Abstract
Interferon γ (IFNγ) inhibits the growth and differentiation of highly purified human erythroid colony-forming cells (ECFCs) and induces erythroblast apoptosis. These effects are dose- and time-dependent. Because the cell surface receptor known as Fas (APO-1; CD95) triggers programmed cell death after activation by its ligand and because incubation of human ECFCs with IFNγ produces apoptosis, we have investigated the expression and function of Fas and Fas ligand (FasL) in highly purified human ECFCs before and after incubation with IFNγ in vitro. Only a small percentage of normal human ECFCs express Fas and this is present at a low level as detected by Northern blotting for the Fas mRNA and flow cytometric analysis of Fas protein using a specific mouse monoclonal antibody. The addition of IFNγ markedly increased the percentage of cells expressing Fas on the surface of the ECFCs as well as the intensity of Fas expression. Fas mRNA was increased by 6 hours, whereas Fas antigen on the cell surface increased by 24 hours, with a plateau at 72 hours. This increase correlated with the inhibitory effect of IFNγ on ECFC proliferation. CH-11 anti-Fas antibody, which mimics the action of the natural FasL, greatly enhanced IFNγ-mediated suppression of cell growth and production of apoptosis, indicating that Fas is functional. Expression of FasL was also demonstrated in normal ECFCs by reverse transcriptase-polymerase chain reaction and flow cytometric analysis with specific monoclonal antibody. FasL was constitutively expressed among erythroid progenitors as they matured from day 5 to day 8 and IFNγ treatment did not change this expression. Apoptosis induced by IFNγ was greatly reduced by the NOK-2 antihuman FasL antibody and an engineered soluble FasL receptor, Fas-Fc, suggesting that Fas-FasL interactions among the ECFCs produce the erythroid inhibitory effects and apoptosis initiated by IFNγ.
HUMAN INTERFERON γ (IFNγ) induces activation of mononuclear phagocytes and lymphocytes and also suppresses normal hematopoiesis in vitro under a variety of cell culture conditions. The inhibitory effects of IFNγ on murine and human granulocyte-macrophage colony-forming units, burst-forming units-erythroid (BFU-E), and colony-forming units-erythroid (CFU-E) in vitro have been reported by many investigators.1-9 Previous experiments performed in this laboratory have shown that intermediate, day-3 to day-6 mature human BFU-E are most sensitive to IFNγ, whereas early BFU-E and more mature erythroid colony-forming cells (ECFCs) are much less sensitive.8
Additional investigations have shown that IFNγ inhibited cell proliferation and produced apoptosis of stromal cells, murine pre-B–cell lines, and also human CD4+ or CD8+thymocytes that had been treated with cyclosporin.10,11Apoptosis of erythroid cells after incubation with IFNγ has been demonstrated in our laboratory by both nuclear condensation and fragmentation plus flow cytometry with in situ end-labeling.8 Whereas reduced cell proliferation was noted by 72 hours, apoptosis was only demonstrated at a later time and was, therefore, quite different from the apoptosis produced by erythropoietin (EP) deprivation, which has been shown by 17 hours with human erythroid cells.12
The process of apoptosis involves many metabolic changes, leading to the final degradation of genomic DNA into nucleosomal fragments, and the regulation of this process involves a large number of genes. Both in vivo and vitro studies have demonstrated that the Fas/Fas ligand (FasL) system performs a critical function in producing apoptosis in several different organ systems after triggering of Fas by FasL.9,13,14 Fas is widely expressed in many cell types, but the FasL has been demonstrated mainly in activated T cells and in a few tissues such as the cornea and testis, plus some tumors. However, Fas/FasL expression on normal erythroid progenitor cells and their role during hematopoietic development has not been clearly defined. No CD95 mRNA expression was detected by reverse transcriptase-polymerase chain reaction (RT-PCR) in CD34+ cells freshly isolated from human bone marrow15,16 and few CD34+ cells from normal bone marrow expressed Fas antigen, but this was markedly increased on bone marrow CD34+ cells from patients with aplastic anemia.17 Immature primitive hematopoietic progenitors from human fetal liver did express CD95, whereas the more mature progenitors showed low CD95 expression.18 Both IFNγ and TNF induced Fas expression on purified human CD34+ cells.9 15 FasL expression in purified hematopoietic progenitors has not been identified.
In this study, we further characterized the inhibitory effect of IFNγ on ECFC proliferation and apoptosis induction. Because binding of FasL, or agonistic anti-Fas antibody, to Fas receptor triggers apoptosis and because ECFCs underwent apoptosis after incubation with IFNγ, we have investigated the expression of Fas and FasL in human ECFCs after incubation with IFNγ and the role of Fas and FasL in IFNγ-induced apoptotic cell death.
MATERIALS AND METHODS
Purification and expansion of human blood ECFCs.
Four hundred milliliters of blood was obtained from normal donors after informed consent approved by the Vanderbilt University and Department of Veterans Affairs Medical Centers (Nashville, TN) Institutional Review Boards. BFU-E were purified by sequential density gradient centrifugation; depletion of platelets, lymphocytes, and adherent cells; and further negative selection of contaminant cells with CD2, CD11b, CD16, and CD45 monoclonal antibodies (MoAbs), as previously described.8,19 The BFU-E were suspended in 20 mL Iscove's modified Dulbecco's medium (IMDM) containing 20% heat-inactivated fetal calf serum, 5% heat-inactivated, pooled, human AB serum, 1% deionized bovine serum albumin (BSA; Intergen Co, Purchase, NY), 5 × 10−5 mol/L 2-mercaptoethanol, 10 μg/mL insulin, 2 U/mL recombinant human (rh) EP, 50 U/mL interleukin-3, penicillin at 500 U/mL, and streptomycin at 40 μg/mL in 50-mL polystyrene flasks to generate ECFCs.19 After incubation at 37°C in 5% CO2/95% humidified air for 4 or 5 days (day-5 or day-6 cells), the cells were collected, further enriched by centrifugation through 10% BSA and over Ficoll-Hypaque, and aliquoted for Northern and flow cytometric analyses plus plasma clot assays for ECFCs. In some experiments, incubation was continued in liquid medium with or without rhIFNγ (4.75 × 107 U/mg; Genzyme Corp, Cambridge, MA) for additional times to observe later effects of rhIFNγ.
Plasma clot assay for ECFCs.
Cells were plated at a concentration of 103/mL in an IMDM mixture containing 20% fetal calf serum, 5% human AB serum, 1% deionized BSA, 10 μg/mL insulin, 2 U/mL rhEP, penicillin plus streptomycin, 2 mg/mL fibrinogen (Sigma, St Louis, MO), and 0.2 U/mL bovine thrombin (Parke-Davis, Morris Plains, NJ) in 48-well flat-bottomed tissue culture plates with 0.2 mL/well. In some experiments, rhIFNγ; anti-Fas antibodies, CH-11 (Immunotech Inc, Westbrook, ME), and/or ZB4 (MBL, Watertown, MA); anti-FasL antibody, NOK-2 (kindly provided by Hideo Yagita, Juntendo University, Tokyo, Japan); Fas-Fc, a fusion protein composed of human Fas and a human Ig constant region prepared as previously described20; control MoAb isotype IgGI (Becton Dickinson, San Jose, CA); or control human IgG Fc fragment (Accurate Chemical and Scientific Corp, Westbury, NY) were added at the indicated concentrations. The clots were fixed on day 15 and stained with 3,3′ dimethoxybenzidine and hematoxylin. Colonies of 2 or more hemoglobinized cells were scored as ECFCs. The ECFC purity was determined by the plating efficiency and data were expressed as the means ± SD with significance calculated by the t-test.
RNA preparation and Northern analysis.
Total RNA was prepared from day-5 to day-8 cells treated or not treated with rhIFNγ using ULTRASPEC or RNAzol (BIOTECX Laboratories, Inc, Houston, TX). Quantification of RNA, formaldehyde gel electrophoresis, blotting onto nylon membranes, and hybridization with32P-labeled DNA probes for FAS and β-actin were performed as previously described.21 Rehybridization of the blots with a similarly labeled probe for actin mRNA was also performed to correct for variation in loading. Actin mRNA is maintained at constant levels in ECFCs in the presence of rhEP during the periods analyzed.21 Fas mRNA expression was quantitated with a laser scanning densitometer and normalized to the amount of total RNA present in the lane by comparison with the 18S band as well as the actin signal. The human β-actin probe was purchased from Calbiochem-Novobiochem International (La Jolla, CA) and the human Fas probe was generated by RT-PCR.
RT-PCR.
Total RNA was extracted as described above. Using a Gene Amp RNA PCR kit (Perkin Elmer, Cetus, Norwalk, CT), single-stranded cDNA was synthesized and RT-PCR was performed. Fas and FasL specific primers were designed according to sequence data published previously.22 23 We used antisense primer 5′-T ACT CAA GTC AAC-3′ (bases no. 873-885), for generation of Fas cDNA, and sense primer 5′ ATG CTG GGC ATC TGG ACC CTC CTA-3′ (bases no. 195-218) plus antisense primer 5′-TGG AGA TTC ATG AGA ACC TTG GTT-3′ (bases no. 810-833) for amplification of Fas cDNA. We used random primers to generate FasL cDNA and sense primer 5′-C AAG TCC AAC TCA AGG TCC ATG CC-3′ (bases no. 517-540) plus antisense primer 5′-CAG AGA GAG CTC AGA TAC GTT GAC-3′ (bases no. 839-862) for amplification of FasL cDNA. Thirty-five cycles of PCR, with denaturation at 94°C for 30 seconds, annealing at 60°C for 30 seconds (Fas) or 60 seconds (FasL), and extension at 72°C for 1 minute, were performed on a programmed-temperature system (Hybaid OmniGene; Midwest Scientific, St Louis, MO). The amplified PCR product was electrophoresed in a 1% agarose gel and ethidium bromide-stained. The gel containing an expected single band was sliced out and DNA was purified using a QIAquick Gel Extraction kit (QIAGEN, Santa Clarita, CA). Both PCR products were verified by sequencing.
Detection of Fas and FasL by flow cytometric analysis.
Data acquisition was performed on a Becton Dickinson FACScan flow cytometer with data saved as listmode files. All incubations were performed on ice. The ECFCs cultured with or without rhIFNγ were collected and washed three times in phosphate-buffered saline (PBS) with 1% BSA. Cell-surface expression of Fas was assayed by direct immunofluoresence flow cytometry. Briefly, 1 × 106cell aliquots in 0.1 mL PBS with 1% BSA from each group were incubated with either fluorescein isothiocyanate (FITC)-murine anti-Fas MoAb (UB2) at 10 μg/mL (Immunotech) or FITC-murine IgG1 (Becton Dickinson) as a control for 30 minutes. The cells were then fixed in PBS with 1% formaldehyde before analysis.
FasL was shown by indirect immunoflourescence. ECFCs were fixed and permeabilized using a commercial Fix & Perm Cell Permeabilization kit (Caltag Laboratories, South San Francisco, CA) before staining. Primary incubations were performed with 106 cells and murine antihuman FasL MoAb at 10 μg/mL (clone G 247-4; PharMingen, San Diego, CA) or murine IgG1 at the same concentration for 30 minutes. These cells were then washed once with PBS/1% BSA. Secondary incubations were performed with FITC-goat antimouse IgG1 at 10 μg/mL (Southern Biotechnology Assoc Inc, Birmingham, AL) for another 30 minutes.
To ensure that the FasL+ cells were ECFCs, dual staining was performed in some experiments combining indirect immunofluorescence for FasL with direct staining using phycoerythrin (PE)-MoAb (5 μg/mL) to CD71 (transferrin receptor; Caltag). After indirect staining as described above, unbound goat antimouse binding sites were blocked with 5 μg/mL normal mouse IgG in PBS for 20 minutes. Direct-labeled, antigen-specific PE-MoAb to CD71 or PE-murine IgG1 (Becton Dickinson) was then incubated with the cells for an additional 30 minutes. The cells were then fixed and analyzed by flow cytometry. Cells were incubated with murine IgG1, indirectly stained with FITC-goat antimouse IgG1, and counterstained with direct-labeled, nonspecific PE-murine IgG1 to show nonspecific fluorescence on both axes. For specific comparison of murine antihuman FasL MoAb on ECFCs, we used indirect murine IgG1 stained with FITC-goat antimouse IgG1 and counterstained with specific PE-MoAb to CD71.
To detect soluble FasL in the cell medium, the sFas Ligand Elisa Kit purchased from MBL was used.
RESULTS
Inhibition of ECFC growth by IFNγ.
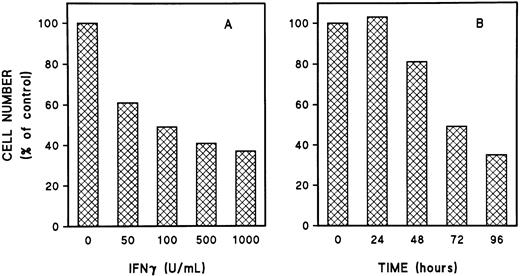
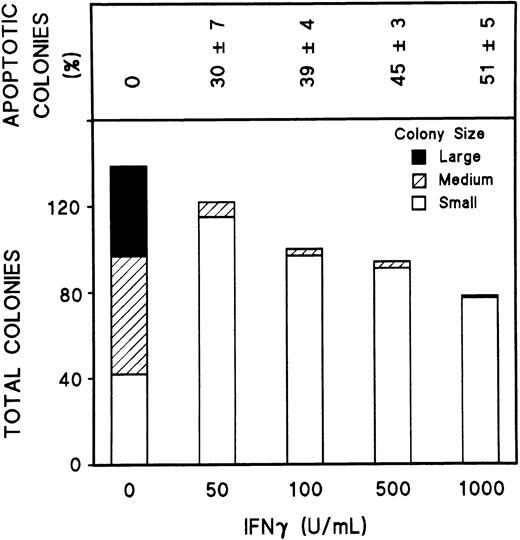
To study the effect of IFNγ on Fas and FasL, we first defined the optimum dose of rhIFNγ and the appropriate duration of incubation with day-5 cells to produce its inhibitory effects. Inhibition of ECFC growth was produced by an rhIFNγ concentration as low as 50 U/mL (Fig 1A) and was clearly evident after 72 hours of incubation with rhIFNγ (Fig 1B). Cytospin preparations of these cells after 96 hours of incubation with 1,000 U/mL of rhIFNγ showed cells with morphologic changes characteristic of apoptosis, such as nuclear condensation, fragmentation, and reduced size, which have correlated with DNA fragmentation demonstrated by in situ end-labeling in ECFCs.8 A marked reduction of the number and size of erythroid colonies and hemoglobin formation was also induced by rhIFNγ, and programmed cell death within the erythroid colonies was enhanced as the concentration of rhIFNγ was increased (Fig 2). Thirty percent of erythroid colonies contained apoptotic cells after incubation with 50 U/mL of rhIFNγ and 50% of erythroid colonies were greatly reduced in size and had an enhanced number of cells with the characteristic morphologic changes of apoptosis at the highest concentration of rhIFNγ. Similar results were obtained in day-6 cells (data not shown).
Dose- and time-dependent inhibition of day-5 ECFCs by IFNγ. Day-5 ECFCs were incubated in liquid medium with rhIFNγ from 0 to 1,000 U/mL. After 96 hours of incubation, the cells were collected and the number of cells was counted (A), or the cells were cultured with or without 1,000 U/mL rhIFNγ and harvested at the indicated times. (B) The number of total cells in each control without rhIFNγ was taken as 100% and was compared with that of the rhIFNγ groups. Each graph represents the mean from two experiments. The purity of the day-5 ECFCs, determined by plasma clot assay, was 54% ± 6% (A) and 56% ± 9% (B).
Dose- and time-dependent inhibition of day-5 ECFCs by IFNγ. Day-5 ECFCs were incubated in liquid medium with rhIFNγ from 0 to 1,000 U/mL. After 96 hours of incubation, the cells were collected and the number of cells was counted (A), or the cells were cultured with or without 1,000 U/mL rhIFNγ and harvested at the indicated times. (B) The number of total cells in each control without rhIFNγ was taken as 100% and was compared with that of the rhIFNγ groups. Each graph represents the mean from two experiments. The purity of the day-5 ECFCs, determined by plasma clot assay, was 54% ± 6% (A) and 56% ± 9% (B).
IFNγ inhibits colony formation and produces apoptosis of erythroid cells. Day-5 cells at 200 cells/well were plated in 0.2-mL plasma clots with or without rhIFNγ. The clots were fixed at day 15 and stained with benzidine-hematoxylin. The size of the colonies was estimated as follows: large colonies, greater than 500 cells/colony; medium colonies, 50 to 500 cells/colony; and small colonies, 2 to 49 cells/colony. The colonies that contained 10% or more cells with the morphologic changes of apoptosis were counted as apoptotic colonies, and the percentage of apoptotic colonies is shown in the top panel.
IFNγ inhibits colony formation and produces apoptosis of erythroid cells. Day-5 cells at 200 cells/well were plated in 0.2-mL plasma clots with or without rhIFNγ. The clots were fixed at day 15 and stained with benzidine-hematoxylin. The size of the colonies was estimated as follows: large colonies, greater than 500 cells/colony; medium colonies, 50 to 500 cells/colony; and small colonies, 2 to 49 cells/colony. The colonies that contained 10% or more cells with the morphologic changes of apoptosis were counted as apoptotic colonies, and the percentage of apoptotic colonies is shown in the top panel.
Expression of Fas on ECFCs.
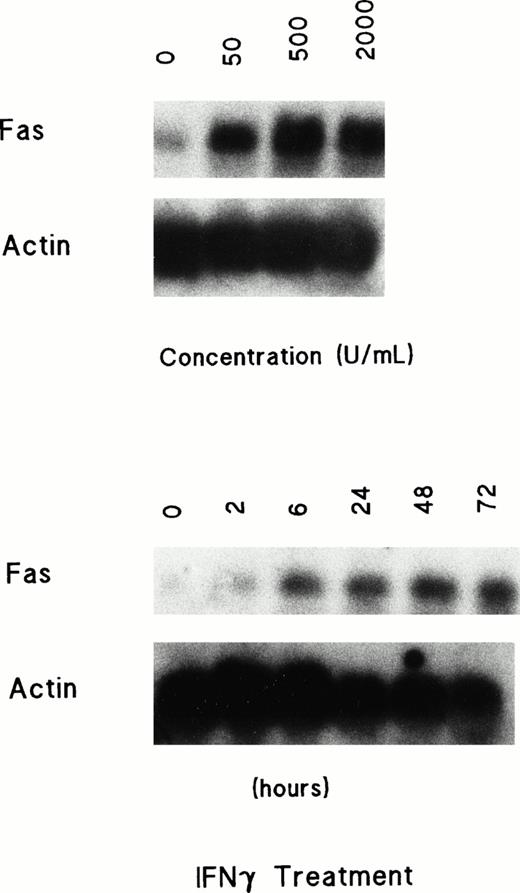
We next determined whether IFNγ treatment induced Fas mRNA expression. Day-5 ECFCs were cultured with rhIFNγ at a variety of increasing concentrations and durations of incubation. The cells were then harvested and Northern analysis for Fas was performed (Fig 3). A very low level of Fas mRNA was detected among the original cells without incubation. After incubation with rhIFNγ, expression of Fas mRNA was markedly enhanced. A greater than fivefold enhancement of Fas mRNA by laser scanning densitometry was evident at an rhIFNγ concentration of 500 U/mL or higher. This induction of Fas mRNA expression by rhIFNγ was clearly increased by 6 hours after incubation with rhIFNγ, and Fas mRNA continued to increase as the time of treatment was extended.
Dose- and time-dependent induction of Fas mRNA in ECFCs treated with rhIFNγ. Day-5 cells were incubated with IFNγ at 0 to 2,000 U/mL for 72 hours or with 2,000 U/mL for 0 to 72 hours, and then the total RNA was prepared. Twenty micrograms of total RNA was loaded in each lane. The blots were hybridized with a labeled probe for Fas and rehybridized with a probe for actin after stripping the initial probe. The ECFC purity at day 5 was 51% ± 9% and 56% ± 8%, respectively, and by day 8 it was 89% ± 6% and 91% ± 8%.
Dose- and time-dependent induction of Fas mRNA in ECFCs treated with rhIFNγ. Day-5 cells were incubated with IFNγ at 0 to 2,000 U/mL for 72 hours or with 2,000 U/mL for 0 to 72 hours, and then the total RNA was prepared. Twenty micrograms of total RNA was loaded in each lane. The blots were hybridized with a labeled probe for Fas and rehybridized with a probe for actin after stripping the initial probe. The ECFC purity at day 5 was 51% ± 9% and 56% ± 8%, respectively, and by day 8 it was 89% ± 6% and 91% ± 8%.
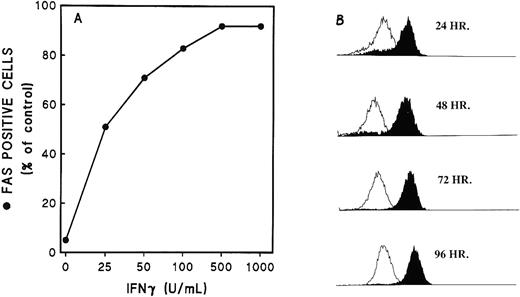
Fas antigen expression on the cell surface was measured by direct immuno-fluorescence with specific FITC-MoAb and flow cytometry. Only a small percentage of Fas+ cells was present among the original cells before incubation, but a very large increase of Fas+ cells was demonstrated after 48 hours of incubation with increasing concentrations of rhIFNγ (Fig 4A). An enhanced expression of Fas antigen on the cells, including both the number of positive cells and the intensity per cell, was clearly apparent at 24 hours and reached a plateau after 48 to 72 hours of incubation with rhIFNγ (Fig 4B). No effect was evident at 6 hours (data not shown). An enhanced concentration of Fas on the surface may be required for inhibition of cell proliferation and induction of apoptosis, because a significant reduction in cell number occurred at 72 hours when the cells had a higher Fas distribution on their surface.
Expression of Fas on ECFCs after incubation with rhIFNγ. Day-5 cells were cultured in liquid medium in the presence of rhIFNγ at 0 to 1,000 U/mL for 48 hours (A) or at 1,000 U/mL for 24 to 96 hours (B). At the indicated times, the cells were incubated with FITC-MoAb to Fas (CD95) or FITC-murine IgG1. Flow cytometric analysis was then performed. The open histogram shows the CD95 fluorescence of the cells incubated without rhIFNγ and the solid histogram shows the CD95 fluorescence of the rhIFNγ-treated cells. The purity of the day-7 cells was 89% ± 9% (A) and 81% ± 7% (B).
Expression of Fas on ECFCs after incubation with rhIFNγ. Day-5 cells were cultured in liquid medium in the presence of rhIFNγ at 0 to 1,000 U/mL for 48 hours (A) or at 1,000 U/mL for 24 to 96 hours (B). At the indicated times, the cells were incubated with FITC-MoAb to Fas (CD95) or FITC-murine IgG1. Flow cytometric analysis was then performed. The open histogram shows the CD95 fluorescence of the cells incubated without rhIFNγ and the solid histogram shows the CD95 fluorescence of the rhIFNγ-treated cells. The purity of the day-7 cells was 89% ± 9% (A) and 81% ± 7% (B).
FasL expression in ECFCs.
Experiments were performed first to demonstrate FasL mRNA by RT-PCR. These studies showed that FasL mRNA was present in cells treated or not treated with rhIFNγ (Fig 5). To investigate the expression of FasL protein in ECFCs, day-5 cells and their descendent day-8 cells, derived from the day-5 cells by incubation with or without rhIFNγ for 72 hours, were fixed and permeabilized for identification of FasL and CD71 with specific MoAb. Although all proliferating cells including activated T and B cells and macrophages also bear CD71, the number of transferrin receptors in erythroid progenitor cells is 15-fold higher than the concentrations associated with other cells.24 The high-intensity CD71+ cells were also larger cells than those with low-intensity CD71+ and ECFCs are large cells. Therefore, when flow cytometric analysis for FasL was performed, large cells with high intensity CD71+ were gated. This group of cells represented more than 80% of the total population. To exclude interference due to staining with two fluorescence markers, not only isotype, normal mouse FITC-IgG with mouse PE-IgG was set as a control, but also mouse FITC-IgG with PE-MoAb to CD71 was used as a second control.
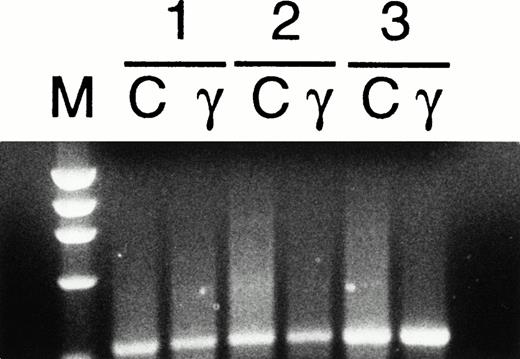
Detection of FasL mRNA by RT-PCR in human ECFCs. Day-5 cells from three donors were cultured in control (C) medium or with 1,000 U/mL rhIFNγ (γ) for 3 days and total RNA was prepared followed by RT-PCR for FasL. The PCR product corresponding to FasL (346 bp) was purified after electrophoresis and verified by sequencing (marker, ◊x174 DNA-Hae III digest).
Detection of FasL mRNA by RT-PCR in human ECFCs. Day-5 cells from three donors were cultured in control (C) medium or with 1,000 U/mL rhIFNγ (γ) for 3 days and total RNA was prepared followed by RT-PCR for FasL. The PCR product corresponding to FasL (346 bp) was purified after electrophoresis and verified by sequencing (marker, ◊x174 DNA-Hae III digest).
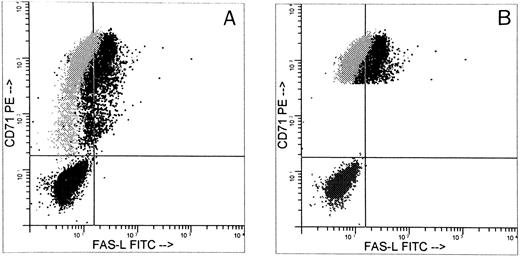
Approximately 50% of the gated CD71+ cells expressed FasL by two-color flow cytometric analysis (Fig6). Figure 6 compares FasL expression on all day-5 CD71+cells (Fig 6A) and high-intensity CD71+ cells (Fig 6B). All CD71+ cells were 47% FasL+, whereas CD71+ bright cells were 64% FasL+. Plasma clot assay showed that at least 65% ± 6% of the cells were ECFCs. This indicated that most of the FasL+ cells were ECFCs. FasL was constitutively expressed in the erythroid progenitors from day 5 to day 8 (Table 1) and was not altered in day-8 cells after 72 hours of incubation with 1,000 U/mL of rhIFNγ (data not shown). When nonpermeabilized cells were used in these experiments, FasL was present on the cell surface in 13% ± 16% of the ECFCs. Day-8 cell culture media from five separate experiments showed an increase in soluble FasL of 83 ± 41 pg/mL compared with undetectable levels in day-8 media incubated without cells. This concentration of soluble FasL is significantly above that of the normal serum level of 58 ± 35 pg/mL (P < .05).
FasL expression in day-5 cells. Day-5 cells were analyzed for FasL by indirect immunofluorescence using a specific murine antihuman FasL MoAb or an isotype control added after permeabilization and were stained with FITC-goat antimouse IgG1. After blocking with mouse IgG in PBS, the cells were incubated with PE-MoAb to human CD71 to identify ECFCs. The histogram shown represents three separate staining procedures with superimposed data: (1) population in lower left quadrant represents fluorescence produced by presence of indirect isotype control plus FITC-goat antimouse IgG1 and murine PE-IgG1; (2) gray represents the fluorescence produced by the presence of indirect isotype control plus FITC-goat antimouse IgG1 and specific PE-MoAb to human CD71; (3) dark black represents fluorescence produced by indirect MoAb to FasL stained with FITC-goat antimouse IgG1 and PE-MoAb to CD71. (A) CD71+ large cell population, representing 84% of all cells; 99% of the cells were CD71+ and 47% were FasL+. (B) Large cells bearing high intensity CD71+ from the same experiment, representing 79% of all cells. Among these cells, 100% were CD71+ and 64% were FasL+. The purity of the ECFCs was 65% ± 6%.
FasL expression in day-5 cells. Day-5 cells were analyzed for FasL by indirect immunofluorescence using a specific murine antihuman FasL MoAb or an isotype control added after permeabilization and were stained with FITC-goat antimouse IgG1. After blocking with mouse IgG in PBS, the cells were incubated with PE-MoAb to human CD71 to identify ECFCs. The histogram shown represents three separate staining procedures with superimposed data: (1) population in lower left quadrant represents fluorescence produced by presence of indirect isotype control plus FITC-goat antimouse IgG1 and murine PE-IgG1; (2) gray represents the fluorescence produced by the presence of indirect isotype control plus FITC-goat antimouse IgG1 and specific PE-MoAb to human CD71; (3) dark black represents fluorescence produced by indirect MoAb to FasL stained with FITC-goat antimouse IgG1 and PE-MoAb to CD71. (A) CD71+ large cell population, representing 84% of all cells; 99% of the cells were CD71+ and 47% were FasL+. (B) Large cells bearing high intensity CD71+ from the same experiment, representing 79% of all cells. Among these cells, 100% were CD71+ and 64% were FasL+. The purity of the ECFCs was 65% ± 6%.
FasL Expression in ECFCs
| Experiment No. . | FasL+Cells (%) . | ECFCs/100 Cells . | ||
|---|---|---|---|---|
| Day 5 . | Day 8 . | Day 5 . | Day 8 . | |
| 1 | 58 | 56 | 62 ± 5 | 86 ± 7 |
| 2 | 61 | 94 | 66 ± 4 | 90 ± 6 |
| 3 | 99 | 69 | 67 ± 7 | 91 ± 10 |
| 4 | 47 | 50 | 65 ± 6 | 93 ± 7 |
| Mean ± SD | 66 ± 22 | 67 ± 19 | 65 ± 4 | 90 ± 3 |
| Experiment No. . | FasL+Cells (%) . | ECFCs/100 Cells . | ||
|---|---|---|---|---|
| Day 5 . | Day 8 . | Day 5 . | Day 8 . | |
| 1 | 58 | 56 | 62 ± 5 | 86 ± 7 |
| 2 | 61 | 94 | 66 ± 4 | 90 ± 6 |
| 3 | 99 | 69 | 67 ± 7 | 91 ± 10 |
| 4 | 47 | 50 | 65 ± 6 | 93 ± 7 |
| Mean ± SD | 66 ± 22 | 67 ± 19 | 65 ± 4 | 90 ± 3 |
Day-5 and derivative day-8 cells incubated without rhIFNγ were analyzed for FasL and CD71 by flow cytometry after permeabilization (experiments no. 2 through 4). In experiment no. 1, only FasL was analyzed. Aliquots of day-5 and day-8 cells were cultured in plasma clots to obtain the ECFC number among the total cells.
Demonstration of functional Fas and FasL.
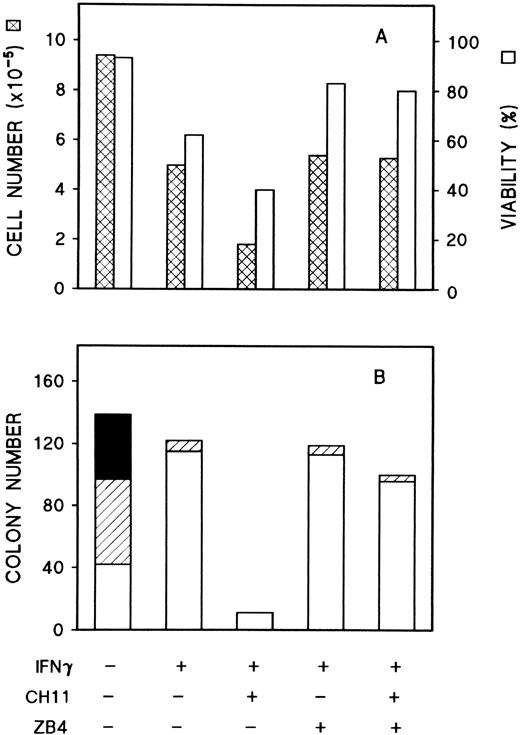
To determine whether IFNγ inhibition of cell growth was produced by the interaction between Fas (induced by IFNγ) and FasL (already present in the cells), the results of ligation of murine antihuman Fas MoAb to ECFCs was studied. Two specific MoAbs to Fas (CH-11 [IgM], which is known to mimic the action of FasL, and ZB-4 [IgG], which blocks the effect of CH-11) were tested.25 Dose-response curves for CH-11 and ZB-4 to determine the optimum concentrations for our experiments were performed and these were compared with the isotype-matched murine Igs to ensure that the effects of the antibody were specific and not related to IgM or IgG addition. When CH-11 or ZB-4 were added to day-5 ECFCs, no effect on colony size, colony number, or hemoglobin concentration was apparent (data not shown). Day-5 ECFCs were then cultured in liquid medium in the presence of a concentration of rhIFNγ known to result in a large increase of Fas, and approximately 50% inhibition of the cell number plus an additional 40% inhibition of viability were noted (Fig 7A). The addition of anti-Fas MoAb, CH-11, to the rhIFNγ-treated ECFCs greatly potentiated the reduction of cell number by rhIFNγ, whereas ZB-4 had little effect. When CH-11 and ZB-4 were added together, the effect of CH-11 was blocked by ZB-4. Fas MoAb-mediated inhibition of colony formation was also observed (Fig7B). CH-11 strongly increased the rhIFNγ inhibitory effect on colony size and number. More than 90% of erythroid colonies were suppressed with the addition of CH-11, even when the concentration of rhIFNγ was quite low (50 U/mL) and ZB-4 neutralized the CH-11 reduction of colony number as well. These data clearly indicated that Fas induced by IFNγ was functional and could be activated.
Effects of anti-Fas MoAbs, CH-11 and ZB4, on IFNγ inhibition of cell growth and erythroid colony formation by day-5 cells. Replicate aliquots of day-5 cells were cultured in liquid medium in the presence of CH-11 (100 ng/mL) and/or ZB4 (500 ng/mL) with or without 50 U/mL rhIFNγ (A). After incubation for 96 hours, the cells were harvested and trypan blue stains were performed. Aliquots of day-5 cells from the same experiment were plated in 0.2-mL plasma clots at 200 cells/well with same concentrations of rhIFNγ and antibodies (B). At day 15, the clots were fixed and stained. (▪) Large colonies, containing more than 500 cells per colony; (▨) medium colonies, containing 50 to 500 cells per colony; (□) small colonies, containing 2 to 49 cells per colony.
Effects of anti-Fas MoAbs, CH-11 and ZB4, on IFNγ inhibition of cell growth and erythroid colony formation by day-5 cells. Replicate aliquots of day-5 cells were cultured in liquid medium in the presence of CH-11 (100 ng/mL) and/or ZB4 (500 ng/mL) with or without 50 U/mL rhIFNγ (A). After incubation for 96 hours, the cells were harvested and trypan blue stains were performed. Aliquots of day-5 cells from the same experiment were plated in 0.2-mL plasma clots at 200 cells/well with same concentrations of rhIFNγ and antibodies (B). At day 15, the clots were fixed and stained. (▪) Large colonies, containing more than 500 cells per colony; (▨) medium colonies, containing 50 to 500 cells per colony; (□) small colonies, containing 2 to 49 cells per colony.
NOK-2, a specific neutralizing MoAb to human FasL,26 was used to confirm the functional activity of FasL in these cells. The results from two experiments are shown in Table 2. In the presence of rhIFNγ at 250 U/mL, the first experiment showed that more than two thirds of the colonies had morphologic apoptotic changes and less than one third looked normal. Erythroid colony size and colony number were greatly reduced. The addition of NOK-2 almost completely restored the number of colonies, although colony size remained small. Apoptosis was not evident in the presence of NOK-2. In the second experiment, the donor cells were more sensitive to rhIFNγ and normal colony formation was almost completely inhibited with rhIFNγ. More than 70% of the colony-forming cells were rescued and had normal morphologic characteristics in the presence of NOK-2. These experimental results were confirmed by similar findings with the addition of Fas-Fc,20 although the latter was not quite as potent (Table 2).
Effect of NOK-2 and Fas-Fc on rhIFNγ-Induced ECFC Inhibition
| . | Normal Colonies* . | Apoptotic Colonies† . | ||
|---|---|---|---|---|
| Large . | Medium . | Small . | ||
| Experiment no. 1 | ||||
| Control | 33 ± 2 | 20 ± 3 | 14 ± 2 | 0 |
| rhIFNγ | 16 ± 2 | 40 ± 2 | ||
| rhIFNγ + NOK-2 | 2 ± 1 | 33 ± 3 | 28 ± 3 | 0 |
| rhIFNγ + Fas-Fc | 14 ± 2 | 43 ± 2 | 7 ± 1 | |
| Experiment no. 2 | ||||
| Control | 20 ± 5 | 25 ± 7 | 24 ± 2 | 0 |
| rhIFNγ | 51 ± 4 | |||
| rhIFNγ + NOK-2 | 6 ± 3 | 42 ± 4 | 13 ± 2 | |
| rhIFNγ + Fas-Fc | 2 ± 1 | 27 ± 2 | 28 ± 2 | |
| . | Normal Colonies* . | Apoptotic Colonies† . | ||
|---|---|---|---|---|
| Large . | Medium . | Small . | ||
| Experiment no. 1 | ||||
| Control | 33 ± 2 | 20 ± 3 | 14 ± 2 | 0 |
| rhIFNγ | 16 ± 2 | 40 ± 2 | ||
| rhIFNγ + NOK-2 | 2 ± 1 | 33 ± 3 | 28 ± 3 | 0 |
| rhIFNγ + Fas-Fc | 14 ± 2 | 43 ± 2 | 7 ± 1 | |
| Experiment no. 2 | ||||
| Control | 20 ± 5 | 25 ± 7 | 24 ± 2 | 0 |
| rhIFNγ | 51 ± 4 | |||
| rhIFNγ + NOK-2 | 6 ± 3 | 42 ± 4 | 13 ± 2 | |
| rhIFNγ + Fas-Fc | 2 ± 1 | 27 ± 2 | 28 ± 2 | |
Day-5 cells were cultured in plasma clots with or without rhIFNγ at 250 U/mL and with or without neutralizing anti-FasL MoAb, NOK-2, or Fas-Fc. After incubation for 10 days, the clots were fixed and stained. NOK-2 (20 μg/mL) was added at the start of incubation and 2.5 μg/mL was added at 24 hours and 48 hours of incubation. Fas-Fc (10 μg/mL) was added at the start of incubation and 5 μg/mL was added at 24 hours of incubation. Equal volumes of media were added to the controls.
Erythroid colony number per 100 cells plated; large colonies, greater than 500 cells per colony; medium colonies, 50 to 500 cells per colony; small colonies, 2 to 49 cells per colony.
Colonies with more than 10% apoptotic cells.
DISCUSSION
The inhibitory effect of IFNγ on the ECFCs was observed both in liquid and plasma clot cultures and inhibition of cell proliferation occurred at a concentration of rhIFNγ as low as 50 U/mL. Evidence that this inhibition is a direct effect of rhIFNγ is provided by the observations that the degree of inhibition did not vary significantly as the ECFCs were purified from 19.8% to 52.6%,27 that ECFCs with a purity of 80% were still inhibited,8 and that ECFCs have rhIFNγ receptors.28 This finding is consistent with past work demonstrating the production of DNA fragmentation in day-6 ECFCs by in situ end-labeling and flow cytometry.8
The Fas/FasL system is a very important cellular pathway responsible for the initiation of apoptosis. Fas is a 45-kD membrane glycoprotein belonging to the tumor necrosis factor (TNF) receptor family. Fas contains a 70 amino acid cytoplasmic domain that is necessary and sufficient for transduction of the apoptotic signal. Fas mRNA is widely expressed in lymphocytes, thymocytes, monocytes, epithelial and endothelial cells, eosinophils, leukemia cells, and many tissues, such as bone marrow, heart, kidney, liver, and ovary.17,22,25,26,29-33 FasL is a 40-kD, type II protein member of the TNF family.23,34 In contrast to Fas, FasL was thought to be relatively restricted in its cell and tissue distribution. It was initially found to be expressed in activated T cells and has a major role in T-cell cytoxicity.23 However, FasL expression in other tissues and cells, such as stroma cells of the eye, Sertoli cells of the testis, thyrocytes, and large granular lymphocytic leukemia cells, as well as in various nonlymphoid carcinoma cells, including colon plus hepatocellular carcinomas and melanoma cells has been recently reported.35-41 This work indicated that expression of FasL was important for maintaining immune privilege and that it appears to have a role in enabling tumor cells to escape from immune attack.
To determine if Fas/FasL expression correlated with IFNγ induction of ECFC apoptosis, we studied the presence of both of these molecules in highly purified ECFCs in the presence or absence of IFNγ. Northern and flow cytometric analyses showed that only a small percentage of normal human ECFCs expressed Fas and that this was at a very low level. Addition of rhIFNγ potently induced Fas expression. Fas mRNA clearly increased by 6 hours after the incubation of ECFCs with IFNγ and at a concentration of IFNγ as low as 50 U/mL. Fas protein on the ECFCs was clearly enhanced by 24 hours after incubation with IFNγ and most of the ECFCs expressed Fas antigen by 48 hours. The amount of Fas on the ECFC surface gradually increased to a maximum after 72 hours of incubation in the presence of IFNγ when the inhibitory effect of IFNγ on the ECFCs clearly appeared. Further experiments showed activation of Fas by the anti-Fas MoAb, CH-11, which is known to functionally mimic FasL. Ligation of Fas with this antibody markedly reduced ECFC viability, the generation of ECFCs in liquid culture and erythroid colony formation, while the anti-Fas MoAb ZB4 corrected the CH-11 inhibition, indicating that Fas expression was not only associated with functional inhibition of the ECFCs, but also, when the Fas system for producing apoptosis was activated, it produced the inhibition.
Nevertheless, CH-11 is an artifactual MoAb added in culture. If apoptosis is mediated via Fas, interaction with an agonistic ligand must occur. Our search for this ligand using flow cytometric analysis showed that approximately 50% of the large CD71+ cells, which were 80% of the total cells, expressed FasL. Because not all the cells in the population were ECFCs, especially at day 5, and because erythroid cells have 15-fold more CD71 than other cells, we performed flow cytometric analysis with the cells gated to include only the high-intensity, CD71+ ECFC. Our results showed that approximately 66% of the large size, high-intensity, CD71+erythroid cells were FasL+ and that FasL was constitutively expressed on ECFCs from day 5 to day 8. Because day-8 cells were almost homogenous, with 90% or more of the day 8 cells proven to be ECFCs by plasma clot assay, most of the FasL+ cells in our cultures are ECFCs and rhIFNγ did not appear to alter FasL expression.
Nok-2, an anti-FasL MoAb, has the biological property of neutralizing FasL.26 When this MoAb was added to the ECFC cultures, the induction of apoptosis and the inhibition of ECFC proliferation and colony formation by IFNγ were partially prevented, indicating that FasL is functional as an actuator of apoptosis in the system. Comparable results were observed with Fas-Fc. Our observations thus suggest a mechanism responsible for at least part of the inhibitory effect of IFNγ on human ECFCs. Because normal ECFCs express negligible amounts of Fas, they do not undergo apoptosis. Simultaneous expression of functional Fas and FasL on ECFCs, the former induced by IFNγ, results in programmed cell death.
Similar findings in Hashimoto's thyroiditis have now been reported.41 FasL is constitutely expressed in normal thyroid cells, but only the cells of Hashimoto's thyroiditis express large amounts of Fas on their cell surface, which is thought to be induced by IL-1β. Coexpression of Fas and FasL also has been thought to contribute to the rapid rate of spontaneous neutrophil apoptosis.42
Because Fas and FasL were coexpressed in ECFCs after incubation with IFNγ, they may commit the cells to an autocrine death as shown with T-cell hybridoma cells20 and/or soluble FasL may function in a paracrine pathway to mediate cell death.43,44Further studies are now in progress to delineate the precise mechanism of this interaction. The mechanism by which IFNγ exerts an inhibitory effect on the cell growth of ECFCs and induces apoptosis may be multifactorial. Our laboratory has found that IFNγ downregulates stem cell factor and EP receptors as well as the mRNA for these receptors.45 Nevertheless, the present study shows that the Fas/FasL system strongly contributes to the inhibition of erythropoiesis by IFNγ.
Supported by a Veterans Health Administration Merit Review Grant and by Grants No. DK-15555, GM-52735, and 5 T32-DK07186 from the National Institutes of Health.
Address reprint requests to Sanford B. Krantz, MD, Department of Medicine/Hematology, Vanderbilt University Medical School MRB II Room 547, Nashville, TN 37232-6305.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal