, CA) and either 5% BSA (US Biochemicals, Cleveland, OH) or 5% nonfat dried milk. Membranes were then incubated with dilutions of the indicated antibodies for 1 hour at room temperature, washed with TBS/Tween-20, incubated with either HRP-conjugated rabbit antimouse or goat antirabbit antibodies (diluted 1:10,000) for 1 hour, washed again, and developed using ECL (Amersham Life Science, Buckinghamshire, UK). When reprobed, membranes were first stripped by incubating in a solution containing 2% sodium dodecyl sulfate, 100 mmol/L 2-mercaptoethanol (2-ME), and 62.5 mmol/L Tris-HCl, pH 6.7, for 30 minutes at 65°C.
Preparation of Nuclear Extracts
After cytokine stimulation, T cells were placed on ice, washed once with cold PBS, resuspended in 5 mL of hypotonic buffer (10 mmol/L Tris, pH 7.4, 10 mmol/L NaCl, 6 mmol/L MgCl2), and incubated on ice for 5 minutes. Thereafter, the cells were centrifuged and resuspended in 0.8 mL of hypotonic buffer containing 1 mmol/L β-mercaptoethanol (βME), 10 μg/mL PMSF, and 1 mmol/L sodium orthovanadate. Cells were disrupted by shearing in a Dounce homogenizer (type b pestle, 25 strokes), and the nuclei were collected by 10 seconds of centrifugation at 12,000g. The nuclear pellet was washed once with hypotonic buffer, resuspended in 3 pellet volumes of high salt buffer (20 mmol/L HEPES, pH 7.9, 420 mmol/L NaCl, 25% glycerol, 1.5 mmol/L MgCl2, 0.2 mmol/L EDTA, 1 mmol/L βME, 1 mmol/L sodium orthovanadate, and 10 μg/mL PMSF), and rocked at 4°C for 30 minutes. Intact nuclei were removed by centrifugation at 12,000g for 3 minutes at 4°C, and the supernatant was recovered.
Electrophoretic Mobility Shift Assay (EMSA)
One microliter of nuclear extract was mixed with 1 ng of a double-stranded 32P-labeled oligonucleotide derived from the IFN responsive factor-1 (IRF-1) promoter (AGCCTGATTTCCCCGAAATGACGGC and its complement) in 5 μL of binding buffer (25 mmol/L HEPES, pH 7.9, 100 μmol/L EGTA, 200 μmol/L MgCl2, 500 μmol/L dithiothreitol, 1 μg/ μL BSA, 0.2 μg/μL poly dI:dC, 1% Ficoll, and 0.1 μg/μL herring testis DNA). The incubation was performed at room temperature for 20 minutes. Where designated, 1 μL of antibody was added at the end of the binding reaction, and incubated at 4°C for an additional 30 minutes. The products of the binding reaction were then separated by electrophoresis on a 4% acrylamide gel in 0.2× tris-borate/EDTA and visualized by autoradiography.
IL-12 Binding Assays
IL-12 was labeled with 125I (DuPont/New England Nuclear, Wilmington, DE) using the Iodo-Bead (Pierce, Rockford, IL) method, as previously described.27 Day-4 activated T cells (2 × 106) were incubated for 2 hours on ice with concentrations of [125I]–IL-12 ranging from 12 nmol/L to 30 pmol/L. To calculate nonspecific binding (routinely 0.1% to 0.3% of total counts added), T cells were first incubated with excess cold IL-12 for 1 hour before adding the radiolabeled IL-12. After incubating cells with radiolabeled IL-12, the bound IL-12 was separated from free IL-12 by pelleting the cells through a mixture of silicone oil and paraffin oil (81:19 ratio) for 1 minute at 10,000g. The amounts of bound and free [125I]–IL-12 were measured using a gamma counter. The number of specific bound counts was calculated by subtracting nonspecific counts bound from total counts bound and converted to molecules bound per cell. IL-12 binding was analyzed using the Scatchard method.
Measurement of IFN-γ, IFN-α, and IL-12
To assess IFN-γ production, PBMC were first activated for 96 hours with PHA and, where indicated, antibodies and/or cytokines. Cells were then washed twice and plated in U-bottom wells at 3 × 104 cells/well with either medium alone or the indicated concentrations of IL-12. Cells were incubated for 72 hours at 37°C, supernatants were harvested, and the IFN-γ concentration was measured using an enzyme-linked immunosorbent assay (ELISA; Endogen, Cambridge, MA). The sensitivity of the IFN-γ ELISA is less than 3 pg/mL.
To determine IFN-α and IL-12 production, PBMC were cultured at a starting concentration of 1 × 106 cells/mL in medium containing either PHA alone or PHA + IFN-γ. Aliquots of culture supernatants were harvested at 24, 48, and 72 hours. The IFN-α concentration was measured using an IFN-α ELISA (Endogen), the sensitivity of which is less than 3 pg/mL. The IL-12 concentration was measured using an IL-12 ELISA (Endogen) that detects only the p70 IL-12 heterodimer. The sensitivity of the IL-12 ELISA is less than 3 pg/mL.
RESULTS
The IL-12–Induced Tyrosine Phosphorylation of STAT1α and STAT1β Is Augmented in T Cells Activated With PHA + IFN-γ
There have been conflicting reports regarding whether IL-12 is able to stimulate the tyrosine phosphorylation of STAT1 in T and NK cells. Using murine CD4+ Th1 cell clones, one study showed that the constitutive tyrosine phosphorylation of STAT1 in these cells was augmented by IL-12,21 although there were no data regarding which STAT1 isoform (STAT1α, STAT1β, or both) was being activated. However, two other studies using either PHA activated human T cells19 or human NK cells28 failed to demonstrate the tyrosine phosphorylation of STAT1 in response to IL-12.
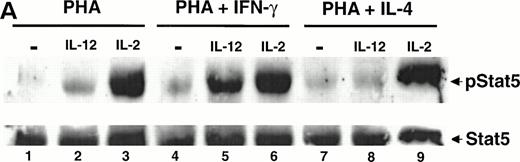
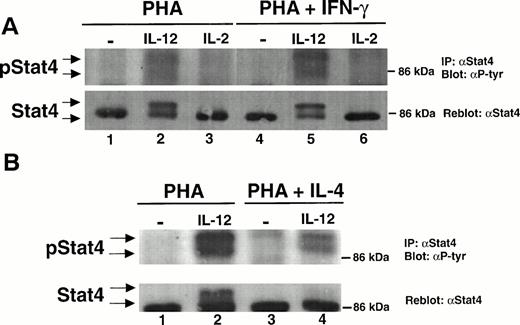
To determine whether IL-12 stimulates the tyrosine phosphorylation of STAT1α and/or STAT1β in T cells, we activated human T cells with PHA for 72 hours, rested them in starvation medium for 18 hours, and then stimulated them with either IL-12 or IL-2. We then looked for STAT1 activation in these stimulated T cells by performing a Western blot on whole cell lysates with an antibody that recognizes the tyrosine-phosphorylated forms of STAT1α, STAT1β, and STAT5. In T cells activated with PHA, there is low-level constitutive activation of STAT1α and STAT1β (Fig 1A and B, lane 1). With the addition of either IL-12 or IL-2, there is a clear increase in the amount of tyrosine-phosphorylated STAT1α and STAT1β (lanes 2 and 3), showing that these cytokines activate both isoforms of STAT1.
IFN-γ augments while IL-4 inhibits the IL-12–induced tyrosine phosphorylation of STAT1α and STAT1β. PBMC were cultured for 72 hours with PHA, PHA + 1,000 U/mL IFN-γ (A), or PHA + 20 ng/mL IL-4 (B); rested overnight in fresh medium containing 2.5% fetal calf serum; and then stimulated for 20 minutes with either medium alone (lanes 1 and 4), 100 pmol/L IL-12 (lanes 2 and 5), or 100 pmol/L IL-2 (lanes 3 and 6). Western blots were performed with antibodies to phospho-STAT1 (A, upper panel, and B) or STAT1 (A, lower panel). Results are representative of five separate experiments.
IFN-γ augments while IL-4 inhibits the IL-12–induced tyrosine phosphorylation of STAT1α and STAT1β. PBMC were cultured for 72 hours with PHA, PHA + 1,000 U/mL IFN-γ (A), or PHA + 20 ng/mL IL-4 (B); rested overnight in fresh medium containing 2.5% fetal calf serum; and then stimulated for 20 minutes with either medium alone (lanes 1 and 4), 100 pmol/L IL-12 (lanes 2 and 5), or 100 pmol/L IL-2 (lanes 3 and 6). Western blots were performed with antibodies to phospho-STAT1 (A, upper panel, and B) or STAT1 (A, lower panel). Results are representative of five separate experiments.
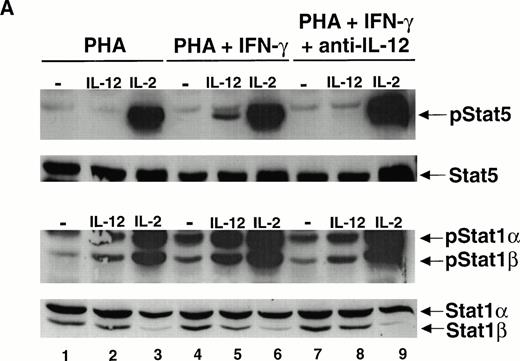
Because human T cells activated with PHA + IFN-γ demonstrate an increase in the number of high-affinity IL-12 receptors and a heightened response to IL-12 compared with T cells activated with PHA alone, we examined whether the ability of IL-12 to activate STAT1 was augmented in these IFN-γ–activated cells. The activation of T cells with PHA + IFN-γ leads to an increase in the constitutive activation of STAT1α and STAT1β (Fig 1A, lane 4). However, even after normalizing for the increased basal activation of STAT1, the tyrosine phosphorylation of STAT1α and STAT1β induced by IL-12 is significantly greater in cells activated with PHA + IFN-γ (lane 5) compared with cells activated with PHA alone (lane 2). IFN-γ does not increase the total amount of STAT1α and STAT1β protein present within the cells, as indicated by reprobing the membrane with a STAT1 antibody (Fig 1A, lower panel). In contrast to IL-12, the activation of STAT1 by IL-2 is only minimally increased in the IFN-γ–treated T cells (lane 6), suggesting that the effect of IFN-γ on cytokine signaling is restricted to IL-12.
T cells activated with PHA + IL-4 fail to upregulate high-affinity IL-12 receptors and respond poorly to IL-12. In these IL-4–treated cells, IL-12 does not induce an increase in the tyrosine phosphorylation of STAT1α and STAT1β (Fig 1B, lane 5). This is not due to the absence of STAT1, because similar amounts of STAT1 protein are present within T cells treated with either PHA alone or PHA + IL-4 (data not shown). The specificity of this IL-4 effect for IL-12 signaling is demonstrated by the finding that IL-4 has no effect on the IL-2–induced tyrosine phosphorylation of STAT1 (lane 6). This supports the observation that the functional response to IL-2 remains intact among T cells activated with PHA + IL-4.12
IL-12 Stimulates the Tyrosine Phosphorylation and DNA Binding of STAT5 in T Cells Activated With PHA + IFN-γ
In addition to activating STAT1 and STAT3, IL-2 induces the tyrosine phosphorylation of STAT5 in T and NK cells.22,28 The phospho-STAT1 antibody not only recognizes the tyrosine-phosphorylated forms of STAT1α and STAT1β, but also cross-reacts with the tyrosine phosphorylated form of STAT5. In cells stimulated with IL-2, a third band migrating slower than STAT1α with a molecular weight of approximately 93 kD is clearly visible (Fig 1A, lanes 3 and 6), and represents the tyrosine-phosphorylated form of STAT5.26However, this same band also appears to be present when cells are stimulated with IL-12 (Fig 1A, lanes 2 and 5), suggesting that IL-12 may also be capable of activating STAT5.
To determine whether IL-12 can induce the tyrosine phosphorylation of STAT5, T cells were activated with PHA for 72 hours, rested in starvation medium for 18 hours, and stimulated with IL-12 or IL-2. A Western blot was then performed on whole cell lysates using an antibody that recognizes the tyrosine phosphorylated form of STAT5. Whereas IL-2 strongly activates STAT5 (Fig 2A, lane 3), IL-12 also activates STAT5 (lane 2) but to a lesser degree than that observed with IL-2. By contrast, in T cells activated with PHA + IFN-γ, there is a striking increase in IL-12–induced STAT5 tyrosine phosphorylation (lane 5), with no significant change in the degree of STAT5 activation by IL-2 (lane 6). Reprobing the membrane with a STAT5 antibody demonstrates that IFN-γ does not increase the total amount of STAT5 present within the activated T cells (Fig 2A, lower panel). Whereas STAT5 tyrosine phosphorylation induced by IL-12 is greatly augmented in T cells activated with PHA + IFN-γ, in cells activated with PHA + IL-4, IL-12 does not induce the tyrosine phosphorylation of STAT5 above the basal level (lanes 7 and 8). The IL-2–induced activation of STAT5 is unaffected by IL-4 (lane 9), showing that this effect is restricted to IL-12 signaling.
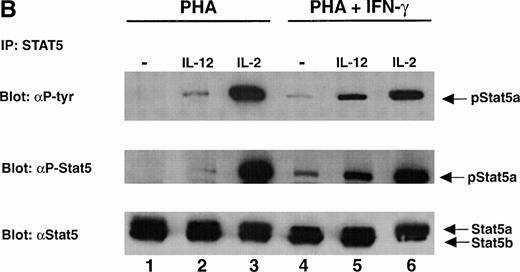
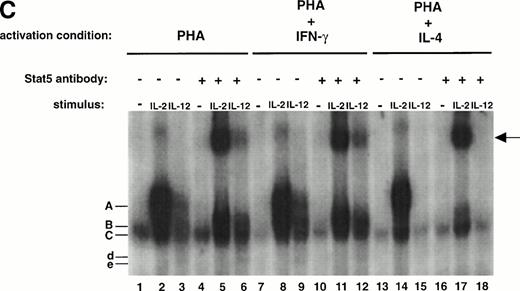
The tyrosine phosphorylation and DNA binding of STAT5 in response to IL-12 is enhanced in T cells activated with PHA + IFN-γ but inhibited in T cells activated with PHA + IL-4. (A) PBMC cultured in either PHA alone, PHA + IFN-γ, or PHA + IL-4 were stimulated with medium alone (lanes 1, 4, and 7), IL-12 (lanes 2, 5, and 8), or IL-2 (lanes 3, 6, and 9). Western blots were performed with antibodies to phospho-STAT5 (upper panel) or STAT5 (lower panel). Results are representative of five separate experiments. (B) PBMC cultured in either PHA alone or PHA + IFN-γ were stimulated with medium alone (lanes 1 and 4), IL-12 (lanes 2 and 5), or IL-2 (lanes 3 and 6). Cell lysates were immunoprecipitated with a STAT5 antibody, and Western blots were performed with antibodies to phosphotyrosine (upper panel), phospho-STAT5 (middle panel), or STAT5 (lower panel). Results are representative of three separate experiments. (C) PBMC were activated with PHA alone, PHA + IFN-γ, or PHA + IL-4 and then stimulated with the indicated cytokine. Nuclear lysates were then prepared and used in an EMSA with a32P-labeled DNA probe consisting of a STAT-binding sequence found within the IRF-1 gene promoter. Where indicated, 1 μL of a STAT5 antibody was added to the binding reaction containing nuclear lysate and DNA probe. The arrow points to supershifted complexes. Similar results were obtained in two separate experiments.
The tyrosine phosphorylation and DNA binding of STAT5 in response to IL-12 is enhanced in T cells activated with PHA + IFN-γ but inhibited in T cells activated with PHA + IL-4. (A) PBMC cultured in either PHA alone, PHA + IFN-γ, or PHA + IL-4 were stimulated with medium alone (lanes 1, 4, and 7), IL-12 (lanes 2, 5, and 8), or IL-2 (lanes 3, 6, and 9). Western blots were performed with antibodies to phospho-STAT5 (upper panel) or STAT5 (lower panel). Results are representative of five separate experiments. (B) PBMC cultured in either PHA alone or PHA + IFN-γ were stimulated with medium alone (lanes 1 and 4), IL-12 (lanes 2 and 5), or IL-2 (lanes 3 and 6). Cell lysates were immunoprecipitated with a STAT5 antibody, and Western blots were performed with antibodies to phosphotyrosine (upper panel), phospho-STAT5 (middle panel), or STAT5 (lower panel). Results are representative of three separate experiments. (C) PBMC were activated with PHA alone, PHA + IFN-γ, or PHA + IL-4 and then stimulated with the indicated cytokine. Nuclear lysates were then prepared and used in an EMSA with a32P-labeled DNA probe consisting of a STAT-binding sequence found within the IRF-1 gene promoter. Where indicated, 1 μL of a STAT5 antibody was added to the binding reaction containing nuclear lysate and DNA probe. The arrow points to supershifted complexes. Similar results were obtained in two separate experiments.
To confirm the results obtained with the phospho-STAT5 antibody, T cells were again activated with PHA or PHA + IFN-γ and stimulated with IL-12 or IL-2. Immunoprecipitations were performed on lysates using an antibody that recognizes both STAT5a and STAT5b, and the precipitated proteins were subjected to Western blotting using a phosphotyrosine antibody. As shown in the upper panel of Fig 2B, the results obtained are identical to those observed using whole cell lysates and the phospho-STAT5 antibody (Fig 2A). Reprobing with the STAT5 antibody (Fig 2B, lower panel) demonstrated that STAT5a is the STAT5 isoform tyrosine phosphorylated in response to IL-12 and IL-2, although a very small amount of STAT5b activation could be detected in some experiments in response to IL-2 (data not shown). This absence of STAT5b activation is not due to diminished STAT5b expression, because activated T cells contain more STAT5b than STAT5a (Fig 2B, lower panel). Reprobing with the phospho-STAT5 antibody (Fig 2B, middle panel) confirmed the specificity of this antibody for the tyrosine-phosphorylated form of STAT5.
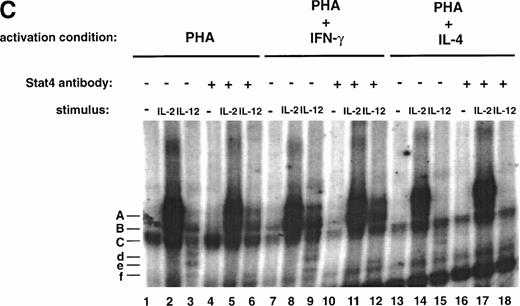
As shown in Fig 2A and B, even in T cells activated with PHA + IFN-γ, the amount of STAT5 activated in response to IL-2 is significantly greater than the amount activated in response to IL-12. To further assess the functional significance of IL-12–induced STAT5 tyrosine phosphorylation, we examined whether IL-12 induces STAT5 binding to a32P-labeled DNA probe consisting of a STAT-binding sequence found within the IRF-1 gene promoter. As shown in Fig 2C, both IL-2 and IL-12 induce the formation of two similar complexes (A and B, lanes 2 and 3) that are not present in the control lane (lane 1). In addition, IL-12 also induces the formation of two faster migrating complexes (d and e, lane 3). In the presence of a STAT5 antibody, complex A induced by IL-2 and IL-12 is supershifted (lanes 5 and 6), demonstrating that complex A contains STAT5. Thus, IL-12 and IL-2 both stimulate not only STAT5 tyrosine phosphorylation, but STAT5 DNA binding as well. Consistent with the results obtained with Western blotting, STAT5 activation by IL-2 assessed through DNA binding is significantly greater than IL-12–induced STAT5 activation. In addition, STAT5 activation by IL-12 is again augmented in T cells activated with PHA + IFN-γ (lane 9) compared with T cells activated with PHA alone (lane 3) and completely abrogated in T cells activated with PHA + IL-4 (lane 15).
The IL-12–Induced Tyrosine Phosphorylation of STAT5 in T Cells Activated With PHA + IFN-γ Is Not Mediated By IL-2
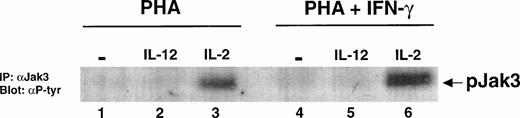
Because IL-2 is known to activate STAT5, it was possible that IL-12 was activating STAT5 indirectly by stimulating the secretion of IL-2 from the IFN-γ–treated T cells. To determine whether IL-2 signaling was responsible for the effect of IL-12 on STAT5 tyrosine phosphorylation, we examined whether IL-12 stimulation of T cells activated with PHA + IFN-γ leads to the tyrosine phosphorylation of Jak3. The activation of this kinase is a proximal event in IL-2 signaling mediated through the γc chain of the IL-2 receptor,18 but it is not a component of signaling through the IL-12 receptor.17 Thus, the absence of Jak3 activation after stimulation with IL-12 would exclude the possibility that STAT activation seen with IL-12 stimulation was due in part to IL-2. IL-2 induces the tyrosine phosphorylation of Jak3 to a slightly greater extent in T cells activated with PHA + IFN-γ compared with T cells activated with PHA alone (Fig 3, lanes 3 and 6). However, whether T cells are activated with PHA alone or PHA + IFN-γ, IL-12 does not stimulate the tyrosine phosphorylation of Jak3 (lanes 2 and 5). This demonstrates that the IL-12–induced tyrosine phosphorylation of STAT5 in IFN-γ–treated cells cannot be due to T cell-derived IL-2.
Jak3 is not tyrosine phosphorylated by IL-12 in T cells activated with PHA + IFN-γ. PBMC cultured with either PHA alone or PHA + IFN-γ were stimulated for 20 minutes with either medium alone (lanes 1 and 4), IL-12 (lanes 2 and 5), or IL-2 (lanes 3 and 6). Immunoprecipitations were performed on whole cell lysates (30 to 40 × 106 cells/lane) with an antibody to Jak3, followed by a Western blot with an antibody to phosphotyrosine. Results are representative of two separate experiments.
Jak3 is not tyrosine phosphorylated by IL-12 in T cells activated with PHA + IFN-γ. PBMC cultured with either PHA alone or PHA + IFN-γ were stimulated for 20 minutes with either medium alone (lanes 1 and 4), IL-12 (lanes 2 and 5), or IL-2 (lanes 3 and 6). Immunoprecipitations were performed on whole cell lysates (30 to 40 × 106 cells/lane) with an antibody to Jak3, followed by a Western blot with an antibody to phosphotyrosine. Results are representative of two separate experiments.
The IL-12–Induced Tyrosine Phosphorylation and DNA Binding of STAT4 Is Not Enhanced in T Cells Activated With PHA + IFN-γ
The mitogen activation of T cells in the presence of IFN-γ greatly augments the ability of IL-12 to stimulate the tyrosine phosphorylation of STAT1 and STAT5 without affecting the activation of these STATs by IL-2. Given this, we examined whether STAT4 activation induced by IL-12 was similarly modulated by IFN-γ. IL-12 induces the tyrosine phosphorylation of STAT4 in PHA-activated T cells (Fig 4A, lane 2), whereas IL-2 does not (lane 3). Two isoforms of STAT4 are visible after activation with IL-12. A previous study has shown that the slower migrating form of STAT4 results from the IL-12–induced serine phosphorylation of STAT4, whereas tyrosine phosphorylation does not affect the electrophoretic mobility of STAT4.20 Both isoforms are tyrosine phosphorylated in response to IL-12 in cells activated with PHA alone (lane 2). The magnitude of STAT4 tyrosine phosphorylation by IL-12 is marginally enhanced in T cells activated by PHA + IFN-γ (lane 5), contrasting with the pronounced augmentation of STAT1 and STAT5 activation in response to IL-12 in PHA + IFN-γ–treated T cells. Furthermore, there appears to be no increase in the IL-12–induced serine phosphorylation of STAT4 in T cells activated with PHA + IFN-γ compared with cells activated with PHA alone (lanes 2 and 5, lower panel). In contrast with IL-12, IL-2 is unable to activate STAT4 in IFN-γ–treated cells (lane 6).
IL-12–induced STAT4 tyrosine phosphorylation and DNA binding is not augmented by IFN-γ but is partially inhibited by IL-4. (A) PBMC were cultured with either PHA alone or PHA + IFN-γ and then stimulated with medium alone (lanes 1 and 4), IL-12 (lanes 2 and 5), or IL-2 (lanes 3 and 6). Immunoprecipitations were performed on whole cell lysates with an antibody to STAT4, followed by Western blots with an antiphosphotyrosine antibody (upper panel) or an antibody to STAT4 (lower panel). Results are representative of three separate experiments. (B) PBMC were cultured with either PHA alone or PHA + IL-4 and stimulated with medium alone (lanes 1 and 3) or IL-12 (lanes 2 and 4). STAT4 was then immunoprecipitated from cell lysates as in (A), and Western blots were performed as described in (A). Results are representative of three separate experiments. (C) PBMC were activated with PHA, PHA + IFN-γ, or PHA + IL-4 and then stimulated with the indicated cytokine. Nuclear lysates were then prepared and used in an EMSA with a 32P-labeled DNA probe consisting of a STAT-binding sequence found within the promoter region of the IRF-1 gene. Where indicated, 1 μL of a STAT4 antibody was added to the binding reaction containing nuclear lysate and DNA probe. Similar results were obtained in two separate experiments.
IL-12–induced STAT4 tyrosine phosphorylation and DNA binding is not augmented by IFN-γ but is partially inhibited by IL-4. (A) PBMC were cultured with either PHA alone or PHA + IFN-γ and then stimulated with medium alone (lanes 1 and 4), IL-12 (lanes 2 and 5), or IL-2 (lanes 3 and 6). Immunoprecipitations were performed on whole cell lysates with an antibody to STAT4, followed by Western blots with an antiphosphotyrosine antibody (upper panel) or an antibody to STAT4 (lower panel). Results are representative of three separate experiments. (B) PBMC were cultured with either PHA alone or PHA + IL-4 and stimulated with medium alone (lanes 1 and 3) or IL-12 (lanes 2 and 4). STAT4 was then immunoprecipitated from cell lysates as in (A), and Western blots were performed as described in (A). Results are representative of three separate experiments. (C) PBMC were activated with PHA, PHA + IFN-γ, or PHA + IL-4 and then stimulated with the indicated cytokine. Nuclear lysates were then prepared and used in an EMSA with a 32P-labeled DNA probe consisting of a STAT-binding sequence found within the promoter region of the IRF-1 gene. Where indicated, 1 μL of a STAT4 antibody was added to the binding reaction containing nuclear lysate and DNA probe. Similar results were obtained in two separate experiments.
Activation of T cells in the presence of IL-4 has the opposite effect from that observed with IFN-γ, leading to the inhibition of STAT4 tyrosine and serine phosphorylation in response to IL-12 (Fig 4B, lanes 2 and 4, upper and lower panels). Whereas STAT4 tyrosine and serine phosphorylation in response to IL-12 is greatly diminished, it is not completely extinguished in these IL-4–treated T cells.
The lack of any augmentation of IL-12–induced STAT4 tyrosine phosphorylation in T cells activated with PHA + IFN-γ was confirmed in an analysis of IL-12–induced STAT4 DNA binding. IL-12 stimulation of T cells activated with PHA alone induces the formation of 2 complexes (Fig 4C, d and e, lane 3) that are not induced in the untreated or IL-2–treated cells (lanes 1 and 2). The addition of a STAT4 antibody results in the loss of these complexes (lane 6), demonstrating that they contain STAT4. The same STAT4-containing complexes are induced by IL-12 in T cells activated with PHA + IFN-γ (lane 9), but the signal intensity is no different from that observed in T cells activated with PHA alone (lane 3). In contrast, the signal intensity of the IL-12–induced STAT5-DNA complex (A) is significantly greater in T cells activated with PHA + IFN-γ (lanes 9 and 12) compared with T cells activated with PHA alone (lanes 3 and 6). Therefore, the analysis of STAT tyrosine phosphorylation and DNA binding in response to IL-12 demonstrates that IFN-γ augments IL-12–induced STAT5 activation but does not affect IL-12–induced STAT4 activation. In addition, the DNA binding studies also confirm that IL-4 completely abrogates IL-12–induced STAT5 activation (lanes 15 and 18) but only partially inhibits IL-12–induced STAT4 activation (lane 15).
IFN-γ Augments and IL-4 Inhibits the IL-12–Induced Activation of Jak2 and Tyk2 in T Cells
In addition to activating STATs, IL-12 induces the tyrosine phosphorylation of the Janus family tyrosine kinases Jak2 and Tyk2. A recent report demonstrated that Jak2 is associated with the cytoplasmic domain of IL-12Rβ2, whereas Tyk2 is associated with IL-12Rβ1.29 The binding of IL-12 to IL-12 receptors presumably leads to the heterodimerization of IL-12Rβ1 + IL-12Rβ2, thereby activating their associated Jaks. These receptor-associated tyrosine kinases would then phosphorylate tyrosine residues on the cytoplasmic domains of one or more IL-12R subunits, allowing certain STATs to dock via their SH2 domains and in turn become phosphorylated on conserved tyrosine residues.30
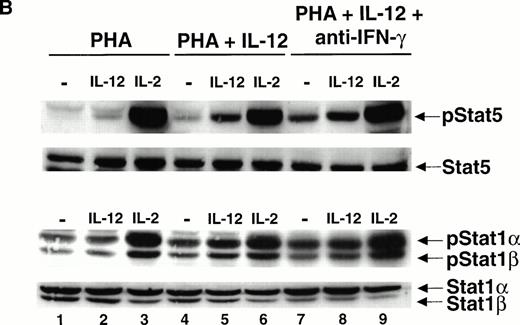
Having shown that IFN-γ augments the IL-12–induced activation of STAT5 and STAT1, whereas IL-4 inhibits the activation of these same STATs, we wanted to determine whether there were similar changes in the IL-12–induced activation of Jak2 and Tyk2. The IL-12–induced activation of Jak2 is greatly augmented in T cells activated with PHA + IFN-γ (Fig 5A, lane 4) compared with T cells activated with PHA alone (lane 2). In addition, the tyrosine phosphorylation of Tyk2 by IL-12 is also significantly increased in the IFN-γ–treated T cells (Fig 5B, lanes 2 and 4). This increase in Jak2 and Tyk2 activation by IL-12 in IFN-γ–treated cells is not due to an increase in the amount of Jak2 or Tyk2 protein (Fig 5A and B, lower panels). In contrast with IFN-γ, the IL-12–induced activation of both Jak2 and Tyk2 is diminished but not abolished in T cells activated with PHA + IL-4 compared with T cells activated with PHA alone (Fig 5C and D).
Activation of T cells with PHA + IFN-γ augments whereas activation with PHA + IL-4 inhibits the IL-12–induced tyrosine phosphorylation of Jak2 and Tyk2. PBMC were cultured with either PHA, PHA + IFN-γ (A and B), or PHA + IL-4 (C and D), and T cells were then stimulated with medium alone (lanes 1 and 3) or IL-12 (lanes 2 and 4). Immunoprecipitations were performed on whole cell lysates with an antibody to Jak2 (A and C) or Tyk2 (B and D) followed by Western blots using an antiphosphotyrosine antibody (upper panels) or antibodies to either Jak2 or Tyk2 (lower panels). Each result is representative of two separate experiments.
Activation of T cells with PHA + IFN-γ augments whereas activation with PHA + IL-4 inhibits the IL-12–induced tyrosine phosphorylation of Jak2 and Tyk2. PBMC were cultured with either PHA, PHA + IFN-γ (A and B), or PHA + IL-4 (C and D), and T cells were then stimulated with medium alone (lanes 1 and 3) or IL-12 (lanes 2 and 4). Immunoprecipitations were performed on whole cell lysates with an antibody to Jak2 (A and C) or Tyk2 (B and D) followed by Western blots using an antiphosphotyrosine antibody (upper panels) or antibodies to either Jak2 or Tyk2 (lower panels). Each result is representative of two separate experiments.
The Effect of IFN-γ on IL-12 Signaling and High-Affinity IL-12 Receptor Expression Is Mediated Indirectly Through IL-12
The activated T cells used in these experiments were derived from PBMC stimulated with PHA alone or PHA plus added cytokines. After 72 to 96 hours of activation, these cultured PBMC are greater than 95% T cells. However, for the first 48 hours, these cultures contain normal complements of NK cells, B cells, and monocytes. This raised the possibility that the effect of IFN-γ on IL-12 signaling in T cells might not be a direct effect of IFN-γ on T cells, but rather could be mediated by another cytokine produced in response to the added IFN-γ. It has been shown that IL-12 and IFN-α can upregulate IL-12Rβ2 gene expression in human T-cell clones, whereas IFN-γ cannot.16 Thus, it was important to determine whether IFN-γ was stimulating IL-12 production (presumably from monocytes) and/or IFN-α production during the activation of PBMC with PHA and, if so, whether these cytokines rather than the IFN-γ were responsible for the observed changes in IL-12 signaling.
No IL-12 could be detected in cultures of PBMC activated with PHA alone, but small amounts (8 to 26 pg/mL, or approximately 0.1 pmol/L) of IL-12 are present in cultures of PBMC activated with both PHA and 1,000 U/mL IFN-γ (Table 1). No IFN-α could be detected in cultures of PBMC activated with PHA alone or PHA + IFN-γ (Table 1). Because activated T cells are capable of responding to as little as 0.1 pmol/L IL-12,31 we next investigated whether neutralization of this small amount of IL-12 would alter the ability of IFN-γ to modulate IL-12 signaling.
IL-12 and IFN-α Production by PBMC During Activation With PHA or PHA + IFN-γ
| Activation Condition . | [IL-12] (pg/mL) . | [IFN-α] (pg/mL) . | ||||
|---|---|---|---|---|---|---|
| 24 h . | 48 h . | 72 h . | 24 h . | 48 h . | 72 h . | |
| PHA | ND | ND | ND | ND | ND | ND |
| PHA + IFN-γ | 8 ± 4 | 26 ± 15 | 8 ± 6 | ND | ND | ND |
| Activation Condition . | [IL-12] (pg/mL) . | [IFN-α] (pg/mL) . | ||||
|---|---|---|---|---|---|---|
| 24 h . | 48 h . | 72 h . | 24 h . | 48 h . | 72 h . | |
| PHA | ND | ND | ND | ND | ND | ND |
| PHA + IFN-γ | 8 ± 4 | 26 ± 15 | 8 ± 6 | ND | ND | ND |
PBMC were cultured at 1 × 106 cells/mL in medium containing PHA alone or PHA + IFN-γ. Aliquots of culture supernatants were harvested at the indicated time points and IL-12 and IFN-α concentration was measured using an IL-12 ELISA (detecting only the p70 IL-12 heterodimer) or IFN-α ELISA. Similar results were obtained in three independent experiments.
Abbreviations: ND, not detectable by IL-12 or IFN-α ELISA (detection limit, <3 pg/mL).
PBMC were cultured with either PHA alone, PHA + IFN-γ, or PHA + IFN-γ + a neutralizing IL-12 antibody, and the ability of IL-12 to activate STAT5 and STAT1 was assessed. Whereas the IL-12–induced tyrosine phosphorylation of both STAT5 and STAT1 is greatly augmented in T cells activated with PHA + IFN-γ compared with T cells activated with PHA alone, this effect of IFN-γ is inhibited by the neutralizing IL-12 antibody (Fig 6A, lanes 5 and 8). The IL-2–induced activation of STAT5 and STAT1 is not affected by the neutralizing IL-12 antibody (lanes 6 and 9). This suggests that during the activation of PBMC with PHA + IFN-γ, a small amount of endogenous IL-12 (on the order of 0.1 pmol/L) is responsible for promoting the development of T cells in which IL-12 can activate STAT5 and STAT1, in addition to STAT4.
The enhancement of IL-12–induced STAT5 and STAT1 activation by IFN-γ is dependent on IL-12. (A) PBMC were cultured with either PHA alone, PHA + IFN-γ, or PHA + IFN-γ + a neutralizing IL-12 antibody (10 μg/mL), and T cells were then stimulated with medium alone (lanes 1, 4, and 7), IL-12 (lanes 2, 5, and 8), or IL-2 (lanes 3, 6, and 9). Western blots were performed with antibodies to phospho-STAT5 and phosphoSTAT1 (upper panels), as well as with antibodies to STAT5 and STAT1 (lower panels). Results are representative of two separate experiments. (B) PBMC were cultured with PHA alone, PHA + IL-12 1 pmol/L, or PHA + IL-12 1 pmol/L + a neutralizing IFN-γ antibody (5 μg/mL). T cells were then stimulated with medium alone (lanes 1, 4, and 7), IL-12 (lanes 2, 5, and 8), or IL-2 (lanes 3, 6, and 9), and Western blots were performed as described in (A). Results are representative of two separate experiments.
The enhancement of IL-12–induced STAT5 and STAT1 activation by IFN-γ is dependent on IL-12. (A) PBMC were cultured with either PHA alone, PHA + IFN-γ, or PHA + IFN-γ + a neutralizing IL-12 antibody (10 μg/mL), and T cells were then stimulated with medium alone (lanes 1, 4, and 7), IL-12 (lanes 2, 5, and 8), or IL-2 (lanes 3, 6, and 9). Western blots were performed with antibodies to phospho-STAT5 and phosphoSTAT1 (upper panels), as well as with antibodies to STAT5 and STAT1 (lower panels). Results are representative of two separate experiments. (B) PBMC were cultured with PHA alone, PHA + IL-12 1 pmol/L, or PHA + IL-12 1 pmol/L + a neutralizing IFN-γ antibody (5 μg/mL). T cells were then stimulated with medium alone (lanes 1, 4, and 7), IL-12 (lanes 2, 5, and 8), or IL-2 (lanes 3, 6, and 9), and Western blots were performed as described in (A). Results are representative of two separate experiments.
To directly demonstrate this effect of IL-12 on T-cell differentiation, PBMC were activated with either PHA alone or PHA + 1 pmol/L IL-12 and assessed for IL-12–induced STAT5 and STAT1 activation. In addition, because it was not clear whether the direct action of both IL-12 and IFN-γ on T cells was required to mediate the observed changes in IL-12 signaling among T cells activated with PHA + IFN-γ, we also activated PBMC with PHA + IL-12 in the presence of a neutralizing IFN-γ antibody to inhibit the biologic effect of any endogenous IL-12–induced IFN-γ. T cells cultured with PHA + IL-12 exhibit a significant enhancement of IL-12–induced STAT5 and STAT1 tyrosine phosphorylation compared with T cells activated with PHA alone (Fig 6B, lanes 2 and 5). The addition of a neutralizing IFN-γ antibody has no effect on that activation (lanes 5 and 8). This indicates that the presence of IL-12 during T-cell activation can enhance IL-12 signaling independent of IFN-γ. T cells activated with PHA + IL-12 or PHA + IL-12 + anti–IFN-γ also display an enhanced basal tyrosine phosphorylation of STAT5 and STAT1 (lanes 4 and 7). This enhancement is most pronounced in T cells cultured with PHA + 1 pmol/L IL-12 + anti–IFN-γ (lane 7).
To determine whether IFN-γ–induced IL-12 was also responsible for the upregulation of high-affinity IL-12 receptor expression observed among T cells activated with PHA + IFN-γ, IL-12 binding studies were performed on T cells activated with PHA, PHA + IFN-γ, or PHA + IFN-γ + anti–IL-12. T cells activated with PHA alone for 4 days have high-affinity (kd = 61 ± 20 pmol/L, 259 ± 59 sites/cell) and low-affinity (kd = 6.0 ± 2.4 nmol/L, 2,775 ± 318 sites/cell) IL-12 receptors (Table 2 and Fig 7). When activated for 4 days with PHA + IFN-γ, the high-affinity IL-12 receptors were altered with respect to both the kd and number of sites per cell, whereas the low-affinity sites remained unchanged. Specifically, there was a 90% increase in the high-affinity kd (increasing from 61 ± 20 pmol/L to 115 ± 21 pmol/L) and an 80% increase in the number of high-affinity sites (increasing from 259 ± 59 to 470 ± 75). In contrast, for T cells activated with PHA + IFN-γ in the presence of the neutralizing IL-12 antibody, the high-affinity kd was increased by only 30% (88 ± 18 pmol/L) and the number of high-affinity sites increased by only 25% (325 ± 49) compared with T cells activated with PHA alone. There was also a modest 20% increase in the number of low-affinity sites with no significant change in the low-affinity kd. This suggests that the effect of IFN-γ on IL-12 binding is mediated largely by IL-12.
The IFN-γ–Induced Upregulation of High-Affinity IL-12 Receptor Expression During T-Cell Activation Is Inhibited by Neutralizing Endogenous IL-12
| Activation Condition . | High-Affinity IL-12R . | Low-Affinity IL-12R . | ||
|---|---|---|---|---|
| kd* (pmol/L) . | Sites/Cell . | kd (nmol/L) . | Sites/Cell . | |
| PHA | 61 ± 20 | 259 ± 59 | 6.0 ± 2.4 | 2,775 ± 318 |
| PHA + IFN-γ | 115 ± 21 | 470 ± 75 | 5.4 ± 1.5 | 2,925 ± 530 |
| PHA + IFN-γ + anti–IL-12 | 88 ± 18 | 325 ± 49 | 5.5 ± 1.4 | 3,325 ± 35 |
| Activation Condition . | High-Affinity IL-12R . | Low-Affinity IL-12R . | ||
|---|---|---|---|---|
| kd* (pmol/L) . | Sites/Cell . | kd (nmol/L) . | Sites/Cell . | |
| PHA | 61 ± 20 | 259 ± 59 | 6.0 ± 2.4 | 2,775 ± 318 |
| PHA + IFN-γ | 115 ± 21 | 470 ± 75 | 5.4 ± 1.5 | 2,925 ± 530 |
| PHA + IFN-γ + anti–IL-12 | 88 ± 18 | 325 ± 49 | 5.5 ± 1.4 | 3,325 ± 35 |
*Mean values and standard deviations for kd and sites/cell are based on data obtained from three separate experiments. Each experiment consisted of radiolabeled IL-12 binding assays performed on T cells from a single donor (using a different donor for each experiment) that were activated under the indicated conditions.
The IFN-γ–induced increase in high-affinity IL-12 receptor expression is mediated by IL-12. PBMC were activated with PHA, PHA + IFN-γ, or PHA + IFN-γ + anti–IL-12. Binding studies using [125I]–IL-12 were performed and the results analyzed using the Scatchard method. Similar results were obtained in three separate experiments.
The IFN-γ–induced increase in high-affinity IL-12 receptor expression is mediated by IL-12. PBMC were activated with PHA, PHA + IFN-γ, or PHA + IFN-γ + anti–IL-12. Binding studies using [125I]–IL-12 were performed and the results analyzed using the Scatchard method. Similar results were obtained in three separate experiments.
T Cells Activated With PHA + IL-12 Produce More IFN-γ in Response to IL-12 Than T Cells Activated With PHA Alone
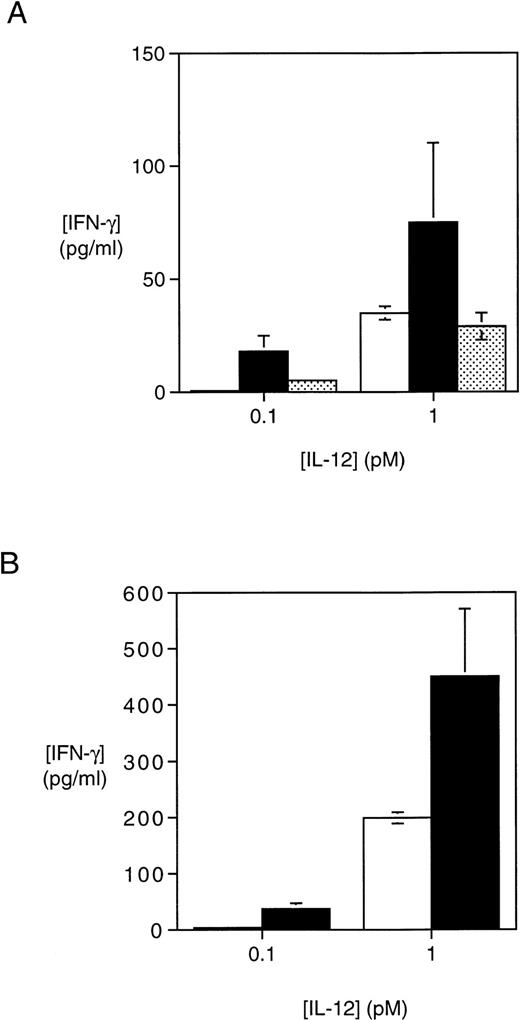
The activation of T cells with PHA + IL-12 significantly augments the expression of high-affinity IL-12 receptors and the ability of IL-12 to activate STAT1 and STAT5 relative to T cells activated with PHA alone. Thus, we examined how these changes impacted on the functional response of T cells to IL-12. We have shown previously that the activation of T cells with PHA + IFN-γ enhances IL-12–induced IFN-γ production to a greater extent than IL-12–induced proliferation.12 In T cells activated with PHA + IFN-γ, there is a twofold increase in IFN-γ production induced by 1 pmol/L IL-12 and a 10-fold increase in IFN-γ production induced by 0.1 pmol/L IL-12 when compared with T cells activated with PHA alone (Fig 8A). Significant changes in IL-12–induced proliferation were not observed (data not shown). When endogenous IFN-γ–induced IL-12 is neutralized during T-cell activation with PHA + IFN-γ, the augmentation of IL-12–induced IFN-γ production is largely abolished (Fig 8A). To confirm that the presence of small amounts of IL-12 during T-cell activation with PHA can enhance subsequent IL-12–induced IFN-γ production, T cells were cultured with either PHA alone or PHA + IL-12 1 pmol/L + anti–IFN-γ and then stimulated for 72 hours with IL-12. PBMC activated with PHA + IL-12 demonstrate similar increases in IL-12–induced IFN-γ production as PBMC activated with PHA + IFN-γ (Fig 8B). Consistent changes of this magnitude in IL-12–induced proliferation were not observed in T cells activated with PHA + IL-12 (data not shown).
T cells activated with PHA + IFN-γ or PHA + IL-12 exhibit an increase in IL-12–induced IFN-γ production. (A) PBMC activated with either PHA alone, PHA + IFN-γ, or PHA + IFN-γ + anti–IL-12 were plated at 3 × 104 cells/well and cultured for an additional 72 hours with the indicated concentrations of IL-12. Supernatants were then harvested and the IFN-γ concentration measured by ELISA. The values shown were obtained after subtracting the amount of IFN-γ produced by the same T cells in response to medium alone for 72 hours. Results are representative of two separate experiments. (□) PHA; (▪) PHA + IFN-γ; (▩) PHA + IFN-γ + anti–IL-12. (B) PBMC activated for 4 days with either PHA alone or PHA + IL-12 + anti–IFN-γ were plated at 3 × 104 cells/well and cultured for an additional 72 hours with the indicated concentrations of IL-12. Supernatants were then harvested and the IFN-γ concentration determined by ELISA as described in (A). Results are representative of two separate experiments. (□) PHA; (▪) PHA + 1 pmol/L IL-12 + anti–IFN-γ.
T cells activated with PHA + IFN-γ or PHA + IL-12 exhibit an increase in IL-12–induced IFN-γ production. (A) PBMC activated with either PHA alone, PHA + IFN-γ, or PHA + IFN-γ + anti–IL-12 were plated at 3 × 104 cells/well and cultured for an additional 72 hours with the indicated concentrations of IL-12. Supernatants were then harvested and the IFN-γ concentration measured by ELISA. The values shown were obtained after subtracting the amount of IFN-γ produced by the same T cells in response to medium alone for 72 hours. Results are representative of two separate experiments. (□) PHA; (▪) PHA + IFN-γ; (▩) PHA + IFN-γ + anti–IL-12. (B) PBMC activated for 4 days with either PHA alone or PHA + IL-12 + anti–IFN-γ were plated at 3 × 104 cells/well and cultured for an additional 72 hours with the indicated concentrations of IL-12. Supernatants were then harvested and the IFN-γ concentration determined by ELISA as described in (A). Results are representative of two separate experiments. (□) PHA; (▪) PHA + 1 pmol/L IL-12 + anti–IFN-γ.
DISCUSSION
In this report, we have shown that the opposing effects of IL-4 and IL-12 on high-affinity IL-12 receptor expression during human T-cell activation with PHA profoundly influence IL-12 signaling. Although IL-4 does not affect the expression of the low-affinity IL-12Rβ1 subunit,12 it does prevent the expression of at least one additional IL-12 receptor subunit (IL-12Rβ2),15,16thereby inhibiting the formation of IL-12Rβ1/IL-12Rβ2 heterodimers that bind IL-12 with high affinity.16 Our demonstration that the activation of Jak2 and Tyk2 by IL-12 is impaired by IL-4 despite the preservation of IL-12Rβ1 expression suggests that the IL-12–induced heterodimerization of IL-12Rβ1 and IL-12Rβ2 is required for both Jak2 and Tyk2 tyrosine phosphorylation. The residual IL-12–induced Tyk2 tyrosine phosphorylation in IL-4-treated cells may be triggered by the binding of IL-12 to IL-12Rβ1 alone, because Tyk2 appears to preferentially associate with IL-12Rβ1, whereas Jak2 associates with IL-12Rβ2.29 However, the observation that there is also residual Jak2 tyrosine phosphorylation suggests that Jak2 and Tyk2 are being activated through a vastly reduced complement of high-affinity IL-12Rβ1/IL-12Rβ2 heterodimers.
The effects of IL-4 on high-affinity IL-12 receptor expression and Jak2/Tyk2 activation by IL-12 are paralleled by the inhibition of STAT activation by IL-12. In this report, we have shown for the first time that IL-12 can induce the tyrosine phosphorylation of STAT1α, STAT1β, and STAT5 in activated human T cells. The tyrosine phosphorylation of STAT1α, STAT1β, and STAT5 induced by IL-12 is completely abrogated in T cells activated with PHA + IL-4, whereas STAT4 activation is greatly diminished but not completely extinguished. It appears, therefore, that, although the expression of IL-12Rβ1 and perhaps a small number of IL-12Rβ1/IL-12Rβ2 high-affinity heterodimers is sufficient to allow for low-level Jak2/Tyk2 and STAT4 activation in response to IL-12 in IL-4–treated T cells, it is not capable of mediating the activation of STAT1 and STAT5. This suggests that, at the level of IL-12 receptor expression, the requirements for STAT4 activation by IL-12 differ from the requirements for STAT1 and STAT5 activation. From a functional standpoint, we have shown previously that human T cells activated with PHA + IL-4 are still capable of proliferating and producing IFN-γ in response to IL-12, although the magnitude of these functional responses is greatly diminished compared with T cells activated with PHA alone.12 Although the preservation of some functional responsiveness to IL-12 in the absence of STAT1 and STAT5 activation may be an indication that STAT4 by itself (or perhaps STAT4 together with STAT3) can mediate the functional effects of IL-12, the fact that STAT1, STAT5, and STAT4 activation by IL-12 are all diminished in IL-4–treated T cells precludes any assignment of the relative importance of these STATs to IL-12 signaling.
In contrast with the demonstration that IL-4 inhibits IL-12 signaling when present during T-cell activation, the activation of T cells with PHA + IFN-γ markedly augments the ability of IL-12 to activate STAT1α, STAT1β, and STAT5. Although the IL-12–induced tyrosine phosphorylation of STAT5 in T cells activated with PHA + IFN-γ remains considerably less than that observed in response to IL-2, it is associated with STAT5 DNA-binding and is therefore likely to be functionally significant. Because we have shown that the effect of IFN-γ is mediated primarily through IL-12, we conclude that IL-12 exerts a positive autoregulatory effect on T-cell IL-12 signaling by stimulating T cells through IL-12 receptors that are upregulated during the early phase of T-cell activation. Several lines of evidence indicate that IL-12 is modulating its signaling pathway through changes in high-affinity IL-12 receptor expression. First, whereas the IL-12–induced activation of STAT5 and STAT1 is augmented in T cells activated with PHA + IFN-γ or PHA + IL-12, the activation of these same STAT proteins by IL-2 is unaffected. This excludes the possibility that IL-12 is nonspecifically augmenting the tyrosine phosphorylation of signaling proteins through, for example, the stabilization of phosphotyrosine moieties. Furthermore, we have shown that IL-12 does not increase the IL-12–induced tyrosine phosphorylation of STAT1 and STAT5 by altering the total amount of STAT1 and STAT5 present within activated T cells. Second, the increased activation of STAT1 and STAT5 in response to IL-12 correlates with strong increases in Jak2 and Tyk2 tyrosine phosphorylation, whereas only weak augmentation in the activation of Jak3 by IL-2 is observed. Because Jak2 and Tyk2 physically associate with subunits of the IL-12 receptor and comprise the most proximal components of IL-12 signaling, it is likely that changes in their level of activation in response to IL-12 reflect changes in IL-12 receptor expression. Finally, we have shown that the increase in high-affinity IL-12 receptor expression (80% increase) observed on T cells activated with PHA + IFN-γ compared with T cells activated with PHA alone is significantly diminished when endogenous IL-12 is neutralized. This correlates with similar decreases in the level of augmentation of IL-12–induced STAT5 and STAT1 tyrosine phosphorylation.
Using a neutralizing IL-12Rβ1 antibody, we have shown previously that all of the high-affinity IL-12 binding on T cells activated with PHA + IFN-γ was dependent on IL-12Rβ1 expression.12 We also demonstrated that IFN-γ did not affect IL-12Rβ1 expression, suggesting that IFN-γ was upregulating high-affinity IL-12 receptor expression by enhancing the expression of one or more additional IL-12R subunits that formed high-affinity complexes with IL-12Rβ1. Although we have presented evidence in this report that indicates that IFN-γ–induced IL-12 is largely responsible for the upregulation of these additional subunits, the identity of these IL-12R subunits is currently undefined. Because IL-12 appears to be capable of promoting IL-12Rβ2 gene transcription,15 16 it is possible that the IL-12–induced increase in high-affinity IL-12 binding sites is due solely to the upregulation of IL-12Rβ2 surface expression. If this is true, it would suggest that a threshold exists for the number of high-affinity IL-12Rβ1/IL-12Rβ2 heterodimers that are required for the optimal activation of STAT5 and STAT1 by IL-12. Alternatively, when present during activation with PHA, IL-12 may be upregulating the expression of a third IL-12R subunit that, like IL-12Rβ2, can form high-affinity heterodimers with IL-12Rβ1. This hypothetical third subunit might be the means through which STAT5 and additional STAT1 molecules can be recruited to the IL-12 receptor and tyrosine phosphorylated in response to IL-12. Interestingly, the kd of the high-affinity IL-12 binding sites on T cells activated with PHA + IFN-γ is twice as large as the kd of high-affinity IL-12 receptors on T cells activated with PHA alone. This supports the hypothesis that the additional high-affinity IL-12 receptors appearing in response to IFN-γ–induced IL-12 might be different from those present after activation with PHA alone.
Whereas the IL-12–induced increase in high-affinity IL-12 receptor expression is associated with an increase in STAT5 and STAT1 activation by IL-12, there is no corresponding increase in STAT4 activation as measured by STAT4 tyrosine phosphorylation and DNA binding. This implies that the complement of high-affinity IL-12 receptors needed for maximal STAT4 activation by T cells differs from that required for optimal STAT5 and STAT1 activation. It also suggests that the observed change in IL-12–induced IFN-γ production among T cells activated with PHA + IL-12 may be mediated through STAT5 and STAT1 rather than STAT4. Although studies with STAT4 knockout mice have demonstrated that STAT4 activation is needed for all of the major functional responses of T cells to IL-12,23 our present findings suggest that STAT5 and STAT1 may play an important role in modulating the magnitude of IL-12–induced IFN-γ production while having little effect on IL-12–induced proliferation. Interestingly, it has been shown that STAT1, STAT4, and STAT5 can all bind to distinct and partially overlapping sequences of DNA within the first intron of the human IFN-γ gene.32 Furthermore, the optimal binding of these STATs to DNA appears to require cooperative interactions between STATs mediated through conserved amino-terminal domains and has a significant impact on gene transcription. It is possible, therefore, that the optimal binding of STAT4 to key regulatory sequences within the IFN-γ gene required for optimal gene transcription depends on cooperative interactions of STAT4 molecules with STAT1 and STAT5, thus dictating that all three STAT proteins must be activated to maximize IFN-γ production.
Our experiments with PBMC cultured with PHA + IFN-γ are notable for demonstrating that the presence of a small amount of APC-derived IL-12 during T-cell activation is sufficient to augment both IL-12 signaling and the functional response of PHA-activated T cells to IL-12. The increase in IL-12–induced IFN-γ production is most pronounced when analyzing the T-cell response to IL-12 at concentrations ranging from 0.1 to 1 pmol/L, lending further support to the hypothesis that the observed increase in high-affinity IL-12 receptor expression plays an important role in the augmentation of IL-12 signaling and IL-12–induced IFN-γ production. A number of reports have shown that the in vitro activation of human monocytes, dendritic cells, and Langerhans cells leads to the production of small amounts of the p70 IL-12 heterodimer, on the order of 0.1 to 1 pmol/L.1-3 This corresponds to what we have observed in our experimental system when PBMC are activated with PHA + IFN-γ. Similar amounts of p70 IL-12 are produced by CD40-stimulated APCs from patients with multiple sclerosis33 and by mycobacterium + IFN-γ–stimulated APCs from patients with tuberculoid leprosy.34 These findings suggest that, during the initial phase of antigen presentation and T-cell activation, the development of a Th1 response may depend on the ability of T cells to respond to small quantities of APC-derived IL-12. The findings in this report suggest that a mechanism has evolved whereby T cells, detecting the presence of small amounts of IL-12 through high-affinity IL-12 receptors, can maximize their response to that IL-12 by upregulating high-affinity IL-12 receptor expression. This may then allow the recruitment and activation of STAT5 and STAT1, which may amplify STAT4-dependent functional responses to IL-12.
Although the correlation observed in this report between changes in IL-12–induced STAT5 and STAT1 activation and IL-12–induced IFN-γ production raises the possibility that the modulation of STAT5 and STAT1 activation can alter specific IL-12 functional responses, it is also possible that STAT5 and STAT1 activation plays no role in mediating the T-cell response to IL-12. In addition to activating the Jak-STAT signaling pathway, IL-12 has also been reported to activate MAP kinase in T cells.35 It is therefore possible that changes in either IL-12–induced MAP kinase activation or in the activation of other undiscovered components of IL-12 signaling are responsible for the change in IL-12 responsiveness in IL-12–activated T cells. To further explore the role of STAT1 and STAT5 in mediating the functional responses of T cells to IL-12, it will be important to examine whether the autoregulatory enhancement of IL-12 responsiveness demonstrated in this report with human T cells exists in mice and, if so, whether it is abrogated in STAT1, STAT5, or STAT1/STAT5 knockout mice. Furthermore, it will be important to determine whether STAT1 and STAT5 activation also correlates with other important functional effects of IL-12, such as Th1 development and cytolytic activity. Because STAT3 is activated by both IL-12 and IL-2 in T cells, determining whether IL-12 and IL-4 can modulate the activation of STAT3 by IL-12 will also help to further elucidate whether changes in the degree of activation of distinct combinations of STATs is one of the mechanisms through which changes in IL-12 receptor expression control specific functional responses to IL-12.
ACKNOWLEDGMENT
The authors thank Genetics Institute for generously providing rhIL-12.
Supported by National Institutes of Health Grant No. CA41619, American Cancer Society Grant No. PRTA-33, and the Dr. Jeanne M. Connors and Adelaide T. Hassett Research Foundation.
Address reprint requests to Jared A. Gollob, MD, Department of Adult Oncology, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.





![Fig. 7. The IFN-γ–induced increase in high-affinity IL-12 receptor expression is mediated by IL-12. PBMC were activated with PHA, PHA + IFN-γ, or PHA + IFN-γ + anti–IL-12. Binding studies using [125I]–IL-12 were performed and the results analyzed using the Scatchard method. Similar results were obtained in three separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/4/10.1182_blood.v91.4.1341/4/m_blod4041007.jpeg?Expires=1769138729&Signature=U8dn3bupmzHDNkVjHwE8zesleHyRfjoEZJ1FZvIWawM46hDDhMYZpA5r7fX5gp7Zb6J00btfbKL4sjJCiYntiaiEfvCB1~tL6-q199uD5pvSir81UFNr-Bic9yYhZXHq3Bjnt-THKisxAFYaOLrZTv06SOCJSoV2HWBXs5cBAGR0WYaFdtWPKoVf--1~dimyIQ9JcO5KFddoWxQ2o55wd48TnhZkd7Lt2gI8MAJlW6FB~C5g8Iu7ECNPAC6MDhHs4l7EIw7tIT8JBeCpEczyxj2deo~7T3fftwrhM7cn~rzdXON~Lm8KgcF-~1uKpp7qwm8AhtIwqxNGjZ7y-2drbA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal