Abstract
Attempts to clarify the pathophysiology of human immunodeficiency virus (HIV)-mediated bone marrow (BM) dysfunction have yielded inconsistent results regarding the susceptibility of BM progenitors to the viral infection. To specifically address this question, we exposed highly purified subpopulations of human BM progenitor cells to various HIV-1 and HIV-2 strains and assessed (pro)viral gene presence and expression in more-committed (CD34+CD38+) as well as most-primitive (CD34+CD38−) cells in long-term BM cultures. Quantitative analysis of long-term culture-initiating cells (LTCIC) failed to demonstrate adverse effects of exposing hematopoietic stem cells to HIV. Our results show that HIV-2, similar to HIV-1, does not infect hematopoietic stem cells in vitro with any significant frequency and infected cells are not present within LTCICs. Cytofluorometric analysis of CD34+ cells for surface molecules that facilitate HIV entry was consistent with the functional assay in that expression of virus receptors was predominantly on the more-committed subsets of BM progenitors. The failure to detect productive or latent HIV in the most-primitive human BM progenitor and stem cells has important implications for future therapeutic strategies, including those dealing with transduction of these cells with protective genes as a treatment modality for AIDS.
A;4 LARGE NUMBER of studies have been conducted to identify and characterize the pathophysiologic mechanisms leading to bone marrow (BM) dysfunction in patients with acquired immunodeficiency syndrome (AIDS).1-3 Understanding of these mechanisms is essential not only for the management of hematologic complications in AIDS but also for successful disease intervention, since T-cell depletion in AIDS is thought to be, at least in part, due to the failure of T-cell development from lymphohematopoietic stem cells. While the results of experiments performed with patient-derived material can be difficult to interpret due to the multifactorial nature of the hematopoietic failure in AIDS,4-13 experiments using ex vivo infected normal BM cells9,14-19 may not properly reflect the pathophysiology operating during disease progression. Generally, the results of all these experiments with regard to both ability of human immunodeficiency virus (HIV) to directly inhibit hematopoiesis7,9-16,19 as well as to the susceptibility of hematopoietic progenitors to HIV infection,4-9,15,17 18have not been uniform and are often contradicting.
Our previous in vitro studies have demonstrated that indirect mechanisms involving release of tumor necrosis factor (TNF-α) from accessory cells in response to HIV-1 envelope proteins could account for hematopoietic suppression observed in vivo.20This and other studies failed to show, however, a direct inhibitory effect of HIV-1 in vitro.1,8,9,21 Since there is no doubt that the therapy-related side-effects, as well as secondary pathophysiologic mechanisms (eg, cytokine disturbances, cachexia, and opportunistic infections) can impair BM function,22 the direct infection of immature hematopoietic cells need not necessarily be postulated to account for the hematopoietic suppression in AIDS.20,23,24-27 In several studies, the infection of phenotypically defined populations of hematopoietic progenitor cells was assessed,4-9,16,18-21,28 and most of them suggest that HIV does not infect a significant portion of progenitor cells.4,5,7-9 28 However, the susceptibility of hematopoietic stem cells to HIV infection is still unclear. Resolution of this central issue could shed light on the pathophysiologic significance of direct virus-mediated cell depletion and the reservoir function of infected hematopoietic cells for the latent virus. Theoretically, integration of proviral DNA into stem cell genomes could lead to the spread of HIV infection through the expansion of infected clones, or conversely, it could interfere with normal stem cell maturation and proliferation resulting in the interruption of normal hematopoiesis.
Bone marrow long-term cultures (BMLTC) and long-term culture-initiating cell (LTCIC) assays offer an opportunity to study human hematopoiesis and quantify the most-immature human hematopoietic cells amenable to in vitro assessment. In BMLTC, the immature hematopoietic progenitor cells are selected over a period of 5 weeks. These cells may subsequently be evaluated for their ability to initiate and form colonies in secondary methylcellulose assays.29-32 The number of colony-forming cells (CFC) can serve as a measure of LTCIC, the best estimate of hematopoietic stem cells in vitro.29-31 Although some strains of HIV-215 and to a lesser degree monocytotropic strains of HIV-1,15,19,33 34 were shown to affect the function and number of LTCIC, the question about the susceptibility of LTCIC to HIV infection has not been systematically studied. Therefore, we investigated the effects of HIV-1 and HIV-2 on the growth of most-primitive human hematopoietic progenitor cells in BMLTC and determined under stringent conditions the ability of HIV to replicate or latently persist in these cells.
MATERIALS AND METHODS
Bone marrow cell preparation.
BM was obtained from healthy donors (age 26 to 42 years) by aspiration from the posterior iliac crest into syringes containing media supplemented 1:10 with heparin (O'Neill and Feldman, St Louis, MO). Informed consent was obtained according to a protocol approved by the Institutional Review Board of the National Heart, Lung and Blood Institute. Mononuclear BM cells (BM MNC) were isolated by density gradient centrifugation using lymphocyte separation medium (Organon, Durham, NC). After washing in Hank's balanced salt solution (HBSS; GIBCO-BRL/Life Technologies, Gaithersburg, MD), cells were resuspended in Iscove's modified Dulbecco's medium (IMDM; GIBCO) supplemented with 20% fetal calf serum (FCS; heat inactivated, Life Technologies).
Separation of CD34+ cells and flow cytometry.
After washing with phosphate buffered saline (PBS) supplemented with 2% human albumin and labeling with anti-CD34 monoclonal antibody (MoAb), BM cells were passed through an affinity column (Cellpro, Bothell, WA) for selection, and the CD34+ cell fraction was eluted with PBS. An aliquot of eluted cells was stained with phycoerythrin (PE)-conjugated anti-CD34 HPCA-2 MoAb (Becton Dickinson, Mountain View, CA) to assess the purity, which usually ranged from 80% to 94%. For higher purity preparations, cells were further fractionated: column-purified cells were stained with fluorescein isothiocyanate-labeled (FITC) anti-CD34+ MoAb, washed with PBS, and sorted by microcytofluorometry (Epics V; Coulter, Hialeah, FL). The purity of cells prepared by combining affinity chromatography and flow cytometry was 97% to 99%. To obtain the most-immature BM progenitor cells from the CD34+ population, cells were stained with anti-CD38 MoAb (PE-labeled, Becton Dickinson) and the negative gate was collected by cytofluorometry.
In several experiments, more detailed analysis of BM cells was performed using FITC-conjugated HPCA-1 MoAb directed against human CD34 antigen (Becton Dickinson), PE-conjugated anti-CD38 MoAb, and PerCP-conjugated anti-CD4 or anti-CD14 MoAb (Becton Dickinson). For analysis of chemokine receptor molecules on CD34+CD38− cells, mouse MoAb to CXCR-4 (12G5, mIgG2a) and CCR-5 (12D1, mIgG2a; both MoAbs obtained from the AIDS Research and Reference Reagent Program, Rockville, MD) were detected with an antimouse-biotin/streptavidin-PE system (DAKO A/S, Denmark, and Molecular Probes). In all flow cytometry experiments, appropriate isotype MoAb conjugate controls were used to determine the levels of background fluorescence.
Long-term bone marrow cultures.
BMLTC and the determinations of the number of LTCIC were performed according to a modification of described methods.29-32Approximately 107 BM MNC were used to initiate stromal cultures. Allogeneic BM cells were grown until they formed a confluent stromal layer. Culture media consisted of stem cell media (MyeloCult H5100, Stem Cell Technologies, Vancouver, BC, Canada) supplemented with 10−6 mol/L hydrocortisone succinate (Sigma, St Louis, MO), 4 mmol/L L-glutamine, 50 U/mL penicillin, 50 μg/mL streptomycin (all Mediatech Inc, Herndon, VA), and the media was renewed to 50% twice a week. After approximately 3 weeks of culture, stromal cells were trypsinized, washed, and placed in 48 well plates. After reestablishment of a confluent cellular layer the plates were irradiated (15 Gy of 250-KV x-rays) to prevent further proliferation of the feeder cells.29 Subsequently to their viral exposure, total BM MNC, isolated CD34+ cells, or sorted CD34+CD38− cells were added to preestablished irradiated stomal feeder layers in duplicate (for each treatment regimen), and cultured for 5 weeks at 35°C in humidified atmosphere, containing 5% CO2. Every 2 days, 50% of the stem cell culture media (as described above, without hydrocortisone) was replaced by fresh media containing stem cell factor (rhSCF, 50 ng/mL, Amgen, Thousand Oaks, CA), rhlL-3 (20 ng/mL, Genzyme, Boston, MA), and rhLIF (20 ng/mL, Biosource International, Camarillo, CA). Starting 1 week after BM cell addition, the number of secondary colonies generated from nonadherent cell fractions of BMLTC was analyzed weekly in triplicate methylcellulose cultures (secondary cultures, performed as described below). After 5 weeks, the remaining cells in BMLTC were harvested and replated in methylcellulose so as to assess the numbers of LTCIC (cells able to form secondary colonies after 5 weeks of BMLTC). Cells and supernatants derived from these cultures were used for determining contents of viral antigen (p24, p27) and proviral DNA (HIV-1 and HIV-2gag).
Secondary hematopoietic methylcellulose cultures.
Hematopoietic CFC in BMLTC were measured in methylcellulose cultures under standard conditions. BMLTC derived cells were plated in methylcellulose (MethoCult H4100, Stem Cell Technologies) in the presence of 50 ng/mL IL-3 (Genzyme), 20 ng/mL granulocyte-macrophage colony stimulating factor (GM-CSF; Boehringer, Indianapolis, IN), 50 ng/mL stem cell factor (SCF), and 2 U/mL erythropoetin (EPO, both Amgen). After 2 weeks, colonies formed were microscopically evaluated for quality and enumerated using a grid.
Tertiary suspension cultures.
In some experiments, 14-day colonies from methylcellulose cultures were dispersed, washed twice in PBS, resuspended in IMDM supplemented with FCS (both GIBCO-BRL) and growth factors (at concentrations used for secondary methylcellulose cultures) and cultured for an additional 14 days. Cells and supernatants derived from these cultures were used for proviral DNA analysis by PCR and viral antigen (p24, p27) measurement by ELISA, respectively.
Virus and recombinant viral proteins.
Viral stocks of HIV-1 strains BAL and RF, or HIV-2 strains ST and ROD were propagated in primary human cells20,35 and cell free supernatants were used for BM infection experiments. Two primary isolates of HIV-1 (passage 9 of isolate MN, passage 3 of isolate 571), which were included in some experiments, were isolated in our laboratory and propagated exclusively in PBMNC as described.20,36 All virus stocks and defined lots were individually titered for infectivity in PHA-P activated PBMNC cultures under standardized conditions.35 Virus incubations were carried out with the viral dose of 50 × tissue culture infectious dose 50 (TCID50), corresponding approximately to 2 × 105cpm/mL of reverse transcriptase activity, for 2 hours at 37°C. For control experiments, mock-virus stocks were prepared by irradiation (20 Gy of 250-KV x-rays) or heat-inactivation for 30 minutes at 59°C. To account for potential transfer of bio-active molecules such as cytokines, chemokines, and growth factors in supernatants, additional controls were generated by exposing peripheral blood (PB) derived MNC fractions to a variety of stimuli. Cells were cultured in IMDM (GIBCO-BRL) containing 10% human AB serum (ABI, Cambridge, MA), and treated with LPS (2 μg/mL; Sigma) or with IL-4 (0.1 μg/mL) and GM-CSF (50 mg/mL), or with rIFN-γ (0.1 μg/mL, all Boehringer) for 3 days. Culture media was changed on day 3 and cell free supernatants were collected on day 6 and stored in aliquots at −80°C until used. Incubations of BM-derived cell fractions with the differently conditioned media were carried out, similar to viral exposure, for 2 hours at 37°C. Commercially available baculovirus derived HIV-1 gp120 (ABI, Cambridge, MA; 5 μg/mL) was added to some control cultures at the concentration of 2 μg/mL. After exposure, cells were thoroughly washed three times with PBS, enumerated by using the trypan blue exclusion method, and placed into cultures.
Semiquantitative PCR.
To determine the amount of HIV-DNA in the infected cells, a quantitative PCR36 was performed using primers complementary to the gag region of HIV-1.37 Plasmid pAC38/93 was used as the internal control.38Both HIV-1 gag sequences were amplified by the same set of primers (SK38 and SK39) and the products were differentiated by size and hybridization to specific probes.37 For quantification, serial dilutions of the positive standard were amplified in parallel with test samples (previously normalized for total DNA using β-globin gene amplification)36 in the presence of 200 copies of the internal control in order to normalize the results. The amplification products were transferred onto nitrocellulose filters, hybridized with 5′-end P32-labeled specific probes in a slot blot format and densitometrically quantified. The limit of detection using this method was at a range of 20 HIV-1 gene copies per 2 × 105cells (1 μg of total DNA). To amplify HIV-2 gag proviral DNA primers SK145 and SK431 (Perkin Elmer) were used in a semiquantitative PCR assay, applying similar reaction conditions as described above. The amplification products (139 bp) were detected with a labeled probe (SK102, Perkin Elmer) and the number of gene copies in test samples was estimated based on a standard curve. Detection limit of the semiquantitative assay was 40 HIV-2 gene copies per 2 × 105 cells.
ELISA.
For determination of p24 (HIV-1) and p27 (HIV-2) Coulter ELISA kits (Coulter, Hialeah, FL) were used according to the protocols supplied. Samples were stored at −80°C until assayed in duplicate determinations using a Bio-Rad microplate reader (Model 3550 Bio-Rad Laboratories, Hercules, CA) and manufacturer's software. The limit of viral core protein detection for the p24 assay was 8 pg/mL and 14 pg/mL for the p27 assay.
RESULTS
Effects of HIV-1 and -2 exposure on total bone marrow as assessed by number of CFC and LTCIC in BMLTC.
The effects of HIV on the colony formation of committed progenitor cells has been previously studied using primary methylcellulose assays.9-13,16,20,28,38 We investigated the long-term effects of a short-term exposure to HIV-1 and HIV-2 by CFC-assays in BMLTC. To minimize the viral effects on stroma formation and function, BM MNC fractions were exposed to HIV-1 and HIV-2 before being placed on preformed irradiated allogeneic stromal layers. Excess of inoculated virus was removed by repeated washing steps. When total BM cells were used in the experiments, differences in the number of secondary CFC were observed in the first weeks of the BMLTC (Table1). Exposure to infectious HIV-1 (strains RF, BaL) as well as HIV-2 (strains ST, Rod) decreased colony formation to 76% ± 10%, 72% ± 8%, 75% ± 6%, and 78% ± 8%, respectively, as compared with controls (paired t-test,P < .05). Mock virus generated by irradiation or heat inactivated viral stocks had similar effects (72% ± 6% and 68% ± 6% of controls, respectively). Consistent with our previous results,20 recombinant viral envelope protein (gp120) alone had a potent inhibitory effect on CFC in the first 2 weeks of BMLTC. Conditioned medium generated from activated PBMNC derived cultures, representing a control for potential transfer of cytokines, chemokines, and growth factors from cell cultures used for virus stock propagation, failed to mediate the suppressive effects on CFC. During the further course of culture, beginning at week 3, the number of colonies derived from BMLTC exposed to the various inactivated (noninfectious) HIV preparations, including gp120, increased moderately (mean 120% ± 5% of controls at weeks 4,P < .05). The number of CFCs obtained in experiments where total BM was exposed to the different infectious viral preparations was lower than in controls throughout the entire period of assessment up to 4 weeks. However, no significant differences in the number of LTCICs were determined (Table 1).
Effects of HIV-1 and HIV-2 Exposure on BM Cells, Measured as the Number of Secondary CFC Derived From BMLTC
| Virus . | % of Control Culture CFC . | ||||
|---|---|---|---|---|---|
| 1 wk . | 2 wk . | 3 wk . | 4 wk . | LTCIC . | |
| HIV-1 RF | 76 | 82 | 76 | 78 | 86 |
| HIV-1 Bal | 72 | 80 | 69 | 78 | 90 |
| HIV-2 ST | 75 | 84 | 72 | 87 | 93 |
| HIV-2 Rod | 78 | 84 | 70 | 72 | 86 |
| HIV-1 Bal mock | 69 | 79 | 117 | 114 | 104 |
| HIV-2 Rod mock | 67 | 82 | 115 | 126 | 105 |
| Sup (LPS) | 104 | 105 | 94 | 115 | 110 |
| gp120 | 64 | 74 | 110 | 125 | 102 |
| Virus . | % of Control Culture CFC . | ||||
|---|---|---|---|---|---|
| 1 wk . | 2 wk . | 3 wk . | 4 wk . | LTCIC . | |
| HIV-1 RF | 76 | 82 | 76 | 78 | 86 |
| HIV-1 Bal | 72 | 80 | 69 | 78 | 90 |
| HIV-2 ST | 75 | 84 | 72 | 87 | 93 |
| HIV-2 Rod | 78 | 84 | 70 | 72 | 86 |
| HIV-1 Bal mock | 69 | 79 | 117 | 114 | 104 |
| HIV-2 Rod mock | 67 | 82 | 115 | 126 | 105 |
| Sup (LPS) | 104 | 105 | 94 | 115 | 110 |
| gp120 | 64 | 74 | 110 | 125 | 102 |
Number of secondary colonies derived from BMLTC initiated with total BM exposed to HIV-1 and HIV-2. CFC are expressed as percentage of control cultures for each time point. All BMLTC were performed in duplicate and each culture was assessed in triplicate methylcellulose cultures to determine the number of CFC. The number of colonies in controls was 2,540 ± 220, 1,080 ± 90, 138 ± 16, 44 ± 6, and 30 ± 4 for 1 to 5 weeks per 3 × 106 cells, respectively. Percentage was calculated based on mean values, SD ≤ 12%. Mock virus stocks used were heat inactivated as described in Materials and Methods.
Effects of HIV-1 and -2 exposure on CD34+ cell subsets assessed by CFC and LTCIC in BMLTC.
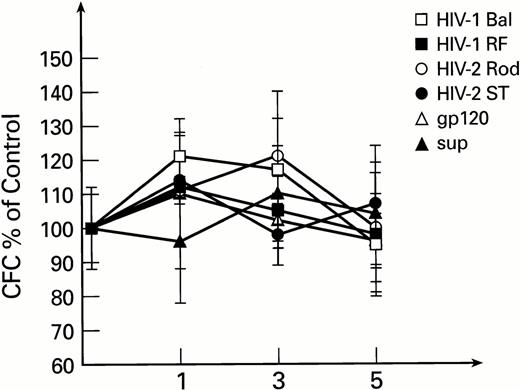
To exclude the possible interaction of monocytes and lymphocytes (contained in total BM preparations, susceptible to infection with HIV-1 and HIV-2) with BM progenitor and stem cells, purified populations of BM-derived CD34+ cells were used in subsequent experiments. Exposure of CD34+ cells to HIV-1 or HIV-2, followed by long-term culture on preformed stromal layers, revealed no inhibitory effects on secondary colony formation. Instead, a moderate increase in CFC was noted after the first week of BMLTC initiation. This effect was not seen with controls exposed to conditioned medium. The number of secondary CFCs for weeks 2 to 5 were unaffected by infectious virus or mock virus controls (Fig1). Similar results were obtained when previously described primary isolates of HIV-1, MN and 571,35 56 were used instead of laboratory strains (data not shown).
Secondary colonies formation as derived from LTBMC initiated with purified CD34+ cells exposed to HIV-1 and HIV-2. CFC are expressed as percentage of control cultures performed with heat inactivated virus for each time point. All cultures were performed in duplicate. The control CFC per 105 cells were 2,890 ± 310, 128 ± 22, and 26 ± 6 for 1, 3, and 5 weeks, respectively.
Secondary colonies formation as derived from LTBMC initiated with purified CD34+ cells exposed to HIV-1 and HIV-2. CFC are expressed as percentage of control cultures performed with heat inactivated virus for each time point. All cultures were performed in duplicate. The control CFC per 105 cells were 2,890 ± 310, 128 ± 22, and 26 ± 6 for 1, 3, and 5 weeks, respectively.
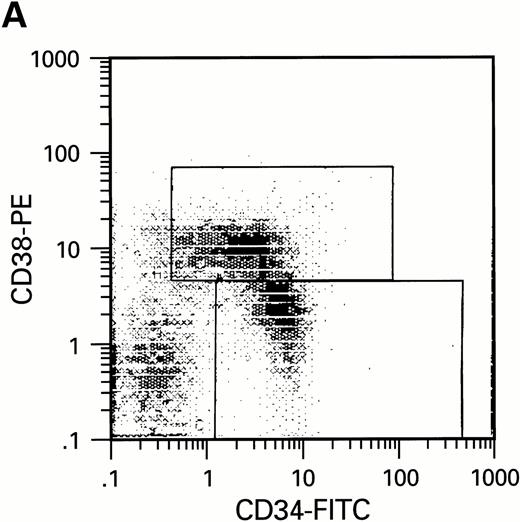
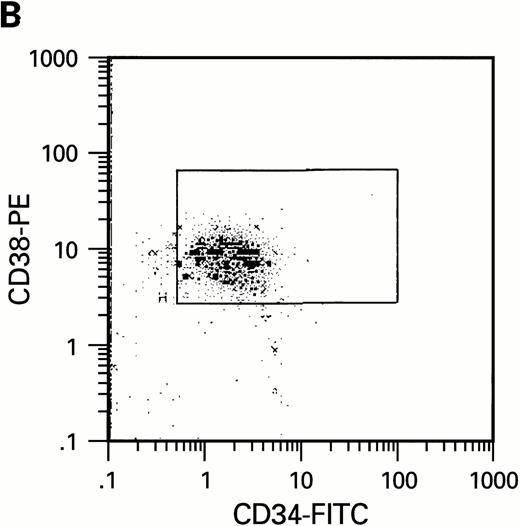
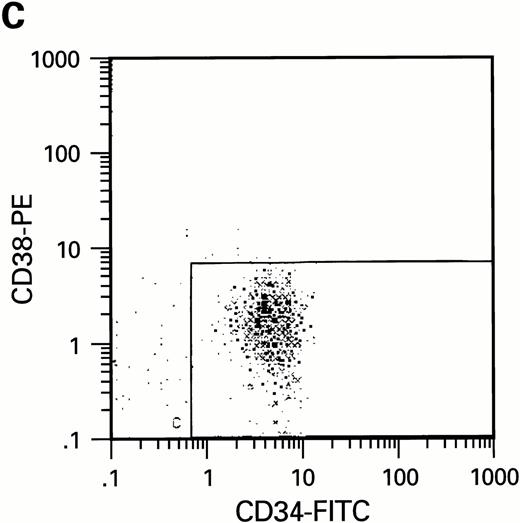
To further analyze the viral influence on immature progenitor and stem cell maintenance and development in vitro, CD34+cells were subdivided based on the expression of CD38 antigen (Fig 2A, B, and C).29-31 While the measurable effects on the more committed CD34+CD38+ BM cells were comparable with those seen in cultures of total CD34+ cells, secondary colony formation by the most-primitive, CD34+CD38− cells exposed to HIV-1 and HIV-2 was unaffected for the entire period of 5 weeks of BMLTC (Table 2). All HIV strains tested showed similar activity.
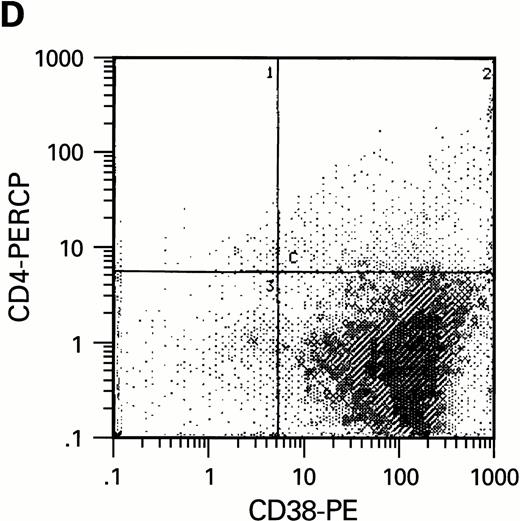
Flow cytometric analysis of CD34+, CD34+CD38+, and CD34+CD38− cell subsets used to initiate BMLTC. (A) Scatter diagram of CD34+ selected cells stained with FITC-labeled CD34 MoAb and PE-labeled CD38 MoAb, in which Log PE fluorescence intensity was displayed versus Log FITC fluorescence intensity. (B) CD34+ cells were gated and sorted. (C) A scatter diagram of gated CD34+ cells sorted for absence of the CD38 activation marker (most-primitive BM stem cells). (D) The log PE fluorescence intensity (CD38) of an affinity column purified CD34+ cell population was displayed versus Log PerCP-fluorescence intensity (CD4). Results of one representative experiment illustrated.
Flow cytometric analysis of CD34+, CD34+CD38+, and CD34+CD38− cell subsets used to initiate BMLTC. (A) Scatter diagram of CD34+ selected cells stained with FITC-labeled CD34 MoAb and PE-labeled CD38 MoAb, in which Log PE fluorescence intensity was displayed versus Log FITC fluorescence intensity. (B) CD34+ cells were gated and sorted. (C) A scatter diagram of gated CD34+ cells sorted for absence of the CD38 activation marker (most-primitive BM stem cells). (D) The log PE fluorescence intensity (CD38) of an affinity column purified CD34+ cell population was displayed versus Log PerCP-fluorescence intensity (CD4). Results of one representative experiment illustrated.
Secondary Colony Formation in the Course of BMLTC Initiated With Purified CD34+ Cells Exposed to HIV-1 and HIV-2
| Time of BMLTC . | CD34+CD38+ Cells . | CD34+CD38− Cells . | ||||
|---|---|---|---|---|---|---|
| 1 wk . | 3 wk . | LTCIC . | 1 wk . | 3 wk . | LTCIC . | |
| gp120 | 96 | 106 | 97 | 104 | 98 | 106 |
| HIV-1 Bal | 94 | 102 | 102 | 103 | 105 | 100 |
| HIV-1 Bal mock (H*) | 102 | 98 | 108 | 109 | 101 | 97 |
| HIV-1 Bal mock (R*) | 98 | 100 | 102 | 106 | 100 | 104 |
| HIV-1 RF | 97 | 98 | 107 | 110 | 102 | 98 |
| HIV-2 Rod | 105 | 96 | 110 | 97 | 102 | 108 |
| HIV-2 Rod mock (R*) | 101 | 98 | 96 | 113 | 94 | 95 |
| Sup (LPS) | 106 | 98 | 109 | 99 | 106 | 98 |
| Time of BMLTC . | CD34+CD38+ Cells . | CD34+CD38− Cells . | ||||
|---|---|---|---|---|---|---|
| 1 wk . | 3 wk . | LTCIC . | 1 wk . | 3 wk . | LTCIC . | |
| gp120 | 96 | 106 | 97 | 104 | 98 | 106 |
| HIV-1 Bal | 94 | 102 | 102 | 103 | 105 | 100 |
| HIV-1 Bal mock (H*) | 102 | 98 | 108 | 109 | 101 | 97 |
| HIV-1 Bal mock (R*) | 98 | 100 | 102 | 106 | 100 | 104 |
| HIV-1 RF | 97 | 98 | 107 | 110 | 102 | 98 |
| HIV-2 Rod | 105 | 96 | 110 | 97 | 102 | 108 |
| HIV-2 Rod mock (R*) | 101 | 98 | 96 | 113 | 94 | 95 |
| Sup (LPS) | 106 | 98 | 109 | 99 | 106 | 98 |
CFC are expressed as percentage of the mean values of control cultures for each time point (SD < 9%). All BMLTC were performed in duplicate and each culture was assessed in triplicate methylcellulose cultures to determine the number of CFC. The number of colonies in controls was 2,980 ± 270, 1,040 ± 110, 158 ± 18, 50 ± 6, and 24 ± 5 for weeks 1, 2, 3, 4, and 5, per 105CD34+ cells, respectively. The control colony numbers for CD34+CD38− sorted cells were 820 ± 58, 510 ± 58, 234 ± 18, 148 ± 11, 92 ± 10 for 1 to 5 weeks per 104 cells. Heat inactivated (H*) and irradiated virus stocks (R*) were used as indicated.
Lack of HIV-1 and -2 propagation in immature hematopoietic cells capable of secondary colony formation after BMLTC.
Previous results suggested that neither HIV-1 nor HIV-2 directly exert significant adverse effects on immature BM progenitor and stem cells as measured in BMLTC. Theoretically, it is possible that HIV-1 or HIV-2 infect these cell types and persist in them or in the progeny generated from these cells relatively inactive, without notably affecting their growth in vitro. To investigate the possibility of a limited HIV replication, we measured the levels of p24 (HIV-1) and p27 (HIV-2) viral protein in BMLTC of purified CD34+ cells as well as in those initiated by CD34+CD38 + and CD34+CD38− cells. While no p24 or p27 production was detected in culture supernatants of most-immature CD34+CD38− cells up to 6 weeks after viral exposure, a low level of HIV core production was detected in the cultures of CD34+CD38+ cells in the first 2 weeks of culture (Table 3). In comparison, levels of viral core protein in BMLTC initiated with total BM cells increased over a period of 3 weeks, reaching p24 and p27 levels of 3.7 ± 1.4 ng/mL and 7.4 ± 5.1 ng/mL, respectively.
Detection of p24 and p27 Core Proteins in BMLTC of CD34+CD38+ Cells Exposed to HIV-1 and HIV-2
| Time of BMLTC . | 1 wk . | 2 wk . | 3 wk . | 4 wk . | LTCIC . |
|---|---|---|---|---|---|
| HIV-1 Bal | 258 ± 49 | 64 ± 18 | 28 ± 6 | ND | ND |
| HIV-1 RF | 180 ± 29 | 110 ± 32 | 20 ± 4 | ND | ND |
| HIV-2 ST | 261 ± 38 | 102 ± 28 | 32 ± 10 | ND | ND |
| HIV-2 Rod | 386 ± 62 | 104 ± 25 | 56 ± 7 | ND | ND |
| HIV-2 Rod mock (H*) | 48 ± 10 | ND | ND | ND | ND |
| Time of BMLTC . | 1 wk . | 2 wk . | 3 wk . | 4 wk . | LTCIC . |
|---|---|---|---|---|---|
| HIV-1 Bal | 258 ± 49 | 64 ± 18 | 28 ± 6 | ND | ND |
| HIV-1 RF | 180 ± 29 | 110 ± 32 | 20 ± 4 | ND | ND |
| HIV-2 ST | 261 ± 38 | 102 ± 28 | 32 ± 10 | ND | ND |
| HIV-2 Rod | 386 ± 62 | 104 ± 25 | 56 ± 7 | ND | ND |
| HIV-2 Rod mock (H*) | 48 ± 10 | ND | ND | ND | ND |
Values were determined in duplicate for each culture and represent concentrations of p24 for HIV-1 and p27 for HIV-2 in pg per mL of media. Results of one representative experiment illustrated.
Abbreviation: ND, not detectable.
Since infections may persist in a “silent,” nonproductive state and since ELISA assays only detect expressed viral gene products, we used a highly sensitive PCR technique to detect HIV-1 and HIV-2 proviral DNA in BMLTC initiated with CD34+CD38+and CD34+CD38− cells (Table 4). HIV-1 and HIV-2 DNA was detected in cultures of CD34+CD38+ cells up to 3 weeks after infection. However, in cultures of CD34+CD38− cells, proviral DNA could only be detected immediately after viral exposure and during the first week in BM-LTCIC, representing most likely a residual contamination by the viral inoculum. No proviral DNA was detectable at any later time point when cultures initiated with CD34+CD38 were analyzed (Table4). In contrast, HIV proviral DNA detected in cultures initiated with total BM reached levels of more than 105 copies per 106 cells.
HIV Proviral DNA PCR Analysis in BM Cells Derived From BMLTC Initiated With CD34+CD38+ and CD34+CD38− Cells
| Time After Initiation of BMLTC . | CD34+CD38+ Cells . | CD34+CD38− Cells . | ||||
|---|---|---|---|---|---|---|
| 1 wk . | 3 wk . | LTCIC . | 1 wk . | 3 wk . | LTCIC . | |
| HIV-1 RF | 2,200 | 120 | ND | 100 | ND | ND |
| HIV-1 Bal | 4,000 | 160 | ND | 150 | ND | ND |
| HIV-2 Rod | 4,500 | 150 | ND | 140 | ND | ND |
| HIV-2 ST | 2,400 | 160 | ND | 120 | ND | ND |
| HIV-2 Rod mock (H*) | 150 | ND | ND | 110 | ND | ND |
| Time After Initiation of BMLTC . | CD34+CD38+ Cells . | CD34+CD38− Cells . | ||||
|---|---|---|---|---|---|---|
| 1 wk . | 3 wk . | LTCIC . | 1 wk . | 3 wk . | LTCIC . | |
| HIV-1 RF | 2,200 | 120 | ND | 100 | ND | ND |
| HIV-1 Bal | 4,000 | 160 | ND | 150 | ND | ND |
| HIV-2 Rod | 4,500 | 150 | ND | 140 | ND | ND |
| HIV-2 ST | 2,400 | 160 | ND | 120 | ND | ND |
| HIV-2 Rod mock (H*) | 150 | ND | ND | 110 | ND | ND |
Values represent proviral genome copies per 200,000 cells as normalized using beta globin gene amplification and based on densitometric quantification. (Values were rounded to the next decimal digit.)
BM stem cells, measured as LTCIC in our experimental system, are present only in a low number even within enriched CD34+CD38− cell populations. To achieve the highest sensitivity of detection of the infection of stem cells in vitro, secondary 5-week colonies derived from LTCIC were harvested and used for further analysis. PCR performed on the viable cell populations failed to demonstrate the presence of HIV-1 or HIV-2 DNA. In attempts to detect low levels of HIV infection, cells derived from secondary colony formation assays initiated after 5 weeks of BM-LTCIC were further subcultured in suspension (tertiary suspension cultures) to achieve their complete differentiation. Neither HIV gene products as assayed in p24 and p27 ELISA nor proviral DNA was found in these cultures (data not shown). This demonstrates that all LTCIC-derived cell cultures, independent from the initially used subpopulation, were not infected by HIV-1 or HIV-2.
Lack of HIV receptor molecules on hematopoietic progenitor and stem cell subpopulations.
To estimate the potential influence of contaminating terminally differentiated cells (macrophages and lymphocytes) on the results of our experiments, the number of these cells was quantified with anti-CD3 and anti-CD14 MoAb using flow cytometry. Preparations of CD34+CD38− cells were virtually free of monocytes and lymphocytes while a low number of CD14+ cells were present in the preparations of CD34+CD38+cells (data not shown). Since CD4, the high affinity receptor for HIV infection, has been reported to be present on purified CD34+ cells free of lymphocytic population,39-41 we studied whether a limited replication of HIV in cultures of CD34+CD38+ cells could be correlated with the presence of receptors required for infection. Using 3-color flow cytometric analysis, CD4, CXCR-4, and CCR-5 molecules were assessed on CD34+ cells. In agreement with our infection assays, CD4 expression could only be found within CD34+CD38+ but not within CD34+CD38− cell populations (Fig2D). Expression of HIV coreceptors such as CXCR-4 and CCR-5 was also not detectable on most-primitive hematopoietic progenitors (CD34+CD38−) by means of flow cytometry. The increase of CD38 molecules on CD34+ BM cells correlates with the presence of HIV receptor molecules CD4, CXCR-4, and CCR-5. On cells purified on the basis of CD34, approximately 6% (±2.6%) of the cell population expressed CD4, 12% ± 5% CXCR-4 and 3.5% ± 2.2% CCR-5, as determined in three independent experiments.
DISCUSSION
The effects of HIV-1 and HIV-2 on committed hematopoietic progenitor cells assayed in methylcellulose cultures have been extensively studied.9-13,16,20,28 38 Fewer reports have addressed the question of whether or not the most-primitive hematopoietic progenitors and stem cells can be infected by the viridae and to what degree the proliferative function of these cells might be affected. Unfortunately, the results of these studies are inconsistent, possibly due to variability of the different methods used and the technical demands of such experiments. However, virus-mediated stem cell depletion or functional impairment due to direct viral cytotoxicity and host immune response may account for some of the hematologic manifestations of AIDS. At least theoretically, the infection of dormant stem cells with retroviridae such as HIV-1 and HIV-2 could be associated with long-term viral persistence and production of a large number of infected progeny. Using highly stringent conditions to select for the most-primitive hematopoietic stem cells in vitro, we demonstrated that these cells are not susceptible to either HIV-1 or HIV-2 infection.
We first investigated the viral effects on the survival and function of the most-immature human hematopoietic progenitor and stem cells in BMLTC. Using purified populations of CD34+CD38− cells (enriched in hematopoietic stem cells), we showed that the number of LTCICs, as assessed by secondary colony formation, remains unchanged after in vitro exposure to HIV-1, or to HIV-2. Because LTCIC assessment is considered the best in vitro assay of human hematopoietic stem cells,29-31these results suggest that the BM stem cells are not directly affected by HIV. Compatible results were reported when the number of LTCIC or cobblestone-area-forming cells (cells functionally comparable with LTCIC) was quantified in the peripheral blood and BM of patients with HIV-1 infection.8,22 These studies suggested that stem cells (measured as LTCIC) are affected by the cytotoxic therapies, opportunistic infections and immune dysregulation rather than by the direct infection with HIV itself. This interpretation is supported by a number of reports where unchanged proliferative capacity of committed progenitor cells in methylcellulose cultures was observed after in vitro challenge with HIV-1.9,18,20,21 However, it is noteworthy that decreased progenitor cell growth was reported under varying experimental conditions which selected for, and assessed, more mature hematopoietic progenitor cells.7,11,13-15,17 28
We have also demonstrated that, in contrast to LTCIC, proliferation of committed progenitor cells (CFCs derived from total BM after 2 to 4 weeks of BMLTC) was decreased after exposure to HIV-1 or HIV-2. Similar findings were reported in previous studies demonstrating the impairment of hematopoietic function in BMLTC after exposure to a variety of HIV strains.19,20,33,34 These results and our own observations are compatible with the theory that the virus may alter the proliferation of hematopoietic progenitor cells indirectly,eg., by the induction of cytokine production such as TNF-α, IFN-γ or TGF-β. A large body of literature provides substantial evidence for cytokine dysregulation in HIV infections. In addition, it was hypothesized that HIV infection may influence the function of BM stromal cells, which support the hematopoietic system.42,43Although our present study was not designed to investigate this issue, several other reports as well as the most recent studies from our laboratory do not sustain this theory, showing that stromal layers derived from AIDS patients adequately support hematopoiesis initiated by normal CD34+ cells.22 44
The second important element of this study is the demonstration that only a limited propagation of HIV-1 and HIV-2 is possible in CD34+ cells. Cells susceptible to HIV-infection were contained within the more committed subpopulation of CD34+CD38+ cells and not in CD34+CD38− cells.
Viral infection and replication was found to be restricted to committed progenitors since no HIV-1 or HIV-2 proviral DNA presence nor viral core protein production could be demonstrated in secondary CFC derived from LTCIC, which represent a readout for the most-primitive stem cell subpopulation. Even continued culture of secondary CFCs in a medium designed to activate HIV-replication (tertiary cultures, supplemented with a cocktail of growth factors) failed to reveal a possible viral presence by any means of detection. These findings are compatible with the demonstration that HIV-1 could be found only in a small subpopulation of CD34+ cells purified from AIDS patient derived BM.5,6 28
In the third part of our study, we demonstrated that the most-primitive hematopoietic stem cells lack the surface molecules CD4, CXCR-4 and CCR-5, which are required for HIV-infection. It has been reported that CD34+ cells can express some level of CD4 molecules.18,39-41 In our study, CD4+ cells were found only within the maturer CD34+CD38+cell populations, explaining their susceptibility to HIV-infection and limited traces of viral replication at the beginning of BMLTC. In contrast, no CD4 expression was detectable on less mature CD34+CD38− cells and no evidence for infection of these cells was found in BMLTC. Several reports using phenotypic rather than functional criteria for the characterization of stem cells support our findings.4,5,7-9,28 Other investigators have described that LTCIC were found within a CD4lowCD34+ cell population and that CD4 expression was higher on CD34+ cells with the CD38− phenotype.41 Although we cannot explain these differences, it is commonly recognized that CD4 expression appears to be generally low and infrequent on CD34+ cells. It is possible that different protocols for obtaining BM cells (marrow aspiration versus leukapheresis) and their storage (fresh cells versus cryo-preservation) may influence the results of phenotype analysis. CXCR-4 or CCR-5 molecules, which represent in conjunction with CD4 a functional unit for high-efficacy HIV entry into CD34+cells, were found to be expressed on the more mature progenitors in our study. This seems somewhat surprising and appears intriguing, since it was suggested that chemokines and their receptor-mediated signals might play an important role in hematopoiesis.45,46 However, the inability to detect HIV receptors on most-immature hematopoietic cells by means of flow cytometry supports our results obtained from stringent long-term culture assessment. The tropism of the different HIV strains used in our experiments should have allowed for an effective entry, if the surface molecules were present on the cells in a sufficient density. The HIV-1 strain Bal represents a non-syncytium inducing (NSI) isolate, which readily infects monocytes/macrophages (monocytotropic). Bal entry into cells is mainly supported by RANTES- receptors, including CCR-5. The primary isolate HIV-1 571 has been found to have very similar characteristics regarding cell tropism.20HIV-1 RF and NM have been demonstrated to induce syncytium in culture (SI) and are preferentially T cells tropic with the ability to use CXCR-4 for entry.47 The HIV-2 strains ST and Rod were reported to use a variety of receptors, including CCR-5 and CXCR-4.48-50
It has been demonstrated that subsets of CD34+CD4+ cells, selected from leukapheresis products, contain mRNA specific for CXCR-4 and CCR-5 as detected by RT-PCR. Sequences for both chemokine receptors were also amplified in some subpopulations of CD34+CD4−cells.51 Theoretically, it is possible that the levels of chemokine receptor mRNA detectable in primitive hematopoietic precursor cells may not necessarily correlate with receptor expression on the cell surface. An alternative explanation for the insusceptibility of BM stem cell subsets to HIV infection could be derived from the hypothesis that the virus would enter, but the immaturity and/or activational stage of the cell may not support the complex process of HIV replication. Clarification of these aspects, however, remains an issue of future investigations.
We have previously demonstrated that exposure of total BM cells to HIV-1 or HIV-2 is associated with TNF-α production.20,52-54 In our experiments, a restricted TNF-α release was also observed in correlation with a temporary modulation of the CFC function (data not shown). The number of LTCICs derived from CD34+ cells was not significantly affected in our experimental system, suggesting that LTCIC are either more resistant to the action of TNF-α (that may have been released in culture) or that continuous or recurrent presence of HIV is required for the sustained levels of TNF-α capable of affecting the function of hematopoietic stem cells. In support of this hypothesis, it was recently demonstrated that continuous exposure of hematopoietic stem cells to IFN-γ was required to induce a decrease of the LTCIC number in BMLTC.55 Similar mechanisms may operate in vivoin AIDS patients. Continuous presence of virus or of viral gene products may cause elevated levels of TNF-α, sufficient to modulate the function of hematopoietic progenitor cells. The stimulus for continuous TNF-α release was not present in BMLTC cultures of CD34+ cells challenged with HIV or gp120, since inoculum was removed through extensive washing and no viral replication was detectable. However, when total BM cells were used to initiate the BMLTC, the decrease in CFC (1 to 3 weeks of BMLTC) correlated with the release of TNF-α, as well as the subsequent detection of viral replication in these cultures.
When purified CD34+ cell preparations were exposed to HIV-1 or HIV-2 viral stocks, a moderate increase in the number of 1-week secondary CFC was noted. Similar findings were reported in other studies.33,34,42 It is possible that virus stocks contain certain growth factors with transient effects on the proliferation of progenitor cells.23 To account for these factors in our experiments, we included controls derived from cell cultures similar to those that were used to propagate viral stocks. These controls showed that growth factors contained in the viral supernatant do not appear to play a relevant role in the observed increase in colony formation seen in the first weeks of cultures. It has been demonstrated that viral gene products such as envelope (gp120, gp41) or Tat represent potent modulators of cell activation and could also trigger the release of a variety of cytokines responsible for the observed differences in colony formation.23,51,56,57 In addition to the proposed indirect mechanisms, some studies suggested a direct effect of viral proteins on CD34+ cells, resulting in inhibition of proliferation and the onset of apoptosis.26,38 58 In our experiments, however, we were unable to observe such a direct effect on LTCIC.
Our results show that HIV-2, similar to HIV-1, does not infect hematopoietic stem cells in vitro with any significant frequency, and that infected cells are not represented within LTCICs. This finding supports the hypothesis that direct infection of hematopoietic stem cells by HIV-1 and HIV-2 does not play a major role in the hematologic pathophysiology of AIDS-associated BM failure, nor are hematopoietic stem cells likely candidates to serve as a significant reservoir of virus in vivo. The failure to detect productive or latent HIV in the most-primitive human BM progenitor and stem cells has important implications for future therapeutic strategies, including those dealing with immune modulation and gene therapy.
Address reprint requests to Frank F. Weichold, MD, PhD, Institute of Human Virology, University of Maryland, 725 West Lombard St, Baltimore, MD 21201.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal