Abstract
Evidence indicates that, at least in the early stage, Kaposi's sarcoma (KS) is a cytokine-mediated disease and that it is consistently associated with a novel herpesvirus termed human herpesvirus-8 (HHV-8). To gain insights into the mechanisms by which cytokines and HHV-8 may cooperate in disease pathogenesis, we examined the phenotype, the Th1 (γ-interferon [γIFN]) and Th2 (interleukin-4 [IL-4]) cytokine profile and the presence of HHV-8 in peripheral blood mononuclear cells (PBMC), tumor-infiltrating lymphocytes (TIL), and spindle cell cultures derived from skin lesions of patients affected by classical KS (C-KS) and acquired immunodeficiency syndrome (AIDS)-associated KS (AIDS-KS). TIL and spindle cell cultures were examined at day 0 or after culture in conditioned media from activated T cells (TCM) that contain the same cytokines increased in KS tissues. No differences were found in the immunophenotype of PBMC from C-KS patients versus controls, except for AIDS-KS patients who showed a T-CD8+ expansion. However, a preferential infiltration of T-CD8+ cells was found in all KS lesions examined, which was maintained after culture of TIL in TCM. γIFN production was found in both PBMC and cultures derived from all KS examined; some IL-4 positive supernatants were found only in three AIDS-KS cases. Uninvolved skin did not show appreciable lymphocyte infiltration or cytokine production. The culture conditions of the lesional skin allowed also the appearance of adherent, spindle-like cells bearing markers of tissue macrophages. Finally, most or all of the PBMC, lesions, and macrophagic cell cultures from the skin lesions were found to be positive for HHV-8 infection by nested polymerase chain reaction (PCR). These findings indicate that patients with KS express a Th1 phenotype with a prevalent γIFN production, likely accounted for by the local T-CD8+ infiltration. By analogy with other viral infections (ie, Epstein-Barr virus), this suggests that in loco recruitment of lymphoid cells and the subsequent γIFN production may be in response to or elicited by HHV-8 that was found in both PBMC and macrophagic cell cultures from the lesions of the same patients.
KAPOSI'S SARCOMA (KS) is an angioproliferative disease involving the skin and mucosas that affects elderly men of Mediterranean origin (classical KS, [C-KS]), as well as transplantanted patients; it is endemic in Africa (endemic KS),1-5 and it is the most common neoplasm of human immunodeficiency virus type 1 (HIV-1) infected homo-bisexual men (acquired immunodeficiency syndrome [AIDS]-KS).6,7 In these patients, KS acquires a more aggressive course than C-KS. However, all of these forms have the same histopathology, including spindle-shaped cells, considered to be the tumor cells of KS, angiogenesis, inflammatory cell infiltration, and edema.1The spindle-shaped cells are composed by an heterogeneous cell population of endothelial cells with an activated cell phenotype, macrophages, and dendritic cells.8 9
Recently, DNA sequences from a new virus called Kaposi-Sarcoma herpesvirus or human herpesvirus-8 (HHV-8), have been found in the majority of the lesions from patients with all forms of KS, and the virus has been propagated from a KS skin lesion, suggesting an epidemiologic association of the virus with KS,10-18although its role in KS pathogenesis is yet unknown. HHV-8 has also been found in peripheral blood mononuclear cell (PBMC) from these patients, as well as in other pathologic conditions.19-21Recent data also indicate that HHV-8 is persistently present in circulating B cells22 and in blood-derived spindle-like cells that are found in KS patients or in HIV-1–infected homosexual men.23,24 In addition, productively infected mononuclear cells including monocytes/macrophages25,26 and latently infected endothelial and spindle cells27-29 are present in KS lesions. The virus, however, is rapidly lost by cultured endothelial spindle cells from KS lesions28 29 (and B.E., unpublished data, June 1995).
The immune system seems to play a major role in KS development. For example, homosexual men, the group of HIV-1–infected individuals at highest risk for KS, show increased signs of immunoactivation, including soluble CD8 and soluble intercellular adhesion molecule (ICAM) even before HIV-1 infection, and KS can represent the first sign of AIDS.30-35 Recent studies indicate that KS itself behaves as a cytokine-mediated disease, since the first observation that conditioned media (CM) derived from retroviruses-infected CD4+ T cells or CM from activated primary T cells or PBMC (TCM), rich in tumor necrosis factor (TNF), γ-interferon (γIFN), interleukin-1 (IL-1), IL-6, and Oncostatin M (OM) were able to support the growth of KS-spindle cells.36-39 KS cells themselves produce a variety of cytokines and angiogenic factors, including IL-1, IL-6, IL-8, basic fibroblast growth factor (bFGF), and vascular endothelial growth factor (VEGF), that mediate the formation of KS-like lesions induced by inoculation of these cells in nude mice40-44 (and F. Samaniego et al, submitted).
KS-like cells with characteristics similar to endothelial and macrophagic spindle cells derived from the lesions have also been found in blood from KS patients with all forms of the disease.23,24,45 Finally, the inflammatory cell infiltration found in KS lesions, particularly in the early stage, is associated with the production of the same inflammatory cytokines found in TCM.42,46-49,49a TCM promote normal endothelial cells to acquire the features of the KS cell phenotype, including the responsiveness to the HIV-1 Tat molecule,37,49-51 that can increase the frequency and aggressiveness of AIDS-KS.43
Despite this body of evidence for the involvement of the immune system in KS development, little or nothing is known on the type of cells infiltrating the lesions and on the Th1 and Th2 cytokine profile in patients with KS and of its correlation with the presence of HHV-8. Similarly, little or no information is currently available on the effects of the cytokines increased in KS on tumor-infiltrating lymphocytes (TIL) and spindle cells present in KS.
Th1 cytokines (IL-2 and γIFN) are involved in the development of delayed type hypersensitivity reactions, whereas Th2 cytokines (IL-4, IL-5, IL-10) are the main regulators of immunoglobulin production.52
In this study, we report that γIFN is found in supernatants derived from both skin lesions and PBMC cultures of all C-KS patients and from the majority of AIDS-KS patients examined. The concomitant immunophenotyping of TIL indicates that the majority of these cells are lymphocytes of the T-cell lineage that are maintained in culture by TCM. TCM also allow the establishment in culture of spindle-shaped cells with markers of tissue macrophages. Finally, the majority of the cultures examined, both at the skin and at the PBMC level, harbor HHV-8, suggesting that T-CD8+ cells producing γIFN may be recruited in KS lesions in response to HHV-8 infected cells.
MATERIALS AND METHODS
Patient population.
Eighteen patients affected by C-KS (men; mean age, 60 years) as well as 22 patients affected by AIDS-KS (21 men and one woman; mean age, 30 years; 18 homosexuals and four heterosexuals) were studied. Patients with C-KS presented a cutaneous involvement only, whereas patients with AIDS-KS presented a cutaneous and visceral (in 16 cases) involvement. In all cases, the diagnosis of KS was supported by conventional histology. A group of 20 normal volunteers was studied as control for immunologic phenotyping of PBMC and a group of 34 patients affected by skin disorders other than KS, such as psoriasis and chronic dermatitis, were studied as control of cytokine production. All of the patients were free of cytokine therapy. Fourteen patients with HIV-1 infection were under antiretroviral therapy with AZT, whereas eight patients were studied at the time of the first KS diagnosis and had refused any prior antiretroviral therapy. Blood samples were obtained after written informed consent.
Isolation of PBMC.
PB from patients was collected by venipuncture and heparinized (10 IU/mL, Liquemin, Hoffman-La Roche Co, Rome, Italy). PBMC were separated by a Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) gradient. Cells were washed twice in Hanks' balanced salt solution, resuspended in RPMI 1640 (GIBCO Laboratories, Grand Island, NY) containing 20% fetal calf serum (FCS) (GIBCO) and plated at a concentration of 106cells/mL in 24-well plates (Falcon, Becton Dickinson, Lincoln Park, NJ). Cells were stimulated with phytohemagglutinin (PHA) (PHA-L, Sigma Chemical, St Louis, MO) at a final dilution of 1 μm/mL for 72 hours at 37°C to assess cytokine production. Control cultures consisted of PBMC plated in the presence of medium alone.
Cultures from KS skin biopsies.
Punch biopsies were performed at the site of cutaneous KS lesions and at the site of apparently uninvolved skin after obtaining from the patients a written informed consent to the biopsy. Tissues were mechanically minced, fragments were washed in medium, and the released lymphoid cells (TIL) were recovered and phenotyped immediately (day 0). The tissue fragments were then cultured in gelatin-coated flasks (1.5% final dilution of gelatin; bovine skin albumin, Sigma) with a culture medium previously used to culture KS spindle cells from the lesions.37 Growth medium consisted of RPMI 1640 medium supplemented with 20% FCS, 1% Nutridoma-HU (100× solution) (Boehringer Mannheim, Milan, Italy), 1% nonessential amino acids (100× solution) (Sigma), 1 mmol/L sodium pyruvate (Sigma), 100 U/mL penicillin G-sodium and 100 mg/mL streptomycin sulfate (Sigma) and 20% CM prepared as described previously from normal donors' PBMC after stimulation with PHA-L for 72 hours (TCM).37,49 A bulk stock of TCM was prepared and used for all of the cultures. TCM contained 96,551 pg/mL of IL-2, 841 pg/mL of γIFN, 234 pg/mL of IL-1α, 60 pg/mL of IL-6, and 0 pg/mL of IL-4 and bFGF. This TCM has differences as compared with previously reported TCM.23,37 49 Specifically, it has a higher content of IL-2 and γIFN and a lower content of IL-6. The culture medium was changed every 3 to 6 days. Supernatants from these passages were filtered and immediately stored at -80°C until cytokine measurements. Some cultures were also grown in the absence of TCM and the supernatants from these cultures were also tested. At day 3 after culture with or without TCM, floating cells (containing the lymphoid population) were harvested and subjected to immunophenotyping by fluorescence-activated cell sorting (FACS) analysis. The adherent cells were maintained in culture for an additional 4 weeks and then assessed for the presence of several cell markers by immunohistochemistry.
Cell flow cytometry.
For double and triple fluorescence analysis, 2 × 105 cells (PBMC or TIL) were examined after staining with the appropriate amounts of monoclonal antibodies (MoAbs) (20 μL of phycoerythrin [PE], fluorescein isothiocyanate [FITC], or peridinin chlorophyle protein [per CP]-conjugated) for 30 minutes on ice. After two washings, cells were resuspended in phosphate-buffered saline (PBS) and analyzed by flow cytometry (Cytoron, Ortho Diagnostics, Raritan, NJ) after electronic gating on lymphocytes. The following MoAbs were used: PE-conjugated CD8, PerCP-conjugated CD4, FITC-conjugated TCRαβ, CD16, CD19, TCRγδ, CD25, LeukoGate (anti-CD14 and anti-CD45). All MoAbs were purchased from Becton Dickinson Immunocytometry System, (Mountain View, CA). TIL were immunophenotyped for cell surface marker analysis immediately after the tissue mincing (day 0) or after 3 days expansion in TCM.
Immunohistochemistry.
Immunohistochemical analyses were performed on adherent cells obtained after 4 weeks of culture in TCM. Cells were detached by ice-cold trypsin-EDTA (GIBCO), as per manufacturer's protocol, resuspended in RPMI 1640, at a concentration of 2 × 106 and then cytocentrifuged smears were made. These were incubated with the following MoAbs: CD68 (Dakopatts, Dako, Golstrup, Denmark), LeuM3 (anti-CD14, Becton-Dickinson), OKT4 (anti-CD4, Ortho), OKT8 (anti-CD8, Ortho), Leu11 (anti-CD16, Becton-Dickinson), CD19 (anti-B, Dakopatts), anti-CD45 (Dakopatts), 11C81 (ICAM-1, British Biotechnology), HLA-DR (Dakopatts), vWF/FVIII-RA (Dakopatts). The sections were then treated with a biotin-conjugated horse anti-mouse Ig MoAbs and later with avidin-biotin peroxidase complex (Vector Laboratories, Burlingame, CA) and with 0.06% 3,3′ diaminobenzidine (Sigma) and 0.03% hydrogen peroxide.
Cytokine measurement.
Cytokines were measured on supernatants from PHA-stimulated PBMC or skin cultures by enzyme-linked immunosorbent assay (ELISA) using manifacturer's protocol (R&D Systems, Minneapolis, MN).
Detection of HHV-8 nucleic acid sequences.
Total DNA was extracted with a Microturbogen DNA extraction kit (Invitrogen, San Diego, CA) from skin specimens as well as from cultures derived from KS skin lesions or uninvolved skin cultured for a period of 4 weeks. Some PBMC samples were also examined. A total of 100 ng of DNA was then amplified for HHV-8 nucleic acid sequences by nested PCR by using as primers KS4 and KS5 for the first round of amplification, followed by a second round with the primers KS1 and KS2, previously described.10 Amplification was performed as follows: 94°C for 3 minutes (1 cycle); 94°C for 1 minute, 58°C for 1 minute, 72°C for 1 minute (35 cycles); 72°C for 5 minutes (1 cycle). Each PCR reaction was performed with 50 pmol of each primer, 1 U of Taq polymerase (Perkin-Elmer, Branchburg, NJ), 200 μmol/L of each deoxyribonucleotide triphosphates (dATP, dTTP, dGTP, dCTP), Perkin-Elmer buffer 1×, 1.5 mmol/L MgCl2, in a final volume of 50 μL. Amplifications were performed with a Perkin-Elmer 9600 Thermocycler. PCR products were analyzed by Southern blot hybridization using the HHV-8 specific probe KS330Bam32P-end–labeled.10 Hybridization was performed at 65°C. The positive control was the DNA extracted from a KS skin biopsy and the negative control was DNA extracted from PBMC previously known to be negative for HHV-8 DNA sequences.
Statistical analysis.
Analysis of variance was performed by the ANOVA test. A multiple comparison of variance was performed by the Newman-Keuls test.
RESULTS
Clinical features of KS patients.
The main clinical features of patients with C-KS and AIDS-KS, as well as data from healthy controls are reported in Table 1. Most of the patients with KS were men. Patients with C-KS were middle- or late-aged in comparison to AIDS-KS patients and presented no risk factors for HIV-1 infection. The majority of AIDS-KS patients were male homosexuals, with occasional experience of intravenous drug use. Healthy controls had no risk factors for HIV-1 infection.
Clinical and Immunological Features of the Study Groups
| Characteristic . | Controls (n = 20) . | C-KS (n = 18) . | AIDS-KS (n = 22) . |
|---|---|---|---|
| Sex (M/F) | 15/5 | 18/0 | 21/1 |
| Age (mean yr ± SD) | 40 ± 10 | 60 ± 8 | 30 ± 10 |
| Risk factor for HIV-1 infection | None | None | 18 Homo 4 hetero |
| CD3+ cells/μL | 1,310 ± 380 | 1,380 ± 298 | 1,298 ± 220 |
| CD16+ cells/μL | 264 ± 12 | 268 ± 12 | 271 ± 20 |
| CD4+ cells/μL | 780 ± 104 | 750 ± 106 | 325 ± 206 |
| CD8+ cells/μL | 319 ± 140 | 324 ± 111 | 750 ± 179 |
| Characteristic . | Controls (n = 20) . | C-KS (n = 18) . | AIDS-KS (n = 22) . |
|---|---|---|---|
| Sex (M/F) | 15/5 | 18/0 | 21/1 |
| Age (mean yr ± SD) | 40 ± 10 | 60 ± 8 | 30 ± 10 |
| Risk factor for HIV-1 infection | None | None | 18 Homo 4 hetero |
| CD3+ cells/μL | 1,310 ± 380 | 1,380 ± 298 | 1,298 ± 220 |
| CD16+ cells/μL | 264 ± 12 | 268 ± 12 | 271 ± 20 |
| CD4+ cells/μL | 780 ± 104 | 750 ± 106 | 325 ± 206 |
| CD8+ cells/μL | 319 ± 140 | 324 ± 111 | 750 ± 179 |
The immunologic profile shows similar values of lymphocyte subpopulations in C-KS patients in comparison to normal donors, whereas AIDS-KS patients show abnormalities (see text for statistical analysis). Results are given as the mean (M) ± standard deviations (SD).
Abbreviations: n, number; yr, years of age.
Immmunological analysis of PBMC and TIL from KS patients: Local infiltration of T-cell receptor (TCR)α/β+CD8+and TCRα/β+CD4-CD8-lymphocytes in KS lesions.
The analysis of lymphocyte subpopulations was performed on freshly isolated PBMC and TIL obtained from biopsies of lesional and uninvolved skin. No statistically significant difference was found among the study groups in regard to circulating CD3+, CD16+, and CD19+ cells (Table 1). As expected, the absolute number of CD4+ cells was significantly reduced in AIDS-KS patients as compared with controls (F = 79.11, P < .001) or C-KS patients (F = 27.02, P < .001). Similarly, no difference was found between healthy controls and C-KS patients. The absolute number of CD8+ cells was significantly increased in AIDS-KS patients as compared with controls (F = 74.47, P < .001) and to C-KS patients (F = 34.84, P < .001), whereas no differences were found between normal controls and C-KS. In contrast, when TIL were phenotyped, immediately after the biopsy and tissue mincing (day 0), a prevalent infiltration of CD8+ T cells was found in the majority of KS patients, irrespective of their seropositivity for HIV-1. The remaining lymphoid population was represented by CD4+ cells. No or few B cells were found (Table 2). No significant lymphoid infiltration was found in uninvolved skin. These findings were confirmed by immunohistochemical stainings of the lesions and they are consistent with those reported in by Fiorelli et al.49a
Immunophenotyping of TIL From KS Lesions and Uninvolved Tissues at Day 0 and After 3 Days Expansion in TCM
| . | KS Lesions % Positive Cells . | Uninvolved Tissue % Positive Cells . | |||
|---|---|---|---|---|---|
| CD4 . | CD8 . | CD19 . | CD4 . | CD8 . | |
| Day 0 | 12 | 15 | 3 | 1.3 | 2 |
| (6-30) | (10-21) | (0-4) | (0-4.2) | (0.4-6.7) | |
| Day 3 | 24 | 36 | 0 | 3.8 | 2.2 |
| (0-60) | (8.2-45) | (0-4.2) | (1.4-6.7) | (2.1-4) | |
| . | KS Lesions % Positive Cells . | Uninvolved Tissue % Positive Cells . | |||
|---|---|---|---|---|---|
| CD4 . | CD8 . | CD19 . | CD4 . | CD8 . | |
| Day 0 | 12 | 15 | 3 | 1.3 | 2 |
| (6-30) | (10-21) | (0-4) | (0-4.2) | (0.4-6.7) | |
| Day 3 | 24 | 36 | 0 | 3.8 | 2.2 |
| (0-60) | (8.2-45) | (0-4.2) | (1.4-6.7) | (2.1-4) | |
Results of the immunophenotyping of TIL are reported. Cells were phenotyped immediately after the biopsy (day 0) and 3 days after expansion in the presence of TCM, as described in Materials and Methods. Cells were analyzed by flow cytometry. The number represents the median of the percentage of positive cells. The range of positive cells is given in parentheses.
To expand and further characterize infiltrating lymphoid cells, biopsies from involved and uninvolved skin from the same patients were then cultured in the presence or absence of TCM. TCM contain mainly Th1 cytokines that are the same found to be increased in KS lesions.42,46-49 49a This system allowed the growth of an adherent cell population with a spindle-shaped morphology, described later, and of a floating cell population with a lymphoid morphology (Fig 1). The latter cells, easily removed by pipetting, were phenotyped after a short-term growth (3 days) in the presence or absence of TCM. Nearly all of the floating cells were found to be lymphocytes, as determined by flow cytometry after electronic gating on lymphocytes and then by the use of a combination of MoAbs to CD14 (recognizing cells of the monocyte lineage) and to CD45 (recognizing cells of leukocyte origin) that distinguish lymphocytes from monocytes and debris (Fig 2A). These cells were mainly of a CD8+ phenotype and were found both in C-KS and AIDS-KS. CD4+ cells were also detected, but to a lesser extent. Very few or no B cells (CD19+) were found (Fig 2B; Table 3). The values of CD4+ and CD8+ cells were variable in different lesions, and this was due to the different stages of the lesions examined, being CD8+ cells detected more easily in early stage lesions.
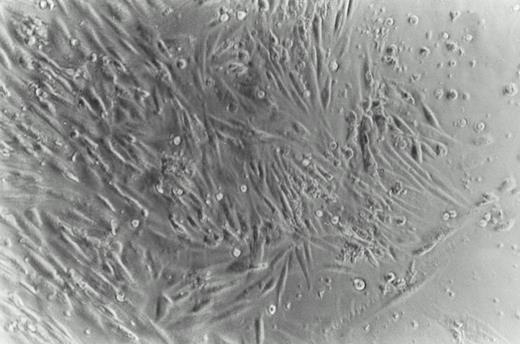
Phase photomicrograph of cultured cells from a KS skin biopsy. Fragments of skin were minced and grown for 4 weeks in culture media containing TCM, as described in Materials and Methods (phase contrast 200×). A double population was obtained: one adherent with a spindle-shaped morphology and another of floating cells with a lymphoid morphology.
Phase photomicrograph of cultured cells from a KS skin biopsy. Fragments of skin were minced and grown for 4 weeks in culture media containing TCM, as described in Materials and Methods (phase contrast 200×). A double population was obtained: one adherent with a spindle-shaped morphology and another of floating cells with a lymphoid morphology.
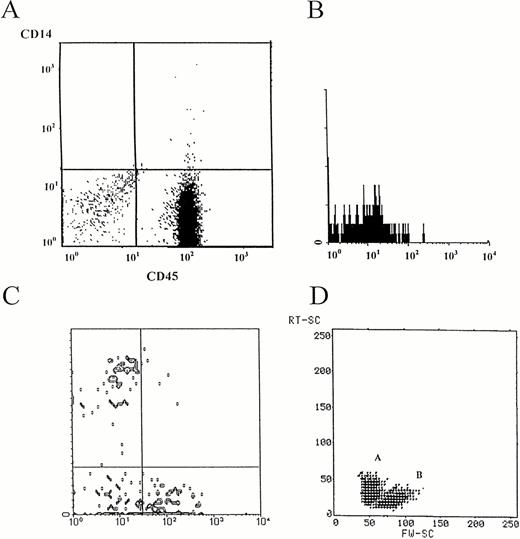
Immunophenotyping of TIL from a C-KS lesion (nodular type). Cells were recovered and cultured in the presence of TCM, as reported in Materials and Methods. Floating cells were phenotyped after 3 days of culture in TCM. (A) Nearly all of the cells (92%) are represented by lymphocytes, as assessed by the LeukoGATE system, which allows the discrimination between these cells, other mononuclear cells, and debris. (B) No CD19+ B cells were found in cultured lesions. Shown is a single immunofluorescence from a representative case. (C) Triple immunofluorescence from a representative case showing that the majority of the cells coexpress the α/β chain of the TCR as well as the CD4+ (24%), the CD8+(39%), or both CD4+CD8+ (13%) markers. Notably 24% of TCRα/β+ cells are CD4-CD8-. The figure represents the CD4 and CD8 staining on α/β positive gated cells. (D) Apoptotic figures are observed by morphologic analysis for orthogonal (y-axis) versus forward (x-axis) light scatter. A, apoptotic region; B, viable lymphocytes. {/CAPT;;;left;stack}
Immunophenotyping of TIL from a C-KS lesion (nodular type). Cells were recovered and cultured in the presence of TCM, as reported in Materials and Methods. Floating cells were phenotyped after 3 days of culture in TCM. (A) Nearly all of the cells (92%) are represented by lymphocytes, as assessed by the LeukoGATE system, which allows the discrimination between these cells, other mononuclear cells, and debris. (B) No CD19+ B cells were found in cultured lesions. Shown is a single immunofluorescence from a representative case. (C) Triple immunofluorescence from a representative case showing that the majority of the cells coexpress the α/β chain of the TCR as well as the CD4+ (24%), the CD8+(39%), or both CD4+CD8+ (13%) markers. Notably 24% of TCRα/β+ cells are CD4-CD8-. The figure represents the CD4 and CD8 staining on α/β positive gated cells. (D) Apoptotic figures are observed by morphologic analysis for orthogonal (y-axis) versus forward (x-axis) light scatter. A, apoptotic region; B, viable lymphocytes. {/CAPT;;;left;stack}
Th1 and Th2 Cytokine Profile in Supernatants From PBMC, Cultured Lesional, and Uninvolved Skin From C-KS and AIDS-KS Patients and From PBMC of Patients With Dermatologic Diseases Other Than KS
| . | IL-4 (pg/mL) . | γIFN (pg/mL) . |
|---|---|---|
| C-KS | ||
| PBMC (n = 18) | 0 | 3,775 ± 638 |
| Lesional skin (−CM) (n = 9) | 0 | 0 |
| Lesional skin (+CM) (n = 9) | 6.5 ± 2.0 | 1,005 ± 369 |
| Uninvolved skin (−CM) (n = 3) | 0 | 0 |
| Uninvolved skin (+CM) (n = 3) | 0 | 0 |
| AIDS-KS | ||
| PBMC (n = 22) | 191 ± 300 | 4,658 ± 3,350 |
| Lesional skin (−CM) (n = 9) | 0 | 0 |
| Lesional skin (+CM) (n = 9) | 59 ± 44 | 1,016 ± 721 |
| Other dermatologic diseases (n = 34) | ||
| PBMC (−CM) | 0 | 4 ± 1.4 |
| PBMC (+CM) | 0 | 600 ± 196 |
| . | IL-4 (pg/mL) . | γIFN (pg/mL) . |
|---|---|---|
| C-KS | ||
| PBMC (n = 18) | 0 | 3,775 ± 638 |
| Lesional skin (−CM) (n = 9) | 0 | 0 |
| Lesional skin (+CM) (n = 9) | 6.5 ± 2.0 | 1,005 ± 369 |
| Uninvolved skin (−CM) (n = 3) | 0 | 0 |
| Uninvolved skin (+CM) (n = 3) | 0 | 0 |
| AIDS-KS | ||
| PBMC (n = 22) | 191 ± 300 | 4,658 ± 3,350 |
| Lesional skin (−CM) (n = 9) | 0 | 0 |
| Lesional skin (+CM) (n = 9) | 59 ± 44 | 1,016 ± 721 |
| Other dermatologic diseases (n = 34) | ||
| PBMC (−CM) | 0 | 4 ± 1.4 |
| PBMC (+CM) | 0 | 600 ± 196 |
Cytokine profile from KS patients and patients with dermatologic diseases other than KS (10 psoriatic patients and 24 with chronic dermatitis). PBMC were isolated, plated at a concentration of 106 cells/mL and stimulated with 1 μg/mL final dilution of PHA for 3 days to assess cytokine production. Lesional and uninvolved skin were cultured in the presence or absence of TCM, as described in Materials and Methods. The supernatants from these cultures were then assessed for cytokine levels. The results are given as M ± SD of the values (pg/mL) and are devoid of the levels present in TCM.
The majority of the T cells detected were TCRα/β+CD8+ or TCRα/β+CD4+, although a high percentage (about one quarter of total TCR α/β+ cells) of TCRα/β+CD8-CD4- cells was also found by triple immunofluorescence (Fig 2C). Very few cells were bearing the γ/δ chains of the T-cell receptor and, after three days activation, a small percentage of the CD4+ and CD8+ cells acquired the IL-2 receptor.
Finally, the FACS analysis showed the presence of a population of lymphoid cells (up to 60% of total lymphocytes) with morphologic characteristics of apoptotic cells, as shown by an increased orthogonal light scatter and a low forward scatter (Fig 2D), which were prevailing in the CD8+ T-cell fraction (data not shown).
Control cultures set up in the absence of TCM did not show viable cells, and the characterization of uninvolved skin did not show appreciable presence of lymphocytes, as assessed by the number of CD4+ or CD8+ cells (Table 2).
Immunohistochemical analysis of KS-derived spindle cells: Adherent cells bear markers of the macrophage lineage.
The adherent cells derived from the skin lesions were grown for 4 weeks in the presence or absence of TCM. These cells acquired a spindle-shaped morphology (Fig 1) and the large majority of them was strongly positive for CD68, HLA-DR, and ICAM-1 (Fig 3), but negative for markers of leukocyte/lymphoid origin, including CD45, CD4, and CD8 (data not shown). The endothelial cell marker vWF/FVIII-related antigen was low or undetectable (data not shown). Thus, the growth conditions allowed the expansion of spindle cells with an immunophenotype of tissue macrophages. Uninvolved skin did not show the appearance of significant amounts of these cells.
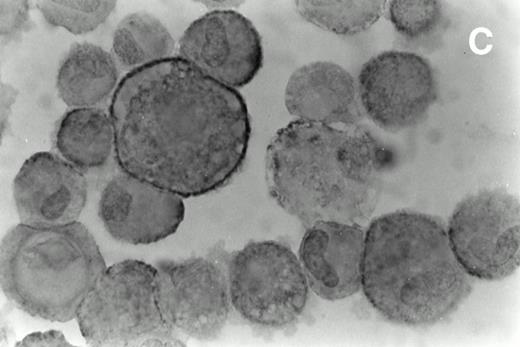
Immunohistochemical analysis of cultured spindle-like cells derived from skin lesions. The cells were removed from the flask using a mild trypsinization cytocentrifuged and immunostained with MoAbs directed against CD68 (A), HLA-DR (B), and ICAM-1 (C) and counterstained with hematoxylin. The staining pattern for CD68 was cytoplasmic and diffuse (A), for HLA-DR, it was mainly granular (B), and for ICAM-1, it was more evident at the cell surface (C). The large majority of the cultured KS cells were HLA-DR positive. Magnifications are (A) ×630, (B) ×700, and (C) ×800.
Immunohistochemical analysis of cultured spindle-like cells derived from skin lesions. The cells were removed from the flask using a mild trypsinization cytocentrifuged and immunostained with MoAbs directed against CD68 (A), HLA-DR (B), and ICAM-1 (C) and counterstained with hematoxylin. The staining pattern for CD68 was cytoplasmic and diffuse (A), for HLA-DR, it was mainly granular (B), and for ICAM-1, it was more evident at the cell surface (C). The large majority of the cultured KS cells were HLA-DR positive. Magnifications are (A) ×630, (B) ×700, and (C) ×800.
Cytokine profile: The majority of KS patients preferentially release γIFN on stimulation.
The Th1 (γIFN) and Th2 (IL-4) cytokine profile was assessed in supernatants from both PHA-stimulated PBMC and skin cultures from KS and uninvolved tissues cultured in the presence or absence of TCM. Cultures established in the absence of TCM did not show appreciable levels of any cytokine tested. In contrast, both C-KS and AIDS-KS patients showed a preferential expression of γIFN, in both PBMC and TCM-cultured skin (Table 3). There was no statistical difference between C-KS and AIDS-KS patients regarding γIFN production (P = .278 for PBMC; P = .968 for cultured skin). However, AIDS-KS patients showed also the emergence of IL-4–producing cells in both PBMC after PHA stimulation and in supernatants derived from cultured skin (Table 3), and this was statistically significant in comparison to C-KS patients (P = .01 for PBMC; P = .003 for cultured skin). No detectable levels of cytokines were found in cultures from uninvolved skin from the same patients (Table 3). IL-2, as well as IL-12, were not found in supernatants from skin cultures from both groups of KS patients (data not shown).
IL-4 and γIFN were also tested in supernatants derived from PBMC obtained from 34 patients with other skin disorders, such as psoriasis and chronic dermatitis, and cultured in the same TCM used to expand TIL from KS patients. In these cases, IL-4 was never found, whereas γIFN was found, but at levels much lower than in KS patients (Table 3).
HHV-8 is detected in PBMC, lesions, and in spindle-like macrophagic cell cultures of lesional skin from KS patients.
Because among all of the infectious agents proposed to play a role in KS, HHV-8 appears to be the only one consistently associated, the presence of HHV-8 DNA sequences was searched by nested PCR in our experimental systems. Viral sequences were consistently detected in 100% (9/9) of the lesions from C-KS patients and in 100% (5/5) of the lesions from AIDS-KS patients, as well as in 88% (8/9) and 66% (6/9) of the PBMC from C-KS and AIDS-KS patients, respectively, but not in the uninvolved skin far from the lesions (Table 4). Some perilesional skin was also found to be positive. In addition, HHV-8 was detected in 88% (8/9) and in 85% (6/7) 4-week-old adherent spindle cell cultures with a macrophage phenotype derived from lesional skin of C-KS and AIDS-KS patients, respectively and established in the presence of TCM (Fig 4; Table 4).
HHV-8 Detection in Specimens From KS Patients
| . | C-KS (%) . | AIDS-KS (%) . |
|---|---|---|
| PBMC | 8/9 (88) | 6/9 (66) |
| Lesional skin | 9/9 (100) | 5/5 (100) |
| Uninvolved skin3-150 | 0/3 (0) | ND |
| Uninvolved skin3-151 | 1/3 (33) | ND |
| Cultured lesions | 8/9 (88) | 6/7 (85) |
| . | C-KS (%) . | AIDS-KS (%) . |
|---|---|---|
| PBMC | 8/9 (88) | 6/9 (66) |
| Lesional skin | 9/9 (100) | 5/5 (100) |
| Uninvolved skin3-150 | 0/3 (0) | ND |
| Uninvolved skin3-151 | 1/3 (33) | ND |
| Cultured lesions | 8/9 (88) | 6/7 (85) |
HHV-8 was detected by nested PCR as reported in Materials and Methods. Freshly isolated PBMC, lesions, or uninvolved skin examined immediately after the biopsy as well as 4-week old skin cultured in the presence of TCM were examined.
Abbreviation: ND, not done.
Uninvolved skin taken from sites far from the lesion.
Apparently uninvolved, perilesional skin.
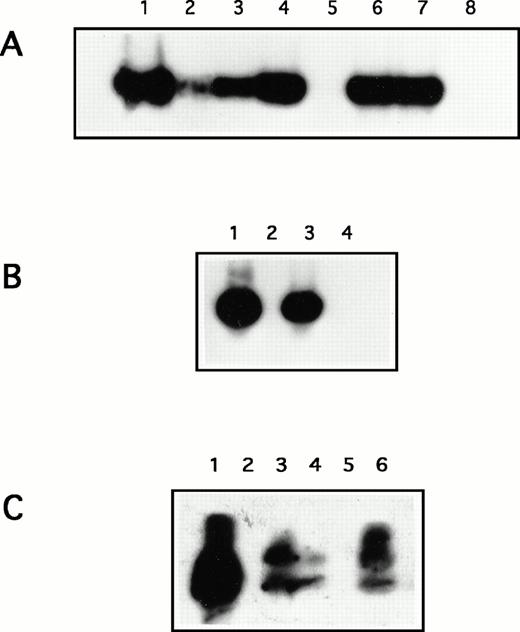
Representative Southern Blot analysis of a nested PCR by using KS1 and KS2 primers to amplify HHV-8 DNA sequences. (A) Lane 1, positive control; lanes 2 to 4 and 6 to 7, lesional skin from C-KS and AIDS-KS patients, respectively; lane 5, uninvolved skin; lane 8, negative control. (B) Lesional skin cultures from C-KS patients: lane 1, positive control; lane 2, negative control; lanes 3 and 4, cultured skin from 2 C-KS patients. (C) PBMC: lane 1, positive control; lane 2, negative control; lanes 3 to 6, PBMC from four C-KS patients; lane 4, PBMC from an AIDS-KS patient.
Representative Southern Blot analysis of a nested PCR by using KS1 and KS2 primers to amplify HHV-8 DNA sequences. (A) Lane 1, positive control; lanes 2 to 4 and 6 to 7, lesional skin from C-KS and AIDS-KS patients, respectively; lane 5, uninvolved skin; lane 8, negative control. (B) Lesional skin cultures from C-KS patients: lane 1, positive control; lane 2, negative control; lanes 3 and 4, cultured skin from 2 C-KS patients. (C) PBMC: lane 1, positive control; lane 2, negative control; lanes 3 to 6, PBMC from four C-KS patients; lane 4, PBMC from an AIDS-KS patient.
DISCUSSION
Experimental observations have demonstrated a role of inflammatory and angiogenic cytokines in KS development. These cytokines are increased in KS lesions from both AIDS-KS and C-KS patients and stimulate the growth of KS cells and the angiogenesis found in KS.36-44 46-49
We report here on the prevalent production of γIFN in PBMC and in cultures established from lesional skin of both AIDS-KS and C-KS patients. In lesional skin, γIFN production is associated with the presence of lymphoid T cells. In contrast, uninvolved skin from the same patients, which did not show a relevant lymphoid infiltration, was negative for cytokine production.
The immunophenotyping of TIL at day 0 or after 3 days in culture with TCM showed that the majority of them are of the T-cell lineage, in particular TCR α/β+CD8+ cells. These cells are consistently found in both AIDS-KS and C-KS. In patients with C-KS, no signs of immunodeficiency or any unbalance of the lymphocyte subpopulations are found at the PBMC level, thus arguing for a local recruitment of T cells in lesional tissue. These data are in agreement with two previous studies53,54 and with the findings of Fiorelli et al49a indicating that γIFN production is high in KS and it is mostly produced by T-CD8+ cells. In addition, an unusual subpopulation of T cells, bearing the TCR α/β+CD4−CD8−phenotype is evident after TCM-culture of TIL. This T-cell subpopulation generally represents a very minor (<2%) subset of normal lymphoid cells that is rarely found expanded in the peripheral blood.55 These cells have been associated with the development of autoimmune diseases56,57 and in the extrathymic maturation and differentiation of lymphocytes,58 even if their precise role remains to be determined. Interestingly, a study describing the emergence of this cell population in a primary immunodeficiency patient, who developed a graft-versus-host disease, has shown that these cells are able of producing γIFN.59
Altogether, our data suggest the presence of a local immune response in KS lesions. This resembles that reported in the peripheral blood during acute viral infections like acute cytomegalovirus infection and infectious mononucleosis60,61 and, locally, in the liver.58 In the latter case, the appearance of TCR α/β+CD4−CD8− and of TCR α/β+CD8+ cells, with apoptotic figures as those observed by us in the skin, have also been found. Th1 cytokines, contained in the TCM used by us to culture TIL, may have amplified the apoptosis of cells primed to die, like host immune cells involved in an antiviral response.62 This has already been described in cultures of lymphocytes from lymphocytic choriomeningitis virus-infected mice expanded in the presence of IL-2.63
Several infectious agents have been associated with KS; however, epidemiologic findings indicate that only HHV-8 is stringently associated with the disease.10-19,22 Recently, a molecular mimicry of human cytokines and cytokines response pathway genes by HHV-8 has been reported and it has been proposed that HHV-8 may interfere with the host immune response.64 We have found HHV-8 in PBMC, KS lesions, and in the short-term (4 weeks) cultures from KS lesions in the majority of the patients studied. Among hematopoietic cells, HHV-8 has been previously detected in B cells22,65 and rarely in T cells.66 However, more recent evidence indicates that the virus is present in circulating monocytes (Colombini et al, submitted) and in mononuclear cells including monocytes/macrophages infiltrating KS lesions.25,26 In addition, peripheral blood-derived KS-like spindle cells with macrophagic cell markers,23,45 which are also detected in KS lesions,67 were recently found in all forms of KS and they were found to be infected by HHV-8.24
In comparison to KS-derived spindle cell lines bearing markers of activated endothelial cells,49 our culture conditions have preferentially allowed the growth of T-TIL, as well as of adherent cells bearing markers of tissue macrophages.68 This is likely due to the higher amount of Th1 cytokines contained in the TCM used to expand our cultures in comparison to the TCM used to establish KS cells with an endothelial phenotype cited in other reports.23,36-43,49 In particular, our TCM is richer in IL-2, and this may account for the stimulation of T-CD8+cells to produce γIFN, which is a powerful activator of macrophages.69 Moreover, activation of the T cells may have induced growth of the adherent cell layer, as also reported in peripheral blood in a similar system, aimed at the long-term culture of human T lymphocytes.70
Our results, therefore, suggest that macrophages present in the adherent layer of our cultures carry HHV-8. In fact, although endothelial and spindle cells of the lesions are latently infected by HHV-8, they lose the virus on culture in the presence or absence of TCM (B.E., unpublished data, June 1995). Because KS lesions contain few or no B cells, but are rich in monocytes macrophages49a that are productively infected with HHV-8,25,26 it is likely that this cell type recruits the virus into tissues. In this context, the presence of TIL and their production of γIFN may represent a physiologic response to HHV-8, as found for other viral infections, including Epstein-Barr virus, a closely associated herpesvirus. In addition, the possibility that HHV-8 encoded chemokines, like viral macrophage inflammatory proteins-I and -II,64 may be chemoattractant for lymphoid cells cannot be ruled out.
Production of γIFN by infiltrating cells may play a pivotal role in KS pathogenesis as described in the accompanying manuscript by Fiorelli et al.49a This cytokine is able to induce normal endothelial cells to acquire a spindle morphology and to express markers similar to those of KS spindle cells. Moreover, among the cytokines detectable in TCM and in KS lesions, only γIFN is capable of inducing alone the release of biologically relevant amounts of bFGF,71,72 promoting the development of KS-like lesions in nude mice (see Fiorelli et al49a). Finally, this cytokine increases the expression of the CD40 antigen on endothelial and spindle cells of KS and this may amplify the tissue inflammation.73It must be underlined that the majority of the adherent cells growing in our skin cultures stained positive for HLA-DR, that is a biological marker of γIFN activity similar to in situ findings presented in the report by Fiorelli et al.49a Finally, it has been demonstrated that γIFN-treated endothelial cells acquire HLA-DR expression, favor proliferation of CD8+ cells that secrete Th1 cytokines, and may acquire antigen-presenting function.74
Our observations should be taken into account when designing clinical trials aimed at treating KS patients, especially if concomitantly affected by AIDS, where a shift to a Th1 response is often desired. In KS patients, the recruitment of lymphoid cells and the release of γIFN may represent a physiologic response to a foreign antigen, such as HHV-8. However, the hyperactivation of these cells may result in disease progression, as already described in an HIV-1 seropositive patient, who developed KS and visceral Leishmaniasis. The administration of γIFN together with anti-Leishmanial therapy, in fact, led to KS progression.75 Similarly, treatment of KS patients with IL-2 and γIFN induced progression of KS.76
ACKNOWLEDGMENT
We thank Dr G. Barillari (Department of Experimental Medicine, University of “Tor Vergata,” Rome, Italy) and Dr Paolo Monini (Laboratory of Virology, Isituto Superiore di Sanità, Rome, Italy) for helpful discussion and A. Lippa for editorial assistance.
Supported by grants from the IX AIDS Project from the Italian Ministry of Health (Rome), Associazione Italiana per la Ricerca sul Cancro (AIRC, Milan), and Ministero Università Ricerca Scientifica (MURST, Rome).
Address reprint requests to Barbara Ensoli, MD, PhD, Laboratory of Virology, Istituto Superiore di Sanità, Viale Regina Elena, 299, 00161 Rome, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal