Abstract
Factor V inhibitors may develop as spontaneous autoantibodies, as alloantibodies after exposure to bovine thrombin preparations, or in factor V–deficient patients after plasma therapy. Clinical manifestations range from asymptomatic laboratory abnormalities to life-threatening hemorrhage. We have characterized the anti–factor V antibodies from 12 patients diagnosed with factor V inhibitors. In 8 patients, hemorrhagic complications (5 autoantibodies and 3 bovine thrombin-induced alloantibodies) developed, and 4 were asymptomatic (2 autoantibodies and 2 alloantibodies). The IgG fractions from all 12 patients immunoprecipitated the factor Va light chain, but only the 8 IgG fractions associated with hemorrhage inhibited factor V activity in a prothrombinase assay. Nine IgG fractions, including the 8 patients with hemorrhage, immunoprecipitated the isolated second C-type domain (C2). The 8 IgG fractions from the symptomatic patients also immunoprecipitated recombinant chimeras containing only the N-terminal third of the factor V C2 domain, and isolated recombinant C2 domain abrogated the inhibitory effect of the antibodies. Five of the inhibitory IgG fractions blocked binding of factor V to phosphatidylserine. These results suggest that inhibitory anti–factor V antibodies are associated with hemorrhagic manifestations and frequently bind to a common region within the C2 domain, whether originating spontaneously or after exposure to bovine thrombin.
COAGULATION FACTOR V is an essential component of the “prothrombinase complex,” accelerating activation of the zymogen prothrombin to thrombin by the serine proteinase factor Xa in the presence of calcium ions and a phospholipid membrane surface.1 Factor V circulates in the plasma as a single-chain protein composed of several domains defined by primary amino acid sequence and arranged as A1-A2-B-A3-C1-C2.1Structurally and functionally, factor V is similar to factor VIII, an essential component of the “factor X-ase complex” that accelerates the activation of factor X by factor IXa in the presence of calcium ions and a phospholipid membrane surface.2
Acquired inhibitors to factor V may develop spontaneously as autoantibodies in previously normal patients, generally after surgical procedures (without exposure to topical bovine thrombin), blood transfusions, or antibiotic administration.3 These autoantibodies are frequently associated with hemorrhagic symptoms, usually mild but occasionally severe.4-6 Some patients are asymptomatic, however, and the antibodies are frequently low titer and transient.3 Factor V inhibitors may also develop in patients with factor V deficiency after plasma infusions for treatment of hemorrhagic complications, although this has been reported in only two cases.7 8
Factor V inhibitors may also develop after exposure to bovine thrombin preparations used as topical hemostatic agents.9-12 Bovine thrombin preparations are frequently used in vascular, orthopedic, and neurosurgical procedures, either applied directly to the bleeding site or as a component of fibrin glue.13 However, these thrombin preparations frequently contain additional bovine proteins, including bovine factor V,10 and several investigators have demonstrated that alloantibodies developing after exposure to bovine thrombin may cross-react with the corresponding human proteins.9 10
Several recent studies suggest that the development of factor V inhibitors after exposure to bovine thrombin may occur considerably more frequently than previously recognized. Bänninger et al14 reported that 11 of 24 patients treated during cardiovascular procedures with a fibrin glue preparation consisting of human fibrinogen and bovine thrombin subsequently developed factor V inhibitors. Similarly, Carroll et al15 observed antibodies to bovine factor V in nine patients after treatment with a bovine fibrin hemostatic agent. Six of the alloantibodies cross-reacted with human factor V, although none of the patients showed the development of hemorrhagic complications.15 In addition, we have observed that more than 80% of patients exposed to topical thrombin preparations during cardiovascular surgery develop a seropositive response to the specific thrombin preparation applied.16The clinical manifestations associated with these cross-reacting alloantibodies are extremely heterogeneous, however, similar to the clinical manifestations observed with spontaneous autoantibodies.11 12
We previously showed that the spontaneously arising factor V inhibitor H1 bound to a discrete region within the second C-type domain, spanning amino acids 2037 through 2087 in the N-terminal region of the domain.17 This inhibitor, which was associated with fatal hemorrhagic outcome,4 blocked binding of factor V to phosphatidylserine.18 The purpose of this study was to determine whether differences in inhibitory activity or epitope specificity of the anti–factor V antibodies isolated from an additional 11 patients diagnosed with factor V inhibitors correlated with the observed clinical phenotypes. In addition, we wanted to determine whether the anti–factor V antibodies that developed as spontaneous autoantibodies differed in inhibitory activity or epitope specificity from the bovine thrombin-induced cross-reacting alloantibodies.
MATERIALS AND METHODS
Materials.
Human thrombin, factor Xa, prothrombin, and bovine prothrombin were from Haemotologic Technologies (Essex Junction, VT).L-(α)-phosphatidylserine (bovine brain) was from Avanti Polar Lipids (Birmingham, AL). The chromogenic substrate S2238 (D-Phe-L-pipecolyl-Arg-p-nitroanilide) was from Chromogenix AB (Mölndal, Sweden). Immunoaffinity-purified polyclonal rabbit anti-human factor V antibodies and the murine monoclonal antibody (MoAb) 6A5 were prepared as described previously.17,19 The murine MoAb HV-1, which binds to the N-terminal region of the factor V C2 domain and blocks binding to phosphatidylserine,17 and all other reagents were from Sigma Chemical Co (St Louis, MO).
Patients.
Plasma or serum samples, or both, were obtained from patients diagnosed with factor V inhibitors. The clinical laboratory criteria used for diagnosis of a factor V inhibitor included (but were not limited to) (1) a prolonged prothrombin time (PT) and activated partial thromboplastin time (aPTT) that did not correct when patient plasma was mixed 1:1 with pooled normal plasma; (2) a decreased or nonmeasurable factor V level; and/or (3) demonstration of an inhibitor to factor V by modification of the Bethesda method.20 For determination of the factor V inhibitor titers, pooled normal plasma was mixed 1:1 with patient plasma and incubated at 37°C for 2 hours. Factor V activity in the patient mixture was then determined and divided by the factor V activity in a control plasma sample, also incubated at 37°C for 2 hours. In this assay, one “inhibitor unit” is defined as the amount inactivating one half of the factor V activity in the patient mixture. Additional clinical laboratory testing performed for certain patients included thrombin clotting times, factor II levels, and fibrinogen levels.11 Several of these patients were also evaluated for a lupus anticoagulant.21 22
Recombinant factor V constructs.
Construction and expression of the recombinant human factor V (rHFV) deletion mutants and chimeras used in this study have been described elsewhere.17,19,23 Specific constructs used for epitope mapping are described in the legends to Figs 1 and 3. Recombinant chimeras substituted exon-size segments of the C2 domain of factor VIII for the corresponding regions of the C2 domain of factor V.17 Only one of these chimeras possessed significant activity in the prothrombinase assay (chimera 5A), and none possessed any activity in a factor VIII chromogenic assay.17
Epitope mapping anti–factor V antibodies with recombinant factor V (rHFV) deletion mutants. Recombinant mutants, depicted schematically on the left side of the figure, included a mutant lacking amino acids 811 through 1491 of the B domain (rHFV des B), and a mutant lacking amino acids 811 through 1491 and residues Gly-2037 through Tyr-2196 of the light chain (rHFV des B/C2). The isolated heavy chain consists of amino acids Ala-1 through Arg-709 (rHFV HC); the isolated light chain consists of amino acids Ser-1546 through Tyr-2196 (rHFV LC); the isolated A3 domain consists of amino acids Ser-1546 through Arg-1877 (rHFV A3); and the isolated C2 domain consists of amino acids Gly-2037 through Tyr-2196 (rHFV C2). The IgG fractions purified by protein A-Sepharose chromatography are listed on the right side of the figure. Immunoprecipitation of [35S]methionine metabolically labeled mutants and analysis by SDS-PAGE was performed as previously described.23 +, the IgG fraction immunoprecipitated the specific factor V mutant; −, it did not. ND, not done.
Epitope mapping anti–factor V antibodies with recombinant factor V (rHFV) deletion mutants. Recombinant mutants, depicted schematically on the left side of the figure, included a mutant lacking amino acids 811 through 1491 of the B domain (rHFV des B), and a mutant lacking amino acids 811 through 1491 and residues Gly-2037 through Tyr-2196 of the light chain (rHFV des B/C2). The isolated heavy chain consists of amino acids Ala-1 through Arg-709 (rHFV HC); the isolated light chain consists of amino acids Ser-1546 through Tyr-2196 (rHFV LC); the isolated A3 domain consists of amino acids Ser-1546 through Arg-1877 (rHFV A3); and the isolated C2 domain consists of amino acids Gly-2037 through Tyr-2196 (rHFV C2). The IgG fractions purified by protein A-Sepharose chromatography are listed on the right side of the figure. Immunoprecipitation of [35S]methionine metabolically labeled mutants and analysis by SDS-PAGE was performed as previously described.23 +, the IgG fraction immunoprecipitated the specific factor V mutant; −, it did not. ND, not done.
Epitope mapping anti–factor V antibodies with recombinant factor V C2 domain chimeras. The complete domain structure of rHFV des B is shown at the top, and the expanded domain structures of the light chain portions of the individual chimeras are shown below. The white boxes indicate factor V–derived sequences, and the shaded boxes indicate factor VIII–derived sequences. Chimera 1A substituted amino acids 2282 through 2332 of factor VIII for amino acids 2149 through 2196 of factor V. Chimera 2A substituted amino acids 2223 through 2332 of factor VIII for amino acids 2088 through 2196 of factor V. Chimera 3A substituted amino acids 2173 through 2332 of factor VIII for amino acids 2037 through 2196 of factor V. Chimera 5A substituted amino acids 2173 through 2222 of factor VIII for amino acids 2037 through 2087 of factor V. Chimera 7A substituted amino acids 2223 through 2281 of factor VIII for amino acids 2088 through 2148 of factor V. The antibodies that immunoprecipitated the factor V C2 domain are listed on the right side. Immunoprecipitation of [35S]methionine metabolically labeled mutants and analysis by SDS-PAGE was performed as described in Materials and Methods. +, the antibody immunoprecipitated the specific factor V mutant; −, it did not.
Epitope mapping anti–factor V antibodies with recombinant factor V C2 domain chimeras. The complete domain structure of rHFV des B is shown at the top, and the expanded domain structures of the light chain portions of the individual chimeras are shown below. The white boxes indicate factor V–derived sequences, and the shaded boxes indicate factor VIII–derived sequences. Chimera 1A substituted amino acids 2282 through 2332 of factor VIII for amino acids 2149 through 2196 of factor V. Chimera 2A substituted amino acids 2223 through 2332 of factor VIII for amino acids 2088 through 2196 of factor V. Chimera 3A substituted amino acids 2173 through 2332 of factor VIII for amino acids 2037 through 2196 of factor V. Chimera 5A substituted amino acids 2173 through 2222 of factor VIII for amino acids 2037 through 2087 of factor V. Chimera 7A substituted amino acids 2223 through 2281 of factor VIII for amino acids 2088 through 2148 of factor V. The antibodies that immunoprecipitated the factor V C2 domain are listed on the right side. Immunoprecipitation of [35S]methionine metabolically labeled mutants and analysis by SDS-PAGE was performed as described in Materials and Methods. +, the antibody immunoprecipitated the specific factor V mutant; −, it did not.
Recombinant C2 domain was obtained for protein purification by subcloning the cDNA encoding the factor V C2 domain into the baculovirus transfer vector pBluebac2. Sf9 cells were cotransfected with rHFV C2 and WT Autographa californica nuclear polyhedrosis virus, and recombinant baculovirus was obtained by plaque purification. Insect cells were infected in spinner flasks, and recombinant C2 domain was purified to homogeneity from conditioned media using cation-exchange chromatography on Mono S (Amersham Pharmacia Biotech, Piscataway, NJ).24
Purification of IgG fractions.
Plasma or serum samples, or both, were fractionated by affinity chromatography on Protein A Sepharose (Amersham Pharmacia Biotech), as previously described.18 The IgG fractions were concentrated using a microconcentrator (Centricon 30; Amicon, Beverly, MA), and both binding and nonbinding fractions were assessed for inhibitory activity.
Purification of anti–factor V C2 domain antibodies.
Two plasma samples (RS and H5) were also fractionated by affinity chromatography with Sepharose to which purified rHFV C2 domain had been coupled. For patient RS, the Protein A Sepharose binding fraction from 0.5 mL of plasma was dialyzed into 10 mmol/L Tris-HCl, pH 7.5/0.15 mol/L NaCl, concentrated, and then applied to a C2 domain affinity column (≈0.9 mL; 8.62 mg/mL). The resin was then serially developed with 100 mmol/L glycine-HCl, pH 2.5; 100 mmol/L triethylamine, pH 11.5; and 50 mmol/L Tris-HCl, pH 7.4/50% ethylene glycol. After each buffer change, ≈10 mL of elution buffer flow-through was pooled, dialyzed into phosphate-buffered saline, pH 7.4, and concentrated to ≈0.5 mL final volume on a CM30 microconcentrator (Amicon). Protein concentration was determined by absorbance at 280 nm. For patient H5, initial fractionation on Protein A Sepharose was not performed because this fractionation step did not isolate significant inhibitory activity from H5 plasma (see Results). Instead, 10 mL of H5 plasma was diluted 1:10 into 10 mmol/L Tris-HCl, pH 7.5, and applied directly to the C2 domain affinity column. The resin was then serially developed as described for patient RS. For a negative control, 10 mL of citrated plasma from a single normal donor was fractionated on the C2 domain affinity column, as described for patient H5.
Immunoprecipitation of factor V deletion mutants and chimeras.
Immunoprecipitation analyses of [35S]methionine metabolically labeled factor V mutants and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were performed as previously described.23 Immunoprecipitation of metabolically labeled factor V mutants with (1) an immunoaffinity-purified polyclonal rabbit anti-human factor V antibody, and (2) a Protein A Sepharose IgG preparation from pooled normal plasma, were used as positive and negative controls, respectively.
Prothrombinase assays.
Factor Va activity in the presence of IgG from inhibitor plasmas was determined using a chromogenic prothrombinase assay, as previously described.18 Briefly, the factor V sample (rHFV des B or Russell's viper venom-activated rHFV) was incubated with patient IgG and then added to 50 mmol/L Tris HCl, pH 7.9, 175 mmol/L NaCl, 5 mg/mL bovine serum albumin (BSA), containing either rabbit brain cephalin (8.0% vol/vol) or 25% phosphatidylserine/75% phosphatidylcholine vesicles (0.8 mmol/L), 0.20 μmol/L prothrombin, and 8.0 mmol/L CaCl2. Preliminary experiments confirmed that inhibition occurred rapidly and was complete within 15 minutes for those IgG fractions containing inhibitory activity. The prothrombinase reaction was subsequently initiated by the addition of 0.2 nmol/L factor Xa and then stopped after 2 minutes by the addition of 20 mmol/L EDTA. Aliquots of the reaction mixtures were transferred to microtiter plate wells, and the amount of thrombin generated during the reactions was determined by adding 0.2 mmol/L S2238 and measuring the change in absorbance at 405 nm using a Vmax microtiter plate reader (Molecular Devices, Menlo Park, CA).18
For inhibition of chimera 5A, conditioned media containing the recombinant chimera (≈13 mU/mL) was preincubated with each of the factor V inhibitors (50 μg/mL) for 15 minutes at 37°C. Residual activity was then determined in the prothrombinase assay using rabbit brain cephalin as the phospholipid membrane source, as described above. For the chromogenic assay, 1 unit is defined as the amount of factor V activity present in 1 mL of activated pooled human plasma.19
Phospholipid binding enzyme-linked immunosorbent assay (ELISA).
The ability of anti–factor V antibodies to interfere with the binding of recombinant factor V to phosphatidylserine (PS) was investigated using a solid-phase ELISA, as previously described.23Briefly, microtiter plate wells were coated overnight with 3 μg/mL PS in methanol and then blocked with 0.5% gelatin.23 Aliquots of conditioned media containing recombinant factor V des B (≈0.7 nmol/L) were first incubated for 15 minutes at 37°C with IgG fractions from the individual patients, an IgG fraction from pooled normal plasma, or the MoAb HV-1, followed by 1-hour incubation of the conditioned media-antibody mixture in the PS-coated microtiter plate wells. The 15-minute preincubation step with the antibodies was included for consistency with the prothrombinase assays. Binding of factor V to PS was determined with either biotin-labeled MoAb 6A5, or biotinylated immunoaffinity-purified rabbit polyclonal anti-human factor V, as previously described.17
Anti–bovine prothrombin ELISA.
Bovine prothrombin (5 μg/mL in PBS, pH 7.4) was coated onto 96-well microtiter plates (Costar EIA, Cambridge, MA; 50 μL/well) and incubated overnight at 4°C. Unbound protein was removed by washing the wells three times with 0.1% Tween 20/phosphate-buffered saline, pH 7.4 (PBS). Wells were then blocked by the addition of 200 μL/well of 1% BSA/0.1% Tween 20/PBS (block buffer) for 2 hours at 22°C. Patient plasma or purified IgG samples were diluted in the block buffer and 100-μL aliquots were incubated in each well for 2 hours at 22°C. Pooled normal plasma was used to determine nonspecific antibody binding. Bound IgG was detected by incubation with a goat α-human IgG peroxidase conjugate (1/500 dilution into block buffer; 100 μL/well; 1 hour at 22°C) followed by peroxidase substrate. Absorbance was measured at 405 nm, as described above.
RESULTS
Patients.
Clinical laboratory results obtained on the 12 patients investigated in this study are summarized in Table 1. Eight patients presented with hemorrhagic manifestations, ranging from oozing around a vascular graft site (EM) to fatal exsanguination (H1). Six of these patients had been treated with antibiotics, five with cephalosporins (patients H3, RB, AR, EM, and RH) and one with an aminoglycoside antibiotic (H1).4 Patient RB initially developed hematuria and gastrointestinal bleeding after a course of therapy with cephradine. The symptoms resolved with discontinuation of the antibiotic, only to recur 2 years later after exposure to cephalexin. Two of the patients had been treated with bovine thrombin, either as a topical hemostatic agent (RS) or as a component of fibrin glue (EM). In a third patient, hemorrhagic symptoms developed after coronary artery bypass grafting (WH), during which bovine thrombin is frequently used.
Clinical and Laboratory Manifestations of 12 Patients With Factor V Inhibitors
| Patient . | Patient (age, sex) . | Factor V Level (%) . | Inhibitor Titer . | Bovine Thrombin Exposure . | Antibiotic Exposure . | Hemorrhagic Manifestations . | Reference . |
|---|---|---|---|---|---|---|---|
| Hemorrhagic anti–factor V antibodies | |||||||
| H1 | 51, M | ND | Inhibitory at >1:1,500-150 | No | Gentamicin, tetracycline, and erythromycin; prior streptomycin | Fatal spontaneous exsanguination | 4 |
| H3 | 67, M | 23-150 | 5 U-150 | No | Cefmetazole | Bloody sputum; bleeding from puncture site | — |
| RS | 64, M | <3.2 | 11 U | Topical thrombin | No | Bleeding from duodenal ulcer when heparin therapy initiated | 11 |
| RB | 82, M | <3.2 | ND | No | Cephradine | Hematuria and gastrointestinal bleeding | — |
| WH | ?, M | <3.2 | ∼35 U | Post-coronary artery bypass grafting | No | “Bleeding diathesis”-151 | — |
| AR | 75, F | >3.2 (inhibitory pattern) | ∼8 U-150 | No | Cephalexin (3 mo before admission) | Hematemesis, hematochezia, and hematuria | 21 |
| EM | 86, M | 58-150 (inhibitory pattern) | ND | Fibrin glue | Cephalexin | Hemorrhage from infected vascular graft site | — |
| RH | 55, M | <3.2 | ND | No | Cephalexin | No hemorrhagic symptoms at time of testing-152 | 22 |
| Nonhemorrhagic anti–factor V antibodies | |||||||
| H4 | 78, F | 19-150 | ND | No | No | None | — |
| H5 | 2, M | Not measurable | ND | Fibrin sealant | Imipenem-cilastatin and amphotericin B | None | 25 |
| MJ | 13, M | <3.2 | 2.2 U | Topical thrombin | No | None | 11 |
| BH | 60, M | <5-150 | 1.05 U-150 | No | No | None | — |
| Patient . | Patient (age, sex) . | Factor V Level (%) . | Inhibitor Titer . | Bovine Thrombin Exposure . | Antibiotic Exposure . | Hemorrhagic Manifestations . | Reference . |
|---|---|---|---|---|---|---|---|
| Hemorrhagic anti–factor V antibodies | |||||||
| H1 | 51, M | ND | Inhibitory at >1:1,500-150 | No | Gentamicin, tetracycline, and erythromycin; prior streptomycin | Fatal spontaneous exsanguination | 4 |
| H3 | 67, M | 23-150 | 5 U-150 | No | Cefmetazole | Bloody sputum; bleeding from puncture site | — |
| RS | 64, M | <3.2 | 11 U | Topical thrombin | No | Bleeding from duodenal ulcer when heparin therapy initiated | 11 |
| RB | 82, M | <3.2 | ND | No | Cephradine | Hematuria and gastrointestinal bleeding | — |
| WH | ?, M | <3.2 | ∼35 U | Post-coronary artery bypass grafting | No | “Bleeding diathesis”-151 | — |
| AR | 75, F | >3.2 (inhibitory pattern) | ∼8 U-150 | No | Cephalexin (3 mo before admission) | Hematemesis, hematochezia, and hematuria | 21 |
| EM | 86, M | 58-150 (inhibitory pattern) | ND | Fibrin glue | Cephalexin | Hemorrhage from infected vascular graft site | — |
| RH | 55, M | <3.2 | ND | No | Cephalexin | No hemorrhagic symptoms at time of testing-152 | 22 |
| Nonhemorrhagic anti–factor V antibodies | |||||||
| H4 | 78, F | 19-150 | ND | No | No | None | — |
| H5 | 2, M | Not measurable | ND | Fibrin sealant | Imipenem-cilastatin and amphotericin B | None | 25 |
| MJ | 13, M | <3.2 | 2.2 U | Topical thrombin | No | None | 11 |
| BH | 60, M | <5-150 | 1.05 U-150 | No | No | None | — |
Abbreviations: M, male; F, female; U, inhibitor units; and ND, not done.
Clinical laboratory studies not performed at Duke University Medical Center.
More definitive information concerning this patient and the hemorrhagic manifestations are unavailable.
At initial presentation, patient RH had “absent” factor V, an inhibitor titer >1,200 U, and severe hemorrhage.22 The sample investigated in this study was obtained at a time when the patient was no longer having hemorrhagic problems, and the inhibitor titer had reportedly decreased to 0.5-1.67 U.22
Four patients presented with abnormal coagulation studies in the absence of any hemorrhagic manifestations (inhibitors H4, H5, MJ, and BH). Two patients (H5 and MJ) had been recently treated with bovine thrombin preparations,11 25 and one (H5) had also been treated with an antibiotic (Table 1). Patient H4 presented with a pleural effusion related to congestive heart failure and underwent thoracentesis without bleeding. Patient BH presented with chest pain and was found to have abnormal coagulation studies during a precatheterization evaluation. Neither patient H4 nor BH had been previously exposed to bovine thrombin or antibiotics. These four patients presented with prolonged PT and aPTT results that did not correct when mixed 1:1 with normal plasma, markedly decreased factor V levels, and low-titer factor V inhibitors by Bethesda analysis in two of the patients (Table 1).
IgG isolated from patients diagnosed with factor V inhibitors contain antibodies that bind to the factor Va light chain.
Protein A Sepharose was used to purify the IgG fractions from these 12 patients. Anti–factor V antibodies were shown in the IgG fraction from each patient by ELISA (data not shown) and by immunoprecipitation analysis (Fig 1). None of the IgG fractions contained antibodies that bound to the heavy chain of factor V (rHFV HC), but all 12 IgG fractions immunoprecipitated recombinant constructs containing an intact light chain (rHFV; rHFV des B; and rHFV LC; Fig1).
Recombinant factor V light chain deletion mutants were used to further delineate the epitopes recognized by these anti–factor V antibodies. All eight IgG fractions from the symptomatic patients (H1, H3, RS, RB, WH, AR, EM, and RH), as well as the IgG fraction from one of the patients without bleeding (BH) immunoprecipitated the isolated C2 domain (rHFV C2). The IgG fraction from one of the patients without hemorrhagic symptoms (MJ) immunoprecipitated the light chain deletion mutant lacking the C2 domain (thrombin-activated rHFV des B/C2) but not the isolated A3 domain (rHFV A3), suggesting a possible epitope in the C1 domain (Fig 1). The other two IgG fractions (H4 and H5) did not immunoprecipitate any construct smaller than the entire light chain (Fig 1).
Factor V activity is inhibited by the IgG fractions from patients with hemorrhagic symptoms.
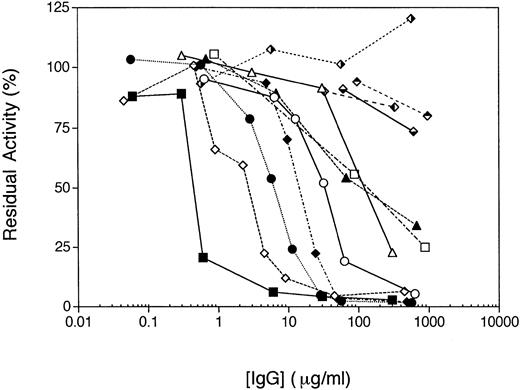
The IgG fractions purified from the eight patients with hemorrhagic manifestations all inhibited the activity of rHFV des B in a prothrombinase assay (Fig 2). In general, the severity of the hemorrhagic complications appeared to correlate with the amount of inhibitory activity in the patient plasma. Three of the IgG fractions did not completely inhibit rHFV des B activity, however, with ≈25% to 50% residual activity at IgG concentrations in excess of 200 μg/mL (patients H3, RH, and EM; Fig 2).
Inhibition of activity of recombinant human factor V des B (rHFV des B) by IgG fractions. The individual IgG preparations were incubated with ≈3 nmol/L rHFV des B in conditioned media at the concentrations shown for 15 minutes at 37°C. The sample was then diluted 1:10 into 50 mmol/L Tris-HCl, pH 7.9, 175 mmol/L NaCl, 5 mg/mL BSA, and residual activity was determined by the chromogenic prothrombinase assay as described in Materials and Methods. The IgG fractions were H1 (▪); H3 (□); RS (⧫); RB (◊); WH (•); AR (○); EM (▴); RH (▵); H4 (); H5 (); MJ (); and BH ().
Inhibition of activity of recombinant human factor V des B (rHFV des B) by IgG fractions. The individual IgG preparations were incubated with ≈3 nmol/L rHFV des B in conditioned media at the concentrations shown for 15 minutes at 37°C. The sample was then diluted 1:10 into 50 mmol/L Tris-HCl, pH 7.9, 175 mmol/L NaCl, 5 mg/mL BSA, and residual activity was determined by the chromogenic prothrombinase assay as described in Materials and Methods. The IgG fractions were H1 (▪); H3 (□); RS (⧫); RB (◊); WH (•); AR (○); EM (▴); RH (▵); H4 (); H5 (); MJ (); and BH ().
By contrast, the IgG fractions purified from the four patients with no hemorrhagic manifestations had essentially no effect on the activity of rHFV des B (Fig 2; patients H4, H5, MJ, and BH), although antibody binding to factor V at concentrations used in the functional assay could be documented by ELISA (data not shown). The Protein A Sepharose nonbinding fractions from these patient plasmas also had no inhibitory effect in this assay, indicating that these patients did not have an IgM (or other non–protein A Sepharose binding isotype) inhibitory antibody (data not shown). Similar results were obtained with these IgG fractions and thrombin-activated factor Va, indicating that the absence of inhibitory activity was not due to a unique property of the B domain deletion mutant (data not shown).
Inhibitory IgG fractions contain anti–factor V antibodies that bind to the N-terminal region of the C2 domain.
We further characterized the nine anti–factor V antibodies that bound to the C2 domain (H1, H3, RS, RB, WH, AR, EM, RH, and BH) by using recombinant factor V/factor VIII C2 domain chimeras (Fig 3). The immunoprecipitation results for patient RS are shown in Fig 4. The IgG fraction from patient BH immunoprecipitated only the WT C2 domain and none of the chimeras (Fig 3). By contrast, the eight IgG fractions associated with hemorrhagic manifestations (H1, H3, RS, RB, WH, AR, EM, and RH) immunoprecipitated those chimeras containing factor VIII sequences substituted for the central and C-terminal thirds of the domain (eg, chimeras 1A and 2A), but not those chimeras that substituted the N-terminal portion of the factor VIII C2 domain for the corresponding segment of the factor V C2 domain (eg, chimeras 3A and 5A; Fig 3). The anti–factor V antibody RH differed slightly from the other inhibitory antibodies in that it did not immunoprecipitate chimera 7A, which substitutes only the central 59 amino acids of the factor VIII C2 domain for the corresponding region of the factor V C2 domain (Fig 3).
Immunoprecipitation of thrombin-activated C2 domain chimeras by IgG fraction from patient RS. Recombinant factor V constructs metabolically labeled with [35S]methionine included rHFV des B, chimera 1A, chimera 2A, chimera 3A, chimera 7A, and chimera 5A. Samples were first incubated with 2 nmol/L thrombin for 1 minute at 37°C and then immunoprecipitated with either the polyclonal rabbit anti-human factor V antibody or the IgG fraction from patient RS, as shown. The immunoprecipitates were analyzed by 6% SDS-PAGE and visualized by autoradiography. A nonspecific, high-molecular-weight band (>200 kD) is seen in all the immunoprecipitations of conditioned media, including the control IgG fraction from pooled normal plasma. Molecular-weight standards are shown on the left, and the positions of the factor Va heavy chain and light chain on the right.
Immunoprecipitation of thrombin-activated C2 domain chimeras by IgG fraction from patient RS. Recombinant factor V constructs metabolically labeled with [35S]methionine included rHFV des B, chimera 1A, chimera 2A, chimera 3A, chimera 7A, and chimera 5A. Samples were first incubated with 2 nmol/L thrombin for 1 minute at 37°C and then immunoprecipitated with either the polyclonal rabbit anti-human factor V antibody or the IgG fraction from patient RS, as shown. The immunoprecipitates were analyzed by 6% SDS-PAGE and visualized by autoradiography. A nonspecific, high-molecular-weight band (>200 kD) is seen in all the immunoprecipitations of conditioned media, including the control IgG fraction from pooled normal plasma. Molecular-weight standards are shown on the left, and the positions of the factor Va heavy chain and light chain on the right.
Recombinant factor V C2 domain neutralizes inhibitory anti–factor V antibodies.
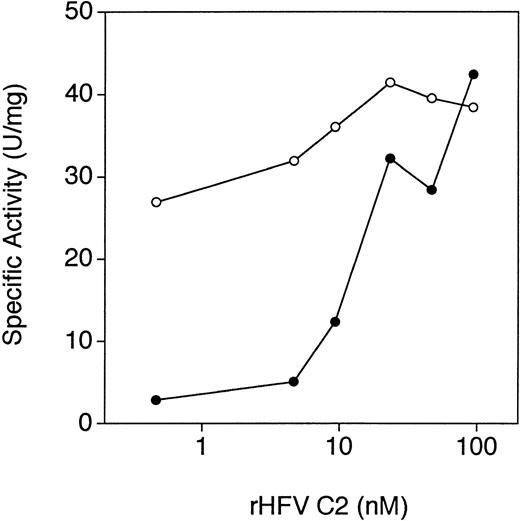
To determine whether antibody binding to the C2 domain in the intact protein resulted in the inhibition of activity, we investigated whether preincubation of the IgG fractions with purified recombinant factor V C2 domain could neutralize the inhibitory activity. As shown in Fig 5, preincubation of the IgG fraction from patient RB with the isolated C2 domain completely abrogated the inhibitory effect of the IgG fraction on the activity of factor V. Similar results were obtained with all of the inhibitory anti–factor V antibodies (data not shown).
Neutralization of the inhibitory anti–factor V antibodies from patient RB by recombinant human factor V C2 domain. Purified IgG from patient RB (≈5 μg/mL) was incubated with purified recombinant factor V C2 domain at the concentrations shown for 15 minutes at 37°C. Conditioned media containing rHFV des B (≈0.5 nmol/L) was then incubated with either the inhibitor IgG/C2 domain mixture (•) or the same concentration of the C2 domain alone (○) for 15 minutes at 37°C. Residual activity was then determined by the chromogenic prothrombinase assay, as described in Materials and Methods.
Neutralization of the inhibitory anti–factor V antibodies from patient RB by recombinant human factor V C2 domain. Purified IgG from patient RB (≈5 μg/mL) was incubated with purified recombinant factor V C2 domain at the concentrations shown for 15 minutes at 37°C. Conditioned media containing rHFV des B (≈0.5 nmol/L) was then incubated with either the inhibitor IgG/C2 domain mixture (•) or the same concentration of the C2 domain alone (○) for 15 minutes at 37°C. Residual activity was then determined by the chromogenic prothrombinase assay, as described in Materials and Methods.
Inhibitory anti–factor V antibodies do not inhibit a recombinant chimera that substitutes the N-terminal portion of the factor VIII C2 domain for the homologous region of the factor V C2 domain.
The recombinant chimera 5A possesses ≈20% of the activity of factor V des B and is resistant to the inhibitory effect of the IgG fraction from patient H1.17 Similarly, this chimera was also resistant to the IgG fractions from all of the patients with hemorrhagic complications (data not shown), consistent with the immunoprecipitation data obtained with the [35S]methionine metabolically labeled factor V mutants (Fig 3). This chimera was also not inhibited by any of the IgG fractions from the nonhemorrhagic patients (H4, H5, MJ, and BH; data not shown).
Inhibition of factor V binding to phosphatidylserine by IgG fractions.
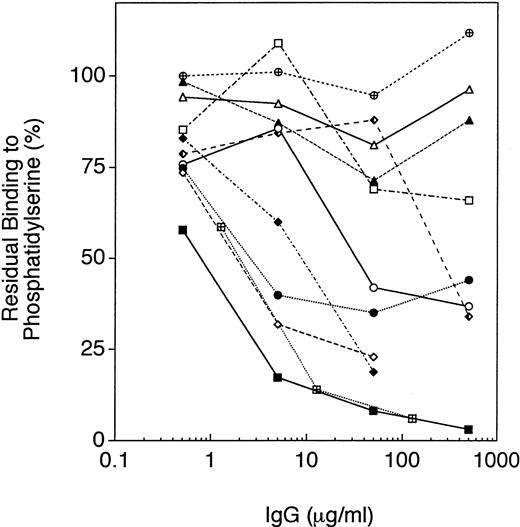
The IgG fractions from patients H1, RS, and RB consistently inhibited binding of rHFV des B to immobilized PS (<25% residual binding at IgG concentrations 50 mg/mL; Fig 6 and data not shown). The IgG fractions from patients WH and AR partially inhibited binding of rHFV des B to immobilized PS (25% to 50% residual binding at IgG concentrations ≥50 μg/mL; Fig 6). By contrast, the IgG fractions from the patients H3, RH, and EM had minimal or no inhibitory effect on binding to PS (>50% residual binding at IgG concentrations ≥50 μg/mL; Fig 6 and data not shown). These three IgG fractions also did not completely neutralize the activity of rHFV des B in the prothrombinase assay (Fig 2). Of the IgG fractions from the patients without hemorrhagic symptoms, H4 partially blocked binding to PS (25% to 50% residual binding at IgG concentrations ≥500 μg/mL; Fig 6). The other three IgG fractions (H5, MJ, and BH), however, had no effect on the binding of factor V to PS (>75% residual binding at IgG concentrations ≥500 μg/mL; data not shown).
Inhibition of rHFV des B binding to phosphatidylserine by IgG fractions. Recombinant factor V des B was incubated with the IgG fractions at the concentrations shown for 15 minutes at 37°C, and then incubated in the PS-coated wells as described in Materials and Methods. Binding to PS was determined by ELISA, as previously described.18 The IgG fractions shown are: H1 (▪); H3 (□); RS (⧫); RB (◊); WH (•); AR (○); EM (▴); RH (▵); and H4 (). Controls include the IgG fraction from pooled normal plasma (⊕) and the murine MoAb HV-1 (⊠).
Inhibition of rHFV des B binding to phosphatidylserine by IgG fractions. Recombinant factor V des B was incubated with the IgG fractions at the concentrations shown for 15 minutes at 37°C, and then incubated in the PS-coated wells as described in Materials and Methods. Binding to PS was determined by ELISA, as previously described.18 The IgG fractions shown are: H1 (▪); H3 (□); RS (⧫); RB (◊); WH (•); AR (○); EM (▴); RH (▵); and H4 (). Controls include the IgG fraction from pooled normal plasma (⊕) and the murine MoAb HV-1 (⊠).
Plasma from patient H5 does not contain inhibitory anti-C2 domain antibodies in low titer.
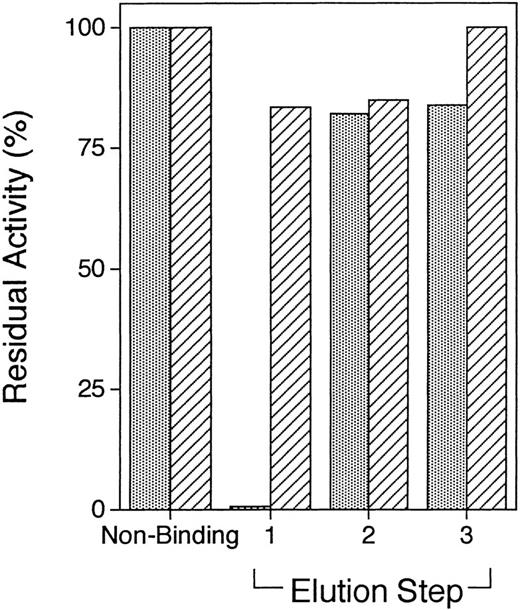
One possible explanation for the differences in the inhibitory activity of the IgG fractions from the hemorrhagic and nonhemorrhagic patients is that the latter simply contain lower titers of inhibitory anti-C2 domain antibodies. To address this possibility, plasma samples from patients RS and H5 were fractionated by C2 domain affinity chromatography, as described in Materials and Methods. For patient RS, all inhibitory activity (from an initial 0.5 mL of patient plasma) bound to the C2 domain affinity column and was eluted with 100 mmol/L glycine-HCl, pH 2.5 (Fig 7). The total amount of bound IgG that was eluted from the C2 column was ≈45 μg, or ≈0.6% of the total IgG loaded on the column.
Inhibition of factor V des B activity by factor V C2 domain-affinity purified RS and H5 fractions. The Protein A Sepharose binding fraction from 0.5 mL RS plasma and 10 mL of unfractionated H5 plasma were applied to ≈1 mL of factor V C2 domain-Sepharose and serially eluted with glycine-HCl (elution step 1), triethylamine (elution step 2), and ethylene glycol (elution step 3), as described in Materials and Methods. A total of 10 ≈1-mL fractions after each buffer change were pooled, concentrated to a final volume of ≈0.5 mL, and then evaluated for inhibitory activity in the prothrombinase assay. Residual factor V activity is plotted against the individual elution steps for the RS (▧) and H5 (▨) fractions.
Inhibition of factor V des B activity by factor V C2 domain-affinity purified RS and H5 fractions. The Protein A Sepharose binding fraction from 0.5 mL RS plasma and 10 mL of unfractionated H5 plasma were applied to ≈1 mL of factor V C2 domain-Sepharose and serially eluted with glycine-HCl (elution step 1), triethylamine (elution step 2), and ethylene glycol (elution step 3), as described in Materials and Methods. A total of 10 ≈1-mL fractions after each buffer change were pooled, concentrated to a final volume of ≈0.5 mL, and then evaluated for inhibitory activity in the prothrombinase assay. Residual factor V activity is plotted against the individual elution steps for the RS (▧) and H5 (▨) fractions.
For patient H5, 10 mL of plasma was diluted into the starting buffer and applied to the C2-domain column. A total of ≈140 μg of protein was eluted with 100 mmol/L glycine-HCl, pH 2.5, but no inhibitory activity was present in this fraction (Fig 7). SDS-PAGE showed predominantly two bands consistent with Ig heavy and light chains, respectively (data not shown). As with patient RS, essentially no protein was eluted with either the triethylamine or ethylene glycol buffers. Fractionation of a second 10-mL plasma sample from patient H5 confirmed the presence of a noninhibitory fraction that could be eluted with the glycine-HCl buffer. By contrast, fractionation of 10 mL of plasma from a normal donor on the C2 domain affinity column resulted in all of the protein remaining in the nonbinding fraction, with no protein being obtained from any of the elution steps (data not shown). Insufficient plasma samples from patients MJ, BH, and H4 were available for similar analyses.
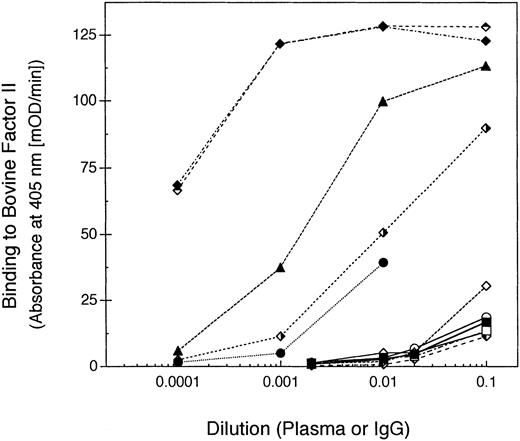
Binding to bovine prothrombin distinguishes spontaneous autoantibodies from topical thrombin-associated alloantibodies.
None of the above data would distinguish patients in whom anti–factor V antibodies developed after exposure to bovine thrombin preparations from patients with spontaneous inhibitors. To determine this, we tested for the presence of antibodies to bovine prothrombin in the patient plasma samples. All four patients in whom anti–factor V antibodies developed after exposure to bovine thrombin also manifested antibodies to bovine prothrombin (Fig 8). In addition, patient WH, who had previously undergone coronary artery bypass grafting (CABG), had antibodies to bovine prothrombin, suggesting that topical bovine thrombin was likely used during this patient's surgical procedure (Fig 8). By contrast, none of the spontaneous anti–factor V antibody plasmas contained antibodies to bovine prothrombin (Fig8).
Anti-bovine prothrombin antibodies in patients with antibodies to factor V. Plasma or sera samples from the patients were diluted into block buffer and incubated in microtiter plate wells coated with bovine prothrombin (for patient WH, a 1:100 dilution of the IgG fraction was used instead of plasma). Antibody binding to bovine prothrombin was detected with a peroxidase-conjugated goat anti-human IgG antibody. The IgG fractions were: H1 (▪); H3 (□); RS (⧫); RB (◊); WH (•); AR (○); EM (▴); RH (▵); H4 (); H5 (); MJ (); and BH ().
Anti-bovine prothrombin antibodies in patients with antibodies to factor V. Plasma or sera samples from the patients were diluted into block buffer and incubated in microtiter plate wells coated with bovine prothrombin (for patient WH, a 1:100 dilution of the IgG fraction was used instead of plasma). Antibody binding to bovine prothrombin was detected with a peroxidase-conjugated goat anti-human IgG antibody. The IgG fractions were: H1 (▪); H3 (□); RS (⧫); RB (◊); WH (•); AR (○); EM (▴); RH (▵); H4 (); H5 (); MJ (); and BH ().
DISCUSSION
The clinical heterogeneity associated with factor V inhibitors was exemplified by the 12 patients included in this study (Table 1). Eight patients manifested hemorrhagic symptoms during their clinical course (H1, H3, RS, RB, WH, AR, EM, and RH), whereas four patients had no bleeding complications (H4, H5, MJ, and BH). Seven patients presented with spontaneous autoantibodies (H1, H3, RB, AR, RH, H4, and BH), whereas in five patients cross-reacting alloantibodies developed after exposure to topical bovine thrombin (RS, WH, EM, H5, and MJ). Seven patients developed antibodies to factor V in the setting of antibiotic therapy (H1, H3, RB, AR, EM, RH, and H5), with one patient (RB) developing a factor V inhibitor on two separate occasions after treatment with different cephalosporin antibiotics (both first-generation cephalosporins that are similar in structure).
All 12 patients clearly had anti–factor V antibodies (Fig 1). However, even though anti–factor V antibodies were present, only the IgG fractions prepared from the patients with hemorrhagic symptoms inhibited the activity of factor V in the prothrombinase assay. By contrast, the IgG fractions (as well as the Protein A Sepharose nonbinding fractions) from the asymptomatic patients were noninhibitory in the prothrombinase assay (Fig 2). IgG binding to factor V at the concentrations used in the prothrombinase assays was confirmed by ELISA for the noninhibitory fractions, but this binding may not have interfered with prothrombinase function or have been weaker than the interactions of factor V with the other components of the prothrombinase complex. Two of the patients with noninhibitory anti–factor V antibodies did have a measurable inhibitor titer, however, as determined by a modified Bethesda assay (patients MJ and BH; Table 1). This may reflect differences in assay methodology (plasma-based v purified proteins) or the fact that plasma samples for this study were not necessarily collected on the same day as determination of the inhibitor titer.
The anti–factor V antibodies from all 12 patients bound to the light chain of factor V (Fig 1). Factor V inhibitors binding to the light chain have also been described in two other patients, both associated with hemorrhagic manifestations.6,9 A spontaneously occurring factor V inhibitor that bound to the heavy chain of factor Va has also been described.26 This spontaneous autoantibody was identified in a patient presenting with extensive ecchymoses, hematuria, and melena.27 None of the patients we studied had anti–factor V antibodies that bound to the heavy chain or to the large connecting region (B domain).
The IgG fractions from the eight patients with hemorrhagic manifestations could be further shown to bind to the N-terminal region of the C2 domain of the light chain, encoded by exon 23 of the factor V gene.28 By contrast, two of the IgG fractions from the four asymptomatic patients immunoprecipitated only the recombinant factor V constructs containing the entire light chain (H4 and H5; Fig 1), and one of the IgG fractions appeared to bind to the C1 domain of the light chain (MJ). The IgG fraction from the fourth patient (BH) immunoprecipitated the isolated C2 domain but did not immunoprecipitate any of the recombinant C2 domain chimeras (Fig 3). Although the IgG fraction from patient H5 did not immunoprecipitate the C2 domain, a C2 domain binding fraction could be obtained from 10 mL of patient plasma by immunoaffinity chromatography on a C2 domain column (Fig 7). Nevertheless, neither the IgG fraction from patient BH nor the C2 domain binding fraction from patient H5 inhibited the activity of factor V in the prothrombinase assay. These results suggest that noninhibitory as well as inhibitory antibodies bind to the C2 domain of factor V.
We have previously shown that binding of factor V to immobilized PS requires the presence of the C2 domain, and that the N-terminal portion of the domain is involved in this binding.17 23 The IgG fractions from five of the patients with hemorrhagic symptoms bound to this region of the C2 domain and at least partially blocked binding of rHFV des B to PS (H1, RS, RB, WH, and AR; Fig 6). The IgG fractions from the other three patients with hemorrhagic symptoms bound to the same region of the C2 domain but did not block rHFV des B binding to PS (H3, EM, RH). These three IgG fractions also did not completely inhibit factor V activity in the prothrombinase assay (Fig 2), which may have been due to lower antibody titers or affinity.
By contrast, although the IgG fraction from patient BH immunoprecipitated the C2 domain, it did not inhibit binding of rHFV des B to PS. The MoAb 6A5 also binds to the C2 domain and does not inhibit binding of rHFV des B to PS, although it is inhibitory in the prothrombinase assay.17 We have previously shown that the IgG fraction from patient H1 and the MoAb 6A5 bind to nonoverlapping epitopes in the C2 domain,17 and it is possible that the IgG fraction from patient BH recognizes a separate region in this domain. Interestingly, although the IgG fraction from patient H4 neither immunoprecipitated the C2 domain nor inhibited factor V in the prothrombinase assay, it did partially inhibit binding of factor V to PS. A second PS-binding site has been identified in the A3 domain of the factor Va light chain,29 but the IgG fraction from this patient did not immunoprecipitate the A3 domain either. Therefore, the mechanism for this inhibition is unknown.
Factor VIII binding to PS also involves the second C-type domain of the light chain, but, in contrast to factor V, the C-terminal portion of the factor VIII C2 domain appears to be involved in binding.30 Factor VIII inhibitors that bind to the C2 domain of factor VIII disrupt binding of factor VIII to anionic phospholipids31 and/or von Willebrand factor.32 Certain factor VIII inhibitors that bind to the C-terminal region of the factor VIII C2 domain recognize an epitope that overlaps with the putative phospholipid-binding site.33 However, we have recently identified several factor VIII inhibitors that bind to the N-terminal region of the C2 domain and interfere with the binding of factor VIII to PS,34 similar to the corresponding region in the factor V C2 domain recognized by factor V inhibitors. Clarification of the mechanism(s) whereby antibodies that bind to the C2 domains of factor V or factor VIII result in inhibition of procoagulant activity and PS binding of the respective cofactors will require fractionation of antibody subsets and detailed functional characterization.
Finally, this study showed that inhibitory anti–factor V antibodies associated with hemorrhagic symptoms bound to a common epitope within the C2 domain of the factor Va light chain, regardless of whether the antibody developed spontaneously or after exposure to bovine thrombin. Nevertheless, bovine thrombin–associated factor V inhibitors could be clearly distinguished from spontaneous autoantibodies by the presence of antibodies to bovine prothrombin (Fig 7). It is possible that the hemorrhagic symptoms manifested by patients exposed to bovine thrombin may require additional cross-reacting alloantibodies to human coagulation proteins, although this has not been previously observed.
ACKNOWLEDGMENT
We thank the Duke Clinical Coagulation Laboratory for performing the factor V assays and inhibitor titers on plasma samples from these patients. We also thank John Brandt, David McGlasson, W. Muntean, and W.A. Dittman for providing us with plasma samples from patients AR, RH, H5, and BH, respectively.
Supported in part by Grant-In-Aid No. NC-93-GS-17 from the American Heart Association North Carolina Affiliate (T.L.O.), a Basil O'Connor Starter Scholar Research Award (No. FY96-0556) from the March of Dimes Birth Defects Foundation (T.L.O.), and by National Institutes of Health Grant No. HL43106 (W.H.K.). T.L.O. is a Pew Scholar in the Biomedical Sciences.
Presented in part at Biomedicine '96, American Federation for Clinical Research, Washington, DC, May 3-6, 1996.
Address reprint requests to Thomas L. Ortel, MD, PhD, Divisions of Hematology and Oncology, Department of Medicine, Duke University Medical Center, Box 3422, Durham, NC 27710.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. Epitope mapping anti–factor V antibodies with recombinant factor V (rHFV) deletion mutants. Recombinant mutants, depicted schematically on the left side of the figure, included a mutant lacking amino acids 811 through 1491 of the B domain (rHFV des B), and a mutant lacking amino acids 811 through 1491 and residues Gly-2037 through Tyr-2196 of the light chain (rHFV des B/C2). The isolated heavy chain consists of amino acids Ala-1 through Arg-709 (rHFV HC); the isolated light chain consists of amino acids Ser-1546 through Tyr-2196 (rHFV LC); the isolated A3 domain consists of amino acids Ser-1546 through Arg-1877 (rHFV A3); and the isolated C2 domain consists of amino acids Gly-2037 through Tyr-2196 (rHFV C2). The IgG fractions purified by protein A-Sepharose chromatography are listed on the right side of the figure. Immunoprecipitation of [35S]methionine metabolically labeled mutants and analysis by SDS-PAGE was performed as previously described.23 +, the IgG fraction immunoprecipitated the specific factor V mutant; −, it did not. ND, not done.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/11/10.1182_blood.v91.11.4188/4/m_blod41133001x.jpeg?Expires=1769799051&Signature=Sd2wpIe8CGiJk4u~2K7E-oqmQoyUuduSBicMkGfcO41lCORIq5uYfEZfYleKHMsPV0F5Brtc4Q5gOhUeWftdHUJ0XOFlkr-tlBzZO3uqEHlk39cWpkEKTuOoJRgIQcEYZku-iXFyuq3PPTv6qDOfOHhKzQKJl5bQ6wfgYl1UPwCUPjpCMJ6o~kHcXJVPAHtw~DUKhhbCSbBWj4oHmjrjN1YIBaR2K0nX87rGVRZVKa0kO4SZLow~7QXc0NH2coV1QTvvsxb6KINBdfpXLTdK5astBDlb~quYrIOhH-M0FKYwYxsJ~71~E-mPVDq9KV8eeGsRnAHAfjO-gO229iPPUQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Epitope mapping anti–factor V antibodies with recombinant factor V C2 domain chimeras. The complete domain structure of rHFV des B is shown at the top, and the expanded domain structures of the light chain portions of the individual chimeras are shown below. The white boxes indicate factor V–derived sequences, and the shaded boxes indicate factor VIII–derived sequences. Chimera 1A substituted amino acids 2282 through 2332 of factor VIII for amino acids 2149 through 2196 of factor V. Chimera 2A substituted amino acids 2223 through 2332 of factor VIII for amino acids 2088 through 2196 of factor V. Chimera 3A substituted amino acids 2173 through 2332 of factor VIII for amino acids 2037 through 2196 of factor V. Chimera 5A substituted amino acids 2173 through 2222 of factor VIII for amino acids 2037 through 2087 of factor V. Chimera 7A substituted amino acids 2223 through 2281 of factor VIII for amino acids 2088 through 2148 of factor V. The antibodies that immunoprecipitated the factor V C2 domain are listed on the right side. Immunoprecipitation of [35S]methionine metabolically labeled mutants and analysis by SDS-PAGE was performed as described in Materials and Methods. +, the antibody immunoprecipitated the specific factor V mutant; −, it did not.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/11/10.1182_blood.v91.11.4188/4/m_blod41133003x.jpeg?Expires=1769799051&Signature=J~AYudAyQlk6I-lPYtl9hZ3M3-rSBBc-RJEN40-T80zit7glnra1~eaOl3Gc3qXpa3~HOh7P9FypVbpKVi5hAgZEnwStcP1SDQeLr91MCA-H9PBKEG1I0mYBLDodN1NpwUISWRnthVfzVhRa5kVce1qkIcv9ouT4urbeBIKA-X0RggDiOfjx9aFj6vTrk-tAxFEoJfkgF0C~lMWys~YMjina6QZICOt2-oResN3wKlZHJBBK1zwCbLxhLFol4-D4vOaqFZGYXyh8lEaUb2Nwu-Yz-FIko7nULmFb7Nb2q0luub1TH1MdA8ooZqfXEaUrtG1xkqQzpBQb--qpWC-OSw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Immunoprecipitation of thrombin-activated C2 domain chimeras by IgG fraction from patient RS. Recombinant factor V constructs metabolically labeled with [35S]methionine included rHFV des B, chimera 1A, chimera 2A, chimera 3A, chimera 7A, and chimera 5A. Samples were first incubated with 2 nmol/L thrombin for 1 minute at 37°C and then immunoprecipitated with either the polyclonal rabbit anti-human factor V antibody or the IgG fraction from patient RS, as shown. The immunoprecipitates were analyzed by 6% SDS-PAGE and visualized by autoradiography. A nonspecific, high-molecular-weight band (>200 kD) is seen in all the immunoprecipitations of conditioned media, including the control IgG fraction from pooled normal plasma. Molecular-weight standards are shown on the left, and the positions of the factor Va heavy chain and light chain on the right.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/11/10.1182_blood.v91.11.4188/4/m_blod41133004w.jpeg?Expires=1769799051&Signature=AUrNXdStOIadYsD8UKsiPZcDGi2uXeqtoZxmUnlVb~JhC15v9dQJWRb-xpYfraaVlEJw7qyEX2wY-n5-xMqXAiv0VjYHDASgHuWucIpFXNdWpYEU-AcWgFXiS7A0gzPik5qWp9O4hx4YpRO5i2oidDn2s0-Cg~TRtWuqJtP22Yi64kqzxWG3y1vFZ11znSEluOvtHOuH325mx4ff5esuRe4tBJnLaQa82enoAWk2k61jLGsaS1e9qdQ-7CKicht9zZwer1U~hKs-vlcjEK2a2HKyOFkNVEyYappzhUIrWuOsV5yfBXmYoUX3h4iGg8rcC0NL4EkiL7hpphuBLzTgfQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal