Abstract
Bcl-2, Bcl-xL, and Mcl-1 are three related intracellular polypeptides that have been implicated as negative regulators of apoptosis. In contrast, the partner protein Bax acts as a positive regulator of apoptosis. Based on the observation that all four of these polypeptides are expressed in a variety of acute myelogenous leukemia (AML) and acute lymphocytic leukemia (ALL) cell lines, cellular levels of these polypeptides were examined by immunoblotting in bone marrow samples harvested from 123 adult AML patients and 36 adult ALL patients before initial antileukemic therapy. Levels of Bcl-2, Mcl-1, Bcl-xL, and Bax each varied over a more than 10-fold range in different pretreatment leukemia specimens. When the 54 AML and 23 ALL samples that contained greater than 80% malignant cells were examined in greater detail, it was observed that pretreatment levels of Bcl-2 and Mcl-1 correlated with each other (R = .44,P < .001 for AML and R = .79,P < .0001 for ALL). In addition, a weak negative correlation between Bax expression and age was observed in AML samples (R = −0.35, P < .02) but not ALL samples. There was no relationship between pretreatment levels of these polypeptides and response to initial therapy. However, examination of 19 paired samples (the first harvested before chemotherapy and the second harvested 23 to 290 days later at the time of leukemic recurrence) revealed a greater than or equal to twofold increase in Mcl-1 levels in 10 of 19 pairs (7 of 15 AML and 3 of 4 ALL) at recurrence. In contrast, 2 of 19 pairs contained twofold less Mcl-1 at the time of recurrence. Approximately equal numbers of samples showed twofold increases and decreases in Bcl-2 (5 increases, 3 decreases) and Bcl-xL (1 increase, 4 decreases) at recurrence. Bax levels did not show a twofold decrease in any patient. These results, coupled with recent observations that cells overexpressing Mcl-1 are resistant to a variety of chemotherapeutic agents, raise the possibility that some chemotherapeutic regimens might select for leukemia cells with elevated levels of this particular apoptosis inhibitor.
INVESTIGATIONS PERFORMED over the past decade indicate that chemotherapeutic agents induce apoptotic cell death in human leukemia cells in vitro.1-5 More recently, apoptosis has been demonstrated in circulating blasts from leukemia patients after therapy in vivo.6,7 Additional experiments have suggested that failure to activate the apoptotic machinery is accompanied by resistance to the cytotoxic effects of multiple chemotherapeutic agents.8-13 These observations raise the possibility that factors regulating the apoptotic process might play a role in drug resistance in the clinical setting.
Members of the Bcl-2 polypeptide family are widely studied regulators of apoptosis.14-18 The prototypic member, Bcl-2, was originally cloned as a consequence of its involvement in the 14;18 translocations that occur in follicular lymphoma.19Subsequent studies revealed that this gene encodes a 26-kD polypeptide that traffics to the outer mitochondrial membrane,20,21 the outer nuclear membrane and endoplasmic reticulum.21Although the exact function of this polypeptide remains unsettled,16,18 overexpression of Bcl-2 has been associated with resistance to a number of treatments that induce apoptosis in vitro, including various chemotherapeutic agents and γ-irradiation.5,16,17,22 Conversely, diminished Bcl-2 expression increases sensitivity to these same treatments.23 24
The expression of Bcl-2 in various hematologic malignancies has been extensively investigated.22 In addition to its well documented expression in many non-Hodgkin's lymphomas and chronic lymphocytic leukemia,25-27 Bcl-2 has been widely detected in specimens of acute leukemia. Bcl-2 is abundant in the blasts from >80% of acute lymphocytic leukemia (ALL) patients28-30but does not appear to affect prognosis in this disease.29,30 Bcl-2 expression has likewise been detected in up to 90% of acute myelogenous leukemia (AML) specimens obtained at diagnosis.31-34 Sensitive flow cytometry techniques can detect Bcl-2 in up to 90% of blasts in each AML sample; but mean Bcl-2 levels can vary as much as 40-fold between different AML samples.34 The relationship between these varied Bcl-2 levels and clinical response to antileukemic therapy has been controversial, with one group reporting a lower remission rate when a higher percentage of blasts expressed detectable Bcl-2,32 a second group reporting a relationship between remission rate and Bcl-2 intensity rather than percentage of blasts expressing Bcl-2,35 and a third group reporting that mean Bcl-2 staining intensity affected remission duration but not remission rate.34 An alternative approach to determining the significance of Bcl-2 expression levels would be the examination of serial samples obtained from individual patients before treatment and at the time of relapse. If elevated levels of Bcl-2 were responsible for resistance of leukemia cells to chemotherapy, then one might predict that elevated expression of Bcl-2 would be observed at the time of relapse in a substantial fraction of patients. In the single study that used this approach, Banker et al observed that Bcl-2 levels at relapse were unchanged from those obtained at diagnosis.36
A number of polypeptides that are structurally and functionally related to Bcl-2 have been identified,14-16,22,37 including Bcl-xL and Mcl-1. The Bcl-x gene, which was originally detected by screening a chicken lymphoid cDNA library with a Bcl-2 probe at low stringency,38 encodes two transcripts, the longer of which yields a 28-kD polypeptide (Bcl-xL) that also localizes to mitochondrial membranes39 and inhibits apoptosis.38,40 Recent studies indicate that Bcl-xL overexpression renders cells resistant to a variety of treatments, including etoposide, doxorubicin, cisplatin, vincristine, bleomycin, paclitaxel and ionizing radiation.40-45 The Mcl-1 gene, which was cloned based on its enhanced expression in phorbol ester-treated ML-1 human myeloid leukemia cells, encodes a 36-kD polypeptide with a carboxyl terminal domain that is 35% identical to Bcl-2.46 In contrast to Bcl-2, which is a relatively long-lived polypeptide, Mcl-1 contains two PEST sequences46 and is thought to have a short half-life. Overexpression studies have revealed that Mcl-1 delays apoptosis,47 including apoptosis induced by etoposide in myeloid leukemia cells.48
The antiapoptotic effects of Bcl-2, Bcl-xL, and Mcl-1 are opposed by a number of proapoptotic Bcl-2 family members.14-18,22,37 The most widely studied is Bax, a 22-kD polypeptide that has the ability to form heterodimers with Bcl-2, Bcl-xL, and Mcl-1.49-51 The mechanism by which Bax induces apoptosis is currently uncertain. Some evidence suggests that Bax can antagonize the anti-apoptotic effects of Bcl-xL even under conditions in which heterodimers cannot form52; and additional observations suggest that Bax might form ion channels in outer mitochondrial membranes.18
In view of this complexity, it might be important to examine multiple Bcl-2 family members in addition to Bcl-2 in order to understand the impact of these apoptotic regulators on the response to antileukemic therapy. Examination of Bcl-xL and Bax polypeptides in acute leukemia samples has been limited53; and there have been no previous reports of Mcl-1 levels in clinical leukemia specimens. Accordingly, we examined the expression of Bcl-2, Mcl-1, Bcl-xL, and Bax in pretreatment AML and ALL bone marrow samples and in a series of paired acute leukemia specimens obtained at diagnosis and at recurrence. Particular emphasis was placed on relating levels of these polypeptides with response to therapy.
MATERIALS AND METHODS
Antibodies and tissue culture cell lines.
Rabbit antipeptide antisera that specifically recognize human Bcl-2, Mcl-1, and Bcl-xL were generated and their specificity for the intended antigens was documented as previously described.54 55 The peptides corresponded to nonconserved regions in the human Bcl-2 (amino acids 41-54), Mcl-1 (121-139), and Bcl-xL (46-66) polypeptides. A rabbit polyclonal antiserum against Bax was purchased from Santa Cruz Biologicals (Santa Cruz, CA). A murine monoclonal IgM recognizing histone H1 was kindly provided by James Sorace (Veteran's Affairs Medical Center, Baltimore, MD).
Human leukemia cell lines were propagated at <1 × 106cells/mL in medium consisting of RPMI 1640, 5% (vol/vol) fetal bovine serum, 100 U/mL penicillin G, 100 μg/mL streptomycin, and 2 mmol/L glutamine. Myeloid (HL-60, KG1a, K562, ML-1) and lymphoid (Molt3, CEM, Raji, Jurkat) lines used in these studies were kindly provided by Richard J. Jones and Michael B. Kastan (Johns Hopkins Oncology Center). CEM/Bcl-2 cells were transfected with Bcl-2 as previously described.56
Buffers.
Buffer A consisted of RPMI 1640 medium supplemented with 10 mmol/L HEPES (pH 7.4 at 21°C). Alkylation buffer contained 6 mol/L guanidine hydrochloride, 250 mmol/L Tris-HCl (pH 8.5 at 21°C), and 10 mmol/L EDTA. Immediately before use, each aliquot of alkylation buffer was supplemented with 1% (vol/vol) β-mercaptoethanol and 1 mmol/L α-phenylmethylsulfonyl fluoride added from a 100 mmol/L stock prepared in anhydrous isopropanol. Sodium dodecyl sulfate (SDS) sample buffer consisted of 4 mol/L deionized urea, 2% (wt/vol) SDS, 62.5 mmol/L Tris-HCl (pH 6.8), and 1 mmol/L EDTA. Blocking solution contained 10% (wt/vol) powdered dry milk, 150 mmol/L NaCl, and 10 mmol/L Tris-HCl (pH 7.4 at 21°C), 100 U/mL penicillin G, 100 μg/mL streptomycin, and 1 mmol/L sodium azide.
Sample preparation.
Between September 1987, and December 1992, patients with newly diagnosed AML or ALL admitted to the Adult Leukemia Service of the Johns Hopkins Hospital were treated on two AML protocols and one ALL protocol approved by the Joint Committee on Clinical Investigation of the Johns Hopkins Medical Institutions in accordance with the policies of the US Department of Health and Human Services. These protocols have been recently described in detail57,58 and are summarized below. In conjunction with those protocols, marrow samples were harvested and prospectively prepared for subsequent SDS-polyacrylamide gel electrophoresis (PAGE). In brief, heparinized bone marrow aspirates were obtained from the posterior iliac crests of these patients before the initiation of chemotherapy. To isolate fractions enriched in blasts, marrows were sedimented on ficoll-Hypaque step gradients (d = 1.079 and 1.119 g/mL) as described.57Cells collected from the upper interface were diluted with buffer A, sedimented at 200g for 10 minutes, and resuspended in buffer A. Aliquots were removed for counting and to prepare Wright's stained cytospins for morphologic examination. Samples were then sedimented at 200g for 10 minutes and immediately solubilized in alkylation buffer.
Western blotting and polypeptide quantitation.
In preparation for SDS-PAGE, samples were sonicated to shear the viscous DNA, treated with iodoacetamide to block free sulfhydryl groups, and dialyzed at 4°C into 4 mol/L urea and then into 0.1% (wt/vol) SDS. Aliquots were lyophilized to dryness, stored at −20°C, and solubilized immediately before electrophoresis by addition of SDS sample buffer to a final dilution of 3 × 107 cell equivalents/mL followed by heating to 65°C for 20 minutes. Aliquots containing 3 × 105 cells were applied to SDS-PAGE, with paired samples being applied to adjacent wells. To provide a standard curve, aliquots containing 0.3 × 105, 0.75 × 105, and 1.5 × 105and 3.0 × 105 HL-60 cells or Molt3 cells were also applied to each gel containing AML or ALL specimens, respectively. After electrophoresis, samples were electrophoretically transferred to nitrocellulose and stained with 0.1% (wt/vol) Fast Green FCF in 50% (vol/vol) methanol-5% (vol/vol) acetic acid to confirm efficient protein transfer. To block nonspecific protein binding sites, blots were treated with blocking solution for ≥6 hours at 21°C. Blots were then reacted overnight with dilutions of anti–Bcl-2, Mcl-1, Bcl-xL, or Bax in fresh blocking buffer; washed; and reacted with peroxidase-coupled goat antirabbit IgG using techniques previously described in detail.59 Binding of the secondary antibody was detected by enhanced chemiluminescence using an ECL kit from Amersham (Arlington Heights, IL). Signals on the resulting x-ray film were scanned on a Kodak UMax Supervista S-12 scanner, quantified (area × intensity) using NIH Image version 1.61 software, and compared with signals resulting from the serial dilution of HL-60 or Molt3 cells on the same blot. To correct for differences in loading and ploidy, blots were reprobed with antibody to histone H1, a polypeptide that is present in constant amounts in each diploid cell. For each pair of samples, the apoptotic polypeptide:histone H1 ratio was determined at diagnosis and again at relapse.
Patient treatment.
Patients with newly diagnosed AML were treated on protocol 8410 or protocol 8902.57 Protocol 8410 involved induction therapy with cytarabine (667 mg/m2/d by continuous infusion [CI], days 1-3), daunorubicin (DNR, 45 mg/m2/d, days 1-3), and amsacrine (200 mg/m2/d, days 8-10). Patients who achieved a complete response (CR) received consolidation therapy on day 60 ± 7, which consisted of cytarabine (2 g/m2/d CI, days 1-3 if age < 55; 667 mg/m2/d CI, days 1-3 if age > 55), DNR (45 mg/m2/d, days 1-3), and more cytarabine (667 mg/m2/d, days 10-12). Patients treated on protocol 8914 received two cycles of therapy that consisted of granulocyte-macrophage colony-stimulating factor (GM-CSF, 5 μg/kg/d CI, days 1-13) along with cytarabine (667 mg/m2/d CI, days 4-6), DNR (45 mg/m2/d, days 4-6), and etoposide (400 mg/m2/d, days 11-13). Patients were then followed without further therapy until relapse.
Patients with ALL received induction therapy on protocol 8802, which consisted of two treatment modules.58 The first consisted of prednisone (60 mg/m2/d orally [po], days 1-21), vincristine (1.4 mg/m2 intravenously [IV], days 1, 8, and 15), etoposide (400 mg/m2/d IV, days 1-3), and L-asparaginase (10,000 U/m2/d, days 10-14). The second module consisted of cytarabine (2 g/m2/d CI, days 22-24) and DNR (45 mg/m2/d, days 22-24 and 30-32). Patients who had evidence of monoclonal lymphoid cells in marrow aspirates on days 60 to 80 after the start of therapy as assessed by Southern blotting of immunoglobulin and T-cell receptor genes received a second course of cytarabine and daunorubicin in the same fashion before the third phase of therapy, which consisted of bone marrow transplantation or maintenance therapy.58
Patients who died before day 10 with leukemia or before day 45 with no evidence of leukemia were considered unevaluable (UE). Patients who had regrowth of leukemia or who died beyond day 45 with persistent aplasia were considered to have no response (NR). Patients who had <5% marrow blasts and reconstitution of normal hematopoiesis that was sustained for at least 30 days beyond discharge were considered to have a CR.
RESULTS
Expression of Bcl-2, Mcl-1, Bcl-xL, and Bax in human leukemia cell lines.
The present study was undertaken to evaluate expression of Bcl-2, Mcl-1, Bcl-xL, and Bax in AML and ALL specimens from cohorts of patients who received relatively uniform chemotherapy at a single institution between 1987 and 1992. Because of concern that transcript levels might not correlate with levels of the corresponding polypeptides,31 60-62 expression was examined by immunoblotting in all samples.
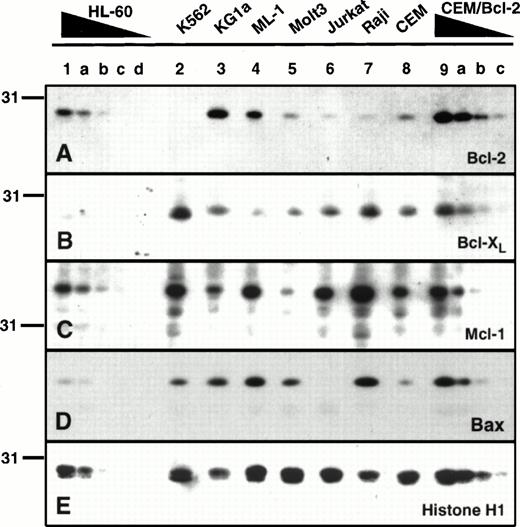
Before studying the clinical specimens, a series of human leukemia cell lines was examined. As indicated in the introduction, Bcl-2 and Bcl-xL were originally described in lymphoid cells; whereas Mcl-1 was initially described in ML-1 myeloid leukemia cells. Examination of a series of human leukemia cell lines revealed that these polypeptides were not limited to the cell types in which they were initially described (Fig 1). For example, Bcl-2 levels were higher in three of the four myeloid lines than in any of the lymphoid lines (Fig 1A). Only K562 cells, which display diminished or delayed apoptosis as a consequence ofbcr/abl expression,63 64 contained undetectable levels of Bcl-2 (Fig 1A, lane 2). In a similar fashion, Bcl-xL and Mcl-1 were not limited to lymphoid or myeloid leukemia lines, respectively, but were instead detected in all eight cell lines (Fig 1B and C). Likewise, Bax was detectable in seven of the eight cell lines examined (Fig 1D). Interestingly, Bcl-2 overexpression in the CEM/Bcl-2 line was associated with elevated levels of the Bax polypeptide (lanes 8 and 9a, Fig 1D).
Expression of Bcl-2, Mcl-1 Bcl-xL and Bax in human leukemia cell lines. Aliquots containing 3 × 105cells from the leukemia cell line (lanes 1 through 9) were subjected to SDS-PAGE followed by blotting with antisera that recognize Bcl-2 (A), Bcl-xL (B), Mcl-1 (C), or Bax (D). As a control for loading and transfer of these samples, the same blots were probed with antibodies to histone H1 (E), a polypeptide that is present in constant amounts in all diploid cells. To provide a basis for comparison of polypeptide levels, serial dilutions of HL-60 cells or CEM cells transfected with Bcl-2 were loaded on the same blots. These samples contained 1.5 × 105, 0.75 × 105, 0.3 × 105, or 0.15 × 105 cells (lanes a through d, respectively).
Expression of Bcl-2, Mcl-1 Bcl-xL and Bax in human leukemia cell lines. Aliquots containing 3 × 105cells from the leukemia cell line (lanes 1 through 9) were subjected to SDS-PAGE followed by blotting with antisera that recognize Bcl-2 (A), Bcl-xL (B), Mcl-1 (C), or Bax (D). As a control for loading and transfer of these samples, the same blots were probed with antibodies to histone H1 (E), a polypeptide that is present in constant amounts in all diploid cells. To provide a basis for comparison of polypeptide levels, serial dilutions of HL-60 cells or CEM cells transfected with Bcl-2 were loaded on the same blots. These samples contained 1.5 × 105, 0.75 × 105, 0.3 × 105, or 0.15 × 105 cells (lanes a through d, respectively).
Expression of Bcl-2, Mcl-1, Bcl-xL, and Bax in AML and ALL samples at the time of diagnosis.
These same antisera were used to examine the expression of Bcl-2, Mcl-1, Bcl-xL, and Bax in clinical specimens of acute leukemia. Samples harvested before initial chemotherapy were available from 123 adults with AML and 36 with ALL. All samples were probed with antisera to Bcl-2, Mcl-1, and Bax. Because of technical limitations (failure of the anti–Bcl-xL serum to react with polypeptides on stored blots), a more limited set of samples was successfully reacted with the anti–Bcl-xL serum.
Results obtained with one group of specimens are illustrated in Fig2. Examination of the immunoblots revealed a wide variety of expression patterns. Some specimens (eg, lanes 6-8) contained all four polypeptides at readily detectable, albeit varying, levels. Others, however, displayed markedly diminished amounts of one (lane 3) or more (lane 2) anti-apoptotic Bcl-2 family members. Bax levels also varied widely between specimens (lanes 2 and 4).
Examination of relative Bcl-2, Mcl-1, Bcl-xL, and Bax polypeptide levels in human AML samples. Samples containing 3 × 105 HL-60 cells (lane 1) or 3 × 105marrow mononuclear cells from nine AML patients (lanes 2 through 10) were subjected to SDS-PAGE followed by Western blotting with the indicated antibody.
Examination of relative Bcl-2, Mcl-1, Bcl-xL, and Bax polypeptide levels in human AML samples. Samples containing 3 × 105 HL-60 cells (lane 1) or 3 × 105marrow mononuclear cells from nine AML patients (lanes 2 through 10) were subjected to SDS-PAGE followed by Western blotting with the indicated antibody.
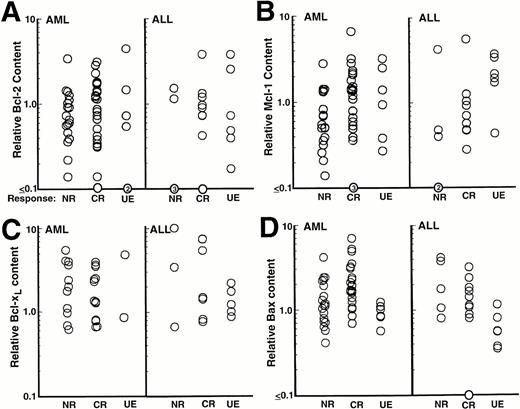
In order to quantitate these results, blots were scanned; the signal (area × intensity) of each polypeptide was determined for each sample; and results were compared with a serial dilution of HL-60 or Molt3 cells that was included on each blot of AML or ALL samples, respectively, as a positive control. To correct for variations in sample loading, the same blots were probed with antibodies to histone H1, a polypeptide that should be present in equal amounts in all diploid cells. Data were recorded as the apoptotic regulator:histone H1 ratio of the sample divided by the apoptotic regulator:histone H1 ratio of the positive control. All further analyses were confined to the 54 samples of newly diagnosed AML and 23 samples of newly diagnosed ALL that contained greater than 80% blasts.
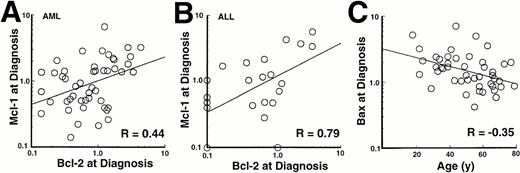
Results of this analysis revealed that levels of Bcl-2, Mcl-1, Bcl-xL, and Bax varied over as much as a 40-fold range (Fig3). When these quantitative results were analyzed, there was a modest correlation (R = .44,P = <.001) between levels of Bcl-2 and levels of Mcl-1 in the AML samples (Fig 4A) and an even stronger correlation (R = .79, P < .0001) between levels of Bcl-2 and Mcl-1 in the ALL samples (Fig 4B). Somewhat weaker correlations were also observed between Bcl-2 or Mcl-1 and Bax levels (Table 1). Interestingly, this analysis also indicated a weak negative correlation between Bax levels and age (R = −.35, P = .02) in the AML samples (Fig 4C), although this relationship was not observed in the ALL samples (R = −.15). There was no correlation between levels of these apoptotic regulators and FAB classification (AML) or immunophenotype (ALL).
Relationship between Bcl-2, Mcl-1, Bcl-xL, or Bax levels and response to initial chemotherapy in patients with AML (left) and ALL (right).
Relationship between Bcl-2, Mcl-1, Bcl-xL, or Bax levels and response to initial chemotherapy in patients with AML (left) and ALL (right).
Relationship between pretreatment Bcl-2 content and pretreatment Mcl-1 content in AML (A) and ALL (B) specimens. (C) Relationship between age and pretreatment Bax content in AML specimens.
Relationship between pretreatment Bcl-2 content and pretreatment Mcl-1 content in AML (A) and ALL (B) specimens. (C) Relationship between age and pretreatment Bax content in AML specimens.
Coefficients of Determination (R2) for Relationships Between Levels of Bcl-2 Family Members and Other Prognostic Factors
| . | AML . | |||
|---|---|---|---|---|
| Bcl-2 . | Bcl-xL . | Mcl-1 . | Bax . | |
| Bcl-xL | .001 | — | — | — |
| Mcl-1 | .19-150 | .04 | — | — |
| Bax | .18-150 | .04 | .15-150 | — |
| WBC | .006 | .08 | .0001 | .02 |
| Age | .003 | .03 | .001 | .12-151 |
| ALL | ||||
| Bcl-2 | Bcl-xL | Mcl-1 | Bax | |
| Bcl-xL | .09-152 | — | — | — |
| Mcl-1 | .62-150 | .17-152 | — | — |
| Bax | .004 | .25-153 | .02 | — |
| WBC | .02 | .07 | .03 | .007 |
| Age | .02 | .002 | .02 | .02 |
| . | AML . | |||
|---|---|---|---|---|
| Bcl-2 . | Bcl-xL . | Mcl-1 . | Bax . | |
| Bcl-xL | .001 | — | — | — |
| Mcl-1 | .19-150 | .04 | — | — |
| Bax | .18-150 | .04 | .15-150 | — |
| WBC | .006 | .08 | .0001 | .02 |
| Age | .003 | .03 | .001 | .12-151 |
| ALL | ||||
| Bcl-2 | Bcl-xL | Mcl-1 | Bax | |
| Bcl-xL | .09-152 | — | — | — |
| Mcl-1 | .62-150 | .17-152 | — | — |
| Bax | .004 | .25-153 | .02 | — |
| WBC | .02 | .07 | .03 | .007 |
| Age | .02 | .002 | .02 | .02 |
Correlation coefficients were calculated for the indicated pairs of continuous variables using data obtained from the 54 pretreatment AML samples or 23 pretreatment ALL samples that contained >80% blasts. WBC, peripheral leukocyte count at diagnosis.
P < .01.
P = .02.
Weak inverse correlations that do not reach statistical significance due to small sample size.
P = .04.
The relationship between levels of these polypeptides and response to initial chemotherapy is shown in Fig 3. There was broad overlap between the levels of Bcl-2, Mcl-1, Bcl-xL, and Bax observed in samples from patients who achieved a CR with initial therapy and those who did not, with no evidence for any relationship between pretreatment expression of these polypeptides and response to initial chemotherapy. Furthermore, when the data were expressed as Bcl-2:Bax, Mcl-1:Bax, or Bcl-xL:Bax ratios, there was still no relationship between protein expression and response to therapy (data not shown).
Elevated expression of Mcl-1 at the time of leukemic relapse.
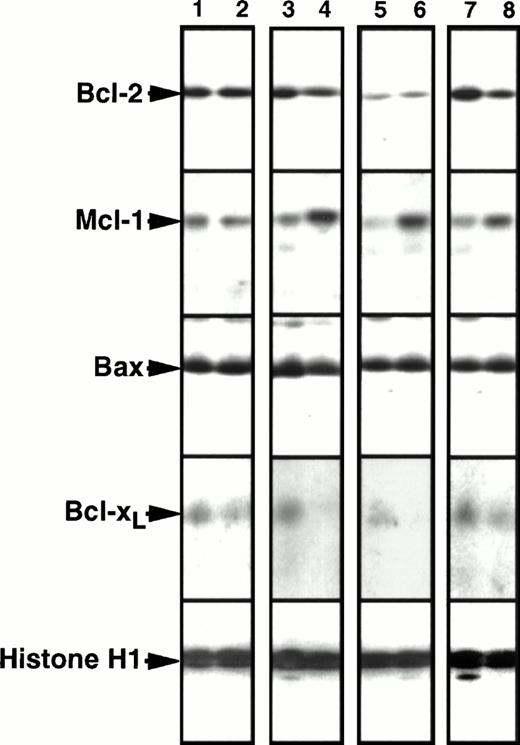
As outlined in the introduction, the analysis of serial samples can sometimes be useful for evaluating the possibility that elevated expression of a particular polypeptide might contribute to drug resistance. A recent analysis of 14 paired samples failed to demonstrate Bcl-2 elevation at relapse.36 To confirm and extend these studies, we examined the expression of Bcl-2, Mcl-1, Bcl-xL, and Bax in paired samples, the first harvested before induction chemotherapy and the second at the time of leukemic recurrence. Samples from 19 adult patients (15 AML and 4 ALL) that contained ≥80% leukemia cells at diagnosis and at recurrence were available for this analysis. Twelve of these patients achieved a CR with their initial therapy, whereas seven did not. The paired samples were obtained a median of 200 days apart (range, 23 to 290 days) and were run in adjacent wells of polyacrylamide gels. Representative immunoblots are presented in Fig 5; and results of this analysis are summarized in Fig6.
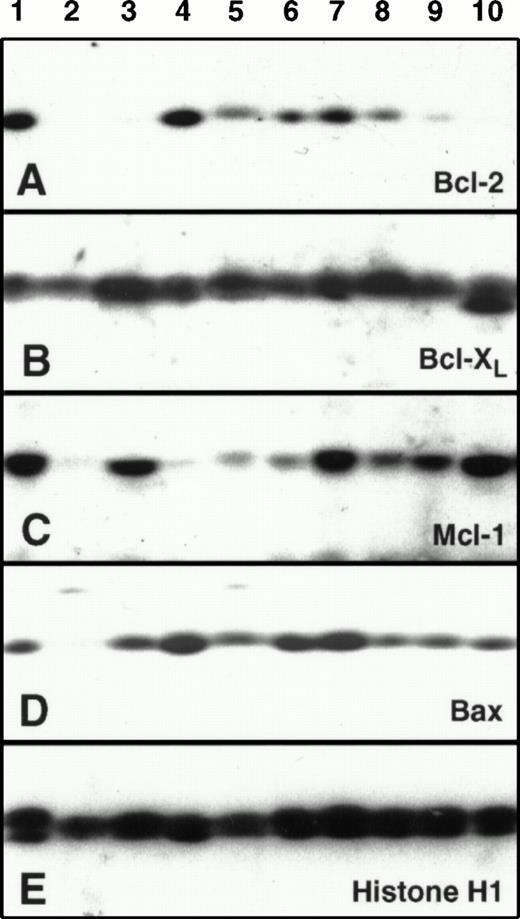
Comparison of Bcl-2, Mcl-1, Bcl-xL, and Bax levels in paired leukemia specimens harvested before chemotherapy and at the time of leukemia recurrence. Odd numbers, pretreatment specimens. Even numbers, corresponding samples at recurrence. To permit quantitation, a serial dilution of HL-60 cells was run on each blot as depicted in Fig 1. Lanes 1 and 2 contain a pair displaying no twofold change in protein expression between diagnosis and relapse. Lanes 3-8 contain pairs displaying more than twofold increases in Mcl-1 expression at relapse. In two cases (lanes 3 through 6) there was no change in Bcl-2 expression; but in one case Bcl-2 expression decreased (lanes 7 and 8).
Comparison of Bcl-2, Mcl-1, Bcl-xL, and Bax levels in paired leukemia specimens harvested before chemotherapy and at the time of leukemia recurrence. Odd numbers, pretreatment specimens. Even numbers, corresponding samples at recurrence. To permit quantitation, a serial dilution of HL-60 cells was run on each blot as depicted in Fig 1. Lanes 1 and 2 contain a pair displaying no twofold change in protein expression between diagnosis and relapse. Lanes 3-8 contain pairs displaying more than twofold increases in Mcl-1 expression at relapse. In two cases (lanes 3 through 6) there was no change in Bcl-2 expression; but in one case Bcl-2 expression decreased (lanes 7 and 8).
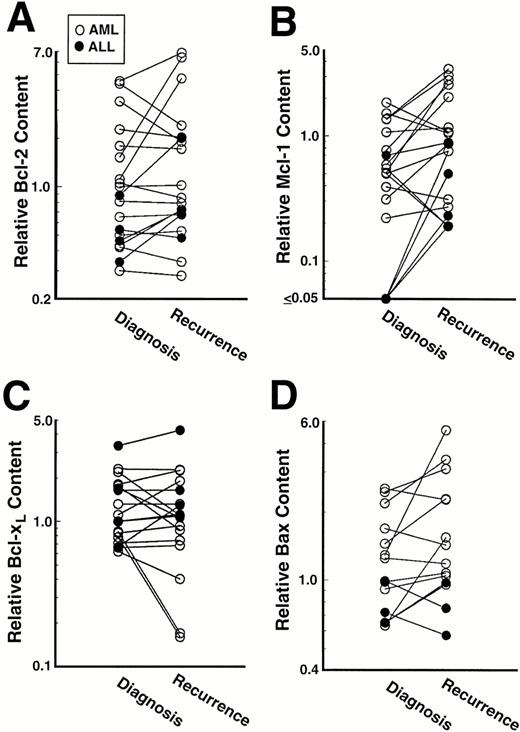
Mcl-1 levels at relapse were ≥200% of pretreatment levels in 10 of 19 pairs, including 7 of 15 AML pairs and 3 of 4 ALL pairs (Fig 6B). Results obtained in three of these 10 pairs are shown in Fig 5, lanes 3 to 8. The largest quantifiable Mcl-1 increase was fivefold. Of the 10 patients whose samples demonstrated this increase in Mcl-1, eight achieved a CR with their initial therapy and then relapsed after a median of 211 days (range 93-245). At the time of recurrence, these 10 patients responded poorly to further therapy (2 of 9 achieved a CR), although the number of patients studied was small and the post-relapse therapy varied. In contrast to these 10 patients, specimens from only two of the 19 patients displayed a ≥twofold decrease in Mcl-1 levels at relapse (Fig 6B).
Bcl-2 levels at relapse were also ≥200% of pretherapy levels in 5 of 19 pairs, the largest increase being fourfold (Fig 6A). Increases at relapse were observed in 2 of 15 AML pairs and 3 of 4 ALL pairs. In contrast, three pairs of AML samples displayed ≥twofold decreases in Bcl-2 levels (Fig 6A) as illustrated in Fig 5, lanes 7 and 8.
In contrast to Mcl-1 and Bcl-2, Bcl-xL levels did not double in any patient (Fig 6C). A smaller increase (just under twofold) was observed in an ALL sample that had twofold increases in Bcl-2 and Mcl-1 as well. In contrast, four pairs showed a ≥twofold decrease in Bcl-xL levels (eg, Fig 5, lanes 3-6). Likewise, although three of the pairs showed a twofold increase in Bax levels, none showed a twofold decrease (Fig 6D).
Overall, a doubling of one or more antiapoptotic Bcl-2 family members was observed in 12 of the 19 paired samples. In one case this was accompanied by a twofold increase in Bax levels. In the other 11 paired samples that displayed Bcl-2 or Mcl-1 increases, Bax levels were unchanged.
DISCUSSION
Studies in tissue culture cell lines have identified Bcl-2, Mcl-1, Bcl-xL, and Bax as important regulators of apoptosis. Previous studies of clinical leukemia specimens have focused almost exclusively on Bcl-2. In the present study, we not only showed that all four Bcl-2 family members are widely expressed in human acute leukemia cell lines, but also examined the expression of these polypeptides in human AML and ALL samples harvested before therapy and, where available, in additional samples obtained at recurrence. These studies lead to several conclusions.
Examination of the leukemia cell lines revealed that Bcl-2, Mcl-1, Bcl-xL, and Bax were all detectable in the majority of the cell lines. This observation is noteworthy from several standpoints. Three of the four myeloid lines contained higher amounts of Bcl-2 than any of the lymphoid lines (Fig 1A). Among these Bcl-2-expressing myeloid lines were HL-60 cells, a cell line that is exquisitely sensitive to chemotherapy-induced apoptosis.5,65,66Conversely, K562 cells, which exhibit diminished or delayed apoptosis in response to many agents,63,64,66 do not express detectable levels of Bcl-2 polypeptide. Not only do these observations suggest that Bcl-2 by itself is a poor predictor of response to chemotherapy in myeloid cell lines, but the latter observation also appears to argue against the recent proposal67 thatbcr/abl inhibits apoptosis by enhancing the expression of Bcl-2.
Examination of Bcl-2, Mcl-1, Bcl-xL, and Bax in pretreatment acute leukemia specimens also leads to several important conclusions. First, one or more of the antiapoptotic Bcl-2 family members examined was detectable in all of the AML and ALL specimens. Second, levels of these polypeptides varied widely between different specimens. As illustrated in Fig 2A and summarized in Fig 3A, levels of Bcl-2 varied over a 40-fold range. This range of Bcl-2 expression is similar to that previously observed using sensitive flow cytometry techniques.34 In our study, immunoblotting indicated that levels of Mcl-1, Bcl-xL, and Bax also varied almost as widely (Figs 2 and 3). Third, there was a correlation between levels of Bcl-2 and levels of Mcl-1 in pretreatment AML and ALL specimens (Fig 4and Table 1). Fourth, there was a weak negative correlation between levels of Bax and increasing age (Fig 4C), raising the possibility that diminished levels of this proapoptotic polypeptide might be one factor that contributes to the diminished response rate generally observed in older AML patients. Despite this finding, there was no relationship between pretreatment levels of Bcl-2, Mcl-1, Bcl-xL, or Bax and response of individual AML patients or ALL patients to initial antileukemic therapy (Fig 3). Likewise, there was no relationship between ratios of Bcl-2:Bax, Mcl-1:Bax or Bcl-xL:Bax and response to therapy. Our results were unchanged when the patients with antecedent myelodysplastic syndrome and secondary leukemia were excluded from analysis (data not shown). Although our observations agree with those of a previous study that examined pretreatment Bcl-2 levels by flow cytometry,34 our results differ from several previous reports indicating that Bcl-2 expression32,35 or the Bcl-2:Bax ratio53 might predict response to antileukemic therapy in AML. Part of the explanation for these differences might lie in the fact that nonquantitative methods were used in some of the previous studies. Alternatively, we cannot rule out the possibility that Bcl-2 levels might predict response to some chemotherapy regimens and not others, an issue that is discussed in greater detail below.
Examination of the paired leukemia specimens led to the observation that one or more of the antiapoptotic Bcl-2 family members assayed were increased at least twofold at recurrence in 12 of 19 cases (Fig 6). Interestingly, 10 of the 19 paired samples displayed a ≥twofold increase in expression of Mcl-1, a polypeptide whose expression has not been previously examined in acute leukemia specimens. Even though pretreatment Bcl-2 and Mcl-1 levels correlated with each other (see above), eight of the ten Mcl-1 increases occurred without any accompanying increase in Bcl-2 (eg, Fig 5). Overexpression of Mcl-1 has recently been shown to convey resistance to apoptosis induced by a number of different treatments, including etoposide, in vitro.47,48 Additional studies have indicated that Mcl-1 expression can be induced within hours in response to a number of DNA damaging agents.68,69 Although these latter observations raise the possibility that the elevated levels observed at relapse might reflect induction of Mcl-1, the short half-life of this polypeptide46 and the long interval between the completion of chemotherapy and harvest of the second sample (13-210 days) argue against this possibility. Instead, it is possible that chemotherapy has selected for cells that overexpress one or more antiapoptotic Bcl-2 family members—especially Mcl-1—in over half of the cases.
A compensatory increase in Bax levels was observed in one of the pairs of samples displaying an increase in Bcl-2. This was not observed in any of the other 11 samples displaying a twofold increase in Bcl-2 or Mcl-1. Although this is similar to results obtained in tissue culture cell lines, in which some cell lines demonstrate elevated Bax levels when Bcl-2 is overexpressed and others do not,56 the explanation for this variation in tissue culture and in the patient samples remains unknown.
In evaluating the present results, several potential limitations must be kept in mind. First, the changes observed in the present study are relatively small in magnitude. Although the 10- to 20-fold increases in Bcl-2 content that occur in virally transduced cells (Fig1A)8,70 are associated with decreases in the sensitivity of leukemia cells to certain chemotherapeutic agents,8 the effect of the smaller changes in expression detected here have not been systematically studied. It must be kept in mind, however, that relatively small changes in drug IC50 values, perhaps on the order of 2- to 10-fold, also might distinguish leukemias that are cured in vivo from those that fail to respond to chemotherapy.71,72 Accordingly, the small magnitude of the changes described in the present study does not necessarily diminish their importance. Second, it is possible that changes in apoptotic regulators are part of a number of changes that occur between diagnosis and recurrence. Studies in tissue culture cell lines have indicated that drug selection can concurrently lead to multiple mechanisms of resistance, some of which involve alterations in apoptotic regulators.42,73 In this context, we have previously reported increased expression of mRNA encoding the multidrug resistance-associated polypeptide MRP (but not mdr1) in some of these same clinical samples of leukemia at relapse.74 Thus, resistance in the clinical setting might be multifactorial; and the individual contributions of different mechanisms might be difficult to assess. Third, it is not clear whether the present results will be representative of Bcl-2 family member expression in all acute leukemias. The practical requirement that samples contain >80% blasts after fractionation might have resulted in a skewed population that contained a relative paucity of certain leukemia subtypes, particularly leukemias that arise in the setting of an antecedent myelodysplastic syndrome. Likewise, the chemotherapy regimens used in the present study might have somehow selected for cells that overexpress Mcl-1 at relapse, whereas other regimens might not. Finally, the paired samples came from a subset of patients with particularly poor outcomes after initial chemotherapy, the longest remission being 258 days. Further study is required to determine whether elevated levels of antiapoptotic Bcl-2 family members—particularly Mcl-1—will be found at the time of relapse in patients who receive different treatments, have <80% blasts in their marrows, or have longer remissions. Nonetheless, the present results indicate that further study of apoptotic regulators in clinical specimens of acute leukemia appears warranted.
ACKNOWLEDGMENT
The excellent technical assistance of Sharon McLaughlin, Sandra Kiesewetter, Timothy Soos, Lisa Prichard, and Christopher Buckwalter; secretarial assistance of Deb Strauss; and advice of Udo Kellner during the course of these studies are gratefully acknowledged. This study was made possible by the skillful care provided by Ken Hall, Louann Morrell, and the attendings, fellows, housestaff, and nurses of the Adult Leukemia Service.
Supported in part by grants from the National Institutes of Health (CA50435, CA69008, CA55164), American Cancer Society Clinical Oncology Career Development Awards to S.H.K. and S.D.G., and Leukemia Society of America Scholar Awards to S.H.K. and J.C.R.
Address correspondence to Scott H. Kaufmann, MD, PhD, Division of Oncology Research, Guggenheim 1342C, Mayo Clinic, 200 First St, SW, Rochester, MN 55905.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal