Abstract
SANORG 34006 is a new sulfated pentasaccharide obtained by chemical synthesis. It is an analog of the “synthetic pentasaccharide” (SR 90107/ ORG 31540) which represents the antithrombin (AT) binding site of heparin. SANORG 34006 showed a higher affinity to human AT than SR 90107/ORG 31540 (kd = 1.4 ± 0.3 v 48 ± 11 nmol/L), and it is a potent and selective catalyst of the inhibitory effect of AT on factor Xa (1,240 ± 15 anti–factor Xa U/mg v850 ± 27 anti-factor Xa U/mg for SR 90107/ORG 31540). In vitro, SANORG 34006 inhibited thrombin generation occurring via both the extrinsic and intrinsic pathway. After intravenous (IV) or subcutaneous (SC) administration to rabbits, SANORG 34006 displayed a long-lasting anti–factor Xa activity and inhibition of thrombin generation (TG) ex vivo. SANORG 34006 was slowly eliminated after IV or SC administration to rats, rabbits, and baboons, showed exceptionally long half-lives (between 9.2 hours in rats and 61.9 hours in baboons), and revealed an SC bioavailability near 100%. SANORG 34006 displayed antithrombotic activity by virtue of its potentiation of the anti–factor Xa activity of AT. It strongly inhibited thrombus formation in experimental models of thromboplastin/stasis-induced venous thrombosis in rats (IV) and rabbits (SC) (ED50values = 40.0 ± 3.4 and 105.0 ± 9.4 nmol/kg, respectively). The duration of its antithrombotic effects closely paralleled the ex vivo anti–factor Xa activity. SANORG 34006 enhanced rt-PA–induced thrombolysis and inhibited accretion of125I-fibrinogen onto a preformed thrombus in the rabbit jugular vein suggesting that concomitant use of SANORG 34006 during rt-PA therapy might be helpful in facilitating thrombolysis and preventing fibrin accretion onto the thrombus under lysis. Contrary to standard heparin, SANORG 34006 did not enhance bleeding in a rabbit ear incision model at a dose that equals 10 times the antithrombotic ED50 in this species and, therefore, exhibited a favorable therapeutic index. We suggest that SANORG 34006 is a promising compound in the treatment and prevention of various thrombotic diseases.
IN THE PAST SEVERAL YEARS, considerable progress has been made in developing ideal antithrombotics.1,2 Recently, SR 90107/ORG 31540, a new pentasaccharide obtained by total chemical synthesis,3which represents the minimal sequence of the heparin chain that interacts with antithrombin (AT), has been shown to display antithrombotic activity by virtue of its potentiation of the anti–factor Xa activity of AT.3,4 This compound, which accelerates the inhibition by AT of factor Xa but not thrombin, possesses antithrombotic efficacy in various thrombosis models without deleterious effects on hemostasis.5-9 When pharmacodynamics of the anti–factor Xa effect and tolerance of SR 90107A/ORG 31540 were investigated in healthy volunteers10 the drug was well tolerated, suggesting that accelerating the inhibition of factor Xa is a promising approach for the treatment of thrombosis. The complex structure of SR 90107/ORG 31540 requires numerous synthetic steps, and, to simplify the synthesis, analogs of SR 90107/ORG 31540 have been synthesized.3 The biochemical and pharmacological activities of one of these compounds, SANORG 32701, a newly developed analog of SR 90107/ORG 31540, have been described recently.11
In an attempt to find new pentasaccharides with longer half-lives, we have designed SANORG 34006, an O-methylated, O-sulfated pentasaccharide. It has been postulated that the specific binding of pentasaccharides to AT in the circulation governs their pharmacokinetic properties.12 In particular, time courses of plasma anti–factor Xa activities for different pentasaccharides could be predicted from their affinities (kd) for AT, the elimination half-life of AT itself, and the plasma AT concentration.12 In the present study we describe the in vitro and in vivo activities of SANORG 34006.
MATERIALS AND METHODS
Drugs
Recombinant human tissue factor (Inovin) was obtained from Dade (Baxter Diagnostics, Deerfield, IL). Tissue thromboplastin and standard heparin (162 IU/mg, sodium salt from pig intestinal mucosa, mean molecular weight 15,000) were purchased from Sigma Chemical Co (L'isle d'Abeau, France). rt-PA (Actilyse) was obtained from Boehringer Ingelheim (Ingelheim, Germany). Human α-thrombin (3,000 IU/mg) was from Centre de Transfusion Sanguine (Strasbourg, France).125I-human fibrinogen (4.1 MBq/mg) was purchased from Amersham (Amersham, UK). Human factor Xa, human AT, human heparin-cofactor II (HC-II), chromogenic substrates Bz-Ile-Glu-(Piperidyl)-Gly-Arg-pNA (S-2222), and D-Phe-Pip-Arg-pNA (S-2238) were from Kabivitrum (Stockholm, Sweden). SR 90107/ORG 31540, a pentasaccharide that represents the minimal sequence on the heparin chains, interacting with AT and SANORG 34006 (Fig 1) were from Sanofi Recherche (Toulouse, France). The pharmaceutical research and development of SR 90107/ORG 31540 and SANORG 34006 is pursued by a partnership agreement between Organon (Oss, The Netherlands) and Sanofi (Gentilly, France). All compounds were solubilized in saline and administered as indicated. All other chemicals and solvents were of reagent grade from Prolabo (Paris, France).
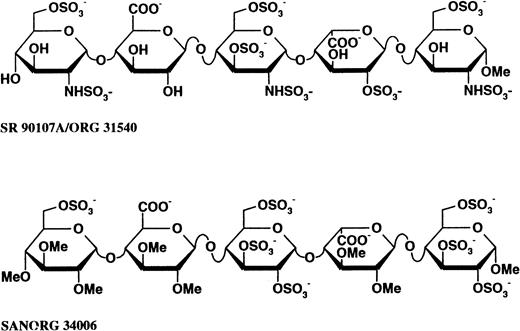
Comparative structures of SR 90107/ORG 31540 and SANORG 34006. SR 90107/ORG 31540 is the α-methyl glycoside of the pentasaccharide which represents the unique sequence in heparin that binds to AT. SANORG 34006 belongs to the so-called “non-glycosaminoglycan” series of analogs of SR 90107/ORG 31540.
Comparative structures of SR 90107/ORG 31540 and SANORG 34006. SR 90107/ORG 31540 is the α-methyl glycoside of the pentasaccharide which represents the unique sequence in heparin that binds to AT. SANORG 34006 belongs to the so-called “non-glycosaminoglycan” series of analogs of SR 90107/ORG 31540.
In Vitro Studies
Inhibitory activity of SANORG 34006 on factor Xa and thrombin.
Anti–factor Xa activities were determined by a modification of the of Teien and Lie procedure13 as recently described.11 Human factor Xa (2.4 nkat/mL) was incubated for 2 minutes with human AT (0.17 U/mL) at 37°C in the presence of various concentrations of the oligosaccharides in Tris/maleate 20 mmol/L buffer pH 7.4, NaCl 150 mmol/L. To measure the residual factor Xa activity, S-2222 (dissolved in 50 mmol/L Tris/HCl buffer, pH 8.4, NaCl 175 mmol/L, EDTA 27.5 mmol/L, polybrene 1 mg/mL) was added (0.25 mmol/L final). The reaction was stopped 2 minutes later by the addition of a 50% aqueous acetic acid solution and the absorbency at 405 nm was read on a spectrophotometer. The percentage of inhibition was then calculated as [% Inhibition = 100 × (Optical Density [OD] Blank − OD Sample)/OD Blank]. The activity per milligram of the compounds was determined by comparison with a calibrated standard (SR 90107/ORG 31540) using Excel 4.0 Software (Microsoft, Redmond, CA).
Affinity for human AT.
Affinity of SANORG 34006 for human AT was determined by fluorescence as described by Atha et al16 using a Perkin Elmer LS-50 type spectrofluorimeter (Perkin Elmer, Norwalk, CT) at a λ excitation = 280 nm and a λ emission = 338 nm at 37°C, under continuous stirring. Oligosaccharides were added into 2 mL of Tris-HCl buffer 0.01 mmol/L pH 7.0 containing NaCl 0.15 mol/L and 5 to 60 nmol/L AT. The ratio and the concentrations of AT-oligosaccharide complex were calculated and dissociation constants (kd) were determined from Scatchard plots using RS/1 software.
Anticoagulant activity.
The activated partial thromboplastin time (APTT) was measured with Actin FS (Dade-Baxter, Dülingen, Switzerland) in a Amelung-Baxter KC10 coagulometer. APTT was expressed in units per milligram, taking unfractionated heparin (180 U/mg) as the standard. The thrombin generation (TG) methodology was adapted from Hemker et al,14 as previously reported.15 TG was triggered either by kaolin (5 μg/mL final concentration) (intrinsic TG) or by rabbit brain thromboplastin (extrinsic TG) in defibrinated human platelet-poor plasma supplemented with 2 μmol/L of cephalin. Aliquots were taken every 15 seconds and the concentration of thrombin was measured using the chromogenic substrate S-2238. The total amount of thrombin generated was quantified by computing the area under the TG curve (AUC).
In Vivo Studies
Ex vivo anti Xa activities in different species.
Pentobarbital anesthetized (30 mg/kg, intraperitoneal [IP]) male Sprague-Dawley rats (275 to 300 g; Iffa-Credo, L'arbresle, France), pentobarbital anesthetized (30 mg/kg, intravenous [IV]) male New Zealand rabbits (2.7 to 3.0 kg; Lago, Vonnas, France), and male conscious baboons (Papio Ursinus, 7 to 9 kg provided by CAPE, Capetown, South Africa) were treated by subcutaneous (SC) or IV injections of SANORG 34006 or SR 90107/ORG 31540. Arterial blood samples (2 mL) were withdrawn in a 3.8% trisodium citrate solution (1/9 vol/vol). Each blood sample was centrifuged (1,800g, 10 minutes) and the platelet-poor plasma was stored at −20°C. The anti–factor Xa activity of SANORG 34006 was determined as described above by measuring the residual activity of factor Xa added to diluted plasma supplemented with human AT.
Stasis-induced venous thrombosis in the rabbit.
Venous thrombosis was induced in pentobarbital anesthetized (30 mg/kg IV) male New Zealand rabbits according to Buchanan et al,17with slight modifications.11 Each jugular vein was isolated and two loose sutures were placed 2 cm apart. Recombinant human tissue factor (1 ng/kg) was injected 5 minutes before the induction of stasis. Both jugular-vein segments were occluded by the distal and proximal sutures and stasis was maintained for 15 minutes. The veins were opened longitudinally, and the thrombus, if apparent, was removed, blotted on filter paper, and weighed. Wet weights of thrombi were averaged for left and right jugular veins. Test compounds or vehicle were administered SC at indicated time points before the IV injection of tissue factor.
Stasis-induced venous thrombosis in the rat.
Thrombus formation by a combination of stasis and hypercoagulability was induced as originally proposed by Vogel et al.5 11 The abdomen of pentobarbital-anesthetized (30 mg/kg, IP) male Sprague-Dawley rats (250 to 300 g) was opened and the vena cava exposed. SANORG 34006 or SR 90107/ORG 31540 were administered IV 5 minutes before thrombosis induction. Two loose sutures were prepared 0.7 cm apart on the inferior vena cava and all collateral veins were ligated. Human tissue factor (1 ng/kg, IV) was injected into the dorsal vein of the penis. Ten seconds after the end of the injection, stasis was established by tightening the two sutures, the proximal and then the distal. The abdominal cavity was provisionally closed and stasis was maintained for 10 minutes. The cavity was then reopened, the ligated segment was opened longitudinally, and the formed thrombus was removed, rinsed, blotted on filter paper, dried overnight at 60°C, and weighed.
125I-fibrinogen accretion on a preformed thrombus in the jugular vein of the rabbit.
The antithrombotic effect of SANORG 34006 was assessed by measuring its ability to inhibit the accretion of 125I-fibrinogen onto autologous nonradioactive venous thrombi preformed in the jugular veins of rabbits as recently described.11 18 Male New Zealand rabbits (2.5 to 3 kg) were anesthetized with sodium pentobarbital (30 mg/kg, IV). Both jugular veins were exposed and a 2-cm segment was isolated on either side. Each jugular vein segment was emptied of blood, and blood flow was temporarily interrupted by proximal and distal clamps. One milliliter of blood was collected from a carotid canula and mixed with 50 μL of human α-thrombin (20 U/mL), and 150 μL of clotting blood was immediately injected into the isolated segment. A 10-cm length silk thread was passed longitudinally through the forming thrombus and the vessel wall to keep the thrombus in place. Blood flow was restored 2 minues later. Fifteen minutes after the thrombus was formed, each animal was injected with 20 μCi of125I-fibrinogen. Five minutes later, indicated doses of SANORG 34006, SR 90107/ORG 31540, or heparin were infused over a 4-hour period with 10% of the dose as an IV bolus. Control animals were infused with the same volume of saline. At the end of the 4-hour infusion period, the venous segments containing the thrombi were tied off, slit open longitudinally, and the remaining thrombi were removed. The specific activity of the whole-blood fibrinogen was estimated from the mean of blood samples taken at hourly intervals throughout the infusion. The ratio of radioactivity of the thrombus to circulating radioactivity was used as an estimate of thrombus size.
Lysis of a jugular vein thrombus in the rabbit.
The thrombolytic effect of rt-PA in association with SANORG 34006, SR 90107/ORG 31540, or heparin was evaluated by the lysis of a standard-sized, preformed 125I-fibrinogen–labeled thrombus produced in the external jugular vein of rabbits.11 19 The jugular vein of pentobarbital-anesthetized male New Zealand rabbits (30 mg/kg, IV) was uncovered, and all tributaries at a distance of 4 cm from the main bifurcation of the external jugular and facial veins were ligated. A silk thread was then inserted through the vessel to anchor the thrombus and avoid embolization. After ligation of the vein with two vessel clamps, the enclosed blood was removed and exchanged with 200 μL of citrated rabbit blood mixed with 0.5 μCi125I-labeled human fibrinogen and 10 IU human thrombin. After clot formation (30 minutes), the surgical clamps were removed and the blood flow was restored. Thrombolysis was performed by infusion of rt-PA via the contralateral marginal ear vein. SANORG 34006 or saline were administered as IV bolus injections at the start of the infusion. At the end of the rt-PA infusion period (4 hours), the thrombus was carefully removed from the vein and weighed. The amount of radioactivity remaining in the clot was determined with a gamma counter (1261 Multigamma counter; Wallac, Turku, Finland). The extent of 125I-fibrino(geno)lysis was calculated at the end of the infusion as the difference between the radioactivity originally incorporated in the thrombus and that remaining in the residual thrombus. Percent decrease of the thrombus-associated radioactivity was calculated with reference to the initial125I-fibrinogen added into the initial clot.
Bleeding test in the rabbit.
Male New Zealand rabbits were anesthetized with sodium pentobarbital (30 mg/kg, IV). SANORG 34006, SR 90107/ORG 31540, or standard heparin were administered IV. Five minutes later, five standardized incisions were made through the ear with a scalpel blade (no. 21; Swann-Norton, Sheffield, UK) as reported.11 Care was taken to avoid any macroscopically visible vessel. The ear was immersed in a 500 mL saline bath at 37°C under continuous stirring. Blood loss was determined 10 minutes later by measurement of the hemoglobin content of the water bath after the addition of hemolizing reagent (Zapoglobin, Coultronics, France).
Statistical Analysis
The results shown are arithmetic means ± SEM. IC50values were calculated using the 4-parameter logistic model and shown with a 95% confidence interval. The adjustment was obtained by nonlinear regression using the Levenberg-Marquard algorithm in RS/1 software (BBN, Cambridge, MA). ED50 values were calculated from the dose-response curves by fitting the logistic equation to the data by means of nonlinear regression. Data were statistically analyzed using the Mann-Whitney test with the Holm-Bonferroni adjustment. A significant difference was accepted at the P < .05 level.
RESULTS
In Vitro Studies
Anti–factor Xa activity of SANORG 34006.
SANORG 34006 inhibited the activity of human factor Xa in the presence of human AT in a dose-dependent manner with an IC50 value of 2.9 ± 0.2 nmol/L (n = 6). Under the same experimental conditions, SR 90107/ORG 31540 showed a slightly lower inhibitory potency (IC50 = 4.1 ± 0.7 nmol/L, n = 6). SANORG 34006 exhibited a specific activity of 1,240 ± 15 anti–factor Xa U/mg versus 850 ± 27 anti–factor Xa U/mg for SR 90107/ORG 31540 and 180 ± 11 anti–factor Xa U/mg for heparin (Table 1). The affinity constant (kd) of SANORG 34006 for human AT was 34-fold higher than that measured for SR 90107/ORG 31540 (Table 1). Neither SANORG 34006 nor SR 90107/ORG 31540 exhibited AT activity in the presence of AT or heparin cofactor II (not shown).
In Vitro Activities of SANORG 34006
| Compounds . | Anti-Xa Activity . | kd for ATIII (nmol/L) . | TG (extrinsic) IC50 (μg/mL) . | APTT (U/mg) . | |
|---|---|---|---|---|---|
| (U/mg) . | (U/nmol) . | ||||
| Standard heparin | 180 ± 11 | ND | 0.27 ± 0.05 | 180 | |
| SR90107A/ORG 31540 | 850 ± 27 | 1.4 | 48 ± 11 | 0.64 ± 0.02 | 3 ± 3 |
| SANORG 34006 | 1,240 ± 15 | 2.1 | 1.4 ± 0.3 | 0.58 ± 0.01 | 1.1 ± 0.16 |
| Compounds . | Anti-Xa Activity . | kd for ATIII (nmol/L) . | TG (extrinsic) IC50 (μg/mL) . | APTT (U/mg) . | |
|---|---|---|---|---|---|
| (U/mg) . | (U/nmol) . | ||||
| Standard heparin | 180 ± 11 | ND | 0.27 ± 0.05 | 180 | |
| SR90107A/ORG 31540 | 850 ± 27 | 1.4 | 48 ± 11 | 0.64 ± 0.02 | 3 ± 3 |
| SANORG 34006 | 1,240 ± 15 | 2.1 | 1.4 ± 0.3 | 0.58 ± 0.01 | 1.1 ± 0.16 |
Means ± SD (n = 9).
Abbreviation: ND, not determined.
Effect of SANORG 34006 on thrombin generation and APTT.
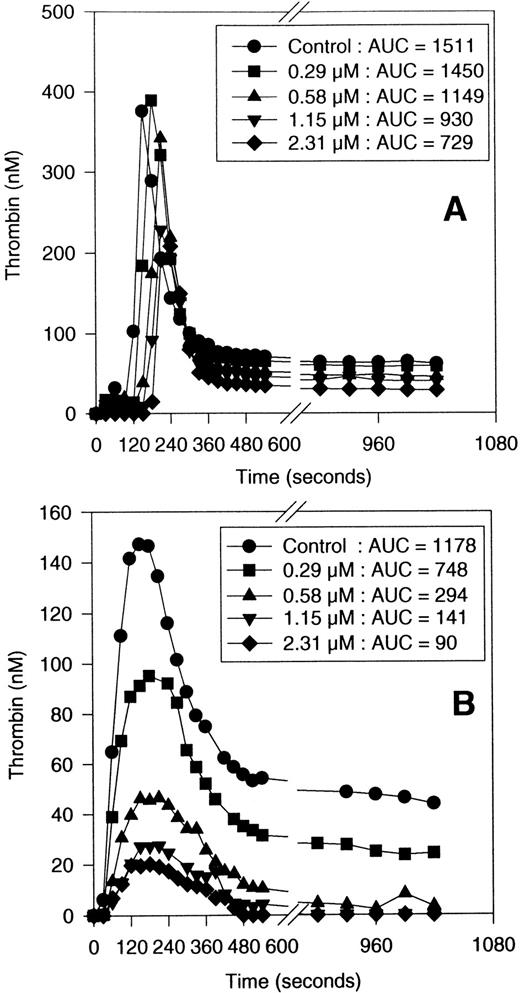
SANORG 34006 inhibited TG in human plasma in a dose-dependent manner (Fig 2). As previously shown for SR 90107/ORG 31540,15 SANORG 34006 impaired TG via the extrinsic pathway more efficiently than via the intrinsic one showing IC50 values of 0.5 (0.3 to 1.1) μmol/L and 1.8 (1.1 to 3.7) μmol/L (means + confidence intervals at 95%), respectively. The potency of SANORG 34006 to inhibit TG was similar to that observed for SR 90107/ORG 31540 and heparin (Table 1). Contrary to heparin, SANORG 34006 and SR 90107/ORG 31540 did not prolong APTT as previously described for this class of compounds11 (Table 1).
Inhibition of TG in human plasma by SANORG 34006. The effects of SANORG 34006 on intrinsic (A) and extrinsic TG (B) are shown. Results shown are the means of three different experiments performed in triplicate. The corresponding AUCs shown in the legend are in nmol/L · min−1. SD bars are not shown for clarity of graph reading.
Inhibition of TG in human plasma by SANORG 34006. The effects of SANORG 34006 on intrinsic (A) and extrinsic TG (B) are shown. Results shown are the means of three different experiments performed in triplicate. The corresponding AUCs shown in the legend are in nmol/L · min−1. SD bars are not shown for clarity of graph reading.
Ex Vivo Studies
Anti–factor Xa and anticoagulant activities of SANORG 34006.
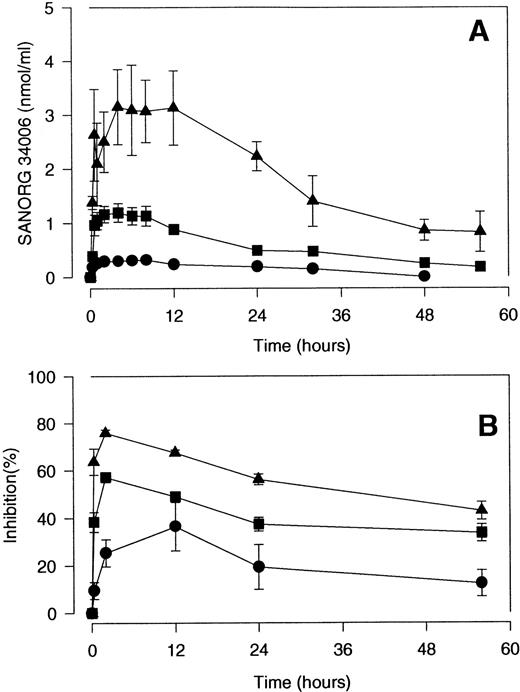
The ex vivo anti–factor Xa and anticoagulant activities of SANORG 34006 after SC injections of 30, 100, and 300 nmol/kg to rabbits were compared in Fig 3. Ex vivo anti–factor Xa activity in plasma increased as a function of the injected dose (Fig3A). SANORG 34006 also inhibited TG ex vivo in a dose-dependent manner (Fig 3B). The kinetics of the ex vivo TG inhibition paralleled the time course of the ex vivo anti–factor Xa activity in plasma (Fig 3).After administration of the lowest dose (30 nmol/kg SC), TG was significantly inhibited for at least 24 hours. At 100 and 300 nmol/kg SANORG 34006 SC, TG was significantly inhibited during the whole investigation period (56 hours). Comparative pharmacodynamics of the anti–factor Xa effect of SANORG 34006 after IV and SC administration of 100 nmol/kg to rats, rabbits, and baboons are shown in Table 2. After IV administration of 100 nmol/kg SANORG 34006, cmax was reached immediately in the three species being slightly lower in baboons compared with rats and rabbits. Half-life and AUC increased with increasing size of the species. Estimated half-lives were 9.2 hours in rats, 22.8 hours in rabbits, and 61.9 hours in baboons (Table 2). Half-lives and AUC were inversely correlated with the measured clearance values in the three species (Table 2). After SC administration of 100 nmol/kg SANORG 34006, cmax was measured after 2.1 hours in rats, 3.4 hours in rabbits, and 1 hour in baboons. Half-life, AUC, and clearance showed the same species dependency as observed for IV SANORG 34006 administration (Table 2). Estimated SC bioavailability was 100% for rats and rabbits and 72% for baboons. After SC administration of SANORG 34006 to rabbits, cmax and AUC showed a quasi-linear dose-dependency, whereas half-life and clearance did not vary as a function of dose. Table 2 also summarizes pharmacodynamic parameters associated with IV and SC administration of SR 90107/ORG 31540 to rats, rabbits, and baboons. The main difference compared with SANORG 34006 is the markedly increased clearance which results in a distinct decrease in half-life and AUC.
Pharmacodynamics of the anti–factor Xa effect of SANORG 34006 in the rabbit. SANORG 34006 was administered SC (•, 30; ▪, 100; ▴, 300 nmol/kg) to rabbits and anti–factor Xa activity (A) or intrinsic TG inhibition (AUC measurement) (B) were determined at indicated time points after administration. Results are expressed as means ± SD (n = 6).
Pharmacodynamics of the anti–factor Xa effect of SANORG 34006 in the rabbit. SANORG 34006 was administered SC (•, 30; ▪, 100; ▴, 300 nmol/kg) to rabbits and anti–factor Xa activity (A) or intrinsic TG inhibition (AUC measurement) (B) were determined at indicated time points after administration. Results are expressed as means ± SD (n = 6).
Pharmacokinetic Parameters Measured for SANORG 34006 and SR 90107 After IV or SC Administration to Rats, Rabbits, and Baboons
| . | Species . | Dose (nmol/kg) . | Tmax (h) . | Cmax (nmol/mL) . | t1/2 (h) . | AUC(0-∞) (h · nmol/mL) . | Clearance (mL/kg · h) . | Distribution Volume (mL/kg) . |
|---|---|---|---|---|---|---|---|---|
| IV | ||||||||
| SR 90107/ORG 31540 | Rat | 100 | 0.08 ± 0 | 1.2 ± 0.8 | 0.7 ± 0.1 | 1.5 ± 0.1 | 23 ± 8 | 123 ± 12 |
| SANORG 34006 | Rat | 100 | 0.08 ± 0 | 2.3 ± 0.3 | 9.2 ± 1.8 | 11.2 ± 2.5 | 12.8 ± 2.4 | 109 ± 41 |
| SR 90107/ORG 31540 | Rabbit | 100 | 0.08 ± 0 | 1.6 ± 0.1 | 2.9 ± 1.4 | 3.4 ± 1.4 | 31.3 ± 7.7 | 104 ± 15 |
| SANORG 34006 | Rabbit | 100 | 0.08 ± 0 | 2.2 ± 0.4 | 22.8 ± 2.0 | 30 ± 9 | 3.5 ± 0.9 | 117 ± 35 |
| SR 90107/ORG 31540 | Baboon | 100 | 0.5 ± 0 | 1.0 ± 0.1 | 4.7 ± 0.9 | 6.1 ± 0.4 | 18.1 ± 2.9 | 122 ± 17 |
| SANORG 34006 | Baboon | 100 | 0.5 ± 0 | 1.6 ± 0.1 | 61.9 ± 7.4 | 62.6 ± 5.3 | 1.6 ± 0.1 | 143 ± 10 |
| SC | ||||||||
| SR 90107/ORG 31540 | Rat | 100 | 0.6 ± 0.3 | 0.6 ± 0.2 | 1.6 ± 0.4 | 1.7 ± 0.3 | 61 ± 9 | 131 ± 17 |
| SANORG 34006 | Rat | 100 | 2.1 ± 0.2 | 0.87 ± 0.01 | 14.7 ± 2.9 | 12.3 ± 0.3 | 8.1 ± 0.1 | 172 ± 34 |
| SR 90107/ORG 31540 | Rabbit | 100 | 2 ± 0 | 0.8 ± 0.1 | 3.3 ± 0.4 | 5.5 ± 0.6 | 16.3 ± 0.4 | 79 ± 10 |
| SANORG 34006 | Rabbit | 30 | 4.3 ± 3.3 | 0.33 ± 0 | 22.7 ± 4.2 | 12.2 ± 1.4 | 2.5 ± 0.3 | 80 ± 7 |
| SANORG 34006 | Rabbit | 100 | 3.4 ± 1.9 | 1.61 ± 0.1 | 16.8 ± 2.5 | 35 ± 10 | 3.2 ± 1.1 | 76 ± 13 |
| SANORG 34006 | Rabbit | 300 | 5.7 ± 4.1 | 4.85 ± 1.7 | 20.8 ± 6 | 140 ± 77 | 2.6 ± 1.1 | 74 ± 19 |
| SR 90107/ORG 31540 | Baboon | 100 | 1.7 ± 1.2 | 0.5 ± 0.1 | 5.4 ± 1.2 | 4.7 ± 0.8 | 21.6 ± 3.8 | 144 ± 25 |
| SANORG 34006 | Baboon | 100 | 1 ± 0 | 0.9 ± 0.1 | 60.4 ± 4.9 | 45.3 ± 4.1 | 2.2 ± 0.2 | 193 ± 17 |
| . | Species . | Dose (nmol/kg) . | Tmax (h) . | Cmax (nmol/mL) . | t1/2 (h) . | AUC(0-∞) (h · nmol/mL) . | Clearance (mL/kg · h) . | Distribution Volume (mL/kg) . |
|---|---|---|---|---|---|---|---|---|
| IV | ||||||||
| SR 90107/ORG 31540 | Rat | 100 | 0.08 ± 0 | 1.2 ± 0.8 | 0.7 ± 0.1 | 1.5 ± 0.1 | 23 ± 8 | 123 ± 12 |
| SANORG 34006 | Rat | 100 | 0.08 ± 0 | 2.3 ± 0.3 | 9.2 ± 1.8 | 11.2 ± 2.5 | 12.8 ± 2.4 | 109 ± 41 |
| SR 90107/ORG 31540 | Rabbit | 100 | 0.08 ± 0 | 1.6 ± 0.1 | 2.9 ± 1.4 | 3.4 ± 1.4 | 31.3 ± 7.7 | 104 ± 15 |
| SANORG 34006 | Rabbit | 100 | 0.08 ± 0 | 2.2 ± 0.4 | 22.8 ± 2.0 | 30 ± 9 | 3.5 ± 0.9 | 117 ± 35 |
| SR 90107/ORG 31540 | Baboon | 100 | 0.5 ± 0 | 1.0 ± 0.1 | 4.7 ± 0.9 | 6.1 ± 0.4 | 18.1 ± 2.9 | 122 ± 17 |
| SANORG 34006 | Baboon | 100 | 0.5 ± 0 | 1.6 ± 0.1 | 61.9 ± 7.4 | 62.6 ± 5.3 | 1.6 ± 0.1 | 143 ± 10 |
| SC | ||||||||
| SR 90107/ORG 31540 | Rat | 100 | 0.6 ± 0.3 | 0.6 ± 0.2 | 1.6 ± 0.4 | 1.7 ± 0.3 | 61 ± 9 | 131 ± 17 |
| SANORG 34006 | Rat | 100 | 2.1 ± 0.2 | 0.87 ± 0.01 | 14.7 ± 2.9 | 12.3 ± 0.3 | 8.1 ± 0.1 | 172 ± 34 |
| SR 90107/ORG 31540 | Rabbit | 100 | 2 ± 0 | 0.8 ± 0.1 | 3.3 ± 0.4 | 5.5 ± 0.6 | 16.3 ± 0.4 | 79 ± 10 |
| SANORG 34006 | Rabbit | 30 | 4.3 ± 3.3 | 0.33 ± 0 | 22.7 ± 4.2 | 12.2 ± 1.4 | 2.5 ± 0.3 | 80 ± 7 |
| SANORG 34006 | Rabbit | 100 | 3.4 ± 1.9 | 1.61 ± 0.1 | 16.8 ± 2.5 | 35 ± 10 | 3.2 ± 1.1 | 76 ± 13 |
| SANORG 34006 | Rabbit | 300 | 5.7 ± 4.1 | 4.85 ± 1.7 | 20.8 ± 6 | 140 ± 77 | 2.6 ± 1.1 | 74 ± 19 |
| SR 90107/ORG 31540 | Baboon | 100 | 1.7 ± 1.2 | 0.5 ± 0.1 | 5.4 ± 1.2 | 4.7 ± 0.8 | 21.6 ± 3.8 | 144 ± 25 |
| SANORG 34006 | Baboon | 100 | 1 ± 0 | 0.9 ± 0.1 | 60.4 ± 4.9 | 45.3 ± 4.1 | 2.2 ± 0.2 | 193 ± 17 |
Means ± SD (n = 4 to 9).
In Vivo Studies
Stasis-induced thrombosis after injection of tissue factor in the rabbit.
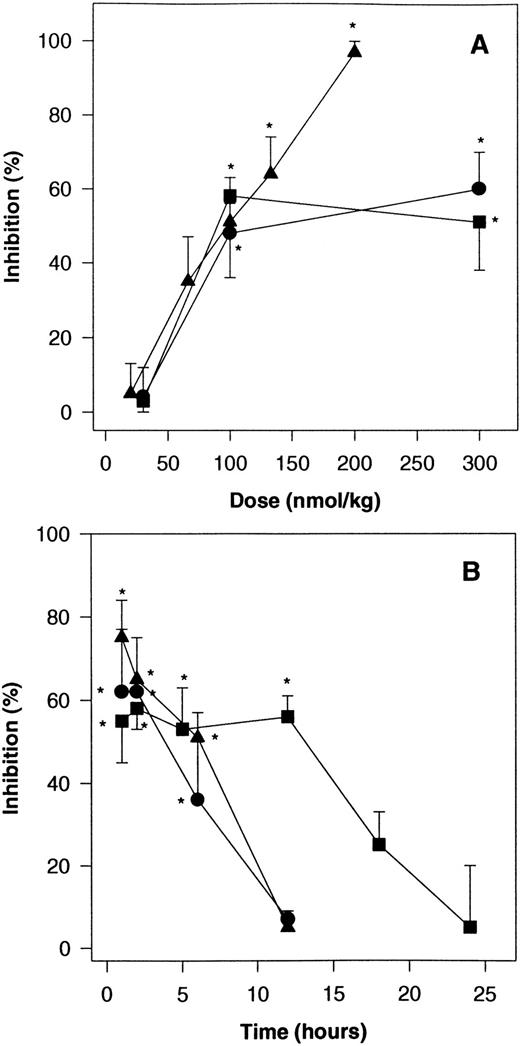
SANORG 34006 was tested for its ability to inhibit thrombus formation in a venous thrombosis model in the rabbit using a combination of a thrombogenic challenge (1.0 ng/kg of tissue factor) and stasis. Thrombus weight under control conditions in this model was 155.9 ± 10.3 mg (n = 10). SANORG 34006 administered sc 2 hours before thrombosis induction displayed a dose-dependent antithrombotic effect. Maximum inhibition of thrombosis (58%) was observed at a dose of 100 nmol/kg (Fig 4A). The ED50value was 105 ± 9.4 nmol/kg (n = 10). In this experimental model, SR 90107/ORG 31540 and standard heparin were as potent as SANORG 34006 showing ED50 values of 74 ± 6.1 and 83 ± 10.2 nmol/kg, respectively, after SC administration (n = 10). However, standard heparin totally inhibited thrombus formation at a dose of 200 nmol/kg SC, whereas SR 90107/ORG 31540 and SANORG 34006 showed lower efficacy (maximum inhibition between 50% and 60% at 100 to 300 nmol/kg SC). The antithrombotic effect of 100 nmol/kg SANORG 34006 SC plateaued at 53% to 58% during 12 hours after administration (Fig4B). This correlates with the pharmacokinetic data that were obtained after SC administration of 100 nmol/kg to rabbits (Table 2). In this rabbit venous thrombosis model, SANORG 34006 exhibited a much longer antithrombotic activity (t1/2 = 16.4 hours) than SR 90107/ORG 31540 and heparin (t1/2 = 1.8 and 2.9 hours, respectively) at doses of 100 nmol/kg SC (Fig 4B).
Effect of SANORG 34006 on stasis-induced venous thrombosis in the rabbit. (A) Dose-response relationships: SANORG 34006 (▪), SR 90107/ORG 31540 (•), or standard heparin (▴) were administered SC 2 hours before stasis and administration of tissue factor as described in Materials and Methods. (B) Effect kinetics: SANORG 34006 (▪), SR 90107/ORG 31540 (•), or standard heparin (▴) were administered SC at the dose of 100 nmol/kg. Their antithrombotic activities were determined at indicated time points after administration. Each point represents the mean ± SEM of 10 animals. Statistical analysis was performed using the Mann-Whitney test: *P < .05 versus controls.
Effect of SANORG 34006 on stasis-induced venous thrombosis in the rabbit. (A) Dose-response relationships: SANORG 34006 (▪), SR 90107/ORG 31540 (•), or standard heparin (▴) were administered SC 2 hours before stasis and administration of tissue factor as described in Materials and Methods. (B) Effect kinetics: SANORG 34006 (▪), SR 90107/ORG 31540 (•), or standard heparin (▴) were administered SC at the dose of 100 nmol/kg. Their antithrombotic activities were determined at indicated time points after administration. Each point represents the mean ± SEM of 10 animals. Statistical analysis was performed using the Mann-Whitney test: *P < .05 versus controls.
Stasis-induced thrombosis after injection of tissue factor in the rat.
Stasis after thrombogenic challenge induced a thrombus with an average weight of 6.1 ± 0.2 mg (n = 20) in this rat model under control conditions. IV injection of SANORG 34006 inhibited thrombus formation in a dose-dependent manner (Fig 5). The ED50 value was 40.0 ± 3.4 nmol/kg. SR 90107/ORG 31540 showed a comparable antithrombotic activity with an IV ED50value of 68.6 ± 8.4 nmol/kg (Fig 5). Standard heparin was more potent than the two pentasaccharides in this rat venous thrombosis model (ED50 = 8.8 ± 2.1 nmol/kg IV). As already observed in the rabbit venous thrombosis model (Fig 4), SANORG 34006 exhibited prolonged antithrombotic activity compared with SR 90107/ORG 31540 and standard heparin (Fig 5B). The half-lives in this rat model after administration of 100 nmol/kg IV were 3.7, 0.7, and 1.25 hours for SANORG 34006, SR 90107/ORG 31540, and standard heparin, respectively.
Effect of SANORG 34006 on stasis-induced venous thrombosis in the rat. (A) Dose-response relationships: SANORG 34006 (▪), SR 90107/ORG 31540 (•), or standard heparin (▴) were administered IV 5 minutes before stasis and administration of tissue factor as described in Materials and Methods. (B) Effect kinetics: SANORG 34006 (▪), SR 90107/ORG 31540 (•), or standard heparin (▴) were administered IV at the dose of 100 nmol/kg. Their antithrombotic activities were determined at indicated time points after administration. Each point represents the mean ± SEM of 10 animals. Statistical analysis was performed using the Mann-Whitney test: *P < .05 versus controls.
Effect of SANORG 34006 on stasis-induced venous thrombosis in the rat. (A) Dose-response relationships: SANORG 34006 (▪), SR 90107/ORG 31540 (•), or standard heparin (▴) were administered IV 5 minutes before stasis and administration of tissue factor as described in Materials and Methods. (B) Effect kinetics: SANORG 34006 (▪), SR 90107/ORG 31540 (•), or standard heparin (▴) were administered IV at the dose of 100 nmol/kg. Their antithrombotic activities were determined at indicated time points after administration. Each point represents the mean ± SEM of 10 animals. Statistical analysis was performed using the Mann-Whitney test: *P < .05 versus controls.
125I-fibrinogen accretion onto a preformed thrombus in the rabbit.
The effects of a 4-hour infusion of SANORG 34006, SR 90107/ORG 31540, and heparin on 125I-fibrinogen accretion onto preformed thrombi are shown in Fig 6. In saline-treated animals, 5.5 ± 0.8 μg (n = 9) of125I-fibrinogen were accreted onto the preformed thrombi during the 4-hour period. SANORG 34006 was much more potent in inhibiting 125I-fibrinogen accretion (ID50value = 14 ± 4 nmol/kg IV) than standard heparin (ID50value = 141 ± 21 nmol/kg IV) and SR 90107/ORG 31540 (ID50 value = 285 ± 12 nmol/kg IV) (Fig 6).
Effect of SANORG 34006 on fibrinogen accretion onto a preformed thrombus in the rabbit jugular vein. A thrombus was formed in the jugular vein of rabbits and 125I-fibrinogen (20 μCi) was administered IV 15 minutes later. The indicated doses of SANORG 34006 (▪), SR 90107/ORG 31540 (•), standard heparin (▴), or saline were then administered as a 4-hour infusion. Radioactivity of the thrombus was determined at the end of the 4-hour infusion period. Results are expressed as percent inhibition of125I-fibrinogen accretion compared with saline-treated control animals. Each point represents the mean ± SEM of 10 animals. Statistical analysis was performed using the Mann-Whitney test: *P < .05 versus controls.
Effect of SANORG 34006 on fibrinogen accretion onto a preformed thrombus in the rabbit jugular vein. A thrombus was formed in the jugular vein of rabbits and 125I-fibrinogen (20 μCi) was administered IV 15 minutes later. The indicated doses of SANORG 34006 (▪), SR 90107/ORG 31540 (•), standard heparin (▴), or saline were then administered as a 4-hour infusion. Radioactivity of the thrombus was determined at the end of the 4-hour infusion period. Results are expressed as percent inhibition of125I-fibrinogen accretion compared with saline-treated control animals. Each point represents the mean ± SEM of 10 animals. Statistical analysis was performed using the Mann-Whitney test: *P < .05 versus controls.
Effect of SANORG 34006 on rt-PA–induced thrombolysis.
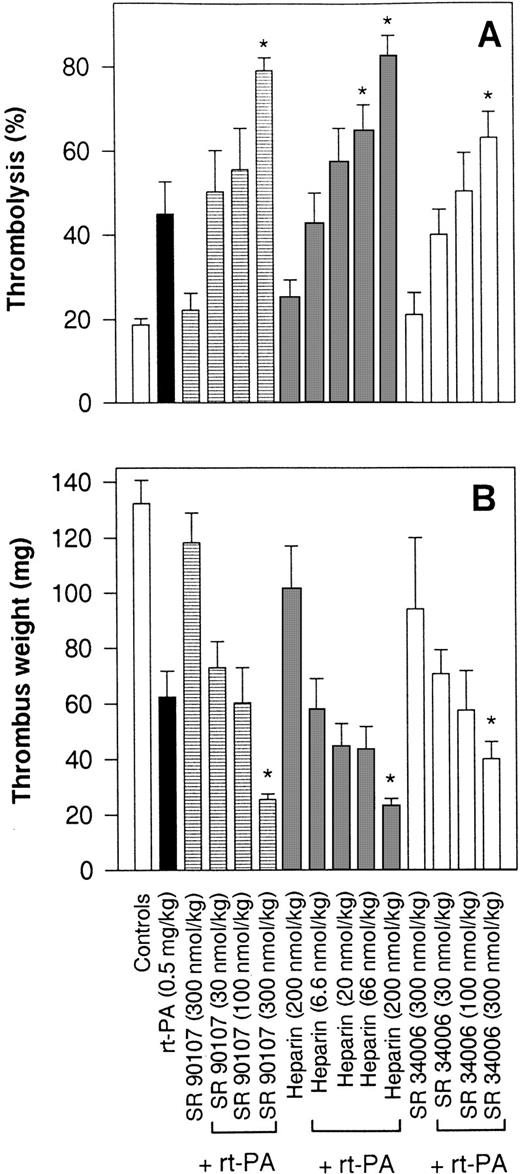
The effects of IV bolus injections of SANORG 34006, SR 90107/ORG 31540, and heparin on rt-PA thrombolysis expressed as isotope recovery balance are shown in Fig 7A. In the control group, infused with saline instead of rt-PA for 4 hours, the average degree of thrombolysis was 18.9% ± 2.0% (n = 8), either as a result of spontaneous lysis and/or washout of unclotted radiolabeled material. This effect was similar to that observed in the absence of rt-PA after single-bolus injections of SANORG 34006, SR 90107/ORG 31540, or heparin (200 or 300 nmol/kg IV). Infusion of rt-PA (0.5 mg/kg) resulted in a 43.1% ± 9.5% (n = 8)125I-fibrino(geno)lysis, which was significantly enhanced (P < .05) by a bolus IV injection of SANORG 34006 (300 nmol/kg) (Fig 7A). A similar efficacy of SANORG 34006 was observed when thrombolysis was expressed as decrease of the thrombus weight (Fig 7B). Thrombus weight decreased from a control value of 134.8 ± 9.1 mg to 61.4 ± 8.6 mg (n = 8) in the rt-PA–treated group. Concomitant treatment with SANORG 34006 further reduced thrombus weights in a dose-dependent manner. SR 90107/ORG 31540 and heparin demonstrated comparable effects in this rt-PA thrombolysis model (Fig 7A and B).
Effect of SANORG 34006 on rt-PA–induced thrombolysis in the rabbit. A 125I-fibrinogen–labeled thrombus was formed in the jugular vein of rabbits. After thrombus formation, blood flow was restored and a 4-hour rt-PA infusion (0.5 mg/kg) was administered. SANORG 34006, SR 90107/ORG 31540, or heparin were administered at indicated doses as a bolus IV injection at the beginning of the rt-PA infusion. At the end of the infusion period, the amount of radioactivity remaining in the clot was determined with a gamma counter (A) and the thrombus was weighed (B). The extent of125I-fibrino(geno)lysis was calculated as the difference between the radioactivity originally incorporated in the thrombus and the remaining radioactivity in the residual thrombus. Values are means ± SEM (n = 8). Statistical analysis was performed using the Mann-Whitney test: *P < .05 versus rt-PA–treated group.
Effect of SANORG 34006 on rt-PA–induced thrombolysis in the rabbit. A 125I-fibrinogen–labeled thrombus was formed in the jugular vein of rabbits. After thrombus formation, blood flow was restored and a 4-hour rt-PA infusion (0.5 mg/kg) was administered. SANORG 34006, SR 90107/ORG 31540, or heparin were administered at indicated doses as a bolus IV injection at the beginning of the rt-PA infusion. At the end of the infusion period, the amount of radioactivity remaining in the clot was determined with a gamma counter (A) and the thrombus was weighed (B). The extent of125I-fibrino(geno)lysis was calculated as the difference between the radioactivity originally incorporated in the thrombus and the remaining radioactivity in the residual thrombus. Values are means ± SEM (n = 8). Statistical analysis was performed using the Mann-Whitney test: *P < .05 versus rt-PA–treated group.
Nonhemorrhagic effect SANORG 34006 in the rabbit.
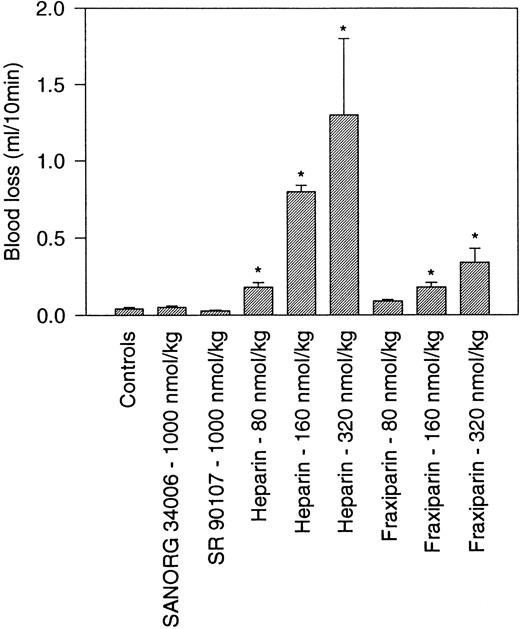
The hemorrhagic risk associated to a treatment with SANORG 34006 was evaluated using a rabbit ear incision model. This model has been shown to be highly sensitive to anticoagulants and to glycoprotein IIb-IIIa inhibitors but not to aspirin and clopidogrel.20 IV administration of standard heparin at doses ≥83 nmol/kg significantly increased blood loss, whereas Fraxiparin, a low-molecular-weight (LMW) heparin, showed a significantly lower efficacy. SANORG 34006 or SR 90107/ORG 31540 did not affect bleeding when administered at a dose of 1,000 nmol/kg IV (Fig 8).
Nonhemorrhagic effect SANORG 34006 in the rabbit. SANORG 34006, SR 90107/ORG 31540, LMW heparin (Fraxiparin), or heparin were administered IV at indicated doses 5 minutes before ear incision. Blood loss were determined during 10 minutes as described in Materials and Methods. Results are expressed as mean volumes ± SEM (n = 10). Statistical analysis was performed using the Mann-Whitney test: *P < .05 versus controls.
Nonhemorrhagic effect SANORG 34006 in the rabbit. SANORG 34006, SR 90107/ORG 31540, LMW heparin (Fraxiparin), or heparin were administered IV at indicated doses 5 minutes before ear incision. Blood loss were determined during 10 minutes as described in Materials and Methods. Results are expressed as mean volumes ± SEM (n = 10). Statistical analysis was performed using the Mann-Whitney test: *P < .05 versus controls.
DISCUSSION
It is now well admitted that the inhibition of factor Xa represents an attractive approach for clinical intervention in various thrombotic disorders.21 Results obtained in this study confirm and extend these observations and clearly show that synthetic analogs of the unique AT binding sequence present on the heparin chains4-9 represent a new class of antithrombotic agents. One of these compounds, SANORG 34006, interacts with human AT with a dissociation constant in the nanomolar range. This affinity for human AT is more than 10 times higher than that observed for the “natural” representative of this class of compounds: SR 90107/ORG 31540.4,11,15 Binding of the pentasaccharides to AT induces a conformational change which results in an accelerated, selective inactivation of factor Xa.22 In that respect, SANORG 34006 is a potent and selective AT-dependent inhibitor of human factor Xa showing an IC50 value which compared favorably with that of SR 90107/ORG 31540. Nevertheless, despite its much higher affinity for AT and a higher anti–factor Xa activity, the resulting overall anticoagulant effect elicited by SANORG 34006 as demonstrated by its effect on thrombin generation appeared to be only slightly enhanced compared with that of SR 90107/ORG 31540. In that respect, SANORG 34006 efficiently impaired thrombin generation in human plasma, with IC50 values in the microgram per milliliter range, the extrinsic pathway being more sensitive to SANORG 34006 than the intrinsic one. Similar results which have been already observed with SR 90107/ORG 3154015 and other pentasaccharides11can be explained by the indirect impairment of factor VIIa formation by such factor Xa–inactivating compounds.23 Alternatively, the recently described AT-mediated inhibition of the factor VIIa–tissue factor complex by the pentasaccharides24 might also account for the observed greater susceptibility of the coagulation extrinsic pathway to inhibition by AT-binding pentasaccharides such as SANORG 34006.
Because these data show that SANORG 34006, in the presence of AT, is a potent inhibitor of factor Xa, we then evaluated the pharmacodynamics of the anti–factor Xa effect of an SC or IV administration of SANORG 34006 by means of measurement of its anti–factor Xa activity in plasma. SANORG 34006 exhibited a strong, dose-dependent anti–factor Xa activity after SC and IV administration to rabbits, rats, and baboons. This anti–factor Xa effect almost completely paralleled the inhibition of thrombin generation measured ex vivo. In contrast to unfractionated and LMW heparin, these pentasaccharides represent single molecular entities with well-defined pharmacological targets. Thus, circulating anti–factor Xa activity may be considered as reflecting the true plasma concentration of this class of compounds. In the range of doses investigated, the elimination half-life of SANORG 34006 (t1/2) was independent of the dose as already shown for other pentasaccharides.12,25 Interestingly, SANORG 34006 was cleared more slowly than SR 90107/ORG 31540 in these three species. Some investigators suggested that this difference could result from the very high affinity of such compounds for AT, accounting for a half-life that tended to be close to that of AT itself.25 Similarly, other investigators suggested that the elimination half-life of structural analogs of the AT-binding pentasaccharide might be correlated to their affinity for AT because they could show a relation between the kd and half-life of elimination in rats.12Based on allometric scaling,26 which gave predictions for SR 90107/ORG 31540 in agreement with the experimental data obtained in a recently published clinical trial in humans,10 the determination of the pharmacodynamics of the anti–factor Xa effect parameters of SANORG 34006 suggested that in humans the terminal half-life for this compound would be around 80 hours. This value, markedly different from the value observed for SR 90107/ORG 31540, will allow a once-a-week administration of the compound. Provided that this extrapolated data will be confirmed in an appropriate clinical study in humans, this compound might therefore be used in several clinical settings where high anti–factor Xa activity is needed with a necessary long-lasting anticoagulation.
The antithrombotic potency of this compound was then evaluated in comparison with that of SR 90107/ORG 31540 and standard heparin. The role of factor Xa bound to fibrin and/or platelets and its subsequent effect on prothrombin activation has been emphasized, showing that these complex phenomena ensure the progressive growth of the thrombus over several hours.27 SANORG 34006 displayed marked dose-dependent antithrombotic activity in different experimental models of thrombosis in rats and rabbits such as stasis-induced venous thrombosis in rats and rabbits where thrombus formation occurs because of a combination of high-grade stenosis and thrombogenic challenge, fibrinogen accretion to a preformed thrombus in the rabbit jugular vein, and potentiation of t-PA–induced thrombolysis of a thrombus implanted in the rabbit jugular vein. Moreover, the curves describing the relationship between the doses, the subsequent plasma concentrations, and the resultant antithrombotic effect were parallel, suggesting that only one mechanism is involved in the antithrombotic property of SANORG 34006, namely its pure anti–factor Xa effects. One important observation was that, compared with SR 90107/ORG 31540, SANORG 34006 exhibited an extended duration of antithrombotic activity as already demonstrated for its anti–factor Xa activity in plasma.
Therefore, these studies clearly show that SANORG 34006 is an efficacious antithrombotic drug after IV or SC administration. This compound, which inhibited several types of experimental venous thrombosis without affecting bleeding, exhibited an extremely long duration of action compared with any other compound of this kind. In contrast with other anticoagulants such as thrombin inhibitors, SANORG 34006, which did not affect thrombin- or adenosine diphosphate (ADP)-induced platelet aggregation (2% and 5% inhibition at 10 μmol/L, respectively), would not compromise the hemostatic response of platelets, thereby indicating the possibility of using it alone or as an adjunctive agent with other antithrombotic drugs without the risk of abnormal bleeding.
Thus, this compound appears a promising antithrombotic agent with reduced bleeding tendency and an extended half-life.
ACKNOWLEDGMENT
We thank F. Roye, A. Hubert, M. Sainte-Marie, C. Gaich, and C. Avril for their technical assistance.
Address reprint requests to J.M. Herbert, PhD, Sanofi Recherche, Haemobiology Research Department, 195 Route d'Espagne, 31036 Toulouse, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal