Abstract
Present data suggest that the primary site of thrombopoietin (TPO) mRNA is the liver. Previously, we reported that specific murine liver endothelial cells (LEC-1) located in the hepatic sinusoids support in vitro megakaryocytopoiesis from murine hematopoietic stem cells suggesting that these cells may be a source of TPO. We report here that TPO and its receptor, c-mpl, are coexpressed on cloned LEC-1. Enzyme-linked immunosorbent assay (ELISA), biological assay, and flow cytometry studies confirmed the expression of both TPO and its receptor, respectively, at the protein level. TPO activity was enhanced in supernatants from LEC-1 treated with tumor necrosis factor (TNF)-α and γ-interferon (INF). Our results show that TPO through its receptor stimulated the growth of LEC-1 in vitro. These observations establish LEC-1 as a novel source of TPO in the liver. To our knowledge, this is the first report that liver endothelial cells express both TPO and its receptor, c-mpl, and our findings indicate that this cytokine constitutes a growth factor for liver endothelial cells in vitro.
RECENTLY, IN A MURINE model of induced hepatic extramedullary hematopoiesis, we showed that hematopoietic cells proliferate and differentiate in close association with a specific type of liver endothelial cells (LEC-1) located in the hepatic sinusoids of adult mice.1-3 In in vitro studies, we showed that cloned LEC-1 support the multilineage differentiation of murine hematopoietic stem cells (ie, Lin− Sca+cells) in long-term cultures (LTC).3 The presence of megakaryocytes in these LTC suggest that LEC-1 may constitute a microenvironment for promoting megakaryocytopoiesis from murine stem cells. Moreover, these findings suggest that LEC-1 may produce some megakaryocyte-stimulating factors.
Although many cytokines have been shown to promote in vivo and in vitro megakaryocytopoiesis,4 the recently cloned thrombopoietin (TPO), also called megakaryocyte growth and development factor, has been identified as the main regulator of megakaryocytopoiesis.5-9 Previous studies show that the liver is the primary source of TPO mRNA.10-12 TPO is also expressed in the bone marrow, spleen, and kidneys from both human and normal mice.13 14 This variety of sources suggests that TPO regulates megakaryocytopoiesis using both endocrine and paracrine mechanisms.
The cell surface receptor for TPO is the product of the proto-oncogene c-mpl, identified as the homolog of the oncogene v-mplpresent in the murine myeloproliferative leukemia virus.15-18 Previous studies have shown that c-mplis expressed in very early hematopoietic progenitors, endothelial cells (EC), and cells of megakaryocytic lineage including the platelet.19 20
Regarding the liver cells responsible for TPO expression, the hepatocytes are the only liver cells in which the TPO message has been reported.11 12 However, because our previous results show that LEC-1 can promote in vitro megakaryocytopoiesis, we hypothesized that these cells could be an alternative source of TPO in the liver. In this study, we tested this hypothesis by examining for the expression and secretion of TPO using a reverse-transcriptase polymerase chain reaction (RT-PCR) technique, enzyme-linked immunosorbent assay (ELISA), and a functional biological assay, respectively. We also examined the expression of TPO receptor (c-mpl) at mRNA and protein levels by RT-PCR and flow cytometry, respectively.
MATERIALS AND METHODS
Animals.
Male C57BL/6 mice (Charles River Laboratories, Wilmington, MA), 8 to 12 weeks of age, were used for this study. All animals were maintained under the standard of the American Association for Accreditation of Laboratory Animal Care (AAALAC).
Reagents.
Metrizamide was purchased from Sigma (St Louis, MO), rat collagen was purchased from Boehringer (Mannheim, Germany), and fluorescent wheat germ agglutinin (WGA-Rhodamine) was from USB (Cleveland, OH).
Cytokines.
Recombinant murine (rm) TPO was a kind gift from Dr Donald Foster (Zymogenetics, Seattle, WA). Recombinant murine gamma-interferon (rmγ-interferon [INF]) was purchased from Genzyme (Cambridge, MA). Recombinant murine tumor necrosis factor (TNF)-α was purchased from R&D Systems (Minneapolis, MN). The cytokines were resuspended in 10% human serum albumin (Sigma) before aliquoting. All cytokines were stored at −20°C, and all were used at the following concentrations: rmγ-INF at 2,000 U/mL and rmTNF at 25 ng/mL.
Antibodies.
Rabbit polyclonal antibodies antimouse c-mpl were generously provided by Dr Donald Foster (Zymogenetics) and phycoerythrin (PE) conjugated goat polyclonal antibodies against rabbit IgG from Southern Biotechnology Associates, Inc (Birmingham, AL). Fluorescein isothiocyanate (FITC)- or PE-conjugated rat monoclonal antibodies (MoAbs) antimouse intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) and biotin-conjugated rat MoAbs antimouse CD34 and streptavidin-PE conjugate were purchased from Pharmingen (San Diego, CA). Rat MoAbs antimouse CD16 used to block nonspecific Fc receptors were from Pharmingen.
LEC-1 isolation and cloning.
LEC-1 were isolated from liver of adult mice, according to previous methods.21 Briefly, the liver from an anesthetized mouse was perfused through the portal vein with Gey's balanced salt solution (GBSS). Tissue digestion was performed by perfusing 10 mL 0.1% Pronase in GBSS at 3 mL/min followed by 15 mL of 0.05% collagenase, 0.05% Pronase, and 0.0003% DNASE in GBSS at 2 mL/min. The liver was then minced and stirred in 20 mL GBSS containing 0.05% collagenase, 0.02% Pronase, and 0.0003% DNASE at 37°C for 10 minutes at a pH of 7.4. The cell suspension was passed through a screen, washed, resuspended, and centrifuged at 300g for 15 minutes in GBSS containing 17.5% (wt/vol) metrizamide. The interface containing LEC-1 was collected, washed in Iscove's modified Dulbecco's medium (IMDM) (BioWhittaker, Walkersville, MD), and counted. The purity of LEC-1 was assayed by von Willebrand factor (VIII/vWF) expression. Once separated, LEC-1 were cultured on collagen-coated 24-well plates in IMDM supplemented with 20% fetal calf serum (FCS) (BioWhittaker) and antibiotics. Cultures were maintained at 37°C in a humidified atmosphere of 5% CO2. To obtain pure endothelial cell cultures, after the fourth passage, when no hepatocytes or Kupffer cells were detected in the cultures, the LEC-1 were cloned by limiting dilution.3 The endothelial nature of LEC-1 clones was assessed by flow cytometry and RT-PCR by the expression of the following specific endothelial cell markers: CD34, VCAM, ICAM-1, VIII/vWF, fms-like tyrosine kinase (flt), and fetal liver kinase-1 (flk-1).22 LEC-1 clones were also characterized by their high WGA binding affinity.21 23 LEC-1 clones were maintained in IMDM-20% FCS and used in the experiments described below, after these cells reached confluence within the wells.
Detection of TPO and c-mpl transcripts in LEC-1 by RT-PCR method.
Total RNA was isolated from monolayers of LEC-1 clones using TRI REAGENT (Molecular Research Center, Cincinnati, OH). One microgram of the total RNA was reverse-transcribed in a total volume of 20 μL in buffer containing 50 mmol/L TRIS-HCL (pH 8.3), 75 mmol/L KCL, 3 mmol/L MgCl2, 10 mmol/L dithiothreitol, 10 mmol/L deoxynucleotide mixture, 100 pmol/L random hexamer oligonucleotides, and 200 U Moloney's murine leukemia virus reverse transcriptase (Perkin Elmer, Branchburg, NJ). PCR amplification of the cDNA was then performed using specific oligonucleotides, which were previously used for the detection of TPO and β-actin transcripts.13 22 The primers for murine TPO were 5′-TCTGTCCAGCCCCGTAGGTC-3′ (sense) and 5′-GTTCCATCCACAGGTCCGTG-3′ (antisense). Primers for β-actin were 5′-CATGATCTGGGTCATCTTTTCACGGTTTGGC-3′ (sense) and 5′-ATGGATGATGATATCGCTGCGCTGGTCGTC-3′ (antisense). Primers specific for the c-mpl message were 5′-GCAGCACCCAGTGGGACATACCAG-3′ (sense) and 5′-GCCATAGCGGAGTTCATGCCTCAGG-3′ (antisense). PCR conditions were 1 minute of denaturation at 95°C, annealing at 55°C for 1 minute, and extension at 72°C for 1 minute for 35 cycles. Analysis of the PCR products was performed by comparing them with the predicted PCR fragment size after ethidium bromide staining of the PCR products separated by electrophoresis in a 1% agarose gel.
TPO determination in LEC-1 supernatants by ELISA.
LEC-1 were grown to 90% confluence, the supernatant removed, and the cells were then cultured in serum-free medium (X-vivo; BioWhittaker) alone or containing γ-INF and TNF-α (2,000 U/mL and 25 ng/mL, respectively) for 24 hours at 37°C in a 5% CO2 atmosphere. Cell supernatants were collected, centrifuged at 700g for 15 minutes, passed through a 0.2 μm filter before use. The supernatants were analyzed by specific ELISA for mTPO (R&D Systems). A standard curve with a TPO-positive control was run in each assay. The sensitivity of these ELISAs was less than 20 pg/mL.
Evaluation of TPO activity of LEC-1–conditioned media on TPO-responsive cells.
LEC-1 were grown to 90% confluence, the supernatant removed, and the cells were cultured in X-vivo medium alone or containing γ-INF plus TNF-α at 2,000 U/mL and 25 ng/mL, respectively, and incubated for 24 hours. After incubation, the conditioned media were collected, centrifuged at 2,000 rpm for 15 minutes, and passed through a 0.2 μm filter before use. Next, we examined the presence of TPO activity in conditioned media from untreated or γ-INF/TNF-α–treated LEC-1 using murine BaF-3 cells, which were transfected with the murinec-mpl receptor (BaF3/MPLR) kindly donated by Dr Donald Foster (Zymogenetics). The response of these cells to purified mTPO was previously described.5 As the control, a parenteral BaF3 cell line, which does not express the murine c-mplreceptor, was included. BaF3/MPLR and BaF3 cells were incubated for 16 hours in X-vivo without cytokines. The cells were washed and plated in 96-well plates at 104 cells per well in 100 μL of X-vivo containing diluted conditioned medium from untreated or γ-INF/TNF-α–treated LEC-1, or from an irrelevant murine myeloma cell line (MPC11) untreated or treated with γ-INF plus TNF-α as described previously. After 24 hours, cell proliferation was measured by a colorimetric assay using a tetrazolium salt/MTS, (Promega, Madison, WI). Briefly, 50 μL of a stock solution of MTS was added to each well. The reduction of XTT by viable cells reflecting the number of cells per well was measured after 3 hours at 490 nm.
Detection of the TPO receptor on LEC-1 clones by flow cytometry.
We evaluated the expression of TPO receptor on the surface of LEC-1 in the following manner. LEC-1 were incubated with unconjugated rabbit polyclonal antibodies against murine c-mpl or with purified control rabbit IgG (Jackson ImmunoResearch Laboratories) and then with rat MoAbs antimouse CD16 to block nonspecific Fc receptors and R-PE–conjugated goat polyclonal antibody against rabbit IgG. Data collection and analysis of the fluorescent intensities were made using a FACSort (Becton Dickinson, San Jose, CA). Ten thousand events were acquired and analyzed using the Lysis II software program.
LEC-1 proliferation assay.
We studied the possible influence of TPO on the proliferation of LEC-1 in the following manner. LEC-1 were plated in 96-well/plates at 5 × 103 cells per well in X-vivo in the presence of various concentrations of rmTPO. After 48 hours of culture, cell proliferation was measured by a colorimetric assay using MTS (Promega), as described above.
Statistical analysis.
Results are reported as mean ± standard error (SE) from triplicate dishes in all assays. We tested the data from the experiments for statistical significance using the analysis of variance (ANOVA) and regression analysis. The level of significance was less than 0.05. All experiments were repeated at least three times and have documented reproducibility.
RESULTS
Isolation and cloning of LEC-1.
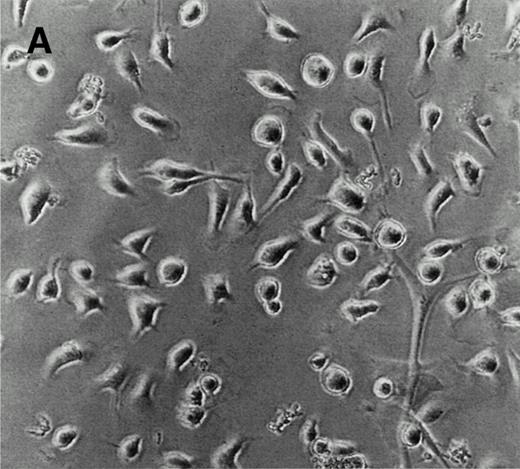
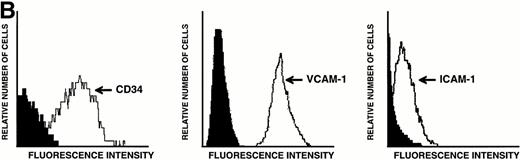
LEC-1 from adult mouse livers were isolated and cultured according to previous methods.3,21 The endothelial fraction of liver nonparenchymal cells, obtained by separation in a metrizamide gradient, uniformly expressed VIII/vWF (>95%) as determined by immunofluorescence microscopy (not shown). After four passages, when no hepatocytes or Kupffer cells were observed, LEC-1 were cloned by limiting dilution.3 Two clones of LEC-1 (A12 and C7) were selected because they maintained stable functional and phenotypical characteristics (described below) after 10 passages.3 A12 and C7 clones showed the typical phenotypic and morphologic characteristics of LEC-1.21,23 LEC-1 clones had, when 50% of confluence, spindle-like morphology and long cytoplasmic extensions (Fig 1A). Flow cytometry analysis showed that these cells also expressed CD34 antigen on their surface (Fig 1B). LEC-1 clones showed a cobblestone growth pattern (not shown). After reaching confluence, LEC-1 reduced their proliferation by contact inhibition. LEC-1 clones were also characterized by the pattern of cellular adhesion molecules (CAM) expressed on their surfaces. LEC-1 express VCAM-1 and ICAM-1 (Fig 1C and D). The endothelial nature of LEC-1 clones was confirmed by RT-PCR analysis of VIII/vWF factor, CD34, flt, and flk-1 expression (not shown). Other characteristics of the LEC-1 clones have been recently reported3 and are summarized in Table 1.
Characterization of LEC-1 clones. (A) Phase contrast microscopy of attached LEC-1 (A12 clone) 2 days after culture in IMDM 20% FCS. Note the spindle like morphology of these endothelial cells (original magnification × 20). Fluorescence-activated cell sorting (FACS) analysis of LEC-1 showing the expression of CD34 (B), VCAM-1 (C), and ICAM-1 (D) (thin line). Negative controls were stained with the respective isotype (black profile). Similar results were obtained with the C7 clone.
Characterization of LEC-1 clones. (A) Phase contrast microscopy of attached LEC-1 (A12 clone) 2 days after culture in IMDM 20% FCS. Note the spindle like morphology of these endothelial cells (original magnification × 20). Fluorescence-activated cell sorting (FACS) analysis of LEC-1 showing the expression of CD34 (B), VCAM-1 (C), and ICAM-1 (D) (thin line). Negative controls were stained with the respective isotype (black profile). Similar results were obtained with the C7 clone.
Characteristics of LEC-1 Clones
| PECAM-1-150 | + |
| VIII/vWF-151 | + |
| CD34-151 | + |
| fms-like tyrosine kinase (flt)-151 | + |
| Fetal liver kinase-1 (flk-1)-151 | + |
| Ulex europaeus I | +++ |
| WGA-binding | +++ |
| Mannose-receptor expression | +++ |
| PECAM-1-150 | + |
| VIII/vWF-151 | + |
| CD34-151 | + |
| fms-like tyrosine kinase (flt)-151 | + |
| Fetal liver kinase-1 (flk-1)-151 | + |
| Ulex europaeus I | +++ |
| WGA-binding | +++ |
| Mannose-receptor expression | +++ |
Additional details were previously published.3
Detected by flow cytometry (as negative staining control, PE-conjugated rat IgG2aκ was used).
Detected by RT-PCR (as negative control, PCR reaction without RNA template was included).
Expression of TPO by LEC-1.
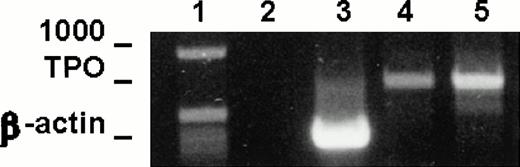
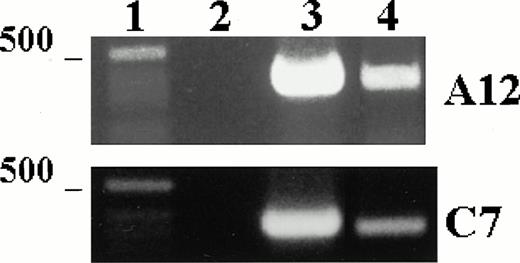
To test the expression of TPO mRNA in LEC-1 clones, we used the RT-PCR technique on total-RNA from A12 and C7 clones cultured in plain medium. The resulting cDNAs were amplified using primers specific for a 717-base sequence of the TPO message. Strong cDNA bands corresponding to the expected size for TPO message were obtained from RNA isolated from A12 and C7 clones (Fig 2). These results show that these cells expressed constitutively TPO transcripts.
TPO mRNA expression in murine LEC-1. RT-PCR was performed on 1 μg total RNA obtained from A12 and C7 LEC-1 clones. Reverse-transcription and amplification were performed as described in Materials and Methods with the primers specific for TPO. Constitutive expression for TPO was detected in A12 and C7 LEC-1 clones. 1: Molecular markers; 2: negative control (PCR reaction without RNA template); 3: internal control, β-actin; 4: A12 clone; 5: C7 clone.
TPO mRNA expression in murine LEC-1. RT-PCR was performed on 1 μg total RNA obtained from A12 and C7 LEC-1 clones. Reverse-transcription and amplification were performed as described in Materials and Methods with the primers specific for TPO. Constitutive expression for TPO was detected in A12 and C7 LEC-1 clones. 1: Molecular markers; 2: negative control (PCR reaction without RNA template); 3: internal control, β-actin; 4: A12 clone; 5: C7 clone.
Detection of TPO in LEC-1 supernatants by ELISA.
We tested the secretion of TPO to the culture medium in untreated or γ-INF/TNF-α–treated LEC-1 clones (A12 clone) by using a specific ELISA for murine TPO. TPO was not detected in the supernatants of untreated LEC-1 clones by ELISA. However, a significant amount of this cytokine was detected in cultures of LEC-1 stimulated with γ-INF/TNF-α (123 ± 0.05 pg/mL). These results confirmed the secretion of TPO by LEC-1.
TPO activity of LEC-1 conditioned media on TPO-responsive cells.
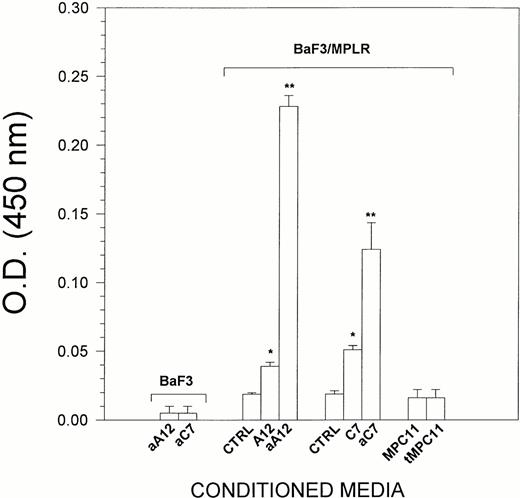
Because LEC-1 secreted TPO, we examined whether conditioned media from these cells exhibited TPO activity. For this purpose, BaF3/MPLR cells, which express the TPO receptor, were cultured in serum-free conditions for 24 hours with supernatants from LEC-1. Although TPO was not detected in the conditioned media from untreated LEC-1 clones, these exerted a significant effect on the growth of BaF3/MPLR (Fig 3). This could be due to a very low concentration of TPO that cannot be detected by ELISA, but exerts a proliferative effect on BaF3/MPLR. The conditioned media from γ-INF/TNF-α–treated A12 and C7 LEC-1 clones at 5% final concentration exerted a strong stimulatory effect on the proliferation of BaF3/MPLR (Fig 3). This effect was equivalent to that exerted by rmTPO at 20 U/mL on BaF3/MPLR (not shown). Conditioned media from untreated or γ-INF/TNF-α–treated LEC-1 clones did not exert any effect on the proliferation of parenteral BaF3 that do not express the c-mpl receptor (Fig 3). Likewise, supernatants from an irrelevant murine cell line (MPC11) untreated or treated with γ-INF/TNF-α did not exert any effect on the proliferation of BaF3/MPLR (Fig 3). Together, these results show the presence of TPO activity on LEC-1–conditioned media.
Effect of LEC-1–conditioned media on BaF3/MPLR proliferation. BaF3/MPLR and the parental BaF3 cells (1 × 104 cells/well) were cultured in serum-free media in the presence of medium alone (control), or conditioned media from untreated A12 and C7 LEC-1 clones (A12, C7) or from γ-INF/TNF-α–treated A12 and C7 LEC-1 clones (aA12, aC7), or from untreated or γ-INF/TNF-α–treated MPC11 (MPC11 and tMPC11, respectively). BaF3/MPLR and BaF3 cells were cultured at 37°C in a humidified atmosphere flushed with 5% CO2. After 16 hours, cell proliferation was assessed using a colorimetric method (see Materials and Methods). The absorbance was read at 490 nm. The results are expressed as the mean ± SE. Results are representative of three separate experiments. The increase in proliferation was statistically significant for A12 and C7 conditioned media with respect to the control (*, P ≤ .05), and for aA12 and aC7 conditioned media with respect to the untreated LEC-1 clones (A12 and C7) (**, P≤ .05).
Effect of LEC-1–conditioned media on BaF3/MPLR proliferation. BaF3/MPLR and the parental BaF3 cells (1 × 104 cells/well) were cultured in serum-free media in the presence of medium alone (control), or conditioned media from untreated A12 and C7 LEC-1 clones (A12, C7) or from γ-INF/TNF-α–treated A12 and C7 LEC-1 clones (aA12, aC7), or from untreated or γ-INF/TNF-α–treated MPC11 (MPC11 and tMPC11, respectively). BaF3/MPLR and BaF3 cells were cultured at 37°C in a humidified atmosphere flushed with 5% CO2. After 16 hours, cell proliferation was assessed using a colorimetric method (see Materials and Methods). The absorbance was read at 490 nm. The results are expressed as the mean ± SE. Results are representative of three separate experiments. The increase in proliferation was statistically significant for A12 and C7 conditioned media with respect to the control (*, P ≤ .05), and for aA12 and aC7 conditioned media with respect to the untreated LEC-1 clones (A12 and C7) (**, P≤ .05).
c-mpl expression by LEC-1.
We examined the expression of the TPO receptor, c-mpl, in the A12 and C7 LEC-1 clones using RT-PCR technique. cDNA obtained by reverse transcription was amplified using specific primers for a 322-base sequence of c-mpl message. A single band corresponding to the expected size (322 bp) was amplified in both A12 and C7 LEC-1 clones (Fig 4). These results show that LEC-1 constitutively express the TPO receptor, c-mpl.
c-mpl mRNA expression in murine LEC-1. RT-PCR was performed on 1 μg total RNA obtained from A12 and C7 LEC-1 clones. Reverse-transcription and amplification were performed as described in Materials and Methods with the primers specific for c-mpl. Constitutive expression for c-mpl was detected in both A12 and C7 LEC-1 clones. 1: Molecular markers; 2: negative control (PCR reaction without RNA template); 3: internal control, β-actin; 4: c-mpl.
c-mpl mRNA expression in murine LEC-1. RT-PCR was performed on 1 μg total RNA obtained from A12 and C7 LEC-1 clones. Reverse-transcription and amplification were performed as described in Materials and Methods with the primers specific for c-mpl. Constitutive expression for c-mpl was detected in both A12 and C7 LEC-1 clones. 1: Molecular markers; 2: negative control (PCR reaction without RNA template); 3: internal control, β-actin; 4: c-mpl.
Surface expression of c-mpl on LEC-1.
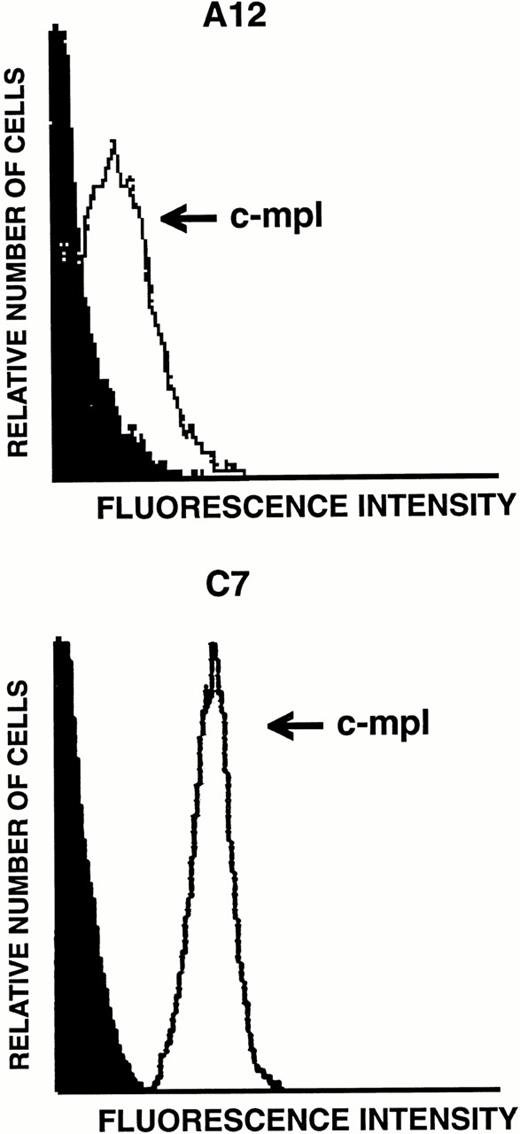
Because transcripts for TPO receptor were detected by RT-PCR in LEC-1, we next examined the levels of expression of this receptor on their surface. The expression of the TPO receptor was investigated by flow cytometry analyses on both A12 and C7 LEC-1 clones using a polyclonal antibody against murine c-mpl. High levels of this receptor were detected on the surface of these two LEC-1 clones (Fig 5). Similar results were obtained with freshly isolated LEC-1 (not shown).
Flow cytometric analysis of c-mpl expression on LEC-1 clones. A12 and C7 LEC-1 clones were incubated with rabbit polyclonal antibodies against c-mpl (thin line) or with purified control rabbit IgG (black profile) followed by R-PE–conjugated goat polyclonal antibodies against rabbit IgG.
Flow cytometric analysis of c-mpl expression on LEC-1 clones. A12 and C7 LEC-1 clones were incubated with rabbit polyclonal antibodies against c-mpl (thin line) or with purified control rabbit IgG (black profile) followed by R-PE–conjugated goat polyclonal antibodies against rabbit IgG.
Effect of TPO on the proliferation of LEC-1.
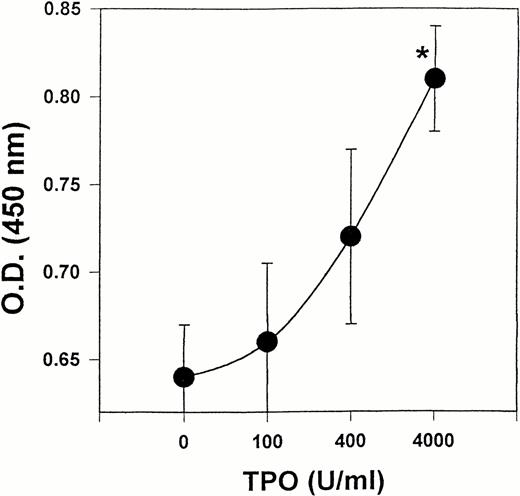
Because our results showed that LEC-1 constitutively express TPO receptor, we examined whether exogenous TPO might stimulate the growth of these cells in vitro. Effectively, exogenous TPO induced a stimulatory effect on the proliferation of LEC-1 cultured in serum-free conditions. This effect was significant at a high dose of TPO (4,000 U/mL) (Fig 6).
Effect of TPO on the proliferation of LEC. LEC-1 (A12 clone) were seeded in 96-wells at 5,000 cells/well in serum-free conditions. Different concentrations of mTPO were added to the cultures. After 48 hours, the proliferation of LEC-1 was assayed using a colorimetric method (see Materials and Methods). The absorbance of the reaction was read at 490 nm. The results are expressed as the means ± SE. Results are representative of three separate experiments. The increase in proliferation was statistically significant for TPO at 4,000 U/mL with respect to the control (*, P ≤ .05). Similar results were observed with C7 clone.
Effect of TPO on the proliferation of LEC. LEC-1 (A12 clone) were seeded in 96-wells at 5,000 cells/well in serum-free conditions. Different concentrations of mTPO were added to the cultures. After 48 hours, the proliferation of LEC-1 was assayed using a colorimetric method (see Materials and Methods). The absorbance of the reaction was read at 490 nm. The results are expressed as the means ± SE. Results are representative of three separate experiments. The increase in proliferation was statistically significant for TPO at 4,000 U/mL with respect to the control (*, P ≤ .05). Similar results were observed with C7 clone.
DISCUSSION
Numerous clinical and experimental observations indicate the liver's involvement in the control of platelet homeostasis. Clinical data show that certain liver diseases and massive hepatic resections are associated with thrombocytopenia.24-26 A low number of platelets are observed in about 30% of patients with hepatic cirrhosis.24,25 Low platelet counts associated with liver injury are reversed after the recovery from liver disease.25 In rodent models, partial hepatectomy results in thrombocytopenia.26 These findings suggested the hypothesis that the liver could produce factors capable of stimulating thrombopoiesis. The discovery of TPO as the main regulating factor of megakaryocytopoiesis5-9 and the liver as a major source of TPO mRNA11 confirmed this hypothesis. However, the liver cells responsible for the production of TPO mRNA are not completely defined. The hepatocyte is often considered the source of TPO because TPO mRNA was found in these cells in the liver, in hepatoma cell lines, and short-term liver cell cultures.11 12 In this study, we show for the first time that specific liver sinusoid endothelial cells (LEC-1) express both TPO and its receptor at mRNA and protein level.
Recently, in situ hybridization experiments show in the human and mouse liver that hepatocytes, but neither portal venule nor sinusoidal endothelial cells, show a significant signal of TPO expression.12-27 There are several possible explanations for the discrepancy between these and our studies that show that LEC-1 cells express TPO. First, the absence of a signal for TPO mRNA in the sinusoidal endothelial cells may be due to the difficulty in getting a good hybridization surface of these sinusoidal endothelial lining cells.28 In fact, it is well documented that morphometric evaluation of liver sinusoidal structures is very difficult due to their fine structure and flat morphology.28 Secondly, in situ hybridization studies could recognize only hepatocytes, as these cells occupy a significant surface area in the liver compared with sinusoidal endothelial cells.28 Hepatocytes could express more TPO than the sinusoidal endothelial cells, thus resulting in a false negative of the signal. Thus, we believe that no clear conclusions regarding the expression of TPO can be drawn from in situ hybridization techniques. In this study, using cloned sinusoidal liver endothelial cell (LEC-1), we show that these cells express and secrete TPO, demonstrating the production of this cytokine by LEC-1.
Our results show that LEC-1 constitutively express TPO mRNA. The fact that conditioned medium from γ-INF/TNF-α–treated LEC-1 contains TPO (detected by ELISA) and shows a strong TPO activity on the BaF3/MPLR cell line confirms the synthesis and secretion of biologically active TPO by these endothelial cells. Moreover, these results suggest that TPO production by LEC-1 cells may be regulated by inflammatory cytokines. As it has been proposed that thrombocytopenia is the stimulus for TPO production,9 we propose that several cytokines may also regulate the production of TPO at transcriptional and posttranscriptional levels. Considering these findings, LEC-1 provide a useful model to study the regulatory mechanisms of TPO production.
The expression of TPO receptors has been reported in various cells of hematogenous and nonhematogenous origin, including endothelial cells.19 20 We show here that LEC-1 also express c-mpl. The constitutive expression of both TPO and its receptor in LEC-1 suggests an autocrine role of this hematopoietic factor in this specific type of endothelial cell. Additional experiments are in progress in our laboratory to study whether the signal generated by TPO through its receptor regulates TPO production at transcriptional level on LEC-1.
The observed in vitro effect of TPO on LEC-1 proliferation indicates that this factor may play a role on the liver endothelial cell repair and regeneration. Our results show that the optimal growth stimulating dose for LEC-1 is 10-fold higher than those doses used for stimulating megakaryocyte growth in vitro, suggesting the existence of a threshold for endothelial cell proliferation over the megakaryocytic regulatory TPO concentrations. An important question suggested by these observations, for both TPO and endothelial cell research, is whether the in vitro effects of TPO on LEC-1 may occur in vivo. Our studies suggest that high levels of TPO would be required to stimulate the growth of the hepatic sinusoidal endothelial cells in vivo. Elevated levels of TPO might be observed during long-term administration of TPO or after administration of suprapharmacologic doses of this factor.29 Another condition in which high levels of TPO might occur is during liver regeneration. In fact, high expression of Ets family of transcription factor associated with the upregulation of TPO mRNA has been reported during liver regeneration.30 The results of studies from these or other conditions associated with elevated levels of TPO could confirm the endothelial growth-promoting properties of TPO on the liver sinusoidal endothelial cells.
In conclusion, LEC-1 constitutively express both TPO and its receptor, c-mpl. The production of TPO by LEC-1 can be modulated by inflammatory cytokines such as TNF-α and γ-IFN. Additionally, these data show that LEC-1 present functional TPO receptors. Activation of c-mpl on LEC-1 may play an important role in endothelial cell repair. Other LEC-1 responses to TPO involving regulation of endothelial cell surface receptors and adhesion molecules, and/or functional interactions with hepatocyte and liver nonparenchymal cells, are now being investigated in our laboratory.
ACKNOWLEDGMENT
We are extremely grateful for the ideas and suggestions provided by Dr Emilio Barberá-Guillem. The authors wish also to thank Wendy Wei, Jenny Kwak, and Heide Kammer for their technical assistance. We also thank Scott West, Ray Morgan, and Mark Mlyniec for their assistance in the preparation of this manuscript.
Supported by Minster Machine Company Foundation, Minister, OH; SBIR Phase II Grant No. 5-R44-AI34817-03; Mathile Family Foundation, Dayton, OH; Sigma Beta Sorority, Inc.; and Consejo Venezolano de Investigaciones Cientificas y Tecnologicas-CONICIT, Caracas, Venezuela.
Address reprint requests to José E. Cardier, MD, PhD, Pharmacology and Toxicology Laboratory, Hipple Cancer Research Center, 4100 South Kettering Blvd, Dayton, OH 45439-2092.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal