Abstract
Retroviral-mediated transduction of human hematopoietic stem cells to provide a lifelong supply of corrected progeny remains the most daunting challenge to the success of human gene therapy. The paucity of assays to examine transduction of pluripotent human stem cells hampers progress toward this goal. By using the beige/nude/xid (bnx)/hu immune-deficient mouse xenograft system, we compared the transduction and engraftment of human CD34+progenitors with that of a more primitive and quiescent subpopulation, the CD34+CD38− cells. Comparable extents of human engraftment and lineage development were obtained from 5 × 105 CD34+ cells and 2,000 CD34+CD38− cells. Retroviral marking of long-lived progenitors from the CD34+ populations was readily accomplished, but CD34+CD38− cells capable of reconstituting bnx mice were resistant to transduction. Extending the duration of transduction from 3 to 7 days resulted in low levels of transduction of CD34+CD38− cells. Flt3 ligand was required during the 7-day ex vivo culture to maintain the ability of the cells to sustain long-term engraftment and hematopoiesis in the mice.
THE CD34+CD38−CELL population represents a subset of human hematopoietic stem cells with a primitive and quiescent phenotype that can be isolated from adult and fetal bone marrow, umbilical cord blood, and fetal liver tissue.1-5 Human CD34+ cells acquire the cell surface marker CD38 with differentiation,6 7 and the CD34+CD38− fraction represents a population enriched in hematopoietic stem cells not yet committed to specific lineages.
It has been shown that the reconstituting ability of the CD34+ cell population lies in the CD38−fraction by using immunoincompetent fetal sheep and NOD/SCID mice as transplant recipients.8,9 Therefore, CD34+CD38− cells would be the ideal target population for gene therapy to repair inborn errors of hematopoiesis caused by single gene defects, such as Gaucher and Hurler's diseases, adenosine deaminase deficiency, and others.10 A small number of CD34+CD38− cells could theoretically be modified by insertion of a normal copy of the affected gene and generate corrected progeny for many years after return to the donor.
Although CD34+CD38− cells are ideal candidates for gene therapy to treat abnormalities of the hematopoietic system, they are not predicted to be easily transduced by retroviral vectors. Moloney murine leukemia-based retroviral vectors presently provide the most efficient method for permanent insertion of genes into mammalian cells, but require target cell division for integration.11 CD34+CD38− cells from bone marrow are largely quiescent and are not rapidly recruited into cell cycle by the cytokines that have been cloned to date,7 12-14 so they might be refractory to transduction by Moloney-based vectors.
Long-lived human hematopoietic progenitors from CD34+populations can be transduced with retroviral vectors, and generate marked progeny in immune-deficient (beige/nude/xid [bnx]) mice for up to 1 year. However, the levels of transduction and engraftment of more highly purified stem cells had not been previously evaluated in the bnx/hu xenograft model of human hematopoiesis.15-17 In the current studies, we used thebnx/hu system to compare engraftment and marking of primitive, reconstituting cells within CD34+ and CD34+CD38− populations isolated from the same bone marrow samples. Cells were transduced ex vivo for 1 hour (used as a baseline control for engraftment) or for 72 hours. After the transduction periods, cells were cotransplanted with IL-3 producing stroma into sibling bnx mice in groups of three to four as previously published.15-17 Nine to 11 months post-transplantation, marrow was harvested from the mice and analyzed for the extents of human hematopoietic cell engraftment, clonogenic progenitor content, and vector marking of tissues and individual T-cell and myeloid clones. In contrast to the CD34+ populations, the CD34+CD38− cells were not transduced to significant levels by the retroviral vector LN in 72-hour incubations.
The period of ex vivo culture and transduction was extended to 7 days, with and without flt3 ligand (FL), a cytokine previously shown to be a maintenance and stimulatory factor for primitive hematopoietic progenitors.18-20 We hypothesized that a longer preincubation with stromal support and FL might permit cell cycle progression in CD34+CD38− cells, enhancing retroviral-mediated transduction. The presence of FL was required in the 7-day cultures to sustain the capacity of the cells to give rise to long-term hematopiesis in the mice. Multiple long-lived, vector-transduced cells were found in two mice that had received human CD34+CD38− cells transduced on days 5, 6, and 7 in the presence of FL. CD34+CD38− cells transduced for the same period of time without FL, or for only 3 days, were not transduced.
Our data suggest that there is a population of long-term engrafting cells in the CD34+ population that is easily transduced and can sustain hematopoiesis for up to 11 months in immune-deficient mice. The more primitive CD34+CD38− cells mediated levels of engraftment comparable to the CD34+ population, but were infrequently marked by retroviral vectors. This system provides a stringent assay that will allow identification of improved methods for ex vivo culture and transduction of multipotent human hematopoietic stem cells.
MATERIALS AND METHODS
Supernatant production from vector-producing fibroblasts.
Marking of human hematopoietic cells was performed by using supernatant from the LN retroviral vector, packaged in the producer cell line PG13.21 Vector-producing fibroblasts (VPF) were grown in Dulbecco's Modified Eagles Medium with high glucose (GIBCO-BRL, Gaithersburg, MD), supplemented with 10% fetal calf serum (FCS). VPF were grown to subconfluency at 37°C in a humidified incubator with 5% CO2, then transferred to 32°C and grown for 48 hours.22 After the vector production period, supernatant was collected, filtered through a 0.45μm cellulose acetate syringe filter (Schleicher & Schuell, Keene, NH), and stored at−70°C. The PG13/LN supernatant had a titer of 5 × 106 infectious virions/mL, assayed on the human cell line HT29 (American Type Culture Collections [ATCC], Rockville, MD) and was determined to be free of recombinant helper virus by marker rescue assay on 3T3 (ATCC) and HT29 cells as described.23
Transduction of human hematopoietic cells.
Human bone marrow cells were collected from screens used to filter marrow during the harvest of normal donors for allogeneic transplantation. Use of the samples was approved by the Childrens Hospital Committee on Clinical Investigations (Los Angeles, CA). CD34+ progenitors were isolated by incubation with the monoclonal antibody (MoAb) HPCA-1 (Becton Dickinson, San Jose, CA), followed by goat antimouse conjugated immunomagnetic beads (Dynal, Oslo, Norway) as described.16CD34+CD38− cells were isolated from human marrow by pre-enrichment of CD34+ cells by using MiniMACS columns (Miltenyi, Auburn, CA), followed by fluorescence-activated cell sorting (FACS) acquisition with a stringent gate (less than half of the fluorescence of the PE isotype control) as previously described.7 Transduction with stromal support was conducted in the presence of the cytokines IL-6 (10 ng/mL, R&D systems, Minneapolis, MN), SCF (c-kit ligand, 50 ng/mL, R&D Systems), and IL-3 (10 ng/mL, Biosource International, Camarillo, CA), with and without inclusion of murine FL (FL, 50 ng/mL, generously donated by DNAX, Palo Alto, CA). Cells were cultured at a maximal concentration of 1 × 105 cells/mL medium, in T-25 vent-cap flasks (Costar, Cambridge, MA). Supernatant from PA317/LN and PG13/LN vector-producing fibroblasts, prepared as described above, was added to the CD34+ and CD34+CD38− cells on stromal support, at 24 hour intervals, on days 1, 2, and 3 of culture versus on days 5, 6, and 7 as described.16 Engraftment controls for designated experiments were CD34+ and CD34+CD38− cells incubated in the transduction medium in suspension culture with vector supernatant for one hour before transplantation into bnx mice. After transduction, a small portion of the CD34+ cells were plated in 14-day methylcellulose colony-forming assay with and without the selective agent G418 (Geneticin, 0.9 mg/mL active: screened lots from GIBCO/BRL), as described.15 The CD34+CD38− cells were not plated in colony-forming assay, because we had previously determined that they are not clonogenic within that time period.7 The remainder of each sample was transplanted into a cohort of immune-deficient (bnx) mice for long-term engraftment and analysis of the marked progeny of primitive human hematopoietic cells.
Transplantation of immune-deficient mice.
Six-week-old bnx mice bred at Childrens Hospital (Los Angeles, CA) were used as transplant recipients in all experiments. Transplantation of transduced human hematopoietic progenitor cells was done as described.15-17 In each experiment, 2,000 CD34+CD38− cells versus 5 × 105 CD34+ cells were transplanted into groups of 3 to 4 mice per arm. Mice were killed by 75% CO2 25% O2 narcosis, 9 to 11 months after transplantation with human cells. Samples of each tissue were taken for DNA preparation for analysis of vector integration. Bone marrow was flushed from the tibiae and femurs of each mouse into 1x phosphate-buffered saline (PBS) and dispersed with a fine needle. The bone marrow cells were then plated at 37°C for 2 to 4 hours in Iscoves Modified Dulbecco's Medium (IMDM) with 20% heat-inactivated FCS to remove murine stromal elements by adherence.
Human-specific colony-forming assay and isolation of clones.
After the adherence step, bnx/hu bone marrow cells were collected, counted, and plated in human-specific methylcellulose-based colony-forming assay with or without G418 as described.15Human IL-3 was added to a concentration of 10 ng/mL in basal methylcellulose medium. This medium had previously been shown to support the exclusive growth of human hematopoietic progenitors, if no murine stromal cells had contaminated the colony-forming unit (CFU) dish.15 Methylcellulose, FCS, and BSA were screened to provide maximal CFU-GEMM development from human CD34+ cells. A total of 5 × 104 and 1 × 105 plastic nonadherent cells from engrafted and control mice were plated in duplicate in 1 mL of medium in gridded culture dishes (Nunc, Naperville, IL), with and without G418 (0.9 mg/mL active compound, screened lots from GIBCO-BRL). Recombinant human erythropoietin (Epoietin alpha [Epo], Amgen Corp, Thousand Oaks, CA) was added to all plates on day 4 of culture to a concentration of 1 U/mL. Colonies were enumerated on day 21, then clones that had attained a size of at least 200 cells in the presence of G418 were plucked from the methylcellulose in a volume of 40 μL, and flushed into 1 mL PBS (Irvine Scientific, Santa Ana, CA) in individual microcentrifuge tubes. The cells were then pelleted and DNA was isolated as described.17
Clonal analysis by inverse polymerase chain reaction (PCR).
Genomic DNA isolated from each tissue sample and individual human CFU recovered from the mice was analyzed for the presence of provirus by PCR for the neo gene as described.15 After confirmation of vector integration, the clonal integration pattern was assessed by subjecting DNA from each colony to amplification in the inverse PCR reaction as described.17 The resulting PCR products were electrophoresed on a 2% gel (1% Seakem LE agarose and 1% Nu-Sieve; FMC Bioproducts, Rockland, ME) and transferred to nylon membrane (Hybond-N+; Amersham, Arlington Heights, IL). Hybridization was done in SSPE Hybridization buffer (10X Denhardt's,24 5X SSPE,24 and 0.5% SDS24) for at least 4 hours at 55°C, with an oligonucleotide probe (5′GGCAAGCTAGCTTAAGT) specific for LTR sequences, end-labeled with γ-32 P-ATP by T4 kinase (GIBCO-BRL). After hybridization, blots were washed twice for 5 minutes each at room temperature in SSPE wash buffer #1 (2X SSPE; 0.1% SDS). Next, blots were washed for 10 minutes at 55°C in SSPE wash buffer #2 (5X SSPE, 0.1% SDS). Exposures to high-performance autoradiography film (Hyperfilm-MP, Amersham) were performed for 5 minutes to 2 days.
Antibody labeling and FACS analysis.
Single-cell suspensions from the marrow and spleens of long-term engrafted bnx mice were preincubated for 15 minutes on ice with unconjugated mouse immunoglobulin (MsIgG; Coulter, Hialeah, FL) before addition of antibody. Directly conjugated antibodies used to identify human-specific cell surface antigens were: HLE-1 (anti-CD45, Becton Dickinson); My9-RD1 (anti-CD33, Coulter); Leu-12 (anti-CD19, Becton Dickinson); Leu-3a (anti-CD4, Becton Dickinson); and Leu-2a (anti-CD8, Becton Dickinson). The antimouse CD45 antibody (Pharmingen, San Diego, CA) was used to identify murine leukocytes. After a 15-minute antibody binding period on ice, cells were depleted of red blood cells by resuspension in Ortho Lysis Buffer (Becton Dickinson), washed, and fixed in 1% paraformaldehyde. Samples were acquired on a Becton Dickinson FACScan and analyzed with the Cellquest software package (Becton Dickinson). Ten thousand events were acquired for each sample. In all experiments, parallel staining and FACS analyses were performed on normal human and nontransplanted bnx mouse bone marrow controls, to confirm that none of the human-specific antibodies cross-reacted with murine cells.
Statistical analyses.
All analyses were performed with the Excel 5.0 software (Microsoft Corp, Seattle, WA). Average values are listed with standard deviations. Standard error of the mean is used in the text in cases where all data points are listed in table format. The significance of each set of values was assessed using the two-tailedt-test assuming equal variance.
RESULTS
Transduction of human colony-forming progenitors.
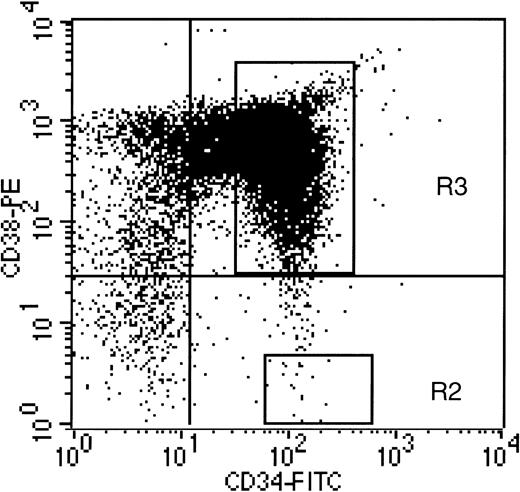
Human CD34+ and CD34+CD38− cells were isolated from normal human bone marrow as described.7,18 The CD34+CD38−cells were acquired by FACS selection as shown in Fig 1. In the first series of experiments (n=4), the engraftment and transduction of CD34+ and CD34+CD38− cells from the same donors were compared after long-term hematopoiesis in immune-deficient mice. A portion of each CD34+ and CD34+CD38− sample was transplanted into the mice after only 1-hour incubation in suspension culture with cytokines and retroviral supernatant. The 1-hour transductions were performed to provide baseline values and engraftment controls to assess the potential extent of loss of long-term hematopoietic capacity in the remainder of each population, after a 72-hour ex vivo culture and transduction period. To accomplish the 72-hour transduction, CD34+ and CD34+CD38− cells were plated onto allogeneic, irradiated human stromal cells in transduction medium including IL-6, IL-3, and SCF. An equal volume of supernatant from the LN vector was added at the initiation of culture, and again 24 and 48 hours later, as described.16 After transduction, a small portion of the CD34+ cells was plated in 14-day methylcellulose colony-forming assay (CFA) with and without the selective agent G418, as described,15 to ensure that the transduction of colony-forming cells had worked adequately. The CD34+CD38− cells were not plated in CFA, because we had previously determined that they were not clonogenic within a 2-week time period.7 No G418-resistant CFUs were obtained from nontransduced marrow plated as controls in these experiments, or from cells cultured in suspension with supernatant addition for only 1 hour. The levels of gene transfer into colony-forming progenitors from the CD34+ populations transduced for 72 hours, assessed by G418-resistant CFU, ranged from 21.3% to 33.4%, with an average of 27.3 ± 5.1 (n=4). These data indicate that the ex vivo culture conditions were favorable for gene transfer into committed, clonogenic progenitors. The levels of transduction of more primitive progenitors, contained within the CD34+ and CD34+CD38− populations, were assessed in the long-term bnx/hu xenograft assay.15-17
Acquisition gate used to define CD34+CD38− cell population. Region R1 was defined as the lymphoid gate containing small, agranular cells, as previously published.7 Quadrants are defined by fluorescein isothiocyanate (FITC) and PE-labeled isotype controls. Region R2 was used to define CD34+CD38− cells for FACS acquisition. This region contains CD34+ cells with PE-CD38 fluorescence less than half of the isotype control.
Acquisition gate used to define CD34+CD38− cell population. Region R1 was defined as the lymphoid gate containing small, agranular cells, as previously published.7 Quadrants are defined by fluorescein isothiocyanate (FITC) and PE-labeled isotype controls. Region R2 was used to define CD34+CD38− cells for FACS acquisition. This region contains CD34+ cells with PE-CD38 fluorescence less than half of the isotype control.
Human cell engraftment in immune-deficient mice transplanted with CD34+ versus CD34+CD38− cells.
The extents of transduction and survival of human cells more primitive than colony-forming progenitors were assessed after long-term engraftment in immune-deficient mice. After 72-hours transduction, the nonadherent cells and the entire adherent population from each transduction flask were combined and transplanted into cohorts of sublethally conditioned bnx mice. The cells were allowed to engraft and to contribute to hematopoiesis in the mice for 9 to 11 months. Then the mice were killed and the marrow was harvested from the hindlegs. The percentage of human CD45+ cells in the marrow of each mouse transplanted with human CD34+ or CD34+CD38− cells was assessed by fluorescent-activated cell sorting (FACS) analysis (Table 1). Significantly higher percentages of human CD45+ cells were obtained in the marrow of mice that had received CD34+ cells cultured ex vivo for one hour (5.9% ± 1.5%, N=4) as opposed to 72 hours (2.7% ± 0.5%, N=10, P = .03). Mice that had received CD34+CD38− cells transduced for 1 hour had an average human CD45+ cell content of 6.2% ± 3.6 % in their marrow (n = 5), and there was an average of 3.2% ± 2.8% in mice that had received human CD34+CD38− cells transduced for 72 hours (n = 14, P = .07). The extent of engraftment obtained from the sets transplanted with CD34+and CD34+CD38− cells was not significantly different. Similar levels of homing and subsequent survival of human cells in the murine bone marrow were obtained from 2.5 logs less CD34+CD38− cells than CD34+cells.
Human Hematopoietic Lineages Recovered From the Bone Marrow of bnx Mice 9 to 11 Months Post-Transplantation
| Experiment No. . | Transduction Conditions . | Months Engrafted . | hu CD45 (%) . | hu CD4 (%) . | hu CD8 (%) . | hu CD33 (%) . | hu CD19 (%) . |
|---|---|---|---|---|---|---|---|
| 1 | CD34+/1 hour | 9 | 2.2 | 0.6 | 0.6 | 1.0 | 0.0 |
| 1 | 9 | 7.2 | 2.6 | 2.5 | 3.0 | 0.0 | |
| 2 | 11 | 9.3 | 3.1 | 2.0 | 4.1 | 0.0 | |
| 2 | 11 | 4.7 | 1.2 | 1.8 | 1.5 | 0.0 | |
| Average = 5.85 ± 1.5 | 1.88 ± 0.59 | 1.73 ± 0.41 | 2.40 ± 0.71 | 0 ± 0 | |||
| 1 | CD34+/72 hours | 9 | 3.0 | 0.9 | 1.0 | 0.9 | 0.0 |
| 1 | 9 | 2.8 | 0.7 | 1.2 | 1.0 | 0.0 | |
| 1 | 9 | 5.1 | 1.1 | 1.9 | 2.0 | 0.0 | |
| 2 | 11 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 2 | 11 | 3.2 | 1.3 | 0.9 | 1.0 | 0.0 | |
| 2 | 11 | 5.7 | 1.2 | 1.5 | 3.2 | 0.0 | |
| 3 | 10 | 2.9 | 0.8 | 1.0 | 0.9 | 0.0 | |
| 3 | 10 | 1.9 | 0.5 | 0.8 | 0.7 | 0.0 | |
| 4 | 9 | 0.3 | 0.0 | 0.0 | 0.3 | 0.0 | |
| 4 | 9 | 1.5 | 0.4 | 0.6 | 0.5 | 0.0 | |
| Average = 2.65 ± 0.57 | 0.69 ± 0.14 | 0.89 ± 0.19 | 1.05 ± 0.29 | 0 ± 0 | |||
| 1 | CD34+CD38− | 9 | 7.1 | 2.9 | 3.2 | 1.1 | 0.0 |
| 1 | 1 hour | 9 | 4.6 | 1.0 | 2.1 | 1.3 | 0.0 |
| 2 | 11 | 2.4 | 0.8 | 0.9 | 0.7 | 0.0 | |
| 2 | 11 | 5.1 | 2.0 | 1.9 | 1.2 | 0.1 | |
| 2 | 11 | 11.9 | 3.3 | 5.7 | 2.9 | 0.0 | |
| Average = 6.22 ± 1.60 | 2.00 ± 0.50 | 2.76 ± 0.82 | 1.44 ± 0.38 | 0.1 ± .05 | |||
| 1 | CD34+CD38− | 9 | 3.3 | 1.1 | 1.0 | 0.9 | 0.0 |
| 1 | 72 hours | 9 | 4.1 | 1.3 | 1.3 | 1.4 | 0.0 |
| 1 | 9 | 2.6 | 0.7 | 1.0 | 0.9 | 0.0 | |
| 1 | 9 | 3.4 | 1.2 | 0.9 | 1.1 | 0.0 | |
| 2 | 11 | 4.0 | 1.5 | 1.2 | 1.3 | 0.0 | |
| 2 | 11 | 1.5 | 0.3 | 0.7 | 0.4 | 0.0 | |
| 2 | 11 | 11.0 | 2.8 | 3.4 | 4.7 | 0.0 | |
| 2 | 11 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 3 | 10 | 6.6 | 1.7 | 2.5 | 2.3 | 0.1 | |
| 3 | 10 | 1.3 | 0.4 | 0.5 | 0.4 | 0.0 | |
| 3 | 10 | 3.6 | 0.8 | 1.3 | 1.1 | 0.0 | |
| 4 | 9 | 0.5 | 0.1 | 0.3 | 0.0 | 0.0 | |
| 4 | 9 | 1.2 | 0.3 | 0.6 | 0.3 | 0.0 | |
| 4 | 9 | 1.8 | 0.4 | 0.4 | 0.7 | 0.0 | |
| Average = 3.21 ± 0.76 | 0.9 ± 0.21 | 1.08 ± 0.24 | 1.11 ± 0.32 | 0.1 ± 0.3 |
| Experiment No. . | Transduction Conditions . | Months Engrafted . | hu CD45 (%) . | hu CD4 (%) . | hu CD8 (%) . | hu CD33 (%) . | hu CD19 (%) . |
|---|---|---|---|---|---|---|---|
| 1 | CD34+/1 hour | 9 | 2.2 | 0.6 | 0.6 | 1.0 | 0.0 |
| 1 | 9 | 7.2 | 2.6 | 2.5 | 3.0 | 0.0 | |
| 2 | 11 | 9.3 | 3.1 | 2.0 | 4.1 | 0.0 | |
| 2 | 11 | 4.7 | 1.2 | 1.8 | 1.5 | 0.0 | |
| Average = 5.85 ± 1.5 | 1.88 ± 0.59 | 1.73 ± 0.41 | 2.40 ± 0.71 | 0 ± 0 | |||
| 1 | CD34+/72 hours | 9 | 3.0 | 0.9 | 1.0 | 0.9 | 0.0 |
| 1 | 9 | 2.8 | 0.7 | 1.2 | 1.0 | 0.0 | |
| 1 | 9 | 5.1 | 1.1 | 1.9 | 2.0 | 0.0 | |
| 2 | 11 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 2 | 11 | 3.2 | 1.3 | 0.9 | 1.0 | 0.0 | |
| 2 | 11 | 5.7 | 1.2 | 1.5 | 3.2 | 0.0 | |
| 3 | 10 | 2.9 | 0.8 | 1.0 | 0.9 | 0.0 | |
| 3 | 10 | 1.9 | 0.5 | 0.8 | 0.7 | 0.0 | |
| 4 | 9 | 0.3 | 0.0 | 0.0 | 0.3 | 0.0 | |
| 4 | 9 | 1.5 | 0.4 | 0.6 | 0.5 | 0.0 | |
| Average = 2.65 ± 0.57 | 0.69 ± 0.14 | 0.89 ± 0.19 | 1.05 ± 0.29 | 0 ± 0 | |||
| 1 | CD34+CD38− | 9 | 7.1 | 2.9 | 3.2 | 1.1 | 0.0 |
| 1 | 1 hour | 9 | 4.6 | 1.0 | 2.1 | 1.3 | 0.0 |
| 2 | 11 | 2.4 | 0.8 | 0.9 | 0.7 | 0.0 | |
| 2 | 11 | 5.1 | 2.0 | 1.9 | 1.2 | 0.1 | |
| 2 | 11 | 11.9 | 3.3 | 5.7 | 2.9 | 0.0 | |
| Average = 6.22 ± 1.60 | 2.00 ± 0.50 | 2.76 ± 0.82 | 1.44 ± 0.38 | 0.1 ± .05 | |||
| 1 | CD34+CD38− | 9 | 3.3 | 1.1 | 1.0 | 0.9 | 0.0 |
| 1 | 72 hours | 9 | 4.1 | 1.3 | 1.3 | 1.4 | 0.0 |
| 1 | 9 | 2.6 | 0.7 | 1.0 | 0.9 | 0.0 | |
| 1 | 9 | 3.4 | 1.2 | 0.9 | 1.1 | 0.0 | |
| 2 | 11 | 4.0 | 1.5 | 1.2 | 1.3 | 0.0 | |
| 2 | 11 | 1.5 | 0.3 | 0.7 | 0.4 | 0.0 | |
| 2 | 11 | 11.0 | 2.8 | 3.4 | 4.7 | 0.0 | |
| 2 | 11 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 3 | 10 | 6.6 | 1.7 | 2.5 | 2.3 | 0.1 | |
| 3 | 10 | 1.3 | 0.4 | 0.5 | 0.4 | 0.0 | |
| 3 | 10 | 3.6 | 0.8 | 1.3 | 1.1 | 0.0 | |
| 4 | 9 | 0.5 | 0.1 | 0.3 | 0.0 | 0.0 | |
| 4 | 9 | 1.2 | 0.3 | 0.6 | 0.3 | 0.0 | |
| 4 | 9 | 1.8 | 0.4 | 0.4 | 0.7 | 0.0 | |
| Average = 3.21 ± 0.76 | 0.9 ± 0.21 | 1.08 ± 0.24 | 1.11 ± 0.32 | 0.1 ± 0.3 |
Bone marrow was recovered from bnx mice 9-11 months after cotransplantation of transduced human hematopoietic cells and IL-3 producing stromal cells. The percentage of human CD45+cells in the marrow (BM) was determined by FACS. The percentages of human cell that were positive for each lineage marker by FACS analysis of the ungated bone marrow cells are shown. In each experiment, normal human peripheral blood and age-matched, nontransplanted bnxmice were analyzed concurrently to verify the species-specificity of the monoclonal antibodies.
Comparison of human hematopoietic lineages recovered from mice transplanted with CD34+ and CD34+CD38− cells.
The human hematopoietic lineages that had developed during the 9 to 11 month engraftment period were determined by incubating samples of marrow from each bnx/hu mouse with a panel of MoAbs. The specificity of each antibody used was determined by testing on human peripheral blood controls and on marrow from nontransplantedbnx mice. Only antibodies that bound specifically to the human cells and did not cross-react with the murine cells were used in the panel. As shown in Table 1, the human hematopoietic lineages that developed (CD4, CD8, and CD33) in mice transplanted with human CD34+ or CD34+CD38− cells did not vary significantly. As we have previously reported,15-17 no human B lymphocytes developed from any human hematopoietic cell population engrafted in the bnx mice.
Analysis of tissue and individual, long-lived, clonogenic human progenitor cell marking.
Human-specific CFAs were plated as described,15 using 3 × 105 marrow cells recovered from eachbnx/hu mouse. The number of human-specific colony-forming progenitors that developed from each sample is shown in Table 2. The highest average number of human clonogenic progenitors was obtained from mice that had received CD34+CD38− cells cultured ex vivo for only 1 hour (84.6 ± 19.5). The number was not significantly higher than the average number of progenitors obtained from the CD34+cells cultured ex vivo for 1 hour (45.8 ± 16.0, P = .18). The numbers of human clonogenic progenitors recovered from mice transplanted with human CD34+ and CD34+CD38− cells cultured ex vivo for 72 hours did not differ significantly from one another (36.4 ± 8.1v 55.6 ± 20.7, P = .46).
Extents of Transduction of Human Hematopoietic Cells Recovered From Long-term Engrafted bnxMice
| Experiment No. . | Cell Type . | Transduction . | G418RCFU . | Total hu CFU . | G418 Resistance (%) . |
|---|---|---|---|---|---|
| 1 | CD34+ | 1 hour | 0 | 80 | 0 |
| 1 | 0 | 16 | 0 | ||
| 2 | 0 | 66 | 0 | ||
| 2 | 0 | 21 | 0 | ||
| Average = 0 | 45.8 ± 16.0 | 0% | |||
| 1 | CD34+ | 72 hours | 6 | 37 | 16.2 |
| 1 | 1 | 18 | 5.6 | ||
| 1 | 9 | 53 | 17 | ||
| 2 | 0 | 0 | 0 | ||
| 2 | 5 | 54 | 9.3 | ||
| 2 | 16 | 89 | 18 | ||
| 3 | 8 | 25 | 22.9 | ||
| 3 | 2 | 24 | 8.3 | ||
| 4 | 0 | 15 | 0 | ||
| 4 | 3 | 49 | 6.1 | ||
| Average = 5 ± 1.6 | 36.4 ± 8.1 | 10.3 ± 2.5% | |||
| 1 | CD34+CD38− | 1 hour | 0 | 107 | 0 |
| 1 | 0 | 131 | 0 | ||
| 2 | 0 | 22 | 0 | ||
| 2 | 0 | 59 | 0 | ||
| 2 | 0 | 104 | 0 | ||
| Average = 0 | 84.6 ± 19.5 | 0% | |||
| 1 | CD34+CD38− | 72 hours | 0 | 95 | 0 |
| 1 | 0 | 43 | 0 | ||
| 1 | 0 | 79 | 0 | ||
| 1 | 0 | 9 | 0 | ||
| 2 | 17 | 240 | 7.1 | ||
| 2 | 2 | 211 | 0.9 | ||
| 2 | 0 | 3 | 0 | ||
| 2 | 0 | 10 | 0 | ||
| 3 | 0 | 16 | 0 | ||
| 3 | 0 | 0 | 0 | ||
| 3 | 0 | 25 | 0 | ||
| 4 | 0 | 9 | 0 | ||
| 4 | 0 | 12 | 0 | ||
| 4 | 0 | 26 | 0 | ||
| Average = 1.4 ± 1.2 | 55.6 ± 20.8 | 0.6 ± 0.5% |
| Experiment No. . | Cell Type . | Transduction . | G418RCFU . | Total hu CFU . | G418 Resistance (%) . |
|---|---|---|---|---|---|
| 1 | CD34+ | 1 hour | 0 | 80 | 0 |
| 1 | 0 | 16 | 0 | ||
| 2 | 0 | 66 | 0 | ||
| 2 | 0 | 21 | 0 | ||
| Average = 0 | 45.8 ± 16.0 | 0% | |||
| 1 | CD34+ | 72 hours | 6 | 37 | 16.2 |
| 1 | 1 | 18 | 5.6 | ||
| 1 | 9 | 53 | 17 | ||
| 2 | 0 | 0 | 0 | ||
| 2 | 5 | 54 | 9.3 | ||
| 2 | 16 | 89 | 18 | ||
| 3 | 8 | 25 | 22.9 | ||
| 3 | 2 | 24 | 8.3 | ||
| 4 | 0 | 15 | 0 | ||
| 4 | 3 | 49 | 6.1 | ||
| Average = 5 ± 1.6 | 36.4 ± 8.1 | 10.3 ± 2.5% | |||
| 1 | CD34+CD38− | 1 hour | 0 | 107 | 0 |
| 1 | 0 | 131 | 0 | ||
| 2 | 0 | 22 | 0 | ||
| 2 | 0 | 59 | 0 | ||
| 2 | 0 | 104 | 0 | ||
| Average = 0 | 84.6 ± 19.5 | 0% | |||
| 1 | CD34+CD38− | 72 hours | 0 | 95 | 0 |
| 1 | 0 | 43 | 0 | ||
| 1 | 0 | 79 | 0 | ||
| 1 | 0 | 9 | 0 | ||
| 2 | 17 | 240 | 7.1 | ||
| 2 | 2 | 211 | 0.9 | ||
| 2 | 0 | 3 | 0 | ||
| 2 | 0 | 10 | 0 | ||
| 3 | 0 | 16 | 0 | ||
| 3 | 0 | 0 | 0 | ||
| 3 | 0 | 25 | 0 | ||
| 4 | 0 | 9 | 0 | ||
| 4 | 0 | 12 | 0 | ||
| 4 | 0 | 26 | 0 | ||
| Average = 1.4 ± 1.2 | 55.6 ± 20.8 | 0.6 ± 0.5% |
Bone marrow was recovered from bnx mice after engraftment periods of 9 to 11 months. Human-specific colony-forming assays were plated from the bone marrow of each mouse (3 × 105bnx/hu bone marrow cells per sample) with and without addition of the selective agent G418 (0.9 mg/mL active). The extent of transduction was calculated as: (G418-resistant/total human CFU) × 100 = % G418 resistance.
The levels of transduction of long-term engrafting, clonogenic progenitors by the neo gene of the LN vector were assessed by determining the percentages of human-specific CFU that were resistant to the selective agent G418 (Table 2). No transduction was observed in cells exposed to the retroviral vector supernatant for only 1 hour. The highest number of G418-resistant clonogenic human cells was obtained from mice that had received human CD34+ cells transduced over a period of 72 hours (Table 2). Eight of 10 mice from this group contained G418-resistant clonogenic human cells. In contrast, only 2 of 14 mice that had received human CD34+CD38− cells transduced under the same conditions harbored G418-resistant human progenitors, with an average percentage of 0.62 ± 0.52, as opposed to 10.34 ± 2.48 in the 72-hour CD34+ group (P = .0003). These data show that, although CD34+ and CD34+CD38− cells engraft and sustain long-term hematopoiesis in immune-deficient mice, only the CD34+ population can be reliably transduced by using stromal support, 25% FCS, and the cytokines IL-3, IL-6, and SCF. Long-lived progenitors from the CD34+CD38−population were only sporadically transduced.
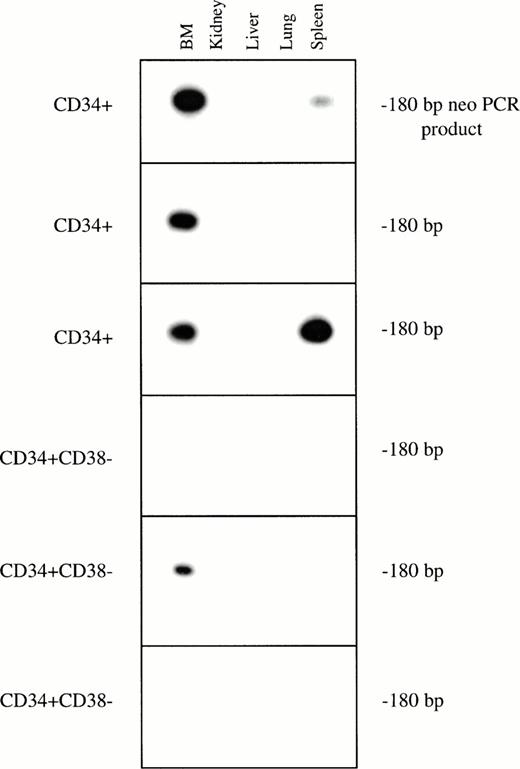
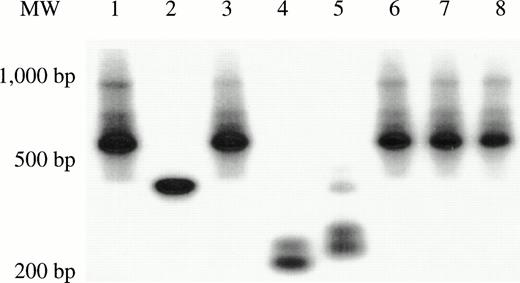
Genomic DNA was extracted from the marrow and tissues recovered from each of the long-term engrafted bnx mice, and subjected to PCR for the neo gene, to determine whether or not gene marking of long-lived human hematopoietic cells had occurred in human hematopoietic progenitors or their differentiated progeny residing within each organ (representative blots are shown in Fig 2). In this set of experiments, marking was observed primarily in the marrow, with no cells positive for theneo gene in the other organs, except for several spleen samples. The results of the PCR of whole tissues were concordant with detection of G418-resistant progenitors.
PCR for the neo gene in bnx/hu bone marrow and tissues. DNA was extracted from the bone marrow and tissues of each long-term engrafted mouse, and subjected to PCR to detect the presence of the neo gene, to determine whether or not gene marking of long-lived human hematopoietic cells had occurred in human hematopoietic progenitors or their differentiated progeny residing within each organ. Hybridization with a neo-specific oligonucleotide probe was done to ensure that the product band had been correctly amplified. Tissues that were positive and negative for the presence of the neo gene were amplified as controls in each reaction. The results from one set of mice are shown.
PCR for the neo gene in bnx/hu bone marrow and tissues. DNA was extracted from the bone marrow and tissues of each long-term engrafted mouse, and subjected to PCR to detect the presence of the neo gene, to determine whether or not gene marking of long-lived human hematopoietic cells had occurred in human hematopoietic progenitors or their differentiated progeny residing within each organ. Hybridization with a neo-specific oligonucleotide probe was done to ensure that the product band had been correctly amplified. Tissues that were positive and negative for the presence of the neo gene were amplified as controls in each reaction. The results from one set of mice are shown.
Transduction of human CD34+ versus CD34+CD38− cells on days 1, 2, and 3 versus 5, 6, and 7 with and without inclusion of FL.
Because the transduction protocols that had been successful for transducing CD34+ progenitors were inadequate to mediate gene transfer into CD34+CD38− cells, we next tried a strategy that would allow more time for the primitive cell population to be induced to cycle. We compared transduction on days 1, 2, and 3 to transduction on days 5, 6, and 7, in the presence and absence of FL. After the designated transduction period, 2,000 cells were transplanted per bnx mouse. Tissues and bone marrow cells were harvested and analyzed after 9 to 11 months engraftment.
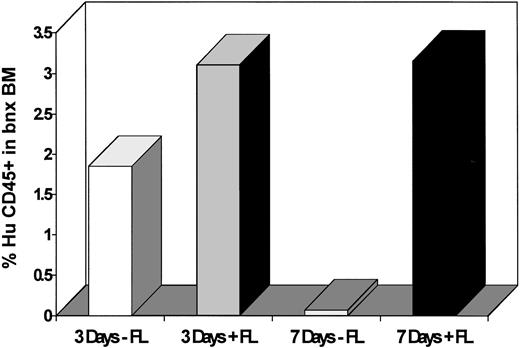
The levels of engraftment and the numbers of transduced and total human colony-forming progenitors recovered from the marrow of each mouse are shown in Table 3. The mean level of human cell engraftment was 1.85% ± 0.54% in mice that had received human CD34+CD38− cells transduced on days 1, 2, and 3 in 3/6/S, and 3.1% ± 0.93% in mice that had received cells transduced on days 1, 2, and 3 in 3/6/S/FL (P = .27, not a significant difference). CD34+CD38− cells maintained in culture for 1 week, and transduced on days 5, 6, and 7, required the presence of FL during culture to sustain the ability to reconstitute the bnx mice. Cells that were cultured for 7 days in 3/6/S gave an average engraftment of only 0.08% ± 0.05% human CD45+ cells, whereas cells cultured 7 days in 3/6/S/FL produced a significantly higher average extent of engraftment (3.15% ± 0.72%, P = .005, Table 3).
Engraftment, Transduction, and Long-Term Clonogenic Capacity of Human CD34+CD38− Cells Transduced on Days 1, 2, and 3 Versus 5, 6, and 7
| Transduction Condition . | % CD45+ in Marrow . | No. G418R CFU . | Total HumanSpecfic CFU . | % G418 Resistance . | Result of PCR for Neo Gene . | No. Integrants: T-Cell Clones† . | No. Integrants: Myeloid Clones . |
|---|---|---|---|---|---|---|---|
| Days 1, 2, 3 - FL | 0.9 | 0 | 10 | 0 | − | − | − |
| 1.5 | 0 | 33 | 0 | − | − | − | |
| 2.6 | 0 | 53 | 0 | − | − | − | |
| 0 | 0 | 0 | 0 | − | − | − | |
| 2.4 | 0 | 83 | 0 | − | − | − | |
| 3.7 | 0 | 30 | 0 | − | − | − | |
| Average = 1.9 ± 0.6 | 0 | 34.8 ± 13.3 | 0 | Total = 0/6 | |||
| Days 5, 6, 7 - FL* | 0 | 0 | 0 | 0 | − | − | − |
| 0.1 | 0 | 3 | 0 | − | − | − | |
| 0 | 0 | 0 | 0 | − | − | − | |
| 0.2 | 0 | 6 | 0 | − | − | − | |
| Average = 0.1 ± 0.1 | 0 | 2.3 ± 1.4 | 0 | Total = 0/4 | |||
| Days 1, 2, 3 + FL | 3.1 | 0 | 193 | 0 | − | − | − |
| 2.8 | 0 | 123 | 0 | − | − | − | |
| 1.4 | 0 | 44 | 0 | − | − | − | |
| 7.3 | 0 | 336 | 0 | − | − | − | |
| 3.2 | 0 | 27 | 0 | − | − | − | |
| 0.8 | 0 | 3 | 0 | − | − | − | |
| Average = 3.1 ± 1.0 | 0 | 121 ± 56.3 | 0 | Total = 0/6 | |||
| Days 5, 6, 7 + FL* | 3.0 | 7 | 293 | 2.4 | + | 2 | 3 |
| 1.9 | 6 | 155 | 3.9 | + | 2 | 1 | |
| 5.2 | 0 | 216 | 0 | − | − | − | |
| 2.5 | 0 | 187 | 0 | − | − | − | |
| Average = 3.2 ± 0.7 | 3.3 | 212.8 ± 29.5 | 1.6 | Total = 2/4 | |||
| Transduction Condition . | % CD45+ in Marrow . | No. G418R CFU . | Total HumanSpecfic CFU . | % G418 Resistance . | Result of PCR for Neo Gene . | No. Integrants: T-Cell Clones† . | No. Integrants: Myeloid Clones . |
|---|---|---|---|---|---|---|---|
| Days 1, 2, 3 - FL | 0.9 | 0 | 10 | 0 | − | − | − |
| 1.5 | 0 | 33 | 0 | − | − | − | |
| 2.6 | 0 | 53 | 0 | − | − | − | |
| 0 | 0 | 0 | 0 | − | − | − | |
| 2.4 | 0 | 83 | 0 | − | − | − | |
| 3.7 | 0 | 30 | 0 | − | − | − | |
| Average = 1.9 ± 0.6 | 0 | 34.8 ± 13.3 | 0 | Total = 0/6 | |||
| Days 5, 6, 7 - FL* | 0 | 0 | 0 | 0 | − | − | − |
| 0.1 | 0 | 3 | 0 | − | − | − | |
| 0 | 0 | 0 | 0 | − | − | − | |
| 0.2 | 0 | 6 | 0 | − | − | − | |
| Average = 0.1 ± 0.1 | 0 | 2.3 ± 1.4 | 0 | Total = 0/4 | |||
| Days 1, 2, 3 + FL | 3.1 | 0 | 193 | 0 | − | − | − |
| 2.8 | 0 | 123 | 0 | − | − | − | |
| 1.4 | 0 | 44 | 0 | − | − | − | |
| 7.3 | 0 | 336 | 0 | − | − | − | |
| 3.2 | 0 | 27 | 0 | − | − | − | |
| 0.8 | 0 | 3 | 0 | − | − | − | |
| Average = 3.1 ± 1.0 | 0 | 121 ± 56.3 | 0 | Total = 0/6 | |||
| Days 5, 6, 7 + FL* | 3.0 | 7 | 293 | 2.4 | + | 2 | 3 |
| 1.9 | 6 | 155 | 3.9 | + | 2 | 1 | |
| 5.2 | 0 | 216 | 0 | − | − | − | |
| 2.5 | 0 | 187 | 0 | − | − | − | |
| Average = 3.2 ± 0.7 | 3.3 | 212.8 ± 29.5 | 1.6 | Total = 2/4 | |||
Bone marrow was recovered from bnx mice after engraftment periods of 9 to 11 months. Human-specific colony-forming assays were plated from the bone marrow of each mouse (3 × 105bnx/hu bone marrow cells per sample) with and without addition of the selective agent G418 (0.9 mg/mL active). The extent of transduction was calculated as (no. G418-resistant/total human CFU) × 100 = % G418 resistance. Genomic DNA was isolated from samples of the bone marrow, and PCR to detect the presence of proviral integrants carrying the neo gene was performed. If positive by neo PCR, the marrow was further subjected to single-cell cloning and single-colony inverse PCR.
Two mice that had received human marrow CD34+CD38− cells cultured for 7 days with and without FL died of natural causes several months after the cotransplantation, and viable marrow could not be recovered for assays. The deaths were unrelated to receiving the human cell xenograft.
Samples in which no neo gene marking in total BM was detectable were not subjected to single-cell cloning and inverse PCR. These samples are shown as (−).
The number of engrafted human CD45+ cells per 1 millionbnx bone marrow cells was calculated from the FACS data obtained (Table 1), and an estimate of the total number of cells that would be engrafted in the marrow of the entire mouse was extrapolated (Table 4). The numbers given assume that all transplanted cells engrafted in the marrow, and that the number of marrow cells per mouse is 8 × 107.9,25,26The fold expansion that the 2,000 transplanted CD34+CD38− cells had undergone to reach the final engraftment levels, as detected by FACS analysis, is given. It is likely that only 10% to 20% of the marrow cells had actually homed to the marrow,27 in which case the fold expansions given in Table 4 would be greatly underestimated. In accordance with observations made in LTCIC and NOD/SCID systems,9,12 28 the capacity for cell proliferation from CD34+CD38− cells is immense, greater than 1,000-fold.
Extent of Human Hematopoietic Cell Content in the Marrow of Long-Term Engrafted bnxMice
| Cell Type and Number . | Duration of Ex Vivo Culture . | Average No. Human Cells/ 1 × 106 bnx BMC3-150 . | Average No. Hu Cells in Total bnx BM3-151 . | Fold Expansion . | Average No. Human CFU per 1 × 106 bnx BM cells3-152 . |
|---|---|---|---|---|---|
| Experiments 1-4 | |||||
| CD34+ 5 × 105 N = 4 | 1 h 3/6/S | 58,675 ± 15,448 | 47.5 ± 12.2 × 105 | 9.5 ± 2.5 | 152 ± 53 |
| CD34+ 5 × 105 N = 10 | 72 h 3/6/S | 26,500 ± 5,773 | 21.2 ± 4.6 × 105 | 3.7 ± 0.8 | 120 ± 27 |
| CD34+38− 2,000 N = 5 | 1 h 3/6/S | 62,380 ± 16,054 | 49.9 ± 12.9 × 105 | 2,495 ± 654 | 279 ± 65 |
| CD34+38− 2,000 N = 14 | 72 h 3/6/S | 32,079 ± 7,581 | 25.7 ± 6.1 × 105 | 1,282 ± 307 | 184 ± 69 |
| Experiments 5 and 6 | |||||
| CD34+38− 2,000 N = 6 | 72 h 3/6/S | 18,500 ± 5,819 | 17.8 ± 4.6 × 105 | 888 ± 227 | 115 ± 43 |
| CD34+38− 2,000 N = 4 | 168 h 3/6/S | 775 ± 484 | 0.6 ± 0.4 × 105 | 31 ± 19 | 8 ± 5 |
| CD34+38− 2,000 N = 6 | 72 h 3/6/S/FL | 31,183 ± 10,151 | 25.0 ± 8.1 × 105 | 1,248 ± 456 | 399 ± 182 |
| CD34+38− 2,000 N = 4 | 168 h 3/6/S/FL | 24,575 ± 10,265 | 19.7 ± 8.2 × 105 | 734 ± 217 | 702 ± 97 |
| Cell Type and Number . | Duration of Ex Vivo Culture . | Average No. Human Cells/ 1 × 106 bnx BMC3-150 . | Average No. Hu Cells in Total bnx BM3-151 . | Fold Expansion . | Average No. Human CFU per 1 × 106 bnx BM cells3-152 . |
|---|---|---|---|---|---|
| Experiments 1-4 | |||||
| CD34+ 5 × 105 N = 4 | 1 h 3/6/S | 58,675 ± 15,448 | 47.5 ± 12.2 × 105 | 9.5 ± 2.5 | 152 ± 53 |
| CD34+ 5 × 105 N = 10 | 72 h 3/6/S | 26,500 ± 5,773 | 21.2 ± 4.6 × 105 | 3.7 ± 0.8 | 120 ± 27 |
| CD34+38− 2,000 N = 5 | 1 h 3/6/S | 62,380 ± 16,054 | 49.9 ± 12.9 × 105 | 2,495 ± 654 | 279 ± 65 |
| CD34+38− 2,000 N = 14 | 72 h 3/6/S | 32,079 ± 7,581 | 25.7 ± 6.1 × 105 | 1,282 ± 307 | 184 ± 69 |
| Experiments 5 and 6 | |||||
| CD34+38− 2,000 N = 6 | 72 h 3/6/S | 18,500 ± 5,819 | 17.8 ± 4.6 × 105 | 888 ± 227 | 115 ± 43 |
| CD34+38− 2,000 N = 4 | 168 h 3/6/S | 775 ± 484 | 0.6 ± 0.4 × 105 | 31 ± 19 | 8 ± 5 |
| CD34+38− 2,000 N = 6 | 72 h 3/6/S/FL | 31,183 ± 10,151 | 25.0 ± 8.1 × 105 | 1,248 ± 456 | 399 ± 182 |
| CD34+38− 2,000 N = 4 | 168 h 3/6/S/FL | 24,575 ± 10,265 | 19.7 ± 8.2 × 105 | 734 ± 217 | 702 ± 97 |
Bone marrow was recovered from bnx mice and the percentage of human CD45+ cells in the marrow (BM) was determined by FACS. The number of human cells per 1 × 106bnx bone marrow cells was calculated. Human-specific colony-forming assays were plated from the marrow of each mouse to determine the number of clonogenic human progenitors recovered from 1 × 106bnxmarrow cells.
The limits of detection by FACS analysis were 100 human hematopoietic (CD45+) cells per 1 × 106bnxbone marrow cells.
Assuming an average of 80 × 106 total bone marrow cells per mouse and 100% seeding efficiency for transplanted cells.
Between 3 × 105 and 9 × 105 bone marrow cells were plated from each mouse, and numbers were normalized to 1 × 106. Therefore, numbers reported as 0 may be as high as two colonies.
The clonogenic capacity of human cells recovered from mice transplanted with human CD34+CD38− cells transduced for 3 versus 7 days with and without FL was assessed next. In accordance with the engraftment results, the cells cultured on stromal support for only three days ex vivo were not affected by the presence or absence of FL. The average number of human CFU recovered per 3 × 105bnx bone marrow cells was 34.8 ± 12.2 from cells maintained ex vivo in 3/6/S and 121 ± 51.7 from those kept in 3/6/S/FL (P = 0.14). In contrast, there was a significant difference in the average number of clonogenic progenitors recovered from mice transplanted with CD34+CD38− cells cultured for seven days with and without FL (Fig 3). The average number of human CFU recovered per 3 × 105bnx bone marrow cells was 2.25 ± 1.4 from cells cultured in 3/6/S and 212.8 ± 29.5 from those cultured in 3/6/S/FL (P = .0004; Table 3). The numbers of clonogenic human progenitors that grew from 1 × 106 bone marrow cells recovered from mice in each transduction group are compared in Table 4. Maintaining the CD34+CD38− cells for 7 days in medium containing IL-3, IL-6, and SCF without inclusion of FL resulted in significantly lower levels of progenitor maintenance than obtained from any other ex vivo culture condition.
Survival of clonogenic human progenitors inbnx mice transplanted with CD34+CD38− cells cultured for 3 versus 7 days with and without inclusion of FL. Human-specific colony-forming assays were plated from marrow recovered from the long-term engrafted mice from experiments #5 and #6. Colonies were counted after 14 to 21 days of growth.
Survival of clonogenic human progenitors inbnx mice transplanted with CD34+CD38− cells cultured for 3 versus 7 days with and without inclusion of FL. Human-specific colony-forming assays were plated from marrow recovered from the long-term engrafted mice from experiments #5 and #6. Colonies were counted after 14 to 21 days of growth.
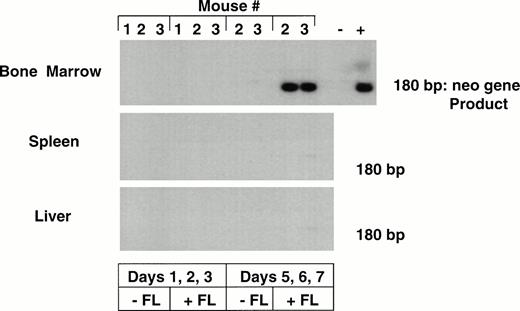
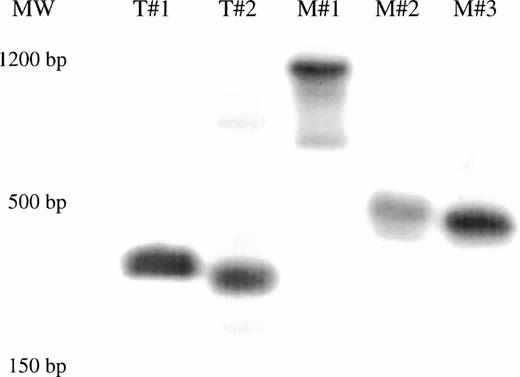
The levels of transduction of human hematopoietic cells engrafted in the different mice were determined by analyzing total marrow and tissues by neo PCR. Marked bone marrow was only detected in two mice that had received the CD34+CD38− cells cultured for 7 days in the presence of FL, with transduction on days 5, 6, and 7 (Table 3). No marking was seen in any tissue from mice that had received cells cultured for 3 days, with or without FL, or from mice that had received human cells cultured for 7 days in the absence of FL. The neo PCR results from selected tissues of one of the sets of mice are shown in Fig 4.
PCR for detection of the LN vector in bnx/hu bone marrow and tissues. DNA was extracted from the bone marrow and tissues of mice from experiments 5 and 6, after 9 to 11 months engraftment. Each sample was subjected to PCR to detect the presence of the neo gene. Hybridization with a neo-specific oligonucleotide probe was done to ensure that the product band had been correctly amplified. Tissues that were positive and negative for the presence of the neo gene were amplified as controls in each reaction. The results from one experiment are shown.
PCR for detection of the LN vector in bnx/hu bone marrow and tissues. DNA was extracted from the bone marrow and tissues of mice from experiments 5 and 6, after 9 to 11 months engraftment. Each sample was subjected to PCR to detect the presence of the neo gene. Hybridization with a neo-specific oligonucleotide probe was done to ensure that the product band had been correctly amplified. Tissues that were positive and negative for the presence of the neo gene were amplified as controls in each reaction. The results from one experiment are shown.
Determination of the number of individual, marked human hematopoietic stem and progenitor cells by inverse PCR.
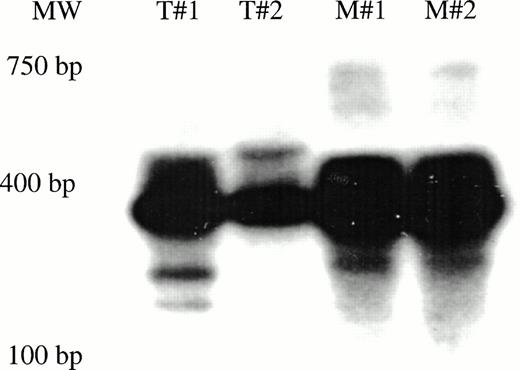
We next determined the number of individual, marked human stem or progenitor cells that had contributed to hematopoiesis over the 9 to 11 month engraftment period, in mice selected from each transduction group. Single-colony inverse PCR, a sensitive technique that can be performed on individual clones containing at least 200 cells, was used.17 Cryopreserved marrow from mice that harbored marked human cells, previously detected by PCR for the neo gene, was thawed and labeled with antibodies to human CD45 plus CD33 to isolate myeloid cells, and antibodies to human CD45 plus CD3 to isolate T lymphocytes. Human T cells and myeloid progenitors (acquired with a lymphoid gate) were then deposited individually into 96-well plates by using the automated cell deposition unit (ACDU) of a FACSVantage (Becton Dickinson), and grown into small colonies as described.17 The clonal integration patterns obtained from human colonies recovered from five mice transplanted with human CD34+ cells transduced for 72 hours in 3/6/S showed oligoclonal marking of long-lived lineage-restricted progenitors with limited expansion within the marrow, as we have previously described.17 29 The mouse that was transplanted with human CD34+CD38− cells cultured for 72 hours in 3/6/S, and that had 17/240 G418-resistant human CFU (shown in Table 2), had clonal integration patterns in individual colonies that revealed that at least four different myeloid progenitors had been transduced (Fig 5A). Two G418-resistant myeloid colonies were obtained from the second mouse transplanted by the same marrow, and both colonies were derived from a fifth progenitor, not detected in the first mouse. No marked T lymphocytes were found in either mouse, and 12 other mice in experiments one through four that had received human CD34+CD38− cells transduced under the same conditions contained no marked human cells 9 to 11 months post-transplantation. These data show that, while occasionally marking of a small portion of a CD34+CD38− cell population can occur on stromal support in 72-hour transductions with IL-3, IL-6, and SCF, the method is not a reliable way to achieve high extents of transduction of this primitive hematopoietic cell population.
Clonal analysis by inverse PCR of marked human T-cell and myeloid colonies recovered from mice transplanted with CD34+CD38− cells. (A) G418-resistant human CFU-GM was recovered from the marrow of 2/14 mice transplanted with CD34+CD38−cells subjected to a 72-hour transduction. The clonal diversity of the individual, marked human myeloid colonies was assessed by single-colony inverse PCR. Colonies shown are from the mouse that had 17/240 G418-resistant progenitors (Table 2). (B) Human T-cell and myeloid colonies were grown from mice transplanted with CD34+CD38− cells transduced for 7 days with FL (Table 3). Colonies from two of the four mice were shown to contain the neo gene by PCR, then were futher subjected to single-colony inverse PCR. Panels B and C show amplified inverse PCR products from marked T-cell and myeloid colonies obtained from each mouse. T = T lymphoid clones. M = myeloid clones.
Clonal analysis by inverse PCR of marked human T-cell and myeloid colonies recovered from mice transplanted with CD34+CD38− cells. (A) G418-resistant human CFU-GM was recovered from the marrow of 2/14 mice transplanted with CD34+CD38−cells subjected to a 72-hour transduction. The clonal diversity of the individual, marked human myeloid colonies was assessed by single-colony inverse PCR. Colonies shown are from the mouse that had 17/240 G418-resistant progenitors (Table 2). (B) Human T-cell and myeloid colonies were grown from mice transplanted with CD34+CD38− cells transduced for 7 days with FL (Table 3). Colonies from two of the four mice were shown to contain the neo gene by PCR, then were futher subjected to single-colony inverse PCR. Panels B and C show amplified inverse PCR products from marked T-cell and myeloid colonies obtained from each mouse. T = T lymphoid clones. M = myeloid clones.
Next, mice transplanted with human CD34+CD38−cells transduced for 7 days in the presence of FL were analyzed. The two mice that had neo-positive cells in their marrow (Fig 4) had both T lymphocytes and myeloid progenitors that bore LN provirus, as determined by single-cell cloning and neo PCR. Inverse PCR from the neo-positive clones recovered from those mice revealed that in the first mouse, at least five different CD34+CD38− cells had been transduced, and had survived in the mice to generate clonogenic progeny after 10 months (Fig 5B). The second mouse harbored marked human myeloid and T-cell colonies derived from at least three different, transduced CD34+CD38− cells (Fig 5C).The blot shown in Fig 5C has one band that is of similar molecular weight in all samples. However, the amplified product of the second LTR and flanking genomic DNA is of a different size in three samples, indicating that those progenitors were derived from unique precursors with different proviral integration sites. No T-cell and myeloid clones bearing the same proviral marker were detected in the current studies.
In conclusion, addition of FL to medium containing IL-3, IL-6, and SCF during 168-hour culture periods was necessary to allow maintenance of the capacity of the human progenitors to sustain subsequent long-term hematopoiesis. The extended transduction with FL allowed multiple progenitors to be marked, an event that had occurred in only one out of 26 mice transplanted with CD34+CD38− cells cultured for only 72 hours (experiments 1-6).
DISCUSSION
From this series of studies, it was shown that CD34+ cells from human marrow contain a population of cells that can be transduced by retroviral vectors, and generate gene-marked progeny in immune-deficient mice for up to 11 months. In contrast, CD34+CD38− cells engraft to comparable extents in the mice, but are not easily transduced by Moloney Murine Leukemia Virus-based vectors in a 72-hour period, in the presence of the cytokines IL-3, IL-6, and SCF. Adding FL to the transduction medium, and increasing the duration of ex vivo culture from 3 to 7 days, with vector addition on days 5, 6, and 7 slightly increased the chance of obtaining transduction of human CD34+CD38− cells capable of sustaining long-term hematopoiesis in the mice. We and others had previously shown that FL is a survival factor for primitive hematopoietic cells.18-20 During a 7-day culture period, FL may be acting to sustain survival of the primitive cells until a small-percentage exit quiescence and become susceptible to transduction by Moloney Murine Leukemia-based retroviral vectors, which require cycling of the target cells to allow integration.11
In support of the theory that FL may be acting at the level of survival of primitive cells, we observed that few human CFU were recovered from long-term engrafted bnx mice transplanted with human CD34+CD38− cells cultured for 7 days with stromal support, in the presence of IL-3, IL-6, and SCF. However, addition of recombinant FL to the same culture conditions supported a significant increase in maintenance of the clonogenic capacity of the CD34+CD38− cells during the 7-day transduction period. The fact that cells cultured ex vivo for 7 days in IL-3, IL-6, and SCF without FL had minimal maintenance of clonogenic capacity, despite the presence of stromal support during the culture period implies that the levels of FL produced by irradiated stroma are insufficient to sustain the long-term clonogenic capacity of cells that are able to give rise to long-term hematopoiesis in mice, during extended culture periods.
The vector titer per cell (multiplicity of infection) was higher when transductions were done by using CD34+CD38−cells, but a higher level of transduction was not achieved. These data indicate that it is not the amount of vector that is rate limiting for effective gene transfer into the primitive population, but rather factors intrinsic to the cells. The CD34+CD38− population has been reported to have lower expression of the receptors that mediate vector entry into the cell.30 The degree of quiescence of the target cell population is also a confounding factor.31 We have previously determined that CD34+CD38−cells with the phenotype used in this study are not easily recruited into cycle by cytokines, but the entry occurs individually over time in culture.7,18 28 This apparently stochastic property of the primitive cell population may explain why there was 1 out of 14 mice that had several long-lived human hematopoietic stem or progenitor cells transduced during a 72-hour culture. Random entry of one primitive cell into cycle could generate several dividing progeny that would each be susceptible to retroviral-mediated transduction.
There are two possibilities to explain the presence in the mice of marked human hematopoietic cells from CD34+ populations and the paucity of marked cells in mice transplanted with transduced CD34+CD38− populations. One possibility is that the primitive CD34+CD38− cells were transduced more efficiently when surrounded by the CD34+/CD38+ cells, which might have provided some accessory factors that enhanced cell cycle progression, allowing retroviral integration. The second possibility is that there is a population of CD34+CD38dim cells that are easily transduced (in contrast to the stringently gated CD38− population used in the current studies), and are able to maintain long-term hematopoiesis in immune-deficient mice. There have been several reports that the CD34+/CD38+ population can mediate only short-term hematopoiesis.8,9 25 However, in those reports, a population expressing CD38 with more intensity than the cells used in our studies was included in the CD38− fraction, and may represent long-lived committed progenitors. The CD34+CD38+ fractions described in the previous publications thus excluded the CD34+/CD38dimfraction, whereas our CD34+ populations included it, and our CD34+CD38− populations stringently excluded it. Future studies will determine whether cells isolated from the CD34+CD38dim region can sustain engraftment for 1 year in bnx mice, and are responsible for the higher level of marking that is obtained from the total CD34+populations.
In the current studies, 2,000 CD34+CD38−cells engrafted bnx mice to levels comparable to 5 × 105 CD34+ cells. Our previous studies had shown the CD34+CD38− population, acquired as shown in Fig 1, to represent 0.02% of the total mononuclear fraction.7 CD34+ cells are present at an average of 1% of the mononuclear cells. The comparable extents of engraftment we obtained from the cell numbers transplanted indicate that the most primitive cells within each population, capable of engrafting bnx mice, might not be present at the frequencies predicted by simple analysis of cell surface expression. The group headed by John Dick has done elegant studies to quantitate the SCID mouse reconstituting cell (SRC) by limiting dilution, and found that they are present at a frequency of one SRC in 617 CD34+CD38− cells.9,25,26 We have observed that 1,000 CD34+CD38− cells give rise to detectable human cell engraftment in 38% of the mice tested (n = 8), whereas 2,000 cells engraft 94% of the mice (n = 34), after a 72-hour transduction period with stroma and cytokines (Dao and Nolta, unpublished data). Calculation of reconstituting cell numbers from our experiments is complicated by the fact that some period of ex vivo culture and transduction is always done, and may alter the capacity of the cells to home to the correct sites or to sustain long-term engraftment as has been shown with murine marrow.27 However, in the current studies, we found that CD34+CD38− cells cultured for 72 hours did not give rise to significantly different levels of CD45+ or clonogenic progenitor cell engraftment than the same population of cells held in suspension culture for only 1 hour before transplantation.
The current data show that CD34+CD38− cells are resistant to transduction with Moloney-based retroviral vectors. Our studies provide a system for evaluating the success of new methods for hematopoietic stem cell transduction. To achieve transduction, the cells must be prompted into mitosis, while maintaining pluripotentiality, or alternatively must be transduced by vectors that integrate into nondividing cells. Alternate cytokines, transduction systems such as fibronectin support to replace the irradiated monolayer of stroma, and vectors such as the lentiviral system described by Naldini et al32-36 may prove to be more useful for transduction of CD34+CD38− cells.
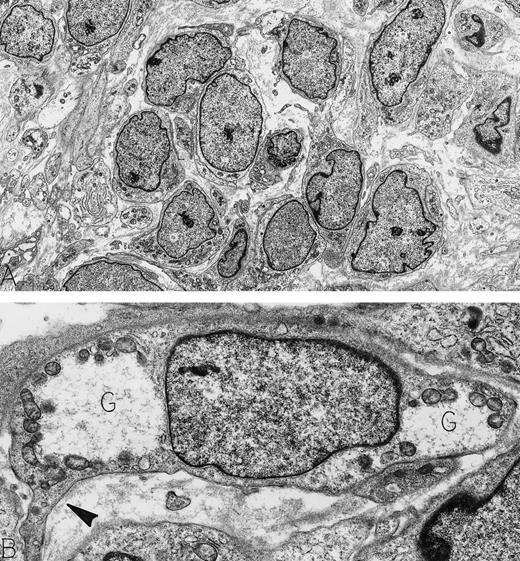
Hemangiopericytoma. An asymptomatic 73-year-old man was found to have a large retroperitoneal mass on routine physical examination. This was confirmed by computer tomography scan; at surgery, the mass did not involve any abdominal organs. Immunoperoxidase stains were positive for vimentin and negative for actin, desmin, neurofilament, keratin, and S-100 protein. Electron microscopy showed a tumor composed of pericytes, illustrated at low magnification in cross-section (A) and at higher magnification in longitudinal section (B). Note the dispersed nuclear chromatin pattern and the paucity of cellular organelles in the cytoplasm. Focal strips of basal lamina beneath extended cytoplasmic processes (arrowhead) and cytoplasmic glycogen pools (G) characterize these neoplastic pericytes. Original magnifications: (A), ×3,500; (B), ×13,000. (Courtesy of Ann M. Dvorak, MD, Department of Pathology, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215.)
Hemangiopericytoma. An asymptomatic 73-year-old man was found to have a large retroperitoneal mass on routine physical examination. This was confirmed by computer tomography scan; at surgery, the mass did not involve any abdominal organs. Immunoperoxidase stains were positive for vimentin and negative for actin, desmin, neurofilament, keratin, and S-100 protein. Electron microscopy showed a tumor composed of pericytes, illustrated at low magnification in cross-section (A) and at higher magnification in longitudinal section (B). Note the dispersed nuclear chromatin pattern and the paucity of cellular organelles in the cytoplasm. Focal strips of basal lamina beneath extended cytoplasmic processes (arrowhead) and cytoplasmic glycogen pools (G) characterize these neoplastic pericytes. Original magnifications: (A), ×3,500; (B), ×13,000. (Courtesy of Ann M. Dvorak, MD, Department of Pathology, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215.)
ACKNOWLEDGMENT
C. H. Hannum at DNAX generously provided the recombinant Flt3 ligand used in these studies. Don Kohn, Ken Weinberg, Craig Jordan, and Robertson Parkman provided useful discussion. This work was made possible by Sally Worttman, head of our animal facility, and Renee Traub-Workman, due to their dedication in maintaining an immaculatebnx mouse colony.
Supported by a grant from the NIH NHLBI (SCOR #1-P50-HL54850-03). J.A.N. was also supported by the John Connell Gene Therapy Foundation. G.M.C. was supported in part by a Translational Research Grant from the Leukemia Society of America (#6360-97) and Grants No. NCI # 2CA14089-21 and 5P01 CA59318-05.
Address reprint requests to Jan A. Nolta, PhD, Division of Research Immunology/Bone Marrow Transplantation, Childrens Hospital Los Angeles, 4650 Sunset Blvd, Mailstop #62, Los Angeles, CA 90027.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal