Abstract
Hereditary hyperferritinemia-cataract syndrome (HHCS) is an autosomal and dominant disease caused by heterogeneous mutations in the iron responsive element (IRE) of the 5′ untranslated flanking region of ferritin L-chain mRNA, which reduce the binding to the trans iron regulatory proteins and make L-chain synthesis constitutively upregulated. In the several families identified so far, the serum and tissue L-ferritin levels are fivefold to 20-fold higher than in nonaffected control subjects, iron metabolism is apparently normal, and the only relevant clinical symptom is early onset, bilateral cataract. Some pathogenetic aspects of HHCS remain obscure, with particular reference to the isoferritins produced by HHCS cells, as well as the mechanism of cataract formation. We analyzed lymphoblastoid cell lines obtained from two nonaffected control subjects and from HHCS patients carrying the substitution A40G (Paris-1), G41C (Verona-1), and the deletion of the residues 10-38 (Verona-2) in the IRE structure. Enzyme-linked immunosorbent assays specific for the H- and L-type ferritins showed that L-ferritin levels were up to 20-fold higher in HHCS than in control cells and were not affected by iron supplementation or chelation. Sequential immunoprecipitation experiments of metabolically-labeled cells with specific antibodies indicated that in HHCS cells about half of the L-chain was assembled in L-chain homopolymers, which did not incorporate iron, and the other half was assembled in isoferritins with a high proportion of L-chain. In control cells, all ferritin was assembled in functional heteropolymers with equivalent proportion of H- and L-chains. Cellular and ferritin iron uptake was slightly higher in HHCS than control cells. In addition, we analyzed the lens recovered from cataract surgery of a HHCS patient. We found it to contain about 10-fold more L-ferritin than control lens. The ferritin was fully soluble with a low iron content. It was purified and partially characterized. Our data indicate that: (1) in HHCS cells a large proportion of L-ferritin accumulates as nonfunctional L-chain 24 homopolymers; (2) the concomitant fivefold to 10-fold expansion of ferritin heteropolymers, with a shift to L-chain–rich isoferritins, does not have major effects on cellular iron metabolism; (3) L-chain accumulation occurs also in the lens, where it may induce cataract formation by altering the delicate equilibrium between other water-soluble proteins (ie, crystallins) and/or the antioxidant properties.
HUMAN FERRITIN IS encoded by the genes for the heavy H-chain (182 amino acids) and the light L-chain (174 amino acids) on chromosome 11 and 19, respectively.1Despite differences in the primary structure (about 50% identity at the amino acid level), the two chains have remarkably similar three-dimensional structures and coassemble in different proportions in the 24-mer protein to originate families of isoferritins.1,2 Both H- and L-chain mRNAs carry iron responsive element (IRE) structures close to the 5′ termini, which interact with the trans iron regulatory proteins (IRPs), leading to an equivalent iron-regulated translational regulation.3,4 The different accumulation of the two chains and messengers in the various cells is mainly attributed to different transcriptional regulations.5 6
The major functional role of ferritin is to store iron into its large cavity, and consequently several in vitro studies analyzed the interaction between iron and ferritin.7 Biochemical and crystallographic studies on the recombinant H-chain homopolymers showed that H-chains contain ferroxidase centers, which promote iron oxidation and accelerate iron incorporation into the ferritin.8,9 The L-chains lack this activity, but are richer in carboxyl groups exposed on the cavity surface, which facilitate iron nucleation and mineralization.10 The complementary functional specificities of the two chains make the heteropolymers more efficient in the in vitro incorporation of iron. For instance, molecules with 30% to 100% H-chains incorporated iron at the same rate, but the ones richer in L-chain were more efficient in iron core formation.11 The finding that L- is more stable than the H-ferritin and that it is predominant in tissues with main iron storage functions suggested that it is designed for long-term iron storage, whereas the H-chain is probably necessary for an active cellular iron sequestration and detoxification. This hypothesis was confirmed by recent studies showing that even a minor overexpression of H-chain in mouse erythroleukemic cell lines induced an iron-deficient phenotype and repression of globin synthesis.12
Some of us recently described a new disease, the hereditary hyperferritinemia-cataract syndrome (HHCS), clinically characterized by the combination of a substantial elevation of serum L-ferritin levels and early onset bilateral cataract, without other apparent symptoms.13 Typically, hyperferritinemia is not related to iron overload and persists even if HHCS patients develop iron-deficient anemia after inappropriate phlebotomies.14 After the descriptions in Italian and French families,13-20 HHCS has been also found in Germany,21 the United Kingdom,22 and the United States (D.G., personal communication, November 1997), suggesting that the disease may be largely distributed in the world and probably not sporadic. This autosomal and dominant genetic disorder is associated with heterogeneous mutations of the L-chain IRE sequences that reduce/eliminate binding to IRPs and make L-chain expression constitutive and noniron regulated. Despite the identification of the genetic basis, not all is clear about the pathophysiology of HHCS, which also offers a unique human model to study ferritin biological roles.13,14 In particular, the structural and functional properties of the isoferritins produced in HHCS cells, as well as the mechanism of cataract formation, remain unclear. A genotype-phenotype correlation has been noted in HHCS, depending on the position of the different mutations in the IRE stem-loop structure and their effect on IRP binding affinity. Mutation in the lower stem has been associated with lower serum ferritin and milder, asymptomatic cataract as compared with mutations in the CAGUGN hexaloop and/or deletion, which determine ferritin levels greater than 1,000 μg/L and severe cataract.15,19 The parallelism between serum ferritin levels, tissue ferritin levels, and severity of the cataract suggested that the three are linked and related to the abnormality of ferritin L-chain regulation.15-17 Unexpectedly, the noniron regulated fivefold to 20-fold increases in L-ferritin levels in serum and did not have significant effects on body iron metabolism, except that some subjects were found prone to develop iron deficiency.14,15 Ferritin is ubiquitous, and all tissues so far analyzed, red blood cells, peripheral monuclear cells, and liver were found to accumulate large excesses of ferritin in the HHCS subjects.15,19 Analysis of lymphoblastoid cell lines of subjects from the Paris-1 family indicated a large unbalance of L-chain synthesis over the H-chain.17
To study in more detail the relationship between ferritin synthesis, cellular iron metabolism, and cataract development, we analyzed cultured lymphoblastoid cell lines from subjects with three different HHCS mutations and a specimen from cataract surgery of one HHCS subject. HHCS lymphoblastoid cells were found to contain a large amount of ferritin rich in L-chain in the form of nonfunctional L-homopolymers and functional L-rich heteropolymers. Cellular iron metabolism, monitored by iron incorporation and IRPs activity, was very similar in control and HHCS cells. A similar modification of ferritin accumulation was found in HHCS lens.
The results indicate that the accumulation of L-chain homopolymers and the shift in isoferritin composition is probably common to most tissues and has minor consequences on iron metabolism. Cataract formation may be due to the indirect effects of L-ferritin overexpression on the solubility of other lens proteins or on antioxidant properties of the lens.
MATERIALS AND METHODS
Reagents.
Cell culture.
The Epstein-Barr virus (EBV)-transformed cultured B lymphoblastoid cell lines were obtained by infection of peripheral blood mononuclear cells with supernatant from EBV-infected B95-8 Marmoset cells (American Type Culture Collection CRL 1612, Rockville, MD). They were grown in RPMI 1640 medium (Dulbecco's modified Eagle's medium; GIBCO, Grand Island, NY) supplemented with 10% fetal calf serum (FCS, GIBCO), 80 μg/mL gentamycin, 2 mmol/L glutamine, 1% sodium pyruvate and maintained at a concentration of 106 cell/mL.
Metabolic labeling and immunoprecipitation.
Cells (2 × 106) were grown for 1 hour in methionine-free MEM medium (GIBCO), 100 mg/mL streptomycin, 100 U/mL penicillin, 2 mmol/L glutamine, 0.5% FCS (GIBCO), 0.5% bovine serum albumin, and then labeled for 2 hours with 50 μCi/mL35S-Met. The cells were collected by centrifugation, washed with phosphate-buffered saline, and then lysed with 500 μL of lysis buffer (20 mmol/L Tris-HCl, pH 8.0; 200 mmol/L LiCl; 1 mmol/L EDTA; 0.5% NP-40). Total radioactivity associated with the soluble proteins was determined by trichloroacetic acid (TCA) precipitation. For immunoprecipitation studies, 2 × 106 cpm of cytosolic lysates were precleared by incubation with 100 μL of protein A-Sepharose 50% vol/vol (Sigma, St Louis, MO) for 1 hour at 4°C with gentle shaking. After centrifugation for 1 minute at 14,000 rpm, the supernatants were added to 30 μL of protein A-Sepharose preincubated with the antiferritin H-chain antibody rH02 at a concentration of 3 mg/mL, incubated 1 hour at 4°C, and precipitated as above. The soluble fraction was further added to 30 μL of protein A-Sepharose preincubated with 3 mg/mL of antiferritin L-chain antibody L03, incubated for 1 hour at 4°C, and precipitated. The immunobeads were washed, resuspended in sodium dodecyl sulfate (SDS) buffer, boiled for 10 minutes, and loaded on 15% SDS-polyacrylamide gel electrophoresis (PAGE). Alternatively, they were resuspended in SDS loading buffer containing 4 mol/L urea, run on 6% polyacrylamide SDS-PAGE containing 4 mol/L urea, and subjected to autoradiography. Under these conditions, ferritin is not denatured.24 The gels were treated with autoradiography image enhancer (Amplify; Amersham), dried, and exposed. The intensity of ferritin subunit bands was quantified by densitometry (Molecular Dynamics, Sunnyvale, CA).
59Fe cellular uptake.
Cells (106) were grown for 18 hours in RPMI supplemented with 10 μCi/mL of 59Fe nitrilo triacetate (59FeNTA) and 200 μmol/L ascorbic acid. The cells were washed and lysed as above, the homogenates centrifuged at 13,000 rpm for 5 minutes at 4°C, and the radioactivity associated with soluble and insoluble fraction measured on a γ-counter (Packard, Downers Grove, IL). The extracts were analyzed on 6% native-PAGE and exposed to autoradiography or subjected to the sequential immunoprecipitation procedure described above and analyzed in 4 mol/L urea on 6% PAGE under nondenaturing conditions.24
Ferritin and protein quantification in cellular extracts.
Ferritin content of 107 cell extracts was measured by enzyme-linked immunosorbent assay (ELISA) based on monoclonal antibodies specific for the H-ferritin (rH02) and the L-ferritin (LF03) calibrated on the corresponding recombinant homopolymers expressed inE coli.23,24 Protein content was evaluated by bicinchoninic acid (BCA) method (Pierce, Rockford, IL) calibrated on bovine serum albumin. Immunoblotting experiments were performed as described in Santambrogio et al.11 IRP activity measurements of cell lysates were determined as in Cairo and Pietrangelo.25
Lens ferritin purification and analysis.
A 25-year-old female HHCS patient belonging to the family Verona-114 recently underwent cataract surgery by means of the phacoemulsification technique with posterior chamber intraocular lens implantation, which is widely used in modern cataract surgery.26 During the procedure, after opening of the anterior capsule, the lens is fragmented by ultrasound and then aspirated in a closed irrigation-aspiration system. The recovered lens material consisted of a suspension in a buffered saline solution. Specimens were obtained from the HHCS patient, as well as from several patients with cataracts unrelated to HHCS, as controls. Soluble homogenates of the solid phases, which were amorphous and not suitable for histological examination, were obtained by incubation in 0.2% NP-40 followed by centrifugation. These samples and the washing solutions were analyzed for ferritin content with the ELISA assays described above. Iron content was measured by electrothermal atomic absorption spectroscopy (EAAS) using a Varian Spectra A 300 (Varian, Sugar Land, TX) equipped with a graphite tube atomizer (Zeeman effect). Ferritin purification was achieved by heating the homogenates at 75°C for 10 minutes followed by anion-exchange column chromatography (20 HQ-Poros). The ferritin fraction was concentrated on Centricon 30 (Amicon, Danvers, MA). To test in vitro functionality, the purified protein (0.5 mg/mL to 0.1 μmol/L) was incubated with 0.1 mmol/L ferrous ammonium sulfate in 0.1 mol/L HEPES, pH 7.0, for 2 hours at room temperature, run on 7.5% polyacrylamide nondenaturing gel electrophoresis, and stained with Prussian blue.10
RESULTS
Ferritin in immortalized lymphocytes.
Figure 1 summarizes the substitutions of human ferritin L-chain IRE sequences, which have been so far associated with HHCS, including the ones analyzed here. We determined ferritin content in the lymphoblastoid cells lines from subjects of HHCS families coded Verona-1,14 Verona-2,15 and Paris-117 cell homogenates with ELISA assays based on monoclonal antibodies for the H- and L-ferritin and calibrated on recombinant human H- and L-homopolymers.23 24 The two control cells had similar levels of ferritin: about 100 ng of the L-type and about 190 ng of the H-type per milligram of total soluble protein. The three HHCS cells had an accumulation of L-ferritin up to more than 2,000 ng/mg of protein (ie, 13-fold to 28-fold higher than controls), and a variable decrease in H-ferritin content (Table 1). In HHCS cells, the L:H ferritin ratios ranged between 15 and 118:1, whereas in control cells, it was approximately 0.5:1.
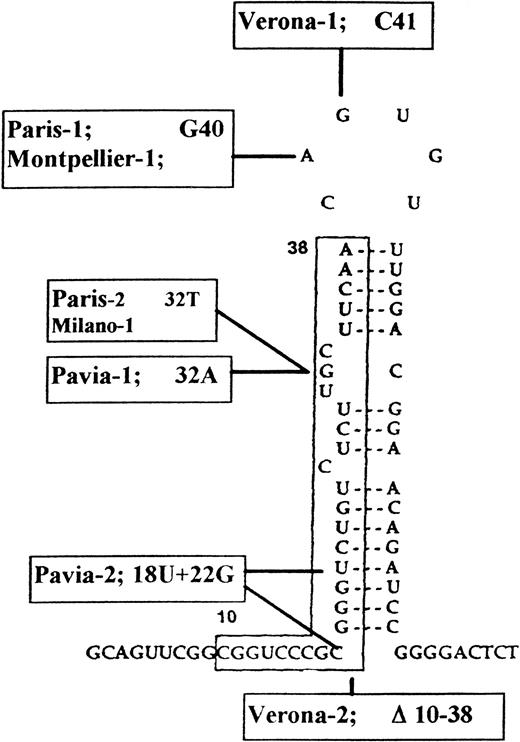
Predicted secondary structure of L-subunit IRE and mutations so far described in HHCS families. Town names indicate the different mutations: Paris-114 is the same as Montpellier-115 and differs from Verona-1,16Verona-2,17 Pavia-1 and Pavia-2,18 and Paris-2 and Milano-1.20
Ferritin Content in Lymphoblastoid Cell Lines
| Cell Lines . | L-Ferritin (ng/mg total protein) . | H-Ferritin (ng/mg total protein) . | L/H Ratio . |
|---|---|---|---|
| C1 | 108 ± 22 | 194 ± 72 | 0.56 |
| C2 | 83 ± 24 | 153 ± 32 | 0.54 |
| Verona-1 | 1,968 ± 439 | 35 ± 12 | 56.26 |
| Verona-2 | 2,372 ± 465 | 20 ± 10 | 118.60 |
| Paris-1 | 1,446 ± 275 | 95 ± 23 | 15.20 |
| Cell Lines . | L-Ferritin (ng/mg total protein) . | H-Ferritin (ng/mg total protein) . | L/H Ratio . |
|---|---|---|---|
| C1 | 108 ± 22 | 194 ± 72 | 0.56 |
| C2 | 83 ± 24 | 153 ± 32 | 0.54 |
| Verona-1 | 1,968 ± 439 | 35 ± 12 | 56.26 |
| Verona-2 | 2,372 ± 465 | 20 ± 10 | 118.60 |
| Paris-1 | 1,446 ± 275 | 95 ± 23 | 15.20 |
To assess the iron-dependent regulation of ferritin expression, the cells were incubated for 18 hours in the presence of 100 μmol/L of the chelator desferrioxamine (DFO) or of 100 μmol/L Fe(III) as ferric ammonium citrate (FAC) (Table 2). In the control cells, iron supplementation determined an ≈ 2-fold increase of both H- and L-ferritins, and iron chelation a 1.3-fold to 2-fold decrease of the ferritins, as expected. In the Verona-1 cells, H-ferritin expression was similarly modulated by iron, while L-ferritin appeared to be almost totally insensitive to either DFO of FAC treatment (Table 2). Similar results were obtained with Verona-2 and Paris-1 cells (not shown).
Ferritin Concentration in Control and Verona-1 Lymphoblastoid Cells With and Without Iron Supplementation or Chelation
| Cell Line . | Treatment . | L-Ferritin . | H-Ferritin . | ||
|---|---|---|---|---|---|
| ng/mg Total Protein . | Relative Change . | ng/mg Total Protein . | Relative Change . | ||
| C1 | — | 123 | — | 192 | — |
| DFO | 77 | 0.6 | 74 | 0.4 | |
| FAC | 230 | 1.9 | 382 | 2.0 | |
| C2 | — | 87 | — | 136 | — |
| DFO | 46 | 0.5 | 41 | 0.3 | |
| FAC | 140 | 1.6 | 302 | 2.2 | |
| Verona-1 | — | 2174 | — | 23 | — |
| DFO | 2345 | 1.1 | 13 | 0.6 | |
| FAC | 2396 | 1.1 | 43 | 1.9 | |
| Cell Line . | Treatment . | L-Ferritin . | H-Ferritin . | ||
|---|---|---|---|---|---|
| ng/mg Total Protein . | Relative Change . | ng/mg Total Protein . | Relative Change . | ||
| C1 | — | 123 | — | 192 | — |
| DFO | 77 | 0.6 | 74 | 0.4 | |
| FAC | 230 | 1.9 | 382 | 2.0 | |
| C2 | — | 87 | — | 136 | — |
| DFO | 46 | 0.5 | 41 | 0.3 | |
| FAC | 140 | 1.6 | 302 | 2.2 | |
| Verona-1 | — | 2174 | — | 23 | — |
| DFO | 2345 | 1.1 | 13 | 0.6 | |
| FAC | 2396 | 1.1 | 43 | 1.9 | |
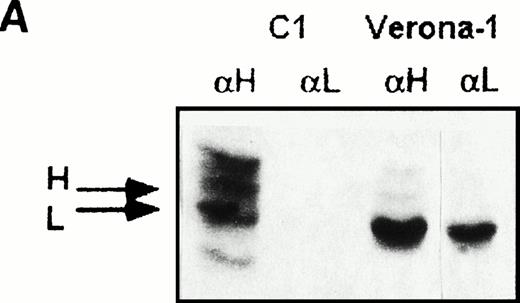
In HHCS cells, the large excess of L- over the H-ferritin (ratio up to 118:1, to be compared with the 23:1 of molecules with a single H-chain ) suggested that at least part of the L-chain accumulated in homopolymers, but the proportion of the ferritin L-homopolymers and the subunit composition of the ferritin heteropolymers remained unknown. To separate the two ferritin populations, we performed sequential immunoprecipitation experiments of soluble homogenates from35S-methionine–labeled cells. The first precipitate, induced by saturating amounts of monoclonal antibody specific for the H-chain, was expected to contain all available H-chains and the associated L-chains, ie, the heteropolymeric fraction of ferritin.24 The supernatant was further incubated with saturating amounts of anti–L-ferritin antibody, which precipitate the possible remaining ferritin L-chains not associated with the H-chain, ie, L-homopolymers. We found that in metabolically-labeled control cells the first precipitate contained comparable amounts of H- and L-chains, plus some nonspecific bands due to the interaction of protein A with endogenous immunoglobulins of the lymphoblastoid cells, and the second precipitate did not contain detectable ferritin subunits (Fig 2A). In contrast, two precipitates from Verona 1 cell homogenates contained comparable amounts of L-chain, the difference being a minor proportion of H-chain in the first precipitate. Similar results were obtained with Verona-2 and Paris-1 cells (not shown). The findings indicate that HHCS cells express two immunologically and structurally distinct ferritin populations of similar size: one composed of L-homopolymers and the other one of heteropolymers with an estimated content of 1-2 H-chains per 24-mer.
Sequential immunoprecipitation experiments of metabolically labeled lymphoblastoid cells with antibodies specific for human ferritin H-chain (αH) and L-chain (αL). (A) Control cells (C1) and Verona-1 cells (Ver-1) were metabolically labeled with35S-methionine, lysed, and 2 × 106 cpm of the soluble fraction of homogenates precipitated with saturating amounts of anti-H chain antibody. The soluble fraction was then precipitated again with saturating amounts of anti–L-chain antibody. The precipitates were analyzed on SDS-PAGE under denaturing conditions and exposed to autoradiography. (B) Verona-1 cells were metabolically labeled with35S-methionine and immunoprecipitated as in (A), the pellets were resuspended in 4 mol/L urea, and separated on 6% polyacrylamide gels containing 4 mol/L urea under conditions that disrupt antibody antigen interactions without affecting ferritin structure. (C) As in (B), except that cells were metabolically labeled with 59FeNTA.
Sequential immunoprecipitation experiments of metabolically labeled lymphoblastoid cells with antibodies specific for human ferritin H-chain (αH) and L-chain (αL). (A) Control cells (C1) and Verona-1 cells (Ver-1) were metabolically labeled with35S-methionine, lysed, and 2 × 106 cpm of the soluble fraction of homogenates precipitated with saturating amounts of anti-H chain antibody. The soluble fraction was then precipitated again with saturating amounts of anti–L-chain antibody. The precipitates were analyzed on SDS-PAGE under denaturing conditions and exposed to autoradiography. (B) Verona-1 cells were metabolically labeled with35S-methionine and immunoprecipitated as in (A), the pellets were resuspended in 4 mol/L urea, and separated on 6% polyacrylamide gels containing 4 mol/L urea under conditions that disrupt antibody antigen interactions without affecting ferritin structure. (C) As in (B), except that cells were metabolically labeled with 59FeNTA.
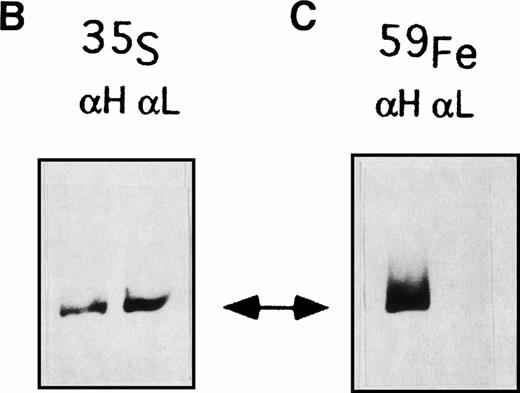
A similar approach was used to analyze the function of the two ferritin populations: cells were metabolically labeled either with35S-methionine or 59FeNTA, the homogenates sequentially immunoprecipitated with anti-H and anti-L ferritin antibodies, as above, and the precipitates resuspended in 4 mol/L urea under conditions that disrupt the association with the antibody without affecting the structural integrity of the highly stable ferritin molecules.24 The samples were then separated on nondenaturing PAGE and exposed to autoradiography (Fig 2B and C). The two precipitates from 35S-methionine–labeled Verona-1 cells showed a similar ferritin content, whereas those from the corresponding 59Fe-labeled cells differed: the first precipitate retained the radioactive iron, and the second remained unlabeled. This result was confirmed on three separate experiments on Verona-1 cells and twice on Verona-2 and Paris-1 cells. The finding implies that although the heteropolymeric and homopolymeric ferritin populations are synthesized in similar amounts, only the heteropolymers can take up iron.
Cellular iron incorporation.
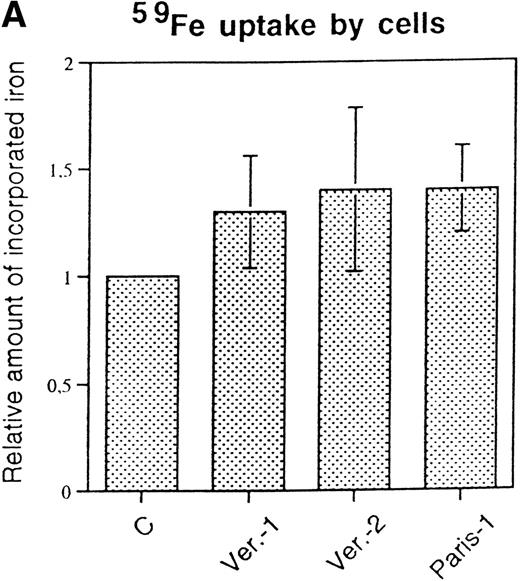
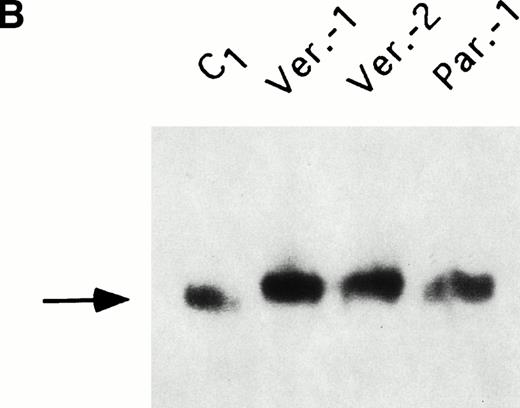
Cells were incubated for 18 hours with 59FeNTA in the presence of 200 μmol/L ascorbic acid and, after washing, the radioactivity associated with cells or with ferritins was determined. Total cellular iron uptake was slightly higher in the three HHCS cells than in control (Fig 3A), but the experiments showed some variability. Autoradiography of59Fe-labeled cellular homogenates separated on nondenaturing PAGE showed that essentially all protein bound radioactivity was associated with ferritin and that band intensities were comparable and slightly stronger in the three HHCS than in the control cells (Fig 3B). Densitometry of various experiments indicated some variability (Fig 3C). It should be noted that59Fe-labeled ferritin in HHCS cells had a slower mobility than that of control cells (Fig 3B), in agreement with the higher L-subunit content. Analyses of IRPs activity performed by band shift assays did not show differences between HHCS and control cells (not shown). It was concluded that despite the large increase in L-ferritin and the dramatic shift in the isoferritin profile, the cellular iron metabolism of HHCS cells is apparently similar to that of controls.
59Fe incorporation in lymphobastoid cells and ferritin. (A) Cells were incubated for 18 hours in RPMI supplemented with 10 μmol/L 59FeNTA and 200 μmol/L ascorbic acid, washed, and the radioactivity in the soluble homogenates counted. The values were normalized on the data of the control cells. Means and standard deviation (SD) of three independent experiments are shown. (B) The soluble homogenates from the same number of cells were subjected to electrophoresis under nondenaturing conditions and exposed to autoradiography. Ferritin mobility is indicated by the arrow. (C) Means and SD of densitometometric quantitation of three independent experiments, as in (B), are shown. Values normalized on the control cells.
59Fe incorporation in lymphobastoid cells and ferritin. (A) Cells were incubated for 18 hours in RPMI supplemented with 10 μmol/L 59FeNTA and 200 μmol/L ascorbic acid, washed, and the radioactivity in the soluble homogenates counted. The values were normalized on the data of the control cells. Means and standard deviation (SD) of three independent experiments are shown. (B) The soluble homogenates from the same number of cells were subjected to electrophoresis under nondenaturing conditions and exposed to autoradiography. Ferritin mobility is indicated by the arrow. (C) Means and SD of densitometometric quantitation of three independent experiments, as in (B), are shown. Values normalized on the control cells.
Ferritin in the lens.
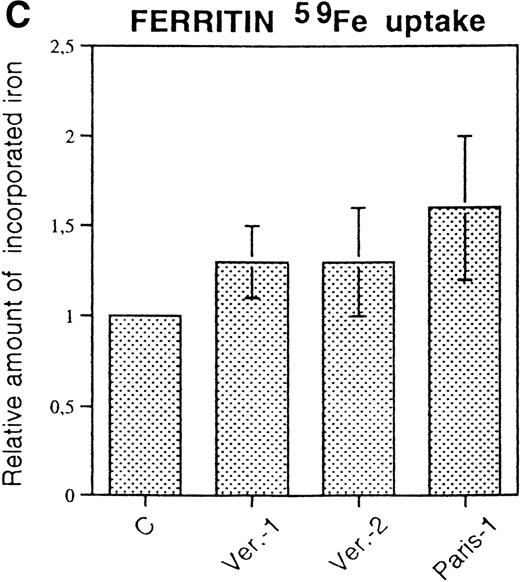
The lens specimens from cataract surgery of an HHCS subject of Verona-1 family were made available to us. The solid samples were too damaged for histological examination and were homogenized in the presence of the mild NP-40 detergent. The ferritin concentrations in the soluble extracts determined with the H- and L-ferritin ELISA assays are reported in Table3. In the control, age-matched cataracts, ferritin was present in detectable amounts (0.01% of total proteins) and the L- was more abundant than the H-ferritin type (L:H ratio 3-6:1). In HHCS lens, L-ferritin accounted for a significant proportion of total proteins (≈ 0.15%), whereas H-ferritin content apparently decreased. Similar data were obtained in the washing solutions obtained from surgery (not shown). EAAS analyses detected similar levels of iron in trace amounts in the homogenates of HHCS and control lens (0.008 μg/mg proteins in HHCS; 0.01 ± 0.002 μg/mg proteins in four controls).
Ferritin Concentration in the Lens of HHCS (Verona-1) and Non-HHCS Subjects (C1 and C2)
| Subject . | L-Ferritin (ng/mg total protein) . | H-Ferritin (ng/mg total protein) . | L:H Ratio . |
|---|---|---|---|
| C1 | 226 ± 29 | 84 ± 14 | 2.7 |
| C2 | 96 ± 13 | 16 ± 2 | 6.0 |
| Verona-1 | 1,500 ± 166 | 7 ± 1 | 214.0 |
| Subject . | L-Ferritin (ng/mg total protein) . | H-Ferritin (ng/mg total protein) . | L:H Ratio . |
|---|---|---|---|
| C1 | 226 ± 29 | 84 ± 14 | 2.7 |
| C2 | 96 ± 13 | 16 ± 2 | 6.0 |
| Verona-1 | 1,500 ± 166 | 7 ± 1 | 214.0 |
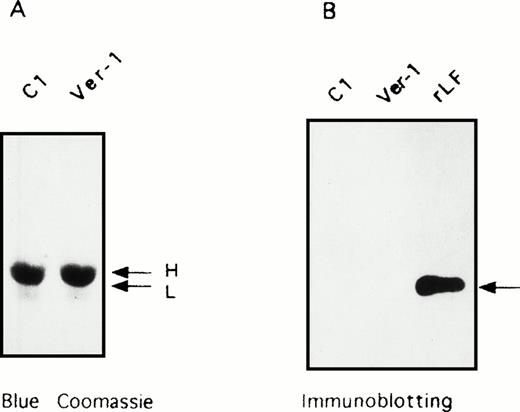
Analysis of the insoluble fraction of lens extracts. SDS-PAGE of the insoluble fraction of homogenates from lens specimens of a control (C1) and Verona-1 subject (Ver-1). (A) Coomassie blue stain of the precipitates. (B) Immunoblotting stained with anti-L ferritin antibody of the two insoluble fractions and a control of recombinant L ferritin (rLF). The arrows indicate the mobility of H and L chains.
Analysis of the insoluble fraction of lens extracts. SDS-PAGE of the insoluble fraction of homogenates from lens specimens of a control (C1) and Verona-1 subject (Ver-1). (A) Coomassie blue stain of the precipitates. (B) Immunoblotting stained with anti-L ferritin antibody of the two insoluble fractions and a control of recombinant L ferritin (rLF). The arrows indicate the mobility of H and L chains.
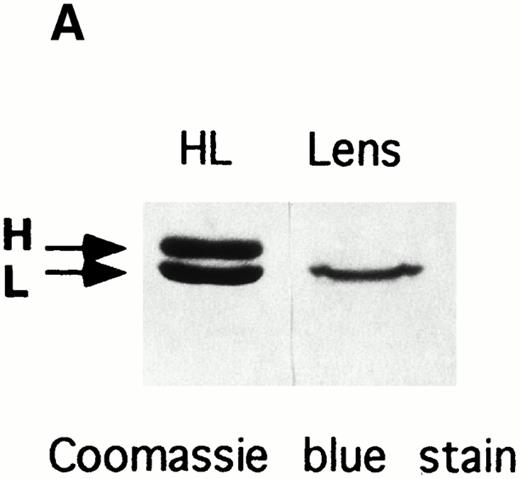
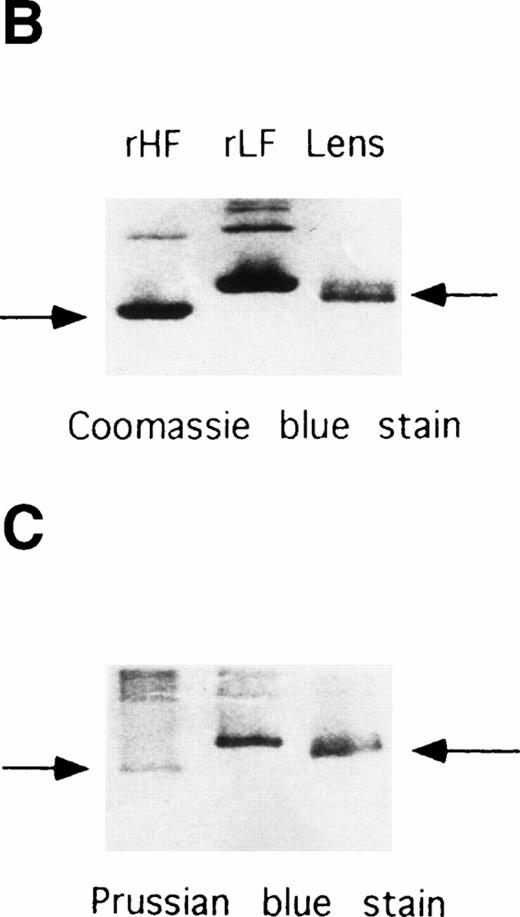
Electrophoretic analyses of ferritin purified from HHCS lens. (A) SDS-PAGE of the purified ferritin compared with human H and L ferritin chains, Coomassie blue stain. (B) Nondenaturing PAGE of the purified lens ferritin compared with recombinant H and L ferritins, Coomassie blue stain. (C) The same as in (B), except that ferritins were preincubated with Fe(II) (1,000 Fe atoms per molecule, pH 7.0) and the gels stained with Prussian Blue.
Electrophoretic analyses of ferritin purified from HHCS lens. (A) SDS-PAGE of the purified ferritin compared with human H and L ferritin chains, Coomassie blue stain. (B) Nondenaturing PAGE of the purified lens ferritin compared with recombinant H and L ferritins, Coomassie blue stain. (C) The same as in (B), except that ferritins were preincubated with Fe(II) (1,000 Fe atoms per molecule, pH 7.0) and the gels stained with Prussian Blue.
SDS-PAGE analysis of the insoluble fractions of the lens homogenates showed a major band of size compatible with α-crystalline (23 kD). Immunoblotting with anti-L antibodies did not show the presence of L-chain in the control and HHCS specimens (Fig 4). Thus, the band was attributed to α-crystalline, and no insoluble ferritin could be detected.
The amount of ferritin in HHCS lens was sufficient to attempt purification, and heat treatment at 75°C of the homogenate yielded a ferritin preparation 90% pure, as judged by SDS-PAGE analysis. It contained a single major band comigrating in SDS-PAGE with human ferritin L-chain (Fig 5A) recognized by anti–L-ferritin antibody (not shown). On nondenaturing PAGE, the protein ran slightly faster than the recombinant L-chain homopolymers (Fig 5B). Similar mobility differences between natural and recombinant ferritins have already been reported and attributed to the lack of blocking of the N-terminal amino group in the proteins expressed from E coli.27 The protein could be stained with Prussian Blue only after in vitro preincubation at pH 7.0 with Fe(II), like the recombinant L-homopolymers (Fig 5C), indicating an iron-poor ferritin. EAAS analyses detected only trace amounts of iron in the preparation from HHCS lens, compatible with a ferritin iron content below 20 Fe atoms per molecule.
DISCUSSION
Ferritin expression in immortalized lymphocytes.
The point mutations of Verona-1, Paris-1, and Pavia-1, which affect residues in the loop and bulge of IRE structure involved in IRPs binding, were shown to abolish the in vitro specific binding to IRPs.14,17,19 The extensive Verona-2 deletion (Fig 1) could be expected to lead to a more severe phenotype; however, the clinical symptoms of the four families were remarkably similar, with serum ferritin in the range of 1,000 to 2,000 μg/L and early onset of bilateral cataracts.15 Accordingly, we found very similar L-ferritin levels in Verona-1 and Paris-1 and Verona-2 lymphoblastoid cells (Table 1). Iron chelation and supplementation treatments modulated H- and L-ferritin accumulation in the control cells, but had no effect on L-ferritin accumulation in HHCS cells, a finding consistent with the observation that even severe iron deprivation did not modify serum ferritin levels in Verona-1 subjects.14These data suggest that the activity of the normal IRP-regulated L-ferritin allele is obscured by the constitutive upregulation of the abnormal allele. However, an analysis of the de novo synthesis of ferritin subunits in the same Paris-1 cells, and under the same conditions of iron supplementation or chelation, showed that L-chain synthesis is iron modulated, albeit slightly,17 indicating that the normal L-chain allele is active.
Our data indicated that H-ferritin tended to be lower in HHCS than control cells possibly caused by L-ferritin overexpression (Table 1). However, the sandwich-type ELISA assays we used need at least two separated antibody binding sites for recognition, and the H-ferritin assay was shown to underestimate the H-chain-poor isoferritins.28 Ferritin heteropolymers in HHCS cells contain very few H-chains (1 or 2 per molecule) (Fig 2A). Thus, the decrease of immunologic H-ferritin in HHCS cells may just reflect a structural shift of isoferritins from H-chain–rich to H-chain–poor molecules, rather than a decrease in H-chain synthesis/accumulation, in agreement with data presented by Beaumont et al,17indicating that H-chain de novo synthesis in control and Paris-1 cells is comparable.
The cellular and total ferritin capacity to incorporate radioactive iron (Fig 3) and IRPs activity were comparable in HHCS and control cells, indicating that the size of the regulatory iron pool and iron metabolism were not modified by L-chain overexpression. This is in agreement with the clinical observation that HHCS subjects have no evident abnormalities in iron metabolism. On the other hand, L-chain overexpression induced the formation of L-homopolymers up to about 50% of total ferritin and caused a dramatic shift in the heteropolymer composition (Fig 2). The strong tendency of ferritin subunits to form copolymers11 favors L-chains coassembly with H-chains until these are available and then form L-homopolymers. This implies that for constant amounts of limiting H-chains, the total amount of isoferritins containing ≈ 5% H-chain (as in HHCS cells) is ≈ 10-fold that of isoferritins with ≈ 50% H-chain (as in control cells), in agreement with the experimental data.
That the L-homopolymers did not take up radioactive iron within the cells (Fig 2C) was expected because these molecules do not incorporate iron during expression in E coli and in vitro under conditions of slow rates of iron autoxidation,6,7 and the natural iron-poor isoferritins are the richest in L-chains.29 More unexpected was the finding that the overall activity of heteropolymeric ferritins in HHCS and control cells was comparable, despite the major differences in the amount and composition. In vitro studies showed that the ferroxidase activity in the heteropolymers increases almost linearly with the increase of H-chain in the range of 4% to ≈ 30% to 50% H-chain.11 30 Because this is the range of isoferritin modification observed in the control and HHCS cells, the total H-chain activity should not be affected by isoferritin reshuffling in the two cell types. In other words, the increased number of active ferritin molecules appears to be compensated by the lower specific activity of the single molecules in HHCS cells.
We think that this interpretation of the data has several implications. First, the overall ferritin iron sequestration activity within the cells is mainly determined by the amount of available H-chain, rather than by isoferritin composition, consistent with the finding that the artificial overexpression of H-chain in mouse erythroleukemic cells induced an iron-deficient phenotype.12 Second, in vitro ferritin iron incorporation increases with H-chain content up to 30% to 50%, then it levels off.11,30 Consequently, an L-chain overexpression may have little effect in tissues where L-chain is predominant, whereas in tissues where H-chain is predominant (such as heart 66%, erythroid cells >70%), it may dilute the H-chains and determine an increase of ferroxidase activity; this is expected to decrease the regulatory iron pool and possibly explain the observation that HHCS subjects are prone to develop iron deficiency. Finally, the major effect of L-chain overproduction is to expand cellular iron storage capacity (ie, the number of ferritin molecules capable of incorporating iron) without major effects on cellular iron metabolism. This observation goes some way to explain why tissues, in particular liver, respond to chronic iron overload in a similar way, by increasing L-chain accumulation with an expansion of total ferritin population and an isoferritin shift towards L-rich molecules,7 a mechanism that increases ferritin iron storage capacity without altering regulatory iron pool. Thus, in vitro and in vivo studies indicate that the L-chain biological role is truly of iron storage, whereas the H-chain has more complex functions to regulate iron metabolism and to store it.
Ferritin in the lens.
The only tissue where L-ferritin overexpression shows clinical manifestation in HHCS is the lens, which becomes opaque. We had the opportunity to analyze specimens from surgical cataract treatment of an HHCS and two control subjects. The procedure involves a harsh treatment of the samples, which cannot be examined by immunohistochemistry. Immunologic evaluation of cellular homogenates showed that ferritin is a significant constituent of non-HHCS lens accounting for about 0.01% of the total soluble proteins, whereas in HHCS lens, it accounted for about 0.15%. In controls, the L:H ferritin ratio was 3-6:1 and increased to 214:1 in HHCS samples. This indicates that in normal lens ferritin is L-rich and becomes even more so in HHCS. Biochemical analysis confirmed the immunologic data: ferritin could be purified from HHCS lens homogenates, but not from control lens and could be stained with Prussian blue after in vitro iron loading, demonstrating a functional iron-poor ferritin. It showed an electrophoretic mobility slightly faster than the control recombinant L-ferritin (Fig 5B and C), a difference fully compatible with previous observations that the exposed N-terminal amino groups are blocked by acetylation in the natural human ferritins and not in the recombinant ferritins from E coli.27 The extra positive charges reduce the electrophoretic mobility of the recombinant proteins in native PAGE. The presence of H-chain in the lens preparation was revealed by sensitive immunoblotting assays (not shown), but not in SDS-PAGE (Fig5A), a finding consistent with the low proportion (<3%) indicated by the immunoassays. It should be noted that on SDS-PAGE, the purified HHCS lens ferritin showed a single peptide corunning with the L-chain, while the glycosylated G-subunit (23 kD), typical of serum ferritin, was undetectable even with immunoblotting stains (not shown). These data support the hypothesis that ferritin in HHCS lens is of the intracellular type, made of L-chains with trace amounts of H-chains, like the one of the corresponding lymphoblastoid cells composed of L-homopolymers and L-rich heteropolymers. This ferritin could not be stained by Prussian blue, and attempts to evaluate Fe concentration by atomic absorption indicated an iron loading below 20 Fe atoms per molecule (in the liver, it is approximately 1,500 Fe atoms per molecule).
Ferritin in HHCS lens is readily soluble and does not form aggregates in vitro, suggesting that ferritin precipitation is not the primary cause of lens opacity. Clearly, the absence of aggregates in vivo can be ruled out only after in situ staining.
Our data do not provide direct indications on the relationship between cataract and L-ferritin overexpression, and we may speculate that this may alter the hydrodynamic equilibrium necessary to maintain lens transparency by affecting either the solubility of other proteins or the antioxidant defense of this tissue. It is known that the ocular lens is exposed to high levels of oxidative stress from oxidants surrounding the lens and from oxidants generated within the lens.31 The aqueous humor and the lens contain high levels of H2O2 and vitamin C, agents that can act as strong prooxidants in the presence of iron,31 and oxidative damage appears a primary cause of cataract formation.31 It was observed that the oxidative damage of lens structural proteins (crystallines) peptides induced by Fe and H2O2not only reduced their solubility, but also decreased the chaperone activity of α-crystalline.32,33 An antioxidant role of ferritin in the lens has been recently proposed by McGahan et al.34
Supported in part by Grant No. E.547 of Telethon-Italy (to D.G.) and by the CNR (Italy)-INSERM (France) collaboration programme.
Address reprint requests to Sonia Levi, PhD, Dibit, H. San Raffaele, Via Olgettina 58, 20132 Milano, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal