Abstract
Capsaicin and its ultrapotent analog resiniferatoxin (RTX) act through specific vanilloid receptors on sensory neurons. The C-type receptor is coupled to 45Ca uptake, whereas the R-type is detectable by [3H]RTX binding. We describe here specific vanilloid responses in murine mast cells (MCs). In the MC lines and in bone marrow-derived mast cells, capsaicin and RTX induced45Ca uptake similarly to that observed for cultured rat dorsal root ganglion neurons (DRGs). This response was antagonized by the antagonists capsazepine and ruthenium red. As in DRGs, pretreatment of MCs with capsaicin or RTX induced desensitization to subsequent stimulation of 45Ca uptake. The potency for desensitization by RTX in the MCs corresponded to that for 45Ca uptake, whereas in DRGs it occurred at significantly lower concentrations corresponding to that for the high-affinity [3H]RTX binding site. Consistent with this difference, in MCs we were unable to detect [3H]RTX binding. Vanilloids were noncytotoxic to the MCs, in contrast to the DRGs. Although vanilloids did not cause degranulation in MCs, in the P815 clone capsaicin evoked selective interleukin-4 release. We conclude that certain MCs possess vanilloid receptors, but only the C-type that functions as a channel. Our finding that MCs can respond directly to capsaicin necessitates a reevaluation of the in vivo pathway of inflammation in response to vanilloids.
A SUBPOPULATION OF primary afferent neurons, located in the dorsal root and trigeminal ganglia, can be defined by their selective susceptibility to the effects of capsaicin,1,2 the major pungent ingredient of hot peppers of the plant genus Capsicum, and to its ultrapotent analog, resiniferatoxin (RTX), a naturally occurring irritant tricyclic diterpene3 that combines structural features of the phorbol ester tumor promoters and of capsaicin.4 Although no exact neurochemical correlation exists, these neurons in general contain calcitonin-gene related peptide (CGRP) and tachykinins such as substance P (SP).5
Upon activation, capsaicin-sensitive nerves both transmit signals to the central nervous system and release neuropeptides such as SP and CGRP in the periphery.6 The role of this latter, efferent function of the sensory neurons is crucial in inducing neurogenic inflammation, a process that can be best modeled by the application of capsaicin and related vanilloids.7
Capsaicin and its analogs act via the stimulation of vanilloid receptors on sensory neurons.8,9 The effects of capsaicin can be described as three consecutive phenomena: first, capsaicin excites neurons bearing the vanilloid receptors; it then desensitizes them to subsequent stimuli; and, finally, depending on its time of action and concentration, it causes neurotoxicity.2 The mechanisms of excitation, desensitization, and neurotoxicity are distinct entities and individually represent the variety of processes elicited by vanilloids. Previous studies have identified two vanilloid receptor subclasses (classified as C- and R-type vanilloid receptors) on dorsal root ganglion neurons (DRGs),10defined by distinct pharmacology and physiology and detected by45Ca uptake and [3H]RTX binding assays, respectively, suggesting that different vanilloid induced mechanisms can be mediated by different receptor subclasses (Table1).
Comparison of the C-Type and R-Type Vanilloid Receptors Found on DRG Neurons
| . | C-Type . | R-Type . |
|---|---|---|
| Capsaicin (Kd) | 316 nmol/L | 4,900 nmol/L |
| Resiniferatoxin (Kd) | 1 nmol/L | 0.04 nmol/L |
| Cooperativity (Hill coefficient) | 0.9-1.2 | 1.5-2.0 |
| Capsazepine (Ki) | 290 nmol/L | 4,000 nmol/L |
| Ruthenium red (IC50) | 800 nmol/L | 14 nmol/L |
| 45Ca uptake | Yes | No |
| Desensitization | Yes | Yes |
| Toxicity | Yes | No |
| . | C-Type . | R-Type . |
|---|---|---|
| Capsaicin (Kd) | 316 nmol/L | 4,900 nmol/L |
| Resiniferatoxin (Kd) | 1 nmol/L | 0.04 nmol/L |
| Cooperativity (Hill coefficient) | 0.9-1.2 | 1.5-2.0 |
| Capsazepine (Ki) | 290 nmol/L | 4,000 nmol/L |
| Ruthenium red (IC50) | 800 nmol/L | 14 nmol/L |
| 45Ca uptake | Yes | No |
| Desensitization | Yes | Yes |
| Toxicity | Yes | No |
See Acs et al.10
The action of capsaicin in inducing neurogenic inflammation involves, among other cell types,2 the activation of mast cells (MCs). This finding is generally attributed to two facts. First, capsaicin-sensitive nerves are in anatomical contact with MCs in a variety of tissues.11,12 Second, neuropeptides released from sensory neurons by capsaicin can induce MC degranulation (resulting in the release of, eg, proteoglycans, histamine, and serotonin) as well as the production and release of a wide array of proinflammatory cytokines including interleukins (ILs) and tumor necrosis factor-α (TNF-α).13,14 These MC mediators then can further stimulate the release of SP and other peptides from sensory nerves, and antidromic stimulation of these neurons can further induce MC activation.15 Thus, this bidirectional MC-sensory neuron autocatalytic loop can amplify the efferent function of sensory neurons, eventually resulting in neurogenic inflammation.16
This hypothesis is supported by a number of reports showing that the induction of neurogenic inflammation by capsaicin in vivo is associated with MC activation. For example, capsaicin induced inflammation of the skin17,18 and gastric mucosa19 have been described to be accompanied by MC degranulation. Furthermore, the inflammatory response to the in vivo administration of capsaicin is reduced by inhibitors of MC degranulation or antagonists of MC products histamine or serotonin.17 20
These actions of capsaicin on MCs have generally been considered to be indirect via the release of SP and other peptides. We show here the existence of functional capsaicin receptors on MC lines, the stimulation of which results in the influx of calcium, desensitization of mast cells to subsequent vanilloid stimulation, and production and release of the proinflammatory cytokine IL-4. We suggest that, in addition to its action on sensory neurons, capsaicin may also act directly on MCs. Moreover, these findings represent the first example of nonneuronal cell lines expressing vanilloid receptors. The presence of the vanilloid receptor on MCs may help to clarify the complex in vivo effects of vanilloids and, furthermore, expand the range of their potential therapeutic applications. In addition, the identification of cell lines containing C-type vanilloid receptors should greatly facilitate the biochemical and molecular analysis of these receptors.
MATERIALS AND METHODS
MC/9, P815, 10P-2, 10P-12, 11PO-1, and RBL-1 cells were purchased from ATCC (Gaithersburg, MD). PT-18 and RBL-2H3 and CFTL-12 cells were generous gifts from Drs J. Rivera and J. Pierce (National Institutes of Health, Bethesda, MD), respectively. [3H]RTX was synthesized by the Chemical Synthesis and Analysis Laboratory, NCI-FCRDC (Frederick, MD). 45Ca (CaCl2) and [3H]5-hydroxytryptamine (serotonin) were purchased from DuPont-New England Nuclear (Boston, MA). Nonradioactive RTX and capsazepine were from LC Laboratories (Woburn, MA). Capsaicin and adenosine-5-triphosphate were from Sigma Chemical Co (St Louis, MO). Ruthenium red was purchased from Research Biochemicals International (Natick, MA).
Cell cultures.
The following media and supplements (GIBCO BRL, Gaithersburg, MD) were used for the culture of mast cell lines: Dulbecco's modified Eagle's medium (DMEM) supplemented with 116 mg/mL L-arginine, 36 mg/mL L-asparagine, 6 mg/mL folic acid, 0.1 mmol/L nonessential amino acids, 1 mmol/L sodium pyruvate, 4 mmol/L L-glutamine, 0.05 mmol/L 2-mercaptoethanol, 10% fetal bovine serum (FBS), and 45% conditioned medium (T-Stim; rat growth factor with concanavalin-A; Collaborative Biomedical Products, Bedford, MA) for MC/9 cells; RPMI-1640 medium supplemented with 10% FBS, 20% T-Stim, 0.1 mmol/L nonessential amino acids, 1 mmol/L sodium pyruvate, 4 mmol/L L-glutamine, and 0.05 mmol/L 2-mercaptoethanol for PT-18 cells; RPMI-1640 medium supplemented with 0.05 mmol/L 2-mercaptoethanol and 10% FBS for 10P-2, 10P-12, 11PO-1, and CFTL-12 cells; DMEM supplemented with 10% FBS for P815 cells; and Eagle's minimum essential medium supplemented with Earle's balanced salt solution, 16% FBS, 0.1 mmol/L nonessential amino acids, and 2 mmol/L L-glutamine for RBL-1 and RBL-2H3 cells. All media contained penicillin and streptomycin at a concentration of 100 IU/mL and 100 μg/mL, respectively.
Preparation of MCs.
Murine mast cell populations, ie, peritoneal mast cells (PMCs) and bone marrow-derived cultured mast cells (BMCMCs), were prepared as described previously.21,22 To obtain PMCs, retired breeder Balb/c mice (The Jackson Laboratory, Bar Harbor, ME) were euthanized by cervical dislocation, and 10 mL sterilized lavage solution was injected into the abdominal cavity. After 3 minutes of abdominal massage, the abdominal cavity was opened and the fluid was collected. Total peritoneal cells from intraperitoneal lavage solution were fractionated on 23% (wt/vol) metrizamide (Sigma). The MC content of mast cell enriched fractions was determined by Toluidine-blue staining (>95% purity)23; viability of all fractions was determined by Trypan-blue dye exclusion (>95% viability). IL-3–dependent BMCMCs were derived from the femoral bone marrow cells of Balb/c mice and were maintained in DMEM supplemented with 10% FBS, 2 mmol/L L-glutamine, 0.05 mmol/L 2-mercaptoethanol, and 20% (vol/vol) supernatants of concanavalin-A–activated spleen cells.22
Measurement of 45Ca uptake by MCs.
Cells were plated into MultiScreen-DV 96-well filtration plates (Millipore, Marlborough, MA) at a density of 5 to 10 × 104 cells/well in 100 μL serum-free DMEM and were then incubated in a total volume of 0.25 mL of serum-free DMEM (containing 1.8 mmol/L CaCl2) in the presence of 0.25 mg/mL bovine serum albumin (BSA; Sigma; included to stabilize the compounds in the aqueous solution), 1 μCi/mL 45Ca, and increasing concentrations of the different compounds for 30 minutes at 37°C.24 25 This incubation period was chosen, because in a control experiment, in which the time course of the capsaicin induced 45Ca uptake was determined, no major differences were found between the values measured on DRGs and mast cells (the45Ca uptake reached its maximum value after 12 and 16 minutes, respectively). Cells were then washed five times with ice-cold serum-free DMEM by filtration using a MultiScreen Vacuum Manifold (Millipore). Filters were dried under a heat lamp and punched out into scintillation vials using MultiScreen disposable punch tips, and the radioactivity was determined by scintillation counting. For each data point in each experiment, five wells were assayed. In the case of desensitization experiments, cells were incubated at 37°C with capsaicin or RTX for the indicated period of time (usually 6 hours) and then challenged with capsaicin or RTX, as indicated.
Analysis of 45Ca uptake data.
The 45Ca uptake experiments were analyzed as described previously25 by computer fit to the Hill equation.26 In the case of the desensitization experiments, desensitization was defined as the difference (in disintegrations per minute [dpm] per well) between the increase in45Ca uptake in these and in control cells upon challenge by capsaicin or RTX. The decrease in the 45Ca uptake induced by vanilloids was plotted against the pretreatment concentration of RTX or capsaicin and the data were fitted to the Hill equation. Data from competition experiments, in which the effect of the desensitizing compound was antagonized by either a competitive (capsazepine) or a noncompetitive (ruthenium red) antagonist, were fitted to the modified Hill equation.27 Data fitting was performed using the computer program MicroCal Origin 3.5 (MicroCal Software Inc, Northampton, MA).
Cytotoxicity (MTT) assay.
Cytotoxicity was measured with the MTT assay kit according to the manufacturer's protocol (Sigma). Briefly, cells were incubated for 6 hours at 37°C with the compound as indicated. Cells were then washed with RPMI-1640 medium without phenol red (GIBCO BRL), resuspended in the same medium at a final concentration of 1 × 105 cells/mL, and plated into 96-well plates. Tetrazolium salt (MTT) was added to each well at the concentration of 0.5 mg/mL, and the absorbance of the developed purple color was measured at 567 nm.
Release of serotonin by MCs.
Degranulation of MCs was assessed by [3H]5-hydroxytryptamine ([3H]-5HT; Dupont-NEN) release as described previously.28 Briefly, cells in 48-well plates (105 cells/well) were preincubated with [3H]-5HT (1 μCi/mL) in Tyrode's buffer for 2 hours at 37°C and then were washed three times with buffer to remove excess radioactivity. The cells were then challenged for 30 minutes at 37°C with different agents and supernatants were collected by centrifugation. Radioactivity released into the supernatants was measured by scintillation counting.
Determination of cytokine secretion.
The production of IL-4, IL-6, and TNF-α was determined by enzyme-linked immunosorbent assay (ELISA) kits (Biosource International, Camarillo, CA). Cells were plated into 48-well plates in serum-free DMEM and incubated with vanilloids for different times (usually 3 hours) at 37°C, after which the supernatant was collected in a final volume of 0.5 mL. ELISAs were performed according to the manufacturer's protocols.
RESULTS
Capsaicin and resiniferatoxin induce 45Ca uptake in MC lines.
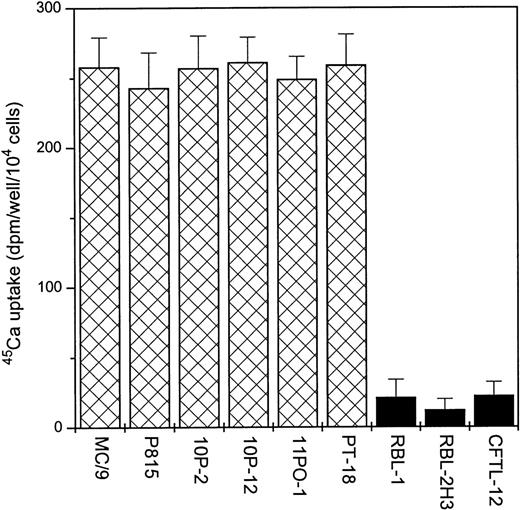
A variety of MC lines were tested for stimulation of 45Ca uptake in response to 3 μmol/L capsaicin (a concentration that induces a maximal response in DRGs10,25). As shown in Fig 1, 6 of 9 mast cell lines examined exhibited significant stimulation of 45Ca uptake after the addition of capsaicin. Adenosine-5-triphosphate (ATP; 500 μmol/L), which has been shown to activate a nonspecific, Ca2+-permeable channel found on many cell types, including mast cells,29 was used as a positive control. ATP induced45Ca uptake in each of the cell lines we examined, including the RBL-1, RBL-2H3, and CFTL-12 cell lines, which showed no sensitivity to capsaicin.
Capsaicin induces 45Ca uptake in different MC lines. Cells were challenged with 3 μmol/L capsaicin for 30 minutes to induce 45Ca uptake. Points represent mean over baseline values from sets of five determinations in at least three experiments in each case; error bars indicate SEM.
Capsaicin induces 45Ca uptake in different MC lines. Cells were challenged with 3 μmol/L capsaicin for 30 minutes to induce 45Ca uptake. Points represent mean over baseline values from sets of five determinations in at least three experiments in each case; error bars indicate SEM.
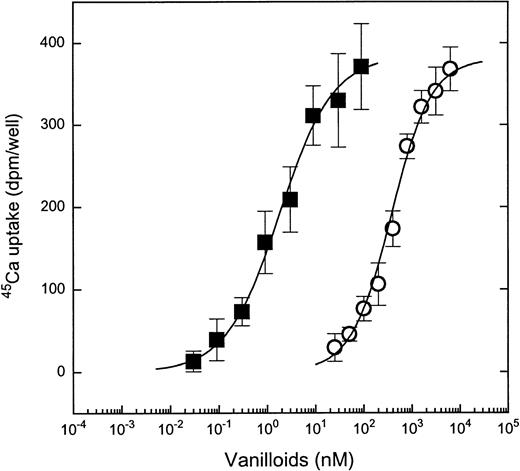
To compare the pharmacologic characteristics of the vanilloid responsiveness of the MC lines with those of DRGs, we determined the dose-response curves for capsaicin- and RTX-induced 45Ca uptake. In the MC/9 cells, capsaicin induced 45Ca uptake in a dose-dependent fashion (Fig 2), with a Kd value of 0.661 ± 0.04 μmol/L (mean ± SEM for 4 experiments). The stimulation of 45Ca uptake by capsaicin was noncooperative, with a Hill coefficient of 0.97 ± 0.01 (mean ± SEM for 4 experiments). Similar Kd and Hill coefficient values were found on the other responsive cell lines; the respective average values for 10P-2 cells were 0.552 μmol/L and 0.967, for 10P-12 cells were 0.494 μmol/L and 1.06, for 11PO-1 cells were 0.683 μmol/L and 0.993, for P815 cells were 0.590 μmol/L and 1.01, and for PT-18 cells were 0.393 μmol/L and 1.03 (2 to 3 experiments for each cell line). These values agreed well with those determined previously on rat DRGs10(Table 2).
Comparison of dose-response curves for induction of45Ca uptake by capsaicin or RTX in MC/9 cells. Cells were challenged with different concentrations of capsaicin (○) or RTX (▪) for 30 minutes. Points represent mean values from sets of five determinations in a single experiment; error bars indicate SEM. In both cases, at least three experiments yielded similar results. The theoretical curves were calculated by fitting the measured values to the Hill equation.
Comparison of dose-response curves for induction of45Ca uptake by capsaicin or RTX in MC/9 cells. Cells were challenged with different concentrations of capsaicin (○) or RTX (▪) for 30 minutes. Points represent mean values from sets of five determinations in a single experiment; error bars indicate SEM. In both cases, at least three experiments yielded similar results. The theoretical curves were calculated by fitting the measured values to the Hill equation.
Comparison of Characteristics of Vanilloid Receptors on DRGs and MC/9 Cells
| . | DRGs . | MC/9 Cells . | ||
|---|---|---|---|---|
| ED50 (nmol/L) . | Hill . | ED50 (nmol/L) . | Hill . | |
| Capsaicin-induced | ||||
| 45Ca uptake | 316 ± 47 | 1.02 ± 0.06 | 661 ± 41 | 0.97 ± 0.01 |
| Desensitization | 445 ± 46 | 0.89 ± 0.15 | 525 ± 12 | 1.06 ± 0.05 |
| RTX-induced | ||||
| 45Ca uptake | 1.24 ± 0.02 | 1.08 ± 0.07 | 2.1 ± 0.18 | 0.82 ± 0.06 |
| Desensitization | 0.081 ± 0.01 | 1.51 ± 0.11 | 2.3 ± 0.65 | 0.84 ± 0.07 |
| [3H]RTX binding | 0.047 ± 0.01 | 1.78 ± 0.12 | No | |
| Capsazepine | 291 ± 31* | 0.98 ± 0.03 | 311 ± 33* | 0.91 ± 0.06 |
| Ruthenium red | 790 ± 40 | NA | 980 ± 69 | NA |
| . | DRGs . | MC/9 Cells . | ||
|---|---|---|---|---|
| ED50 (nmol/L) . | Hill . | ED50 (nmol/L) . | Hill . | |
| Capsaicin-induced | ||||
| 45Ca uptake | 316 ± 47 | 1.02 ± 0.06 | 661 ± 41 | 0.97 ± 0.01 |
| Desensitization | 445 ± 46 | 0.89 ± 0.15 | 525 ± 12 | 1.06 ± 0.05 |
| RTX-induced | ||||
| 45Ca uptake | 1.24 ± 0.02 | 1.08 ± 0.07 | 2.1 ± 0.18 | 0.82 ± 0.06 |
| Desensitization | 0.081 ± 0.01 | 1.51 ± 0.11 | 2.3 ± 0.65 | 0.84 ± 0.07 |
| [3H]RTX binding | 0.047 ± 0.01 | 1.78 ± 0.12 | No | |
| Capsazepine | 291 ± 31* | 0.98 ± 0.03 | 311 ± 33* | 0.91 ± 0.06 |
| Ruthenium red | 790 ± 40 | NA | 980 ± 69 | NA |
*In the case of capsazepine, numbers represent Kivalues.
Abbreviation: NA, not applicable.
RTX induced 45Ca uptake into mast cells with markedly higher potency than did capsaicin (Kd = 2.1 ± 0.18 nmol/L, mean ± SEM for 4 experiments on MC/9 cells; Fig 2). As was the case with capsaicin, the stimulation of 45Ca uptake by RTX was noncooperative (Hill coefficient, 0.82 ± 0.06; mean ± SEM for 4 experiments on MC/9 cells). Once again, these values agreed well with those determined on DRGs (Table 2 and Acs et al10).
Vanilloid-induced 45Ca uptake in MCs is inhibited by vanilloid antagonists.
In DRGs, 45Ca uptake in response to vanilloids can be blocked by the competitive antagonist capsazepine30,31 and the noncompetitive antagonist ruthenium red.32 In MC/9 cells (and also in the other responding cell lines; data not shown) capsazepine likewise inhibited the 45Ca uptake by 3 μmol/L capsaicin (Fig 3). The Ki for inhibition by capsazepine was 0.311 ± 0.03 μmol/L (mean ± SEM for 4 experiments) and reflected noncooperative kinetics (Hill coefficient, 0.91 ± 0.06; mean ± SEM for 4 experiments). The noncompetitive inhibitor ruthenium red also inhibited the 45Ca uptake induced by capsaicin (Fig 3); the ED50 was 0.98 ± 0.07 μmol/L (mean ± SEM for 3 experiments). Similar values were obtained for the inhibition of45Ca uptake induced by 4 nmol/L RTX by capsazepine and ruthenium red (Ki of 0.291 ± 0.05 μmol/L and ED50 of 0.91 ± 0.11 μmol/L for capsazepine and ruthenium red, respectively; mean ± SEM for 3 experiments in each case). These values once again showed good agreement with those found for rat DRGs (Table 2 and Acs et al10).
Vanilloid antagonists inhibit the capsaicin-induced45Ca uptake in MC/9 cells. Cells were challenged with 3 μmol/L capsaicin in the presence of increasing concentrations of capsazepine (○) or ruthenium red (▪). Points represent mean values from sets of five determinations in a single experiment; error bars indicate SEM. Two additional experiments in each case yielded similar results. The theoretical curves were calculated by fitting the measured values to the Hill equation.
Vanilloid antagonists inhibit the capsaicin-induced45Ca uptake in MC/9 cells. Cells were challenged with 3 μmol/L capsaicin in the presence of increasing concentrations of capsazepine (○) or ruthenium red (▪). Points represent mean values from sets of five determinations in a single experiment; error bars indicate SEM. Two additional experiments in each case yielded similar results. The theoretical curves were calculated by fitting the measured values to the Hill equation.
Pretreatment of MCs with capsaicin or RTX results in desensitization.
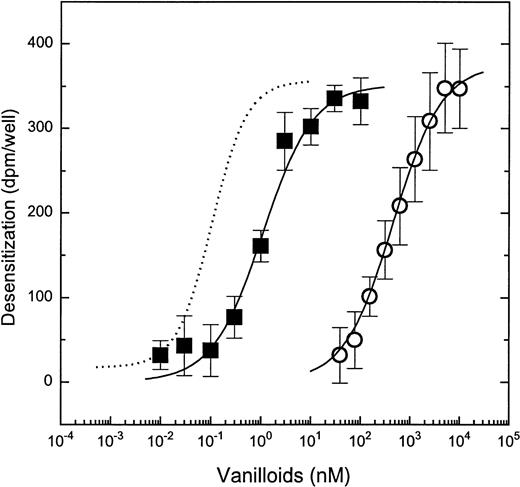
In DRGs, stimulation of 45Ca uptake by capsaicin is followed by desensitization of subsequent stimuli.2,10 We therefore examined the effect of vanilloid pretreatment on vanilloid-induced activation of MCs. MC/9 cells were pretreated with different concentrations of capsaicin for 6 hours (a time that was shown to induce maximal desensitization on rat DRGs10) and challenged with 3 μmol/L capsaicin to evoke 45Ca uptake. Desensitization was determined as a decrease in the capsaicin induced response compared with control (solvent-treated) cells. Pretreatment of the cells with capsaicin resulted in the dose-dependent loss of45Ca uptake induced by challenge with 3 μmol/L capsaicin (Fig 4). The ED50 and Hill coefficients were 0.525 ± 0.01 μmol/L and 1.06 ± 0.05, respectively (mean ± SEM for 3 experiments). These values are similar to those for induction of desensitization on DRGs. Likewise, they resemble those for the acute induction of45Ca uptake by capsaicin10 (Table 2).
Pretreatment with capsaicin or RTX results in the desensitization of capsaicin-induced 45Ca uptake in MC/9 cells. Cells were pretreated with different concentrations of capsaicin (○) or RTX (▪) for 6 hours and then were challenged with 3 μmol/L capsaicin for 30 minutes. For better comparison, the dose-response for RTX-induced desensitization on DRGs is also plotted (dotted line). Desensitization was defined as the difference (in dpm per well) in45Ca uptake between pretreated and control cells when challenged with capsaicin. Points represent mean values from sets of five determinations in a single experiment; error bars indicate SEM. In both cases, at least three experiments yielded similar results. The theoretical curves were calculated by fitting the measured values to the Hill equation. The dose-response curve for RTX-induced desensitization on DRGs is a single experiment, yielding similar values to those found by us previously.10
Pretreatment with capsaicin or RTX results in the desensitization of capsaicin-induced 45Ca uptake in MC/9 cells. Cells were pretreated with different concentrations of capsaicin (○) or RTX (▪) for 6 hours and then were challenged with 3 μmol/L capsaicin for 30 minutes. For better comparison, the dose-response for RTX-induced desensitization on DRGs is also plotted (dotted line). Desensitization was defined as the difference (in dpm per well) in45Ca uptake between pretreated and control cells when challenged with capsaicin. Points represent mean values from sets of five determinations in a single experiment; error bars indicate SEM. In both cases, at least three experiments yielded similar results. The theoretical curves were calculated by fitting the measured values to the Hill equation. The dose-response curve for RTX-induced desensitization on DRGs is a single experiment, yielding similar values to those found by us previously.10
When pretreated for 6 hours with increasing concentrations of RTX, MC/9 cells also exhibited desensitization to subsequent challenge with 3 μmol/L capsaicin (Fig 4). The ED50 and Hill coefficient values for desensitization by RTX were 2.3 ± 0.65 nmol/L and 0.84 ± 0.07, respectively (mean ± SEM for 4 experiments). The potency of RTX thus was markedly different from that determined on rat DRGs for desensitization (ED50 of 81 ± 5 pmol/L; see Acs et al10). However, it was similar to that for induction of 45Ca uptake by RTX on MCs (ED50 of 2.1 ± 0.18 nmol/L; see above). In addition, RTX induced desensitization in a noncooperative manner, once again similar to the induction of45Ca uptake by RTX in these cells but in contrast to the positive cooperativity found on DRGs (Hill coefficient, 1.51 ± 0.11; see Acs et al10).
MC lines express the C-type but not the R-type vanilloid receptor.
Previously, we have shown high-affinity specific binding of [3H]RTX on DRGs cultured in vitro25 and on DRG membrane preparations.8,33 Using the same protocols, we were unable to detect specific binding on any of the mast cell lines examined, either on intact cells or on membranes. The measured value on membranes was 0.69 ± 0.99 fmol/mg protein (this value did not differ significantly from 0; P > .5), in contrast to the 198 ± 13 fmol/mg protein on DRG membranes.25 The lack of binding is consistent with the lack of the high-affinity binding site. The failure in detection of the low-affinity, although obviously existing, C-type receptor with this binding assay was predictable and could be attributed to the combination of lower affinity, nonspecific binding, and low Bmax on mast cells (see also in Discussion).
Table 2 summarizes the characteristics of the vanilloid receptors found on MC/9 cells and compares them with those described on rat DRGs.10 Our findings strongly argue that MCs possess the C-type receptor that can be detected by the 45Ca uptake assay but not the R-type receptor that can be assayed by [3H]RTX binding.
Vanilloid-mediated activation of MCs is not associated with cytotoxicity.
On DRGs, the capsaicin-induced Ca2+ influx is thought to be a major factor mediating vanilloid cytotoxicity.9,34Because of the relative resistance of mast cells to vanilloid toxicity in vivo,35 36 we wished to evaluate whether the mast cell vanilloid receptor confers cytotoxicity in the MCs similar to its action on DRGs. Using the MTT assay, we found that the exposure of MC/9 cells to capsaicin for 6 hours was nontoxic up to a concentration of 10 μmol/L (data not shown); likewise, RTX showed no toxicity up to 20 nmol/L.
Vanilloids do not induce degranulation in MCs.
To assess whether vanilloid-induced activation of mast cells is associated with degranulation, we determined the release of [3H]5-HT (serotonin) from MC lines upon vanilloid treatment. Neither capsaicin nor RTX induced significant [3H]5-HT release from any of the MC lines examined at concentrations corresponding to those that induced 45Ca uptake (data not shown). Up to 10 μmol/L capsaicin (and 20 nmol/L RTX), the maximal increase was approximately 4% to 5% of the total, which was not statistically significant (P > .5, Student'st-test) and did not exceed the level of the spontaneous release. It thus seems that, in the MC lines, the vanilloid receptor and the mediated influx of calcium are not directly coupled to degranulation.
Capsaicin induces 45Ca uptake in BMCMCs but not in PMCs.
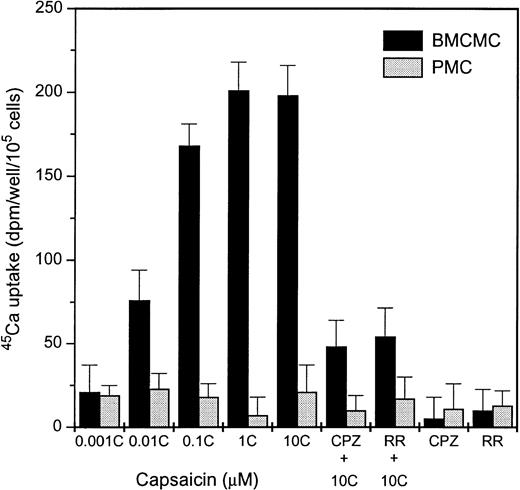
We have also tested the effect of vanilloids on different mast cell populations isolated from mice. Capsaicin did not induce45Ca uptake in PMCs (at concentrations up to 20 μmol/L) but was effective on BMCMCs (Fig 5). The effect of capsaicin on BMCMCs was inhibited by the vanilloid antagonists capsazepine and ruthenium red (10 μmol/L and 5 μmol/L, respectively), also showing its vanilloid receptor specificity. It is important to note that the estimated receptor density (Bmax) on BMCMCs was approximately 10% of the level found on mast cell lines (thus 1% of that found on DRGs), presumably reflecting in part the extremely small size of the BMCMCs. On the other hand, capsaicin was unable to induce degranulation at this (high nanomolar to low micromolar) concentration range on either BMCMCs or PMCs, similar to its ineffectiveness on MC lines (see above). It should be noted that capsaicin did induce significant histamine release from the cells at concentrations of greater than 50 μmol/L, and, furthermore, its actions at these high concentrations were not antagonized by capsazepine (data not shown); it therefore seems probable that these latter effects reflect nonspecific, non–vanilloid-mediated actions of the compound.
Capsaicin induces 45Ca uptake in BMCMCs but not in PMCs. Cells were challenged with different concentrations of capsaicin (C) for 30 minutes to induce 45Ca uptake. The vanilloid specificity of the capsaicin-induced response was determined by using 10 μmol/L capsazepine (CPZ) or with 5 μmol/L ruthenium red (RR) as vanilloid antagonists. Points represent mean over baseline values from sets of five determinations in three experiments in each case; error bars indicate SEM.
Capsaicin induces 45Ca uptake in BMCMCs but not in PMCs. Cells were challenged with different concentrations of capsaicin (C) for 30 minutes to induce 45Ca uptake. The vanilloid specificity of the capsaicin-induced response was determined by using 10 μmol/L capsazepine (CPZ) or with 5 μmol/L ruthenium red (RR) as vanilloid antagonists. Points represent mean over baseline values from sets of five determinations in three experiments in each case; error bars indicate SEM.
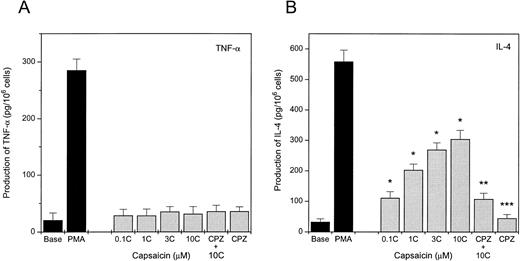
Capsaicin induces the differential production of IL-4.
Because activated MCs are known to produce and secrete a wide array of proinflammatory cytokines,37 we have assessed the production of TNF-α, IL-4, and IL-6 by MCs. As seen in Fig 6A, capsaicin failed to induce the release of TNF-α from P815 cells, although 100 nmol/L phorbol 12-myristate 13-acetate (PMA) was found to be effective; similarly, capsaicin was ineffective in producing IL-6 (data not shown). On the other hand, capsaicin did induce the production of IL-4 from P815 cells (Fig 6B) at concentrations as low as 0.1 μmol/L. The response was blocked by capsazepine at concentrations from 0.1 to 10 μmol/L (the latest is plotted in Fig 6), supporting its vanilloid specificity. Capsazepine alone was ineffective in inducing IL-4 production.
Capsaicin induces the production of IL-4 but not TNF-α in P815 cells. Cells were treated with different concentrations of capsaicin (C) for 3 hours. Solvent (Base) or 100 nmol/L PMA-treated cells were used as controls. The inhibitory effect of capsazepine (CPZ) was assessed by incubating one group of cells with 10 μmol/L CPZ in the presence of 10 μmol/L capsaicin. Supernatants were then collected and the produced TNF-α (A) or IL-4 (B) content was determined in duplicates by ELISA kits according to the manufacturer's protocol. Each of the measured doses of capsaicin caused significant release of IL-4 (*P < .01). CPZ significantly (**P < .05) decreased the release of IL-4 by capsaicin, whereas it did not modify the release (***P > .5) when applied alone (Student'st-test). Points represent mean values of three individual experiments; error bars indicate SEM.
Capsaicin induces the production of IL-4 but not TNF-α in P815 cells. Cells were treated with different concentrations of capsaicin (C) for 3 hours. Solvent (Base) or 100 nmol/L PMA-treated cells were used as controls. The inhibitory effect of capsazepine (CPZ) was assessed by incubating one group of cells with 10 μmol/L CPZ in the presence of 10 μmol/L capsaicin. Supernatants were then collected and the produced TNF-α (A) or IL-4 (B) content was determined in duplicates by ELISA kits according to the manufacturer's protocol. Each of the measured doses of capsaicin caused significant release of IL-4 (*P < .01). CPZ significantly (**P < .05) decreased the release of IL-4 by capsaicin, whereas it did not modify the release (***P > .5) when applied alone (Student'st-test). Points represent mean values of three individual experiments; error bars indicate SEM.
DISCUSSION
We report here for the first time that a variety of murine mast cell lines as well as BMCMCs express functional vanilloid receptors. We have also shown that the MC vanilloid receptor is similar to the C-type vanilloid receptor described on DRGs10 and that its activation leads to Ca2+ uptake, desensitization, and differential cytokine production in MCs. Our findings that vanilloids, in addition to the activation of a subset of sensory neurons, also can act directly on nonneuronal cell types suggest the need to reexamine the complex in vivo effects of vanilloids, particularly with respect to neurogenic inflammation.
In our previous studies,10 we had shown the existence of two pharmacologically defined classes of vanilloid receptors (the R- and the C-type) on rat DRGs (Table 1). In MCs, the vanilloid-induced 45Ca uptake showed similar pharmacologic behavior to that found on DRGs (Table 2), suggesting that (1) certain MCs possess the C-type vanilloid receptor (a receptor subtype that functions as a ligand-gated, Ca2+ permeable channel9) and (2) the characteristics of the C-type receptors found on MC lines are very similar to those on DRGs.10 On the other hand, we failed to detect any specific [3H]RTX binding on MCs (either on intact cells or on membrane preparations) with techniques optimized for [3H]RTX binding on DRGs. This result, in addition to the similar affinities of RTX on inducing 45Ca uptake and desensitization, compared with its orders of magnitude higher potency to induce desensitization (and binding) than 45Ca uptake on DRGs10,25 (see also in Tables 1 and 2), suggests that MCs do not express the R-type vanilloid receptor. The existence of the C-type and lack of the R-type receptor on mast cells further supports this subclassification of vanilloid receptors. Our failure to detect the low-affinity [3H]RTX binding to the C-type receptor is presumably attributable to several factors, including the relatively low density of C-type receptors on mast cells (the capsaicin-induced45Ca uptake in mast cells is approximately 10% of that in DRGs and even lower [<1%] on BMCMCs) and the predicted 45-fold weaker binding affinity to the C-site (the ratio of the ED50s for 45Ca uptake and [3H]RTX binding on DRGs, 2.1/0.047 ≈ 45). These two factors suggest a 450-fold less favorable ratio of specific to nonspecific binding for the C-type receptor on mast cells than for the R-type receptor on DRGs.25 Finally, the [3H]RTX binding assay is significantly improved by the use of α1-acid glycoprotein to remove nonspecifically bound [3H]RTX, relying on the slower off rate of the specifically bound [3H]RTX than of the nonspecifically bound ligand.38 A faster off-rate of the ligand from the C-type receptor would prevent the use of α1-acid glycoprotein, further degrading the detection of specifically bound [3H]RTX.
In DRGs, the prolonged action of capsaicin results in the degeneration of neurons due to calcium-mediated cell toxicity.9,34However, 6 hours of treatment of MCs with capsaicin up to 10 μmol/L showed no toxicity (Fig 5). This result is in good accord with the in vivo finding that neonatal capsaicin treatment, which causes the degeneration and ablation of sensory neurons,4 does not decrease the number of mast cells in the gastric mucosa,19dura mater,36 lung, or spleen.35 The lack of toxicity after the activation of the capsaicin-sensitive channel and the concomitant increase of intracellular Ca2+concentration is probably due to the different Ca2+handling mechanisms in mast cells.
Interestingly, capsaicin was effective in inducing 45Ca uptake in most, but not all, of the MC lines examined. Although the reason for the latter observation remains to be determined, our findings clearly show that distinct murine MC populations express the C-type vanilloid receptor. We also found that BMCMCs, but not PMCs of Balb/c mice, exhibited 45Ca-uptake after stimulation with capsaicin. These findings may indicate that the expression of the vanilloid receptor is developmentally and/or microenvironmentally regulated. BMCMCs (considered to be similar to mucosal-type MCs) differ from connective-tissue type MCs isolated from the serosal peritoneum of mice in numerous characteristics, including mediator content, responses to pharmacologic agents, and sensitivity to agents that induce proliferation, activation, or mediator release.39 For example, IL-3 has been shown to induce proliferation of BMCMCs but not PMCs isolated from Balb/C mice,40 whereas stem cell factor (SCF) reportedly induces degranulation of murine PMCs but not BMCMCs.41 42Accordingly, it will be of interest to assess the expression of vanilloid receptors and the response to vanilloids in various distinct MC populations of various maturation states.
To address the biologic significance of capsaicin-induced45Ca uptake in MCs, we next assessed whether activation of the MC vanilloid receptor would induce the release of preformed, granule-associated MC mediators or the production of proinflammatory cytokines. To our surprise, neither capsaicin nor RTX caused vanilloid receptor-mediated degranulation in any of the responsive MC lines or MCs. Only when stimulated with high micromolar to millimolar concentrations of capsaicin did MCs exhibit significant release of serotonin, which, taken together with the ineffectiveness of capsazepine in reducing this response, suggests an unspecific, nonvanilloid receptor-mediated mechanism of MC activation at these high vanilloid concentrations.
In contrast to its lack of effect on degranulation, capsaicin induced production of IL-4 in the P815 clone. This response was dose-dependent and observed at the same concentrations of capsaicin as45Ca uptake. In addition, coincubation with antagonists capsazepine or ruthenium red significantly inhibited IL-4 production, further supporting a vanilloid receptor-mediated mechanism of MC activation. Given the ability of MC-derived cytokines to influence diverse biologic responses,43 the identification of agents that induce MC cytokine production in the absence of (potentially counterproductive) degranulation is of particular interest. To our knowledge, only lipopolysaccharide, prostaglandin E2, cholera toxin, and SCF have been shown to induce selective stimulation of MC cytokine production independently of histamine release.42 Moreover, MCs produced IL-4 upon challenge with capsaicin that resulted in little or no detectable release of TNF-α or IL-6. This observation confirms previous reports suggesting different regulatory mechanisms in MCs for the expression of different groups of cytokines. Stimulation of BMCMCs with protein kinase C activators such as PMA reportedly induces the expression of TNF-α and IL-6 but not of IL-2, IL-3, IL-4, IL-5, granulocyte-macrophage colony-stimulating factor (GM-CSF), or interferon-γ (IFN-γ), and SP has been shown to differentially stimulate the secretion of TNF-α but not that of IL-1, IL-3, IL-4, IL-6, or GM-CSF in freshly isolated murine PMCs.13,21 44
Although the in vivo relevance of our findings remains to be elucidated, the observation that capsaicin in vitro can induce MCs to produce significant amounts of IL-4, but not TNF-α or IL-6, in the absence of degranulation (but with electrophysiological alterations, ie, 45Ca uptake) and, furthermore, that capsaicin selectively affects different MC populations (ie, BMCMCs vPMCs) offers a new look at the potential physiologic functions of MCs. Vanilloid receptor signaling of MCs may play a role in MC-directed regulation of antibody production and inflammation and the development of effector T-cell responses, as well as in the autocrine modification of MC functions.45,46 Moreover, the lack of in vitro toxicity of vanilloids on MCs versus DRGs and the differential in vivo action of capsaicin on MCs (namely neonatal capsaicin treatment affects exclusively MCs expressing markers of MMCs35 36) suggests the possible involvement of vanilloid receptors in the development and differentiation of MCs.
The molecular cloning of the vanilloid receptor would have great impact on the detailed evaluation of vanilloid-mediated mechanisms. A major obstacle has been the lack of a convenient source of RNA and DNA. The establishment of neuronal hybrid cell lines derived from DRGs47 48 has not proved to be a good solution, because these cell lines tend to lose their vanilloid sensitivity. Thus, our description of cell lines expressing the C-type vanilloid receptors should greatly facilitate aspects of the molecular characterization of these receptors.
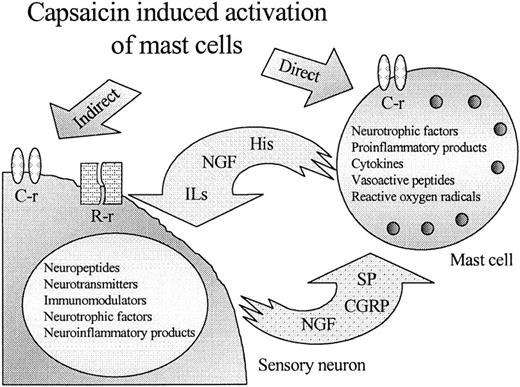
The existence of vanilloid receptors on MCs (and other nonneuronal cell types, characterization of which is currently in progress; T. Bı́ró, manuscript in preparation) necessitates the reevaluation of our understanding of the in vivo pathways of vanilloid action (Fig 7). Previously, capsaicin and related compounds were believed to activate exclusively neurons expressing the vanilloid receptors, and the modification of the functions of nonneural cell types, including MCs, was regarded as an indirect process via the release of peptide transmitters.2,18,19 Our demonstration that capsaicin can act directly on MCs in parallel with the activation of sensory neurons shows a potential new aspect of the processes induced by vanilloids, eg, neurogenic inflammation7 and stimulation of murine hair growth in vivo.49 In addition, the identification of vanilloid receptors on easily cultured cells should be a valuable experimental tool in the effort to identify the putative endogenous ligand(s) for the vanilloid receptor.
A new model for the action of capsaicin and related vanilloids on MCs. Capsaicin, in addition to its sensory neuron-mediated effect via C- and R-type vanilloid receptors (C-r and R-r), can also directly act on mast cells expressing functional (C-type) vanilloid receptors.
A new model for the action of capsaicin and related vanilloids on MCs. Capsaicin, in addition to its sensory neuron-mediated effect via C- and R-type vanilloid receptors (C-r and R-r), can also directly act on mast cells expressing functional (C-type) vanilloid receptors.
These findings might have therapeutic implications. Capsaicin is widely used in therapy in various inflammatory conditions, including rheumatoid arthritis, psoriasis, neuropathy of different origins, prurigo, urticaria, and atopic eczema, in which an increased number of mast cells and/or their altered, pathognomic function have been described.50,51 The efficacy of capsaicin in these conditions might be augmented by the accompanied modification of the function of MCs. In addition, the presence of a new receptor on MCs may further help in understanding the role of MCs as a central component of the neuroimmune axis52 and to clarify the functional significance of vanilloid receptor-dependent signaling in health and disease.
Supported in part by a grant from the Deutsche Forschungsgemeinschaft (DFG Pa 345/6-1) to R.P.
Address reprint requests to Peter M. Blumberg, PhD, MMTP/LCCTP/NCI, Bldg 37, Room 3A01, 37 Convent Dr MSC 4255, Bethesda, MD 20892-4255.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal