Abstract
Antibodies associated with heparin-induced thrombocytopenia/thrombosis (HITT) are now thought to be specific for complexes formed between heparin and platelet factor 4 (PF4), a basic protein found normally in platelet alpha granules. How these antibodies cause thrombocytopenia and, in some patients, thrombosis, is not fully understood, in part because purified antibodies that could be labeled and used as probes to characterize target epitopes have not been available. We developed a novel method for antibody purification involving binding to and elution from PF4 complexed to heparin immobilized by end-linkage (EL) to a solid phase. Isolated antibodies were functional and after biotinylation, reacted with heparin: PF4 complexes in the same manner as unlabeled antibodies. Using these probes, we found that antibodies from 11 patients with HITT recognized two, and probably three, distinct sites on heparin: PF4 complexes. The antibodies did not bind to PF4 complexed with heparin immobilized by multiple chemical cross-linkages, suggesting that the heparin molecule must be in a flexible, relatively unconstrained state to react with PF4 in such a way as to create sites for HITT antibody binding.
BETWEEN 1% AND 10% of patients treated with unfractionated heparin develop thrombocytopenia and a subset of these experience arterial or venous thromboembolism.1,2These complications appear to be caused by an unusual type of antibody that induces platelet activation in the presence of pharmacologic concentrations of heparin,3,4 but is difficult to detect in conventional assays that measure binding of immunoglobulin to intact platelets.5 Recent reports indicate that these antibodies are specific for complexes formed between heparin and platelet factor 4 (PF4), a heparin-binding protein normally found in platelet alpha granules.6-9 Immune complexes consisting of PF4, heparin, and these antibodies, formed on or in close proximity to the platelet membrane, interact with platelet Fc receptors to induce platelet activation and are thus important in the pathogenesis of heparin-induced thrombocytopenia/thrombosis (HITT).7
The epitopes on heparin: PF4 complexes recognized by antibodies associated with HITT have not yet been characterized, and it is uncertain whether a preferred site on the complex is targeted. This question is of more than academic interest because not all antibodies specific for heparin: PF4 are associated with adverse symptomatology,10-12 and a fuller understanding of the epitopes they recognize could help to explain why only some are pathogenic.
Purified antibodies that can be directly labeled with a tag such as biotin could provide useful probes with which to address this question, but to date, such reagents have not been available. We addressed this problem by developing a novel method for isolating antibodies from plasma of four patients who experienced HITT and found that they recognize at least two, and probably three distinct sites on heparin: PF4 complexes.
MATERIALS AND METHODS
Patients.
Large amounts of plasma were available from four patients (designated 1 through 4) who were treated by plasma exchange after developing heparin-induced thrombocytopenia with thrombotic symptoms. Each patient had been treated with unfractionated porcine heparin for 3 to 5 days after major surgery before developing thrombocytopenia and venous and/or arterial thromboses. In each case, serum was positive for heparin-dependent antibodies in both the serotonin release13 and heparin: PF4 enzyme-linked immunosorbent assay (ELISA)7 assays. Two patients (1 and 4), whose course was complicated by disseminated intravascular coagulation, had a fatal outcome. Smaller amounts of plasma were available from seven additional patients (designated 5 through 11) who met the same diagnostic criteria. All of these patients survived.
Buffers.
PB: Phosphate buffer 0.02 mol/L, pH 7.2; phosphate-buffered saline (PBS): PB containing 0.145 mol/L NaCl; PBS-Tw: PBS containing Tween-20, 0.05%.
Heparin: PF4 ELISA.
PF4 was isolated from human platelets and purified as described previously.7 Unfractionated heparin (specific activity, 169 IU/mg) was obtained from ESI Pharmaceuticals, Cherry Hill, NJ. PF4: heparin complexes were produced by incubating PF4 at a concentration of 10 μg/mL with heparin at a concentration of 0.4 IU/mL in PBS for 10 minutes at room temperature (RT). Fifty-microliter aliquots of the mixture were incubated overnight at 4°C in the wells of a polystyrene microtiter plate (Easywash; Corning, Corning, NY). The trays were washed three times with PBS-Tw and were blocked for 30 minutes at RT with PBS-Tw containing 20% fetal bovine serum (Hyclone Labs, Logan, UT). We previously showed that complexes formed at this ratio of PF4 to heparin are optimal for detection of antibodies associated with HITT.7
Determination of antibody isotype and subclass.
Antibodies specific for heparin: PF4 complexes were detected by adding 50 μL of plasma, usually diluted 1:50 in PBS, to each well and incubating for 60 minutes at RT. After three washes with PBS-Tw, bound IgG, IgA, and IgM was detected with alkaline phosphatase-labeled murine monoclonal antibodies specific for these Ig classes (Zymed Labs Inc, San Francisco, CA) as previously described.7,14 IgG subclass was determined with alkaline phosphatase-labeled monoclonal probes (Zymed).14 The predominant antibody species were found to be IgG1 (patients 2 and 3), IgG3 (patient 1), and IgA (patient 4) (Table 1).
Class and Subclass Specificity and Titer of Antibodies From Patients 1 Through 4
| Patient . | Immunoglobulin Class/Subclass . | ||||
|---|---|---|---|---|---|
| G1 . | G2 . | G3 . | A . | M . | |
| 1 | 0* | 10 | 500 | 10 | 0 |
| 2 | 250 | 0 | 100 | 25 | 100 |
| 3 | 100 | 0 | 0 | 10 | 0 |
| 4 | 50 | 0 | 10 | 500 | 0 |
| Patient . | Immunoglobulin Class/Subclass . | ||||
|---|---|---|---|---|---|
| G1 . | G2 . | G3 . | A . | M . | |
| 1 | 0* | 10 | 500 | 10 | 0 |
| 2 | 250 | 0 | 100 | 25 | 100 |
| 3 | 100 | 0 | 0 | 10 | 0 |
| 4 | 50 | 0 | 10 | 500 | 0 |
*Values shown are the highest dilution of plasma giving a positive reaction in ELISA.
Isolation of immunoglobulins.
Total immunoglobulins were initially purified from 100 mL of plasma from patients 1 through 4 on T-gel (Pierce, Rockford, IL), a thiophilic adsorption agarose gel specific for immunoglobulins of all classes and subclasses.15
Unidirectional immobilization of heparin fragments on diamino-dipropylamine (DADPA) agarose (end-linked [EL] heparin).
Controlled digestion of heparin with nitrous acid was performed as described by Hoffman et al.16 One gram of porcine heparin (specific activity, 176 IU/mg) (Sigma Chemical Co, St Louis, MO) was dissolved in 20 mL of distilled water and the pH was adjusted to 2.5 with 0.1 mol/L HCl. A sodium nitrite solution (15 mg sodium nitrite in 1 mL of distilled water) was added dropwise over a period of two minutes and the mixture was incubated for 2 hours at RT with stirring. The pH was then adjusted to 7.0 with 0.1 mol/L NaOH and the mixture was dialyzed twice against 5 L of water using a Spectra/POR membrane (Spectrum, Laguna Hills, CA) having a molecular weight cutoff of 3.5 kD. The dialyzed solution, containing heparin fragments larger than 3.5 kD, was passed through a 0.2 μm filter and the concentration of heparin was measured by a modified Toluidine blue assay.17
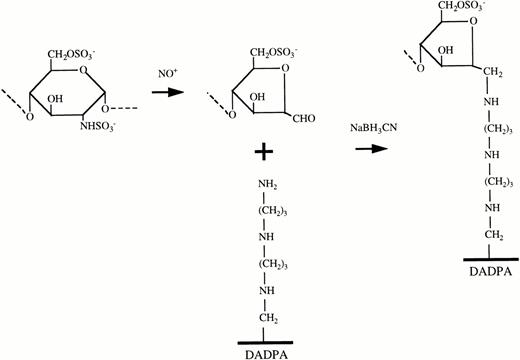
Each fragment produced by nitrous acid digestion of unfractionated heparin carries a formaldehyde residue at the new reducing terminus, which can be linked to a tertiary amine by reductive amination16 as shown schematically in Fig 1. To distance the immobilized heparin fragments from the solid surface, we linked them to DADPA residues immobilized on cross-linked 4% beaded agarose (Pierce). DADPA is a linear molecule containing seven carbon and two nitrogen residues and a terminal amino group. It has a loading capacity of 16 to 20 μmol of available amino groups per mL of gel and a particle size ranging from 45 to 165 μm diameter (median, 90 μm). A total of 2 mL of DADPA gel in a 4-mL slurry was washed twice with 0.1 mol/L sodium phosphate buffer, pH 7.4. A total of 60 mg of digested heparin in a 10-mL solution of 0.1 mol/L sodium cyanoborohydride was added and the mixture was adjusted to pH 5.0 with 1 N HCl. After incubation for 4 hours at RT with continuous agitation, the gel was washed three times with 0.02 mol/L PBS, pH 7.2 and was stored at 4°C overnight.
Reactions involved in immobilization of heparin by EL. The GAG chain is cleaved into smaller fragments, each bearing a formaldehyde (CHO) group at one end by controlled digestion with nitrous acid. The heparin fragments are then attached through these formaldehyde groups to free amino groups displayed on the solid phase by the Schiff base reaction.
Reactions involved in immobilization of heparin by EL. The GAG chain is cleaved into smaller fragments, each bearing a formaldehyde (CHO) group at one end by controlled digestion with nitrous acid. The heparin fragments are then attached through these formaldehyde groups to free amino groups displayed on the solid phase by the Schiff base reaction.
Agarose beads coated with heparin attached by multiple chemical cross-linkages (XL heparin) were obtained from Sigma. The quantity of heparin immobilized on the two types of beads was measured by the Toluidine blue method.17 EL and XL beads contained 6.3 and 0.79 mg heparin per mL of packed beads, respectively. The larger quantity of heparin on the EL beads (eight times the amount on the XL beads) was expected because each molecule is tethered to the surface only by a single terminal saccharide residue, allowing the remainder to extend away from the surface. In contrast, XL heparin is covalently linked by way of free amino residues randomly along the entire length of the saccharide chain and more or less follows the configuration of the surface to which it is bound. Accordingly, a molecule of XL heparin occupies a much larger surface area than a molecule of EL heparin.
Measurement of PF4.
PF4 bound to immobilized heparin was measured with an ELISA. A measured aliquot of beads, preincubated with different quantities of PF4 and then washed, was incubated for 1 hour at RT with a 1:100 dilution (saturating concentration) of rabbit antiserum specific for PF4 (Celsus Laboratories, Cincinnati, OH). After three washes with PBS containing 0.05% Tween 20 (PBS-Tw), the beads were incubated for 1 hour with alkaline phosphatase-labeled goat antirabbit IgG (H+L chain specific) (Zymed Labs) at a dilution of 1:500. After washing four times with PBS-Tw, bound probe was detected with PNPP (p-nitro phenyl phosphate) substrate (Zymed Labs). After 30 minutes, the mixture was rapidly centrifuged. The supernatant was transferred to wells of a microtiter plate and absorbance was determined at 405 nm, using 650 nm for reference values.
Protein G and B affinity purification of immunoglobulin.
Immunopure columns containing 2 mL of immobilized protein G were obtained from Pierce Chemical. A protein B column was prepared by cross-linking 1 mg of protein B (CalBioChem, La Jolla, CA) to 1 mL Sepharose CL-4B (Sigma) activated with cyanogen bromide (CNBR) according to the manufacturer's instructions. Protein G binds all subclasses of IgG, but not IgA or IgM,18 while protein B binds IgA, but not IgG or IgM.19
Biotin labeling of affinity purified antibodies.
Affinity-purified antibodies were labeled with biotin as described by Furihata et al.20 In brief, 0.5 mg of antibody in 0.4 mL PBS was dialyzed overnight against 0.1 mol/L NaHCO3. The dialyzed preparation was labeled with NHS-LC-biotin (Pierce) at 0.25 mmol/L final concentration in 0.5 mL total volume by incubating at RT for 4 hours on an orbital shaker. The final preparation was dialyzed against 2 L of PBS and was stored at 4°C in 0.05% NaN3until used. Biotin-labeled antibody binding was determined using alkaline phosphatase-conjugated streptavidin (Zymed) followed by addition of PNPP substrate.
Competitive inhibition of the binding of biotinylated antibody to immobilized heparin-PF4 complexes.
Saturating quantities of unlabeled, affinity-purified immunoglobulin or plasma containing antibody were added in triplicate to microtiter plate wells containing immobilized heparin: PF4 complexes for 60 minutes at RT. A subsaturating quantity of biotin-labeled antibody was then added, and the mixture was incubated for an additional 60 minutes. The plates were then washed three times with PBS and bound biotin was detected with alkaline phosphatase-conjugated streptavidin and PNPP substrate.
Peptide synthesis.
Peptides corresponding to the C-terminal 15 (PF4-15 = QAPLYKKIIKKLLES) and 26 (PF4-26 =LKNGRKICLDLQAPLYKKIIKKLLES) amino acids (AA) of the PF4 monomer and a scrambled tail 26-mer (scrambled at the 11 N-terminal residues) (PF4-26-Scr = GDRNLCLKLIKQAPLYKKIIKKLLES) were synthesized by the Protein Biochemistry Core Laboratory of the Blood Research Institute using a 950 plus peptide synthesizer (Millipore, Bedford, MA) and were purified on a C-18 column in reverse phase high-performance liquid chromatography (HPLC). Purity of the final product was determined to be greater than 95% by mass spectrometry.
RESULTS
Beads coated with heparin by end-linkage (EL) and cross-linkage (XL) bind PF4 equally well relative to their heparin content.
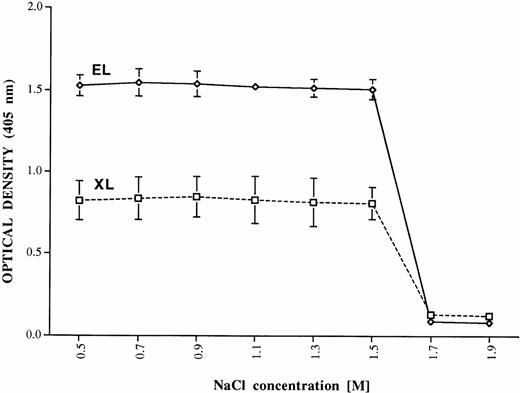
The interaction of PF4 with heparin immobilized by EL and XL to the solid phase is shown schematically in Fig 2. In preliminary studies, binding of PF4 to the two types of heparin was compared by incubating EL and XL beads with various quantities of PF4, washing, and measuring bound PF4 by ELISA. EL and XL beads were capable of binding 2.0 and 0.25 μg of PF4 per μL of beads, respectively at saturation (data not shown). Because the EL beads carried about eight times as much heparin as the XL beads, this indicates that EL and XL heparin bind PF4 equally well on a molar basis. To compare their avidity for PF4, EL and XL beads were saturated with PF4 and washed with isotonic buffer. Aliquots of the saturated beads were washed three times with PB containing various concentrations of NaCl and PF4 remaining on the beads was measured by ELISA. As shown in Fig 3, significant amounts of PF4 eluted from the beads only when they were exposed to NaCl at a concentration greater than 1.5 mol/L. Thus, EL and XL heparins are similar in respect to both their molar capacity and their avidity for PF4.
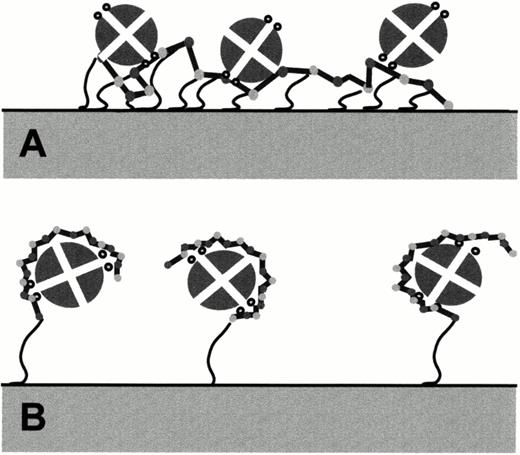
Probable modes of interaction between the PF4 tetramer and heparin immobilized by multiple cross-linkages (A) and by EL (B). Small circles on each side of the PF4 tetramer denote (in cross section) alpha helical regions known to be important for heparin binding. The schemes shown are consistent with experimental observations, but the exact nature of heparin: PF4 interaction is not yet known.
Probable modes of interaction between the PF4 tetramer and heparin immobilized by multiple cross-linkages (A) and by EL (B). Small circles on each side of the PF4 tetramer denote (in cross section) alpha helical regions known to be important for heparin binding. The schemes shown are consistent with experimental observations, but the exact nature of heparin: PF4 interaction is not yet known.
Elution of PF4 bound to heparin immobilized by EL and XL at various concentrations of sodium chloride. PF4 (ordinate) was eluted from both preparations by treatment with NaCl 1.7 mol/L, but not 1.5 mol/L. Brackets indicate ± 1.0 standard deviation (SD).
Elution of PF4 bound to heparin immobilized by EL and XL at various concentrations of sodium chloride. PF4 (ordinate) was eluted from both preparations by treatment with NaCl 1.7 mol/L, but not 1.5 mol/L. Brackets indicate ± 1.0 standard deviation (SD).
HITT antibodies bind to PF4 complexed with EL, but not XL heparin.
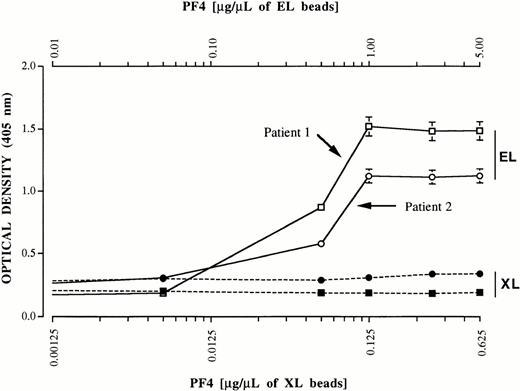
The ability of PF4 immobilized on EL and XL heparin to bind antibodies was investigated in a pilot study. A total of 20 μL of EL beads and 160 μL of XL beads (containing equal amounts of immobilized heparin) was incubated with PF4 at concentrations ranging from subsaturating to 2.5 times saturation in a final volume of 400 μL PBS. After 1 hour, purified total immunoglobulins (1.0 mg) from patients 1 and 2 were added to each mixture in a final volume of 0.5 mL and incubated for 2 hours with gentle agitation. After three washes with PBS containing 0.05% Tween-20 (PBS-TW), immunoglobulin bound to the beads was detected with alkaline phosphatase-labeled goat antihuman Ig (H+L chain specific) (Zymed Laboratories) and ELISA as described for measurement of PF4 (Materials and Methods). As shown in Fig 4, immunoglobulin bound readily to PF4 associated with EL, but not XL heparin. On the basis of these findings, we concluded that EL beads coated with 1.0 μg PF4 for each 1.0 μL of beads were optimal for the specific binding of HITT antibodies and that PF4 bound to XL heparin was incapable of binding antibody.
Reactions of IgG antibodies (ordinate) from patients 1 (squares) and 2 (circles) with various quantities of PF4 (abscissa) bound to heparin immobilized by EL and XL to agarose beads. A ratio of 1 μg PF4/μL of EL beads was optimal for antibody binding. Antibody failed to bind XL heparin beads, even at a ratio of 0.625 μg PF4/μL of beads, about 2.5 times the saturating concentration. Values shown are the average of duplicate determinations. No detectable immunoglobulin binding occurred when either preparation was incubated with a total immunoglobulin fraction from normal plasma (not shown).
Reactions of IgG antibodies (ordinate) from patients 1 (squares) and 2 (circles) with various quantities of PF4 (abscissa) bound to heparin immobilized by EL and XL to agarose beads. A ratio of 1 μg PF4/μL of EL beads was optimal for antibody binding. Antibody failed to bind XL heparin beads, even at a ratio of 0.625 μg PF4/μL of beads, about 2.5 times the saturating concentration. Values shown are the average of duplicate determinations. No detectable immunoglobulin binding occurred when either preparation was incubated with a total immunoglobulin fraction from normal plasma (not shown).
Purified, functional antibody was obtained by elution from PF4 bound to EL heparin.
Purification of specific antibodies from plasma of patients 1 through 4 was accomplished by adding 50 mg of total immunoglobulin from each patient to 1 mL of EL beads previously treated with 1.0 mg of PF4. After incubation for 4 hours at RT with gentle agitation, the beads were washed three times with PBS, and bound antibody was eluted together with PF4 by adding 1.6 mol/L NaCl in PB. No residual antibody was detected in the absorbed immunoglobulin preparations. The eluates were diluted sixfold with PB to lower the NaCl concentration to 0.25 mol/L and were applied to columns containing protein G (patients 1 through 3) or protein B (patient 4). The columns were washed with PBS to remove residual PF4 and bound Ig was eluted with 0.5 mol/L acetate buffer, pH 3.0. Eluted fractions were immediately neutralized with 1.5 mol/L TRIS-Cl, pH 8.8. Fractions containing antibody (determined by absorbance at 280 nm) were dialyzed against 5 L of PBS followed by adsorption with 70 mg Ecteola cellulose equilibrated in PBS to remove any residual heparin and heparin-PF4 complexes7 and were concentrated with a Centricon-30 concentrator (Amicon-Millipore, Bedford, MA).
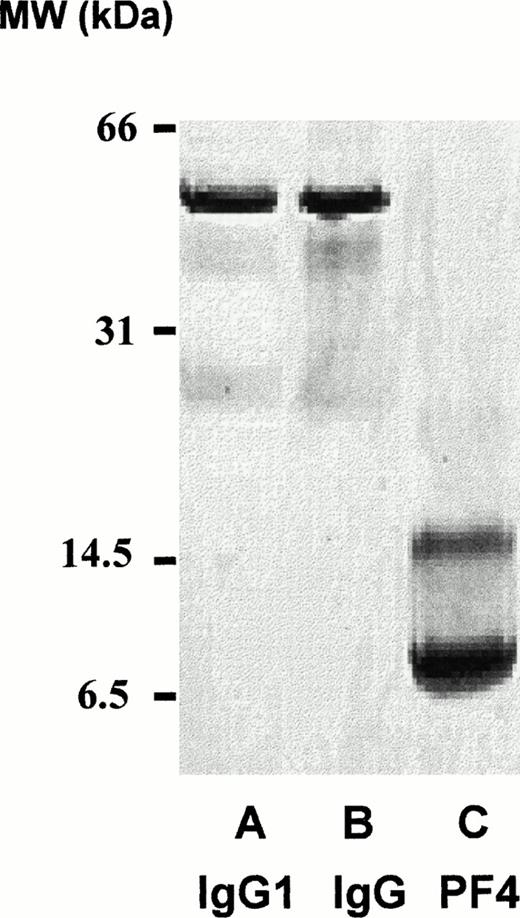
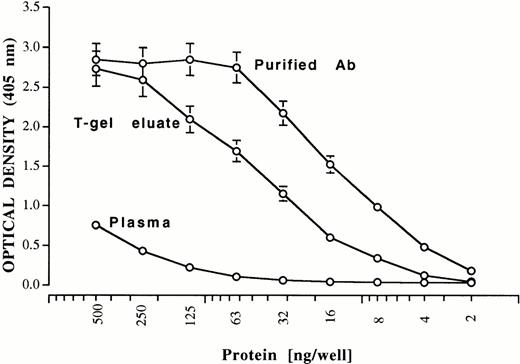
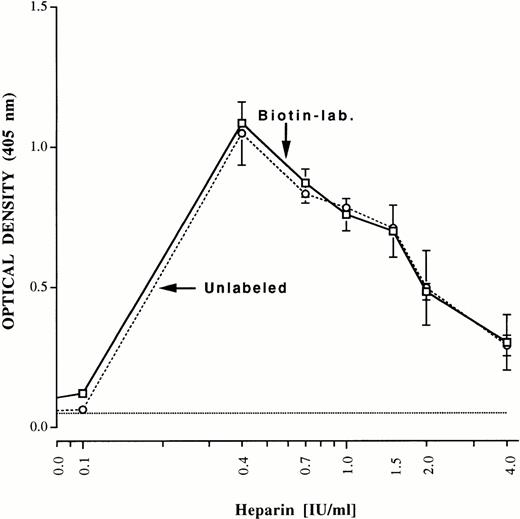
Aliquots of the final preparations were reduced, electrophoresed in 20% sodium dodecyl sulfate (SDS), and subjected to silver staining. Only bands corresponding to immunoglobulin, heavy and light chains, which were free of PF4 contamination, were observed as shown in Fig 5 for patient 2. Quantities of purified immunoglobulin (determined by the bicinchoninic acid (BCA) assay, Pierce) obtained from each patient (IgG for patients 1 through 3 and IgA for patient 4) were 1.12 mg (patient 1), 0.68 mg (patient 2), 0.39 mg (patient 3), and 0.58 mg (patient 4). As little as 4 ng of purified antibody gave a significant positive reaction in the heparin: PF4 ELISA (shown for patient 1) (Fig 6). Purification achieved relative to the protein content of starting plasma was about 125-fold. An aliquot of each of the purified antibody preparations was labeled with biotin and the ability of the labeled antibodies to bind to heparin: PF4 complexes was compared with that of unlabeled antibody using alkaline phosphatase-labeled goat antihuman Ig (H+L chain specific, Zymed). As shown in Fig 7 for patient 1, reactions of biotin-labeled and unlabeled antibody from the same patient were indistinguishable. Complexes optimal for antibody detection were formed by mixing heparin 0.4 IU/mL and PF4 10 μg/mL in confirmation of previous studies.7
Twenty percent SDS gel electrophoresis (reduced, silver stain) of affinity-purified antibody from patient 2 (lane A), purified normal IgG (lane B), and purified PF4 (lane C).
Twenty percent SDS gel electrophoresis (reduced, silver stain) of affinity-purified antibody from patient 2 (lane A), purified normal IgG (lane B), and purified PF4 (lane C).
Reactions of antibody in plasma, T-gel eluate, and PF4/EL heparin eluate from patient 1 against immobilized heparin: PF4 complexes. Brackets indicate ± 1.0 SD. On the basis of protein concentration, antibody was purified about 125-fold relative to starting plasma.
Reactions of antibody in plasma, T-gel eluate, and PF4/EL heparin eluate from patient 1 against immobilized heparin: PF4 complexes. Brackets indicate ± 1.0 SD. On the basis of protein concentration, antibody was purified about 125-fold relative to starting plasma.
Reactions of unlabeled (dashed line) and biotin-labeled (solid line), purified antibody from patient 1 with immobilized heparin: PF4 complexes prepared at various ratios of the two reactants. For each determination, the indicated quantity of heparin was incubated with 10 μg/mL PF4 to form complexes that were immobilized in microtiter trays. Complexes optimal for antibody detection were formed at a ratio of heparin 0.4 IU/mL to PF4 10 μg/mL. Brackets indicate ± 1.0 SD.
Reactions of unlabeled (dashed line) and biotin-labeled (solid line), purified antibody from patient 1 with immobilized heparin: PF4 complexes prepared at various ratios of the two reactants. For each determination, the indicated quantity of heparin was incubated with 10 μg/mL PF4 to form complexes that were immobilized in microtiter trays. Complexes optimal for antibody detection were formed at a ratio of heparin 0.4 IU/mL to PF4 10 μg/mL. Brackets indicate ± 1.0 SD.
The biotin-labeled antibodies recognized different sites on heparin: PF4 complexes.
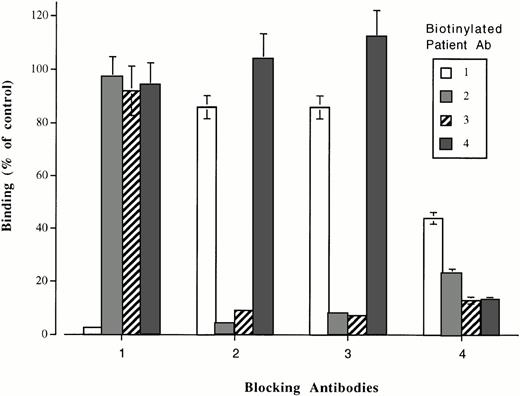
The ability of plasma from patients 1 through 4 to block binding of the biotin-labeled antibodies to heparin: PF4 complexes was then investigated. In these studies, the blocking antibodies were used in saturating concentrations and the biotinylated antibodies were used at the highest dilution that gave an optical density (OD) value about 90% of maximum. As shown in Fig 8, binding of biotinylated antibody was completely blocked by plasma from the same patient in each case. Plasma from patient 1 failed to affect the binding of any of the other three biotinylated antibodies; plasma from patients 2 and 3 blocked each other, but not antibody from patients 1 and 4, and plasma from patient 4 completely blocked the binding of antibody from patient 3 and partially blocked the binding of antibodies from patients 1 and 2. No inhibition of antibody binding occurred when nonimmune immunoglobulin or normal plasma was substituted for patient samples.
Reactions of biotinylated antibodies from patients 1 through 4 with immobilized heparin: PF4 complexes previously saturated with unlabeled (“blocking”) antibody from the same patients. Antibody 1 was blocked by itself and (partially) by antibody 4, but not by antibodies 2 and 3. Antibodies 2 and 3 blocked each other, but not antibody 1 or 4. Antibody 4 blocked itself, antibody 3, and (partially) antibodies 1 and 2. Values (ordinate) are expressed as percent of controls in which normal plasma, rather than patient plasma was used for “blocking” and are the average of triplicate determinations ± 1.0 SD.
Reactions of biotinylated antibodies from patients 1 through 4 with immobilized heparin: PF4 complexes previously saturated with unlabeled (“blocking”) antibody from the same patients. Antibody 1 was blocked by itself and (partially) by antibody 4, but not by antibodies 2 and 3. Antibodies 2 and 3 blocked each other, but not antibody 1 or 4. Antibody 4 blocked itself, antibody 3, and (partially) antibodies 1 and 2. Values (ordinate) are expressed as percent of controls in which normal plasma, rather than patient plasma was used for “blocking” and are the average of triplicate determinations ± 1.0 SD.
We then studied the ability of plasma from seven other patients (patients 5 through 11), available only in small quantities, to block the binding of biotinylated antibodies from patients 1 and 2. Plasma from patients 5 through 7 totally inhibited the binding of antibody 1, but inhibited antibody 2 only weakly (about 30%). Plasma from patients 9 through 11 inhibited antibody 2 completely, but had little effect on antibody 1. The seventh plasma (patient 8) partially inhibited (46% to 51%) both antibodies 1 and 2.
The antibodies failed to react with C-terminal PF4 peptides, whether or not they were complexed with heparin.
Reactions of IgG from patients 1 through 4 and plasma from patients 5 through 11 with peptides corresponding to the C-terminal 15 (PF4 15) and 26 (PF4 26 ) AA of the human PF4 monomer (known to contain the major heparin-binding domain) and with a scrambled 26-mer (scrambled at the 11 N-terminal AA) in the presence and absence of heparin were also studied. No antibody reacted detectably in ELISA with any of the peptides immobilized in the absence of heparin or to complexes of heparin and the 26-mer and scrambled 26-mer formed by incubating 2 to 20 mg/mL of peptide with heparin at concentrations ranging from 0.01 to 2.0 IU/mL. The 15 mer was unable to bind heparin as assessed with tritiated heparin (data not shown). Antibodies from patients 1 through 11 also failed to recognize human PF4 reduced with 10 mmol/L dithiothreitol and alkylated with 30 mmol/L iodoacetamide, whether or not heparin was present.
DISCUSSION
Our findings show that heparin-induced antibodies associated with HITT bind readily to complexes formed between PF4 and heparin fragments attached by EL to agarose beads through a DADPA spacer arm and that the antibodies can be isolated from these beads in a purified, functional state. In contrast, the antibodies failed to bind to PF4 complexed with heparin molecules that were immobilized by multiple cross-linkages (XL heparin). Physical measurements of heparin have shown that, in aqueous solution at neutral pH, the chain configuration is that of a “free-draining coil,” in which state the molecule is highly flexible.21 Heparin fragmented by nitrous acid digestion can be tethered to the solid phase through a single terminal (reducing) saccharide (EL heparin). By virtue of their strong negative charges, the immobilized molecules can be expected to repel each other and to extend away from the surface, where they can interact freely with PF4. In contrast, cross-linked heparin is immobilized on agarose through free amino groups randomly distributed along the saccharide chain and thus is highly constrained. These observations suggest that the saccharide chain making up the heparin molecule must be in a flexible, relatively unconstrained state to react with PF4 in such a way as to create the epitopes for which HITT antibodies are specific.
The findings are consistent with a model in which heparin wraps itself around the PF4 tetramer to form compound epitopes consisting of saccharide plus protein or to induce conformational changes elsewhere in the PF4 molecule (neoepitopes) that are recognized by antibody. Stuckey et al22 have described a model based on the crystalline structure of bovine PF4 (closely homologous to human PF4) and experimental observations by Talpas et al23 in which the anionic polysaccharide chain binds to a ring of positive charges extending around the molecule perpendicular to alpha helical structures located on both sides of the tetramer. Mayo et al,24 using normal and mutagenized human PF4 and nuclear magnetic resonance (NMR) analysis, drew similar conclusions. Our results with PF4 mirror findings made with the regulatory protein, antithrombin III (ATIII), in which it was found that complexes formed between ATIII and immobilized EL heparin inactivate thrombin more efficiently than complexes formed between PF4 and XL heparin.25,26Glycosaminoglycan (GAG) molecules associated with endothelial cells are also end-linked to core protein structures (syndecans).27We have previously shown that PF4 bound to endothelial cell GAG readily binds HITT antibodies and have suggested that this interaction may cause vessel wall injury predisposing some patients to thrombosis.7
From findings made in the present study using biotin-labeled, purified HITT antibodies, it is clear that the antibody from patient 1 recognizes a site or sites distinct from the site(s) recognized by the antibodies of patients 2 and 3 (Fig 8). Because binding of antibody 4 was not inhibited by antibodies 1, 2, or 3, it appears to recognize yet a third site on heparin: PF4. Alternatively, it might contain two antibodies of such high-affinity that they can displace previously attached antibodies 1, 2, and 3. This is unlikely because the blocking antibodies were used in saturating quantities, while biotinylated antibody 4 was added at a subsaturating concentration. Antibody 4 also inhibited the binding of antibody 3 and partially inhibited the binding of antibodies 1 and 2. Antibody 4 is of the IgA class and it is possible that steric hindrance related to its large size may account, in part, for the inhibition of antibodies 1, 2, and 3. Studies with Fab fragments should help to resolve this question. Results obtained with plasma from patients 5 through 11 provide further evidence for the distinctness of the epitopes recognized by antibodies 1 and 2, as three of them (5 through 7) inhibited antibody 1, but not antibody 2, while three others (9 through 11) gave the reciprocal inhibition pattern. Partial inhibition of both antibodies 1 and 2 by plasma from patient 8 is consistent with the possibility that it contains two different antibodies recognizing the binding sites for antibodies 1 and 2, respectively.
Only two previously reported studies have attempted to map epitopes on heparin: PF4 complexes recognized by HITT antibodies. Horsewood et al28 studied a total of 29 antibodies from patients with HITT that were positive in the heparin: PF4 ELISA and in the serotonin release test. Five of these antibodies also reacted with PF4 that had been reduced and alkylated before being allowed to interact with heparin. The same five sera recognized a peptide containing the 19 C-terminal amino acid residues of the PF4 monomer, a region that encompasses an alpha helical domain thought to be critical for heparin binding.29 However, neither reduced PF4 nor the C-terminal peptide was able to inhibit binding of HITT antibodies to heparin: PF4 complexes, even at high concentrations. Therefore, the clinical significance of the five antibodies was not established. Antibodies from our patients 1 through 4 failed to bind to reduced PF4 and did not recognize peptides comprising the 26 or 15 C-terminal AA residues of the PF4 monomer in the presence or absence of heparin. Amiral et al30 studied a subgroup of 15 patients thought to have HITT who had antibodies that were positive in a platelet aggregation test, but were negative in heparin: PF4 ELISA. Nine of these patients had antibodies that recognized neutrophil-activating peptide 2 (NAP-2) and/or interleukin-8 (IL-8), two members of the CXC chemokine family that are homologous to PF4. These findings are of interest because five of the nine patients had thrombotic episodes. However, reactions to these antibodies against NAP-2 or IL-8 in their normal configurations (not immobilized on plastic) were not described, and the relationship of these antibodies to those that recognize heparin: PF4 complexes is uncertain.
Our findings demonstrate that antibodies isolated from four patients with HITT recognize two and possibly three distinct determinants on complexes of heparin and PF4 formed at optimal ratios of the two reactants. Because the PF4 molecule is a nearly symmetrical tetramer,31 an epitope recognized by a single antibody could be expressed four times on each heparin: PF4 heterodimer. This creates the potential for even a single antibody to react with four sites on a heparin: PF4 complex. Studies from our group12and others10,11 have shown that, although antibodies reactive with heparin: PF4 complexes are nearly always present in patients with HITT, not all patients who form such antibodies experience thrombosis, or even thrombocytopenia. Factors that could predispose antibody formers to develop the HITT syndrome include the formation of unusually potent (high-titer) antibodies14 and associated conditions, congenital or acquired, that predispose to thrombosis. It can be speculated that antibodies recognizing certain sites on heparin: PF4 complexes form immune complexes that are particularly effective in activating platelets. The same antibodies might be more likely to promote vessel injury when they bind to PF4 complexed with GAG on endothelial cells.
Use of PF4 complexed to end-linked heparin fragments is an effective way to purify HITT antibodies on a large scale. These reagents should prove useful for preparation of Fab fragments that can be used to map epitopes on heparin: PF4 with greater resolution and for studying the mechanism(s) by which these antibodies activate platelets and cause thrombocytopenia.
ACKNOWLEDGMENT
We thank Trudy Holyst for the expert technical assistance in the synthesis of peptides and Rhonda Geoffrey for expert technical assistance. We are indebted to the Word Processing Department of The Blood Center for outstanding assistance in preparing this manuscript.
Supported by Grants No. HL-13629, HL-44612, and K08-HL-03464 from the National Heart, Lung, and Blood Institute, Bethesda, MD.
Address reprint requests to Gian P. Visentin, MD, Blood Research Institute, Blood Center of S.E. Wisconsin, PO Box 2178, Milwaukee, WI 53201-2178.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal