Abstract
The role of T lymphocytes in the control of chronic myeloid leukemia (CML) after bone marrow transplantations has been clearly shown. This effect closely correlates with graft-versus-host disease (GVHD). A specific graft-versus-leukemia (GVL) effect separate from GVHD has been postulated but has been difficult to show. One possible target for specific GVL activity is the bcr-abl fusion protein characteristic of CML. We have investigated the use of normal peptide-pulsed dendritic cells for the generation of cytotoxic, bcr-abl–specific T cells from normal donors. T cells (CD3+, CD8+, TCRαβ+, and NK receptor-negative) generated from a normal donor (HLA A24, B52, B59, Cw1) after stimulation with autologous dendritic cells, primed with a 16 mer peptide spanning the b3a2 breakpoint of bcr-abl, lysed CML cells from the peripheral blood of seven patients with CML with the b3a2 breakpoint. CML cells from four patients with only the b2a2 breakpoint were not lysed. Phytohemagglutinin (PHA) blasts derived from peripheral blood of patients with CML were not lysed, suggesting that cytotoxicity was not due to alloreactivity. Blocking experiments with anti–HLA-A,B,C indicated that cytotoxicity was dependent on recognition of major histocompatibility complex (MHC) class I molecules, although cytotoxicity was not MHC-restricted because not all patients shared HLA types with the T-cell donor. Specificity for bcr-abl and absence of alloreactivity was confirmed by the presence of lytic activity against autologous and allogeneic class I HLA-A matched monocytes pulsed with the 16 mer bcr-abl fusion peptide, but not against unpulsed monocytes or monocytes pulsed with other peptides. These results show that bcr-abl–specific T cells with marked cytotoxic activity against CML cells can be generated and amplified from normal donor peripheral blood. Recognition of HLA molecules is essential for cytotoxicity but strict HLA identity is not required.
THE IN VITRO generation, amplification, and subsequent in vivo administration of cytotoxic T-cell lines and clones that can specifically kill tumor cells represents a potentially powerful therapeutic approach to treating malignancy. Theoretically, the junction-spanning sequences of oncogene fusion proteins make ideal targets for immunotherapy because they are not present in normal cells. Malignancies in which translocations result in fusion peptides include acute myeloid leukemia (AML) [t(15;17) in AML M3; t(8;21) in AML M2; and inv 16 in AML M4] and chronic myeloid leukemia (CML). CML is characterized by the reciprocal t(9;22) translocation that results in a bcr-abl fusion gene encoding a chimeric 210 kD fusion protein. Individual patients with CML have one or both of the two common chimeric p210 bcr-abl proteins (b2a2 and b3a2) that differ according to whether the breakpoint is between exons b2 and b3 or between exons b3 and b4.1,2 In addition to having amino acid sequences not normally combined in the same protein, the potential for immunogenicity of the bcr-abl fusion protein is enhanced by the presence, at the fusion point, of a codon for an amino acid not derived from either the bcr or abl proteins.3 A cellular immune therapeutic approach is particularly attractive in CML because donor lymphocytes have been clearly shown to be active in inducing complete remission in patients who relapse after bone marrow transplantation (BMT).4 5 It has been difficult to separate graft-versus-host disease (GVHD) due to alloreactivity, from a specific graft-versus-leukemia (GVL) effect targeting disease-specific antigens. However, there is increasing in vitro evidence that specific immune responses can be generated against bcr-abl expressing cells.
For the induction of a CML-specific cellular immune response, peptides only present in leukemic cells (such as bcr-abl) must be presented on the cell surface by HLA molecules. In vitro studies have confirmed that peptides derived from the bcr-abl fusion region can bind HLA class I molecules with high affinity allowing bcr-abl peptides to be presented on the cell surface by HLA molecules.6 The resulting major histocompatibility complex (MHC)-peptide complex must be sufficiently immunogenic to provoke an effective but specific immune response and T cells with the capacity to respond must be in the immune repertoire. Immunogenicity of b2a2 and b3a2 has been shown in vitro by the generation of bcr-abl–specific T-cell lines and clones.7-9Allogeneic, peptide-specific CD4+ T-cell proliferative responses against bcr-abl peptide-expressing cells confirm peptide presentation, immunogenicity, and the existence of T cells specific for bcr-abl in the T-cell repertoire of normal individuals.
An effective specific antileukemic effect also requires CML-specific T cells capable of killing leukemic cells either by direct cytotoxicity or by the induction of apoptosis. It has been difficult to show T cells with specific cytotoxic activity against leukemia cells expressing the fusion protein. There are few reports showing cytotoxic activity of leukemia-specific T cells against CML cells from clinical samples.10,11 Choudhury et al showed that T cells of patients with CML, generated by stimulation with autologous Philadelphia chromosome-positive dendritic cells (DCs), were cytotoxic against autologous CML cells.10 In addition, these cells inhibited growth of CML clonogenic precursors. The presence, in the peripheral blood of normal individuals, of cytotoxic bcr-abl–specific T cells capable of killing CML cells has not previously been shown.
In the studies described here, we show that CD8+ cytotoxic effector cells specific for bcr-abl fusion peptides can be generated from normal peripheral blood. These cells have direct cytotoxic activity against cells expressing bcr-abl, including fresh leukemia cells from patients with CML, and no significant alloreactivity. Our studies illustrate how potent antigen presenting cells, including DCs can be used to generate an effective immune response in situations in which the primary malignant cells have failed to do so.
MATERIALS AND METHODS
Separation of peripheral blood DCs and monocytes.
Peripheral blood DCs were obtained by using a series of density gradient centrifugation steps, including Ficoll, Percoll, and Metrizamide, that specifically enriches for DCs while removing monocytes and other mononuclear cell populations.12Peripheral blood mononuclear cells (PBMNCs) were separated with Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) before centrifugation through discontinuous Percoll (Pharmacia) gradients (Percoll 30%, 40%, 50%, and 75%). Monocyte depleted PBMNCs were recovered from the interface between Percoll 50% and 75% and cultured at 37°C in 5% CO2. After 24 hours DCs were separated from high-density lymphocytes by sequential centrifugation through 15% and then 14% Metrizamide. Purity of DC fractions was confirmed by assessment of morphology under phase contrast microscopy and by immunophenotyping (including lack of expression of CD3, CD14, CD20, and CD56). Cells were 91% HLA-DR++/CD14−; 0.0% HLA-DR+/14+; 0.0% HLA-DR−/CD14+; and 9% HLA-DR−/CD14−. Cells in the HLA-DR−/CD14− fraction were CD3+ (3%), CD20+ (1%), and CD56+(5%). The monocyte-rich fraction was obtained from the interface between Percoll 40% and 50%, cryopreserved in aliquots, and later thawed for use as autologous feeder cells and as target cells for cytotoxicity assays. The purity of this monocyte-rich fraction determined by CD14 positivity by flow cytometry was 70% to 85%. The same method of monocyte enrichment was used to obtain allogeneic monocytes from four healthy donors (HLA A24, HLA A24, HLA A2/A11, and HLA A2/A26) for use as target cells after peptide pulsing.
Establishment of CD8+ T-cell clones.
Normal donor T cells (1 × 105) were enriched from PBMNC and cultured with 5 × 103 irradiated autologous DCs. T-cell enrichment, resulting in 85% CD3+ cells by flow cytometry, was by monocyte depletion of PBMNCs followed by panning to deplete FcR+ cells with γ-immunoglobulin. Cultures were continued for 10 days in the presence of defined bcr-abl peptides as antigen (10 to 20 μg/mL). Culture medium (CM) was AIM-V (Gibco BRL, Gaithersburg, MD) supplemented with 10% normal human AB serum. Two 9 mer peptides GFKQSS-K-AL (P3) and SS-K-ALQRPV (P4) and a 16 mer peptide TGFKQSS-K-ALQRPVAS (P2) spanning the breakpoint of the b3a2 bcr-abl fusion protein were used as antigens in separate cultures. The specific breakpoint and the joining amino acid lysine are indicated by -K-. Donor HLA type was A24, B52, 59, Cw1 and was taken into account during peptide design. Of the possible 9 mer peptides that span the breakpoint of b3a2, P3 and P4 were predicted to be the most likely to be optimally presented by A24 and A2 respectively,13,14 although there is evidence that even these theoretically optimal peptides have suboptimal binding affinity for these HLA types.6,15 A 9 mer peptide Mart-1 (AAGIGILTV) specific for melanoma and melanocytes was used as a control peptide.16 Peptide purity, assessed by reverse-phase high-performance liquid chromatography, was greater than 95%.
After 10 days culture, CD8+ T cells (purity 98% to 99%) were separated with miniMACS (Miltenyi Biotec, CA) and restimulated with autologous antigen-pulsed monocytes in CM with added IL-1β (2.5 U/mL) and IL-2 (0.5 U/mL). After this secondary stimulation the in vitro primed T cells were restimulated weekly with autologous antigen-pulsed monocytes in CM with added IL-2 (2.5 U to 5 U/mL) and IL-4 (3 U/mL to 5 U/mL). After 6 to 8 weeks the resulting CD8 positive T-cell lines were analyzed for cytotoxic T lymphocyte (CTL) activity. Some of the T-cell lines were cloned after six stimulations with a limiting dilution method in 96-well microcloning plates.
Phenotypic analysis of established cytotoxic clones/lines.
The immunophenotype of T-cell clones/lines was determined by two-color flow cytometry with combinations of fluorescein- and phycoerythrin-conjugated monoclonal antibodies (MoAbs). MoAbs used were: anti-CD3, anti-CD4, anti-CD8, anti-TCRαβ, anti-TCRγδ, and MoAbs to p58 receptors (GL183 and EB6) that function as specific NK receptors for two different groups of the C locus.
Cytotoxic assays.
Cytotoxic assays were performed with a standard 4 to 6 hour51Chromium release assay. Effector target ratios were 10:1. All assays were performed in triplicate. Spontaneous release from target cells in the absence of CTL was less than 15% of maximum release. Peptide-specific lytic activity was first determined by using normal autologous and allogeneic monocytes (matched at HLA-A) pulsed with P2, P3, or P4 as targets. Controls were autologous and allogeneic monocytes pulsed with the 9 mer Mart-1 peptide. Allogeneic monocytes tested were HLA-A matched (A24, n = 2) and HLA-mismatched (A24 negative, n = 2). HLA-A, B, and C types are shown in Table1. The role of HLA-A24 in peptide recognition by T cells was assessed by performing cytotoxicity assays in the presence of anti-A24 MoAb.
HLA Restriction of BCR/ABLb3a2 Peptide P2-Specific T-Cell Clone (P218) by Using Monocytes Pulsed With P2
| Targets . | HLA-A . | HLA-B . | HLA-C . | % Specific Lysis αA2/A24MoAbs . | |
|---|---|---|---|---|---|
| (−) . | (+) . | ||||
| HLA-A24 positive monocytes | |||||
| NG (Auto) | 24 | 52/59 | 1 | 23 ± 3-150 | NT |
| JN (Allo) | 24 | 51/61 | 10 | 25 ± 2-150 | NT |
| NY (Allo) | 24 | 52/61 | 10 | 20 ± 3-150 | 0 |
| HLA-A24 negative monocytes | |||||
| OY (Allo) | 2/11 | 13/51 | 10 | 3 ± 2 | 1 ± 1 |
| KD (Allo) | 2/26 | 7/46 | 1/7 | 1 ± 1 | 0 ± 1 |
| Targets . | HLA-A . | HLA-B . | HLA-C . | % Specific Lysis αA2/A24MoAbs . | |
|---|---|---|---|---|---|
| (−) . | (+) . | ||||
| HLA-A24 positive monocytes | |||||
| NG (Auto) | 24 | 52/59 | 1 | 23 ± 3-150 | NT |
| JN (Allo) | 24 | 51/61 | 10 | 25 ± 2-150 | NT |
| NY (Allo) | 24 | 52/61 | 10 | 20 ± 3-150 | 0 |
| HLA-A24 negative monocytes | |||||
| OY (Allo) | 2/11 | 13/51 | 10 | 3 ± 2 | 1 ± 1 |
| KD (Allo) | 2/26 | 7/46 | 1/7 | 1 ± 1 | 0 ± 1 |
Results are expressed as % specific lysis at an E:T ratio of 10:1.
Abbreviation: NT, not tested.
Significant lysis P < .0002.
Cytotoxic activity against cells expressing the complete bcr-abl fusion protein with the b3a2 breakpoint was determined by using the K562 cell line that has the 9;22 translocation and constitutively expresses b3a2 bcr-abl. K562 cells express little HLA class I in the basal state but DNA typing confirmed the HLA type to be A 1101/3101 and B 1801/4001. Cytotoxicity assays with K562 cells were performed in an identical manner to the other cytotoxicity assays described above. The effect of interferon on cytotoxicity was determined by incubating K562 cells with interferon-γ (1,000 U/mL) for 4, 8, 24, and 48 hours. At each time point the level of HLA expression was assessed as K562 cells normally have little or no HLA class I expression. To further assess the role of class I molecules and to address some of the other molecular interactions involved in bcr-abl–specific cytotoxicity, MoAb blocking experiments were performed with anti-CD4, anti-CD8, anti-HLA-A,B,C, and anti-A2/A24 MoAbs (The Japanese Red Cross, Tokyo, Japan). Cytotoxic activity of cell lines and clones was determined with and without addition of blocking antibodies (20 μg/mL) to the51Chromium release assay cultures.
Cytotoxic activity against native CML cells from patients with chronic-phase CML (n = 11) was determined by using peripheral blood either fresh or after cryopreservation in 10% DMSO. Patient HLA types and degree of Ph1 positivity at the time of testing are shown in Table 2. Breakpoints, determined by reverse transcriptase polymerase chain reaction (RT-PCR), were b3a2 (one also with b2a2) in seven patients and exclusively b2a2 in four patients. To control for nonspecific lytic activity due to HLA or other allogeneic differences, bcr-abl negative control targets (T-cell PHA blasts) were generated from each patient studied by stimulating peripheral blood CD3+ T cells, purified using miniMACS (Miltenyi Biotec, CA) with phytohemagglutinin (PHA; 2 μg/mL) and IL-2 (10 U/mL, Boehringer, Mannheim, Germany) for 5 to 7 days. As an additional control for nonspecific killing due to allogeneic differences, effector T cells with the same immunophenotype and generated by stimulation with the same antigen presenting cells, but pulsed with Mart-1 rather than bcr-abl, were tested for cytotoxicity against CML cells. Cytotoxicity was also tested after 24-hour culture of PBMNC and T-cell PHA blasts from CML patients in the presence of interferon (IFN)-α (3,000 U/mL).
Lytic Activity of CD8+ T-Cell Clone (P210) Against Leukemia Cells
| Patient . | b3a2 . | b2a2 . | Sample . | HLA . | Ph1 Positivity % . | Lytic Activity (%) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukemia Cells . | CD3+ PHA Blasts . | ||||||||||
| A . | B . | DR . | IFN(−) . | IFN(+) . | IFN(−) . | IFN(+) . | |||||
| AS | + | − | PB | 2402 | 4006/1518 | 0406/1101 | 100 | 66 ± 3 | 80 ± 6 | 0 | 1 |
| HM | + | − | BM | 2402/3303 | 5401/3501 | 0406/0410 | 100 | 51 ± 5 | 46 ± 4 | 0 | 0 |
| SA | + | + | BM | 2402/0201 | 4403/5401 | 0405/1302 | 100 | 27 ± 3 | 44 ± 5 | NT | NT |
| AM | + | − | BM | 2402/0301 | 4402/5401 | 0403/1301 | 96 | 14 ± 2 | 20 ± 3 | 1 | 1 |
| FS | + | − | PB | 1101/3101 | 5101/1501 | 0406/0802 | 32 | 17 ± 2 | 36 ± 4 | 0 | 1 |
| MW | + | − | PB | 0201/2902 | 4403/5101 | 0407/0701 | 83 | 28 ± 3 | 36 ± 3 | 0 | 0 |
| KT | + | − | BM | 0201/2603 | 0702/4403 | 0101/1302 | 90 | 11 ± 2 | 21 ± 3 | NT | NT |
| BC | − | + | PB | 0301 | 3501 | 0101 | 50 | 4.0 ± 0.4 | 3.0 ± 1.2 | NT | NT |
| JA | − | + | PB | 2402/0301 | 0702/1402 | 0102/0701 | 100 | 2.1 ± 0.4 | 3.7 ± 0.0 | NT | NT |
| AO | − | + | PB | 2402/0201 | 0801/4402 | 0301/0401 | 30 | 1.2 ± 0.9 | 1.7 ± 0.4 | NT | NT |
| KP | − | + | PB | 0301 | 2703/3501 | 0404 | 25 | 2.4 ± 0.3 | −3.6 ± 2.1 | NT | NT |
| Patient . | b3a2 . | b2a2 . | Sample . | HLA . | Ph1 Positivity % . | Lytic Activity (%) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukemia Cells . | CD3+ PHA Blasts . | ||||||||||
| A . | B . | DR . | IFN(−) . | IFN(+) . | IFN(−) . | IFN(+) . | |||||
| AS | + | − | PB | 2402 | 4006/1518 | 0406/1101 | 100 | 66 ± 3 | 80 ± 6 | 0 | 1 |
| HM | + | − | BM | 2402/3303 | 5401/3501 | 0406/0410 | 100 | 51 ± 5 | 46 ± 4 | 0 | 0 |
| SA | + | + | BM | 2402/0201 | 4403/5401 | 0405/1302 | 100 | 27 ± 3 | 44 ± 5 | NT | NT |
| AM | + | − | BM | 2402/0301 | 4402/5401 | 0403/1301 | 96 | 14 ± 2 | 20 ± 3 | 1 | 1 |
| FS | + | − | PB | 1101/3101 | 5101/1501 | 0406/0802 | 32 | 17 ± 2 | 36 ± 4 | 0 | 1 |
| MW | + | − | PB | 0201/2902 | 4403/5101 | 0407/0701 | 83 | 28 ± 3 | 36 ± 3 | 0 | 0 |
| KT | + | − | BM | 0201/2603 | 0702/4403 | 0101/1302 | 90 | 11 ± 2 | 21 ± 3 | NT | NT |
| BC | − | + | PB | 0301 | 3501 | 0101 | 50 | 4.0 ± 0.4 | 3.0 ± 1.2 | NT | NT |
| JA | − | + | PB | 2402/0301 | 0702/1402 | 0102/0701 | 100 | 2.1 ± 0.4 | 3.7 ± 0.0 | NT | NT |
| AO | − | + | PB | 2402/0201 | 0801/4402 | 0301/0401 | 30 | 1.2 ± 0.9 | 1.7 ± 0.4 | NT | NT |
| KP | − | + | PB | 0301 | 2703/3501 | 0404 | 25 | 2.4 ± 0.3 | −3.6 ± 2.1 | NT | NT |
Lytic activity of CD8+ cell clone (P210) is shown against leukemia cells from patients with chronic-phase CML and PHA blasts before (IFN−) and after (IFN+) incubation with IFN.
RESULTS
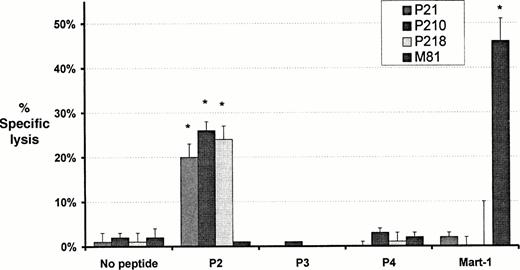
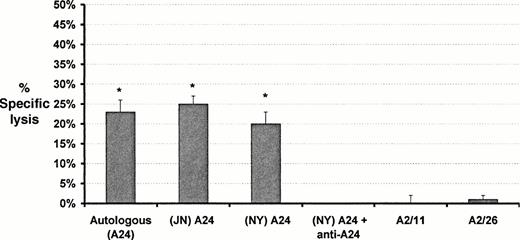
Induction of cytotoxic T-cell lines and clones by primary in vitro stimulation with bcr-abl fusion peptides. CD8+T-cell lines were generated in response to P2 (P21), P3 (P31), P4 (P41), and Mart-1 (M81). The cells were CD3+, CD8+, TCR αβ+, and negative for CD4, TCR-γδ, and NK receptors p58 (GL183 and EB6). Cloning of the P21 cell line produced two clones, P210 and P218. The P210 and P218 clones generated by using P2 showed significant lytic activity against autologous and allogeneic A24 positive monocytes from normal individuals pulsed with P2, but not against monocytes from the same sources pulsed with P3, P4, or Mart-1 (Figs1 and 2; Table1). Lytic activity was inhibited by the presence of anti-A 24 MoAb (Fig2). No lytic activity was observed against P2-pulsed monocytes from individuals who were not HLA-A24 positive (Fig 2; Table 1). The M81 cell line showed lytic activity against Mart-1–pulsed autologous monocytes but not against autologous or allogeneic monocytes pulsed with P2, P3, or P4 (Figs 1 and 2). Cell lines P31 and P41, generated against the shorter 9 mer peptides P3 and P4 respectively, did not show lytic activity against monocytes pulsed with these peptides despite the prediction that P3 was the optimal peptide for presentation for HLA-A24 (data not shown).
Cytotoxic activity of T-cell lines and clones against autologous monocytes pulsed with peptide. Monocytes pulsed with either a bcr-abl–derived peptide (P2, P3, or P4) or the melanoma peptide Mart-1 were used as targets for the cell lines P21 and M81 (generated against bcr-abl and Mart-1 respectively) and cell clones P210 and P218 derived from P21. Data shown represents the mean ± SD percent specific lysis in chromium release assays performed in triplicate with an E:T ratio of 10:1. *Significant lysis (P < .0002).
Cytotoxic activity of T-cell lines and clones against autologous monocytes pulsed with peptide. Monocytes pulsed with either a bcr-abl–derived peptide (P2, P3, or P4) or the melanoma peptide Mart-1 were used as targets for the cell lines P21 and M81 (generated against bcr-abl and Mart-1 respectively) and cell clones P210 and P218 derived from P21. Data shown represents the mean ± SD percent specific lysis in chromium release assays performed in triplicate with an E:T ratio of 10:1. *Significant lysis (P < .0002).
Effect of HLA-A type and HLA blocking antibodies on cytotoxic activity of the bcr-abl–specific T-cell clone (P218). Target cells in each case were monocytes pulsed with the 16 mer bcr-abl peptide P2. Results are expressed as mean ± SD specific lysis at an E:T ratio of 10:1. *Significant lysis (P < .0002). Cytotoxicity is shown against autologous and HLA-A matched monocytes but not against HLA-A mismatched monocytes. Addition of anti-HLA-A24 antibodies to assays blocked cytotoxic activity.
Effect of HLA-A type and HLA blocking antibodies on cytotoxic activity of the bcr-abl–specific T-cell clone (P218). Target cells in each case were monocytes pulsed with the 16 mer bcr-abl peptide P2. Results are expressed as mean ± SD specific lysis at an E:T ratio of 10:1. *Significant lysis (P < .0002). Cytotoxicity is shown against autologous and HLA-A matched monocytes but not against HLA-A mismatched monocytes. Addition of anti-HLA-A24 antibodies to assays blocked cytotoxic activity.
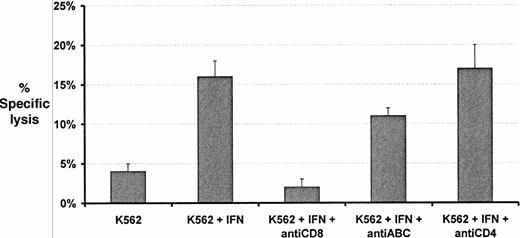
Incubation of K562 cells with IFN-γ resulted in an increase in class I expression with time, varying from less than 1% of cells positive at baseline to 50% positive after 48-hours incubation. Lytic activity was observed against K562 cells by clones P210 and P218 but only after HLA class I expression was induced on K562 cells by incubation with IFN (Fig 3). Lytic activity progressively increased as class I expression increased. The Mart-1–specific cell line M81 did not lyse K562 cells even after class I induction with IFN (Fig 3). Most of the lytic activity shown by P210 against IFN treated K562 cells was prevented by incubation with anti-CD8 whereas anti-CD4 did not influence lysis (Fig 4). The addition of anti-HLA class I MoAbs (w6/32) specific for nonpolymorphic determinants produced a minor reduction in lytic activity from 16 ± 2% lysis to 11 ± 1% lysis (mean ± SD).
Effect of increasing duration of culture with IFN-γ on expression of MHC class I and cytotoxicity against K562 cells by bcr-abl–specific clones (P210 and P218) and Mart-1–specific (M81) T cells. The percentage of K562 cells expressing HLA-A,B,C at each time interval in culture with IFN-γ is shown. K562 cells express minimal HLA class I in the basal state and are not lysed by bcr-abl–specific clones (P210 and P218). With increased time in culture with IFN the percentage of K562 cells expressing class I increases as does the mean number of class I molecules on each HLA-A,B,C positive cell as determined by fluorescence intensity. Specific cytotoxicity increased with duration in culture. Cytotoxicity data represents the mean ± SD percent specific lysis in chromium release assays performed in triplicate at an E:T ratio of 10:1. *Significant lysis (P < .006).
Effect of increasing duration of culture with IFN-γ on expression of MHC class I and cytotoxicity against K562 cells by bcr-abl–specific clones (P210 and P218) and Mart-1–specific (M81) T cells. The percentage of K562 cells expressing HLA-A,B,C at each time interval in culture with IFN-γ is shown. K562 cells express minimal HLA class I in the basal state and are not lysed by bcr-abl–specific clones (P210 and P218). With increased time in culture with IFN the percentage of K562 cells expressing class I increases as does the mean number of class I molecules on each HLA-A,B,C positive cell as determined by fluorescence intensity. Specific cytotoxicity increased with duration in culture. Cytotoxicity data represents the mean ± SD percent specific lysis in chromium release assays performed in triplicate at an E:T ratio of 10:1. *Significant lysis (P < .006).
Effects of blocking antibodies on the cytotoxic activity of P210 CD8+ T cell clones against K562 target cells in chromium release assays. The increase in cytotoxicity in response to IFN is partly abrogated by incubation with anti–HLA-A,B,C. Cytotoxicity is largely blocked by anti-CD8 but unaffected by anti-CD4. Cytotoxicity data represent the mean ± SD percent specific lysis in chromium release assays performed in triplicate at an E:T ratio of 10:1.
Effects of blocking antibodies on the cytotoxic activity of P210 CD8+ T cell clones against K562 target cells in chromium release assays. The increase in cytotoxicity in response to IFN is partly abrogated by incubation with anti–HLA-A,B,C. Cytotoxicity is largely blocked by anti-CD8 but unaffected by anti-CD4. Cytotoxicity data represent the mean ± SD percent specific lysis in chromium release assays performed in triplicate at an E:T ratio of 10:1.
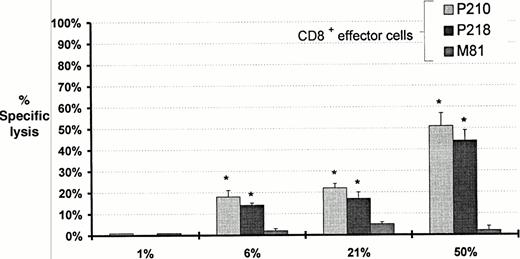
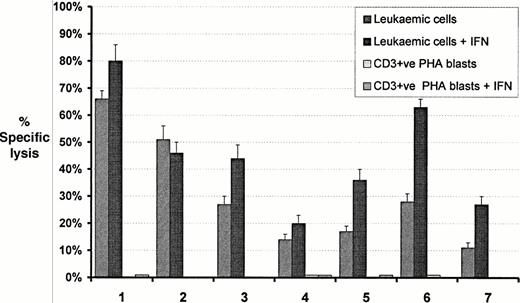
The lytic activity of P218 was tested against peripheral blood MNC from seven patients with CML and the b3a2 breakpoint and four patients with only the b2a2 breakpoint. Substantial lytic activity occurred against all seven patients with the b3a2 breakpoint but no lysis occurred against the patients with the b2a2 breakpoint (Fig5 and Table 2). Lytic activity was increased in six out of the seven b3a2 positive patients after treatment of the leukemic cells with IFN-α. Lytic activity was not observed against T-cell PHA-stimulated blasts from any of the patients expressing b3a2, even after treatment of the blasts with IFN. Blocking experiments using anti-HLA-A24 were performed on clinical samples from two HLA-A24 positive CML patients (AS and HM) resulting in complete blockade of cytotoxicity (data not shown).
Comparison of cytotoxic activity of the bcr-abl–specific clone P218 against peripheral blood MNC from patients with CML expressing transcripts for b3a2 and T-cell PHA blasts from each patient. Cytotoxicity of CML cells and each patient's control cells before and after incubation with interferon are shown. HLA types for each patient are listed in Table 1. Data shown represent the mean ± SD percent specific lysis in chromium release assays performed in triplicate at an E:T ratio of 10:1.
Comparison of cytotoxic activity of the bcr-abl–specific clone P218 against peripheral blood MNC from patients with CML expressing transcripts for b3a2 and T-cell PHA blasts from each patient. Cytotoxicity of CML cells and each patient's control cells before and after incubation with interferon are shown. HLA types for each patient are listed in Table 1. Data shown represent the mean ± SD percent specific lysis in chromium release assays performed in triplicate at an E:T ratio of 10:1.
DISCUSSION
In this study we show that cytotoxic T lymphocytes specific for bcr-abl and with little or no reactivity against alloantigens are present in the immune repertoire of normal individuals. These cells can be expanded in vitro while retaining antigen specificity, cytotoxicity, and the ability to lyse bcr-abl positive leukemic cells from patients with CML.
A number of previous studies confirmed that specific CD4+and CD8+ T cells from normal individuals can proliferate in response to cells bearing bcr-abl peptides.7-9 Earlier studies did not address cytotoxic activity of the responding cells. More recently, cytotoxic T cells able to lyse autologous bcr-abl expressing CML cells were generated from patients with CML by using DCs derived from the malignant Ph1 positive clone as antigen presenting cells.10 We extend these observations by showing that allogeneic, CD8+ cytotoxic T cells specific for bcr-abl and active against leukemic cells from patients with CML, can be generated from normal donors.
We used several strategies to confirm that cytotoxicity was bcr-abl–specific and not due to alloreactivity or nonspecific cytotoxicity. Autologous and allogeneic HLA class I matched monocytes could only be killed by the bcr-abl–specific cell lines and clones after pulsing with 16 mer bcr-abl breakpoint peptides. Unpulsed monocytes and monocytes pulsed with other peptides were not lysed. To exclude alloreactivity in clinical samples, T-cell PHA blasts from patients with CML were used as targets. These cells should carry the same alloantigens as leukemia cells but usually not bcr-abl. PHA blasts were not lysed (Table 2; Fig 5). In contrast we observed effective lysis of peripheral blood mononuclear cells from patients with predominantly Ph1 positive hemopoiesis. It could be argued that PHA blasts may be less susceptible to lysis in the culture conditions used than myeloid cells and therefore suboptimal as a control for alloreactivity. Although a direct comparison between leukemic myeloid cells and PHA blasts may not be completely valid, absence of lysis of PHA blasts in standard cytotoxicity assays suggests there is little alloreactivity. The lack of lysis against unpulsed monocytes and against cells only expressing b2a2 provides further evidence that alloreactivity is minimal. The marked reduction in cytotoxicity against peptide-pulsed monocytes and minor reduction in cytotoxicity against K562 cells produced by incubation with anti-class I antibodies (Figs 2 and 4) confirms a role for class I in the cytotoxic interactions.
DCs derived from normal peripheral blood were used as antigen presenting cells for primary stimulation of presumed naive T cells. Previous studies indicate that DCs are the most potent initiators of primary antigen-specific immune responses involving activation of naive T cells.17 Short peptides spanning the breakpoint of the bcr-abl fusion protein were used to prime dendritic cells. To optimize antigen presentation by use of the intracellular processing pathways, a 16 mer peptide spanning the breakpoint of bcr-abl was used. This allows selection by the antigen presenting cell of the optimal 9 mer sequence for presentation. Previous studies show that after processing, 9 mer is the optimal size for embedding within MHC-class I molecules for presentation to CD8+ T cells.18 It is unlikely that 16 mer peptides can be directly presented by HLA molecules already present on the cell surface.18
We also investigated pulsing antigen presenting cells with 9 mer peptides. Theoretically, these peptides can bind directly to vacant sites on surface MHC molecules without the requirement for incorporation into the cell and intracellular processing. A potential disadvantage of this approach is the requirement for prior knowledge of the optimal 9 mer sequence for each HLA type unless a pool of different peptides is used. A pool of peptides, many of which are unsuitable, may through competition reduce the potency of antigen presentation. Cell lines P31 and P41, generated against the shorter 9 mer peptides P3 and P4 respectively, did not show lytic activity against monocytes pulsed with these peptides (data not shown) although P3 had been predicted to be the optimal peptide for presentation by HLA-A24. CD8+ T cells generated against the 16 mer peptide P2 were also not lytic against monocytes pulsed with the P3 and P4 peptides, but were lytic against monocytes pulsed with the longer P2 peptide. These results suggest either specificity for sequences not represented in these two shorter peptides or poor presentation of exogenous 9 mer peptides compared with exogenous 16 mer peptides. These negative findings also support earlier reports that neither P3 nor P4 are presented by A24.6 15 The sequence of the peptide effectively presented as a result of intracellular processing of P2 is unknown.
Of particular interest is the apparent lack of MHC restriction in the cytotoxicity against leukemic cells from patients with CML. Randomly selected native CML cells were killed by bcr-abl–specific T cells despite lack of class I identity between donor and patient. The highest levels of cytotoxicity were observed against HLA-A matched CML targets. Matching at HLA-A was required for cytotoxicity when bcr-abl primed monocytes were used as models for bcr-abl expressing cells. An explanation for these observations is that when the density of presented antigen is high, as might occur in cells constitutively expressing bcr-abl, the otherwise weak interaction between the bcr-abl–specific T-cell receptor and the bcr-abl peptide plus MHC complex may be sufficient to induce cytotoxicity. Low level expression, as may be expected in the macrophage model (in which exogenous peptides compete weakly for sites on MHC molecules), is sufficient for killing only when the target cells share at least one HLA allele. Support for this concept comes from the observations with K562 cells which are also mismatched with the cytotoxic T cells at HLA-A and B. As the number of MHC molecules on the surface of K562 target cells increases after incubation with IFN (indicated by increasing fluorescence intensity on flow cytometry) there is the potential for increased density of MHC complexes bearing bcr-abl. This would predict for the observed proportional increase in cytotoxicity against K562 cells.
An additional possibility for lack of MHC restriction of cytotoxicity was that cytotoxicity was not mediated through recognition of antigen-MHC complexes by T-cell receptor (TCR) as may occur with NK-mediated killing. This seems to have been excluded by our observations by using blocking antibodies that indicate that recognition of MHC molecules is important for cytotoxic activity against macrophages (Fig 2) and clinical samples (2 patients studied, data not shown) and to a lesser extent K562 cells (Fig 4). Cytotoxicity against bcr-abl–pulsed monocytes and patient samples could be completely or substantially abrogated by blocking MHC class I molecules. The presence of T-cell–specific markers including CD3 and TCR, and the absence of the NK receptor p58 confirm that the cytotoxic cells isolated are T cells and not NK cells. The absence of cytotoxicity against K562 cells before the addition of interferon is functional evidence that the cell lines and clones do not share NK characteristics.
The importance of CD8 in the interactions between TCR and MHC-antigen complexes has recently been emphasized.19 It has been suggested that CD8 molecules dramatically increase the interaction between low-affinity TCR/MHC class I pairs by enhancing complementarity at the TCR-MHC/peptide complex interface. Our findings that cytotoxicity is substantially diminished when CD8 is blocked by anti-CD8 antibodies supports these previous observations.
In conclusion, our results indicate that cytotoxic T cells specific for bcr-abl and with little or no alloreactivity can be expanded from the peripheral blood of normal individuals. This shows that, at least in vitro, GVL can be separated from GVHD. If these results can be confirmed in systems scaled up for clinical use, therapeutic trials in suitable donor recipient pairs are warranted.
ACKNOWLEDGMENT
We are particularly grateful to Prof Jill Hows (University of Bristol, Bristol, UK) for helpful suggestions and discussions. We also thank the medical and scientific staff of The Japanese Red Cross, Central Blood Center (Tokyo, Japan) for providing normal apheresis products.
Address correspondence to Mie Nieda, PhD, Department of Research, The Japanese Red Cross Central Blood Center, Hiroo 4-1-31, Shibuya-ku, Tokyo, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 17.34 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal