PART I
THE ENDOTHELIUM has long been viewed as an inert cellophane-like membrane that lines the circulatory system with its primary essential function being the maintenance of vessel wall permeability. Shortly after the first description of circulating blood by William Harvey in 1628, the existence of a network of vessels arose from studies of Malphigi, who described the physical separation between blood and tissue.1 In the 1800s, von Reckingausen established that vessels were not merely tunnels bored through tissues but were lined by cells. The strength of Starling's experiments and his law of capillary exchange proposed in 1896 served to solidify the belief that the endothelium was principally a selective but static physical barrier, not withstanding Heidenhahn's description in 1891 of the endothelium as an active secretory cell system. However, electron microscopic studies of the vessel wall by Palade in 1953 and physiological studies by Gowan in 1959 describing the interaction between lymphocytes and endothelium of postcapillary venules stimulated numerous subsequent studies that led to the current view of the endothelium as a dynamic, heterogeneous, disseminated organ that possesses vital secretory, synthetic, metabolic, and immunologic functions.1
The endothelial cell (EC) surface in an adult human is composed of approximately 1 to 6 × 1013 cells, weighs approximately 1 kg, and covers a surface area of approximately 1 to 7 m2.2 ECs line vessels in every organ system and regulate the flow of nutrient substances, diverse biologically active molecules, and the blood cells themselves. This gate-keeping role of endothelium is effected through the presence of membrane-bound receptors for numerous molecules including proteins (eg, growth factors, coagulant, and anticoagulant proteins), lipid transporting particle (eg, low-density lipoprotein [LDL]), metabolites (eg, nitrous oxide and serotonin), and hormones (eg, endothelin-1), as well as through specific junctional proteins and receptors that govern cell-cell and cell-matrix interactions.
The endothelium also plays a pivotal role in regulating blood flow. In part, this results from the capacity of quiescent ECs to generate an active antithrombotic surface that facilitates transit of plasma and cellular constituents throughout the vasculature. Perturbations, such as those that may occur at sites of inflammation or high hydrodynamic shear stress, disrupt these activities and induce ECs to create a prothrombotic and antifibrinolytic microenvironment. Blood flow is also regulated, in part, through secretion and uptake of vasoactive substances by the endothelium that act in a paracrine manner to constrict and dilate specific vascular beds in response to stimuli such as endotoxin.
Detailed study of endothelial function first became feasible with the development in the 1970s of techniques to culture ECs in vitro.3-5 Limitations of this approach have become apparent recently with the realization that cell culture perturbs ECs from their quiescent in vivo state (0.1% replications per day) to an activated phenotype (1% to 10% replications per day) with loss of specialized functions associated with diverse vessels and organ systems. More complex analytic systems now exist that incorporate changes in EC properties imparted by plasma and cellular blood elements, by rheologic factors, and by cell-cell interactions that occur within the vessel wall. Genetic recombination studies in mice are likely to advance understanding of ECs in both their physiologic and pathologic roles in thrombosis, atherosclerosis, tumor metastasis, and organ rejection.
The purpose of this review is to provide a broad overview of EC participation in several biological processes judged to be relevant to clinical hematologists and investigators of vascular biology. Part I will principally examine the known physiologic roles of the endothelium, whereas Part II will discuss the interactions between ECs and blood cells and emphasize the contribution of the EC to the pathogenesis of specific diseases. Because of the introductory nature of this review, many topics and important contributions have been omitted, including the involvement of ECs in hematopoiesis, neuroendocrinology, cell aging, cellular integrins and matrix interactions, vascular permeability, lipid metabolism, the lymphatic vasculature, and the endothelium as a target for gene therapy. We hope that the abbreviated bibliographies will serve as an introduction to readers who wish to further investigate EC biology.
VASCULOGENESIS AND ANGIOGENESIS
Overview of early vascular development.
Recently developed techniques that permit alteration of genomic sequences and manipulation of developing embryonic tissues have provided important insights into molecular and genetic elements that regulate vascular development.6 These studies show that the cardiovasculature is the first system to form in the gastrulating embryo. The de novo organization of ECs into vessels in the absence of any pre-existing vascular system is referred to as vasculogenesis and only occurs in the early embryo. Angiogenesis, the continued expansion of the vascular tree as a result of ECs sprouting from existing vessels, occurs in avascular regions of the embryo and is repeated many times in the mature animal, most commonly during wound healing and tumor metastasis7 (Fig 1). It remains uncertain how the pattern of the vascular tree is established or which factors govern the site of sprouting or the route taken by migrating ECs during angiogenic expansion.
The formation of new vessels during vasculogenesis and angiogenesis. Vasculogenesis, the de novo organization of ECs into vessels in the absence of preexisting vascular structures, takes place during embryogenesis in the blood islands of the yolk sac (pictured) and in the embryo through expression of growth factors, in particular fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF). The tyrosine receptor kinases, VEGFR-1 (flk-1) and VEGFR-2 (flt-1), are expressed on mesenchymal cells and newly formed ECs, respectively, and are essential for the generation and proliferation of new ECs and the formation of tubal EC structures. Angiogenesis, the continued expansion of the vascular tree, is mediated through the expression of additional tyrosine kinase receptors, tie-2 (tek), which binds to Ang1 and Ang2 (angiopoietins), resulting in the maintenance of mature vessels, the development of new vessels, and the regression of formed vessels in processes dependent on a combination of factors, most notably the presence or absence of growth factors.
The formation of new vessels during vasculogenesis and angiogenesis. Vasculogenesis, the de novo organization of ECs into vessels in the absence of preexisting vascular structures, takes place during embryogenesis in the blood islands of the yolk sac (pictured) and in the embryo through expression of growth factors, in particular fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF). The tyrosine receptor kinases, VEGFR-1 (flk-1) and VEGFR-2 (flt-1), are expressed on mesenchymal cells and newly formed ECs, respectively, and are essential for the generation and proliferation of new ECs and the formation of tubal EC structures. Angiogenesis, the continued expansion of the vascular tree, is mediated through the expression of additional tyrosine kinase receptors, tie-2 (tek), which binds to Ang1 and Ang2 (angiopoietins), resulting in the maintenance of mature vessels, the development of new vessels, and the regression of formed vessels in processes dependent on a combination of factors, most notably the presence or absence of growth factors.
Origin of the vascular endothelium.
Molecular events involved in EC differentiation from the early mesoderm remain uncertain. Vascular and hematopoietic tissues develop together, beginning shortly after implantation with the formation of blood islands within the primitive yolk sac8 (Fig 1) composed of two cell types: (1) angioblasts that form the outer layer of ECs encasing the blood island; and (2) hematopoietic stem cells, in the inner cluster, from which the first embryonic blood cells develop. Angioblasts committed to EC differentiation are found primarily in embryonic mesoderm,9 whereas the early epiblast also contains a subpopulation of hematopoietic stem cells.10Recently, angioblasts have been identified in the adult as well.11 The endoderm is the initial stimulus for angioblast formation.8,9 Within the embryo proper, the first angioblasts arise from the lateral mesodermal plate and cardiac crescent8,9; some cells migrate into the forming brain, whereas others assemble into the endocardium of the early heart tube. Other angioblasts form a plexus of ECs at the base of the primitive heart tube that assemble into the vitelline vessels, allowing blood cells from the yolk sac to circulate within the body of the embryo.8 The vasculature of the viscera is formed from ECs that differentiate directly from the surrounding mesenchyme incorporated into the angiogenic extensions of invading vessels.12 For example, as the airway of the developing lung expands, endodermally derived cells of the branching airway provide directional queues for advancing branchial arteries and induce formation of angioblasts that become part of the pulmonary vasculature.13 Vasculogenic activity of early organ rudiments was shown through engraftment experiments using pieces of early quail lung and chick embryos. These experiments lead to the hypothesis that endoderm, but not ectoderm, induces vasculogenesis but that both endoderm and ectoderm can support angiogenesis14; recent experiments suggest additional sources of endothelial growth factors may exist.15
Genetic programs regulating EC differentiation and early vascular development.
The best insight into molecular events required to initiate and maintain vascular development has come from detailed analyses of mouse embryos in which the genes for specific polypeptide growth factors or their transmembrane receptor tyrosine kinases (RTKs) have been inactivated. Such experiments show that initiation of vascular development requires both basic fibroblast growth factor (βFGF) and vascular EC growth factors (VEGF; see Beck and D'Amore16for a detailed discussion of growth factors and vascular development). Three alternatively spliced isoforms of VEGFs, members of the platelet-derived growth factor family (VEGF, VEGF-B, and VEGF-C17), interact with specific tyrosine kinase receptors. The growth factor-receptor interactions include VEGFR-1 (also known as flt-1 or fms-like tyrosine kinase-1) with VEGF and a related placenta growth factor (PlGF); VEGFR-2 (known alternatively as flk-1, fetal liver kinase-1; or Kdr, kinase-inserted domain containing receptor) with both VEGF and VEGF-C; and VEGFR-3 (originally designated flk-4) with VEGF-C. All VEGFs stimulate receptor autophosphorylation and EC replication and migration. The crucial role of this ligand in early vasculogenesis is demonstrated by the fact that loss of the VEGF gene results in embryonic death. Subsequent assembly of ECs into vessels requires activation of VEGFR-1 on the surface of the newly differentiated cells.18,19 The decision for a vessel to become a vein or an artery appears to be under the control of yet another growth factor, VEGFR-3,20 that is expressed later in development only on ECs that will become veins or lymphatic vessels.
Expansion of the vascular tree, continued endocardial and ventricular development, and formation of the vascular wall is controlled by two members of a second family of RTKs and their ligands,19tie-1 (tyrosine kinase with Ig and epidermal growth factor homology domains) and tie-2 or tek (tunica interna EC kinase). Two ligands, termed angiopoietin-1 and angiopoietin-2,21,22 are specific for tie-2 and are synthesized by cells surrounding the developing vessels. Ligand binding results in autophosphorylation of tyrosine residues in the intracellular domain of tie-2, but does not lead to EC replication or tube formation, as is the case for other endothelium-associated receptor-ligand interactions. Interestingly, angiopoietin-2 appears to function as an antagonist for angiopoietin-1, blocking its binding to tie-2. Targeted mutations of the genes for either tie-2 or angiopoietin-1 result in embryos with abnormal hearts and vessels with poorly formed walls.23 This has led to the suggestion that angiopoietin-1 acts via its receptor on ECs to stimulate the production of growth factors that, in turn, stimulate the differentiation of surrounding mesenchyme into pericytes or smooth muscle cells required for vessel wall formation.24 This is consistent with the phenotype of a Tie-2 mutation in humans that leads to smooth muscle deficiencies around small vessels and microaneurysms.25 These observations suggest that carefully regulated activity of tie-1 and 2 is required for continued vascular branching and vessel remodeling. Thus, the assembly of the early vascular tree depends on the programmed expression of at least two sets of RTKs and their ligands, one set for EC differentiation and initiation of vessel formation and the other for subsequent branching, establishment of capillary beds, and vessel wall formation.
Although genetic manipulations now possible in the mouse have provided important insights, there is much that we do not know about vascular development. Random genetic mutations introduced into the zebrafish have also generated many surprising and fascinating cardiovascular anomalies.6 26 For example, zebrafish can be induced to develop with hearts that do not contain an endocardium although the remainder of the vascular system appears functional. Thus, it is likely that a combination of genetics and developmental biology, unencumbered by previous assumptions, will continue to show new genes and suggest new paradigms that will advance our understanding of vascular development.
Extracellular matrix and matrix adhesion receptors in vascular development.
The ability of ECs to form capillary-like tubes is regulated not only by specific cytokine/receptor combinations, but also by the extracellular matrix. For example, human umbilical vein ECs (HUVECs) exposed to transforming growth factor-β (TGF-β) grow as a rapidly dividing monolayer if cultured on a flat surface coated with type I collagen.27 However, under similar TGF-β exposure, but within a type I collagen gel, ECs spontaneously organize into capillary-like tubules and continue to divide. Several kinds of molecules on the EC surface act together to mediate cell-extracellular matrix (ECM) interactions, including proteoglycans as well as proteins. The best studied family of receptors that mediate cell-matrix interactions is the integrins,28 which serve both a tethering and an information transfer function. Integrin-ligand binding triggers cytoskeletal organization at specific sites on the surface membrane to facilitate cell movement or maintain tissue stability. Binding also activates intercellular pathways that can result in either cell replication or programmed cell death.29
Cells express more than one integrin and the combination of integrins expressed during embryonic development is constantly changing, suggesting that specific combinations are required as development proceeds. Experimental results in the developing mouse embryo suggest that functional compensation by integrins can occur during embryogenesis. It is also possible that receptors required for angiogenesis in early development may differ from those required for collateral vessel formation or tumor angiogenesis or that both gene inactivation and the introduction of inhibitory agents have unknown secondary effects. Similarly, mouse embryos continue to develop normally when genes for certain ECM components have been inactivated, whereas inactivation of other genes, such as the abundant fibronectin, results in early embryonic lethality.30 Similarly, knocking out the gene for a fibronectin receptor subunit (α5β1) also results in a poorly developed heart and vascular system and early embryonic death.30Again, other integrins, most likely αvβ3, appear to compensate for the absence of α5β1 during preimplantation development and early gastrulation, but are unable to do so as development proceeds.
Endothelial cell-cell interactions and vessel formation.
Angioblasts and ECs must contact like cells for vessels to sprout and lengthen. Such cell-cell adhesion is mediated by a distinct series of cell surface receptors that includes platelet EC adhesion molecule (PECAM-1),31 a member of the Ig superfamily, and vascular endothelial (VE)-cadherin.8 ECs express two isoforms of PECAM that mediate cell adhesion that differ in their requirement for divalent cations and sulfated proteoglycans. VE-cadherin, also known as cadherin-5, found almost exclusively on ECs, promotes cell-cell adhesion by a calcium-dependent homotypic mechanism,32 ie, in the presence of calcium, VE cadherin from one cell binds to the VE-cadherin expressed on an adjacent cell. As the vessel matures, more classic junctional complexes, such as tight junctions and gap junctions, form depending on the function of the particular vascular bed. Thus, during vascular development, junction formation initially involves rather weak adhesion complexes, likely required for cell-cell recognition, that facilitate the assembly of additional junctional complexes. However, the factors that determine the organ-specific nature of junction formation remain unknown.
Proteinases and vascular development.
Vascular development may be regulated by some of the same factors that are involved in the control of blood clotting and capillary formation. EC movement through the ECM is tightly regulated and requires integrin mediated cell-matrix adhesion complex formation and subsequent disassembly. This involves repetitive cycles of reversible integrin/matrix binding, assembly, and disassembly of cytoskeletal elements as well as matrix degradation restricted to the advancing edge of the moving cell.33 This is accomplished through the organization of active molecular complexes that approximate integrins and integrin ligands with matrix metalloproteinases and plasminogen activators with their respective substrates and inhibitors at sites of cell-matrix interaction.34 It has long been hypothesized that, when ECs are exposed to angiogenic stimuli, plasminogen activation is initiated through the binding of plasminogen and urokinase to their receptors. This leads to the formation of plasmin that activates prometalloproteases, degrades noncollagenous components of the matrix, provides a path for cell migration, and releases peptides that can promote or inhibit continued angiogenesis.35 Two peptides, angiostatin and endostatin,36,37 are currently being evaluated for clinical use in reversing tumor angiogenesis.36 However, much of the dogma regarding the role of plasmin in angiogenesis may have to be revisited in light of recent data indicating that transgenic animals with targeted disruption in the genes for tissue-type plasminogen activator (t-PA), urokinase (u-PA), the urokinase receptor (u-PAR), and plasminogen appear to develop a normal vasculature in the absence of trauma or other stressors (see below).
Perspective.
The induction of embryonic angioblasts to differentiate into ECs, organize into a vascular network, and subsequently populate the specialized vascular bed of an organ results from a complex genetic program, the details of which are only now emerging. This program is not only sensitive to the composition and structure of the ECM but is influenced by cell-cell contact as well as angiogenic and angiostatic growth factors and peptides generated by vascular expansion itself. Many of these same events are recapitulated after injury or as part of an inflammatory response and, if allowed to proceed unchecked (eg, tumor angiogenesis and diabetic retinopathy), can have serious consequences for the organism. Insight into the molecular and genetic programs involved in vascular differentiation may suggest better approaches to minimize ischemic tissue damage, avoid tissue rejection, stimulate wound healing, and inhibit tumor growth. This section has focused on the development of the vascular endothelium; however, the formation of the basement membrane, the induction and differentiation of vascular smooth muscle cells, and the complex process of assembling the elastic lamina are all essential to vessel formation and are under separate regulation. Ultimately, prevention of devastating effects from congenital abnormalities and facilitation of normal vascular function will, in a large part, be influenced by our ability to manipulate molecular mechanisms involved in vascular development.
EC HETEROGENEITY
Many human vascular diseases are exquisitely restricted to specific types of vessels. For example, the contribution of platelets to the pathogenesis of arterial and venous thrombosis differs as does the susceptibility of these two types of vessels to atherosclerosis. It is also common for vasculitis to show marked predilection for specific arteries, veins, or capillaries or for certain organs. Tumor cells may show similar predilection to metastasize through particular vascular beds.38 Even when systemic risk factors are clearly evident, such as is the case with inherited disorders of lipoprotein metabolism or proteins that control coagulation, there is marked regional variation in disease expression. Furthermore, clinical events such as thromboses are generally episodic and often localized to single vessels. The basis for variation is poorly understood, but may lie, in part, in the heterogeneity of ECs themselves (see Augustin et al2 and McCarthy et al39 for reviews).
To date, appreciation of EC function has been largely based on the behavior of cultured umbilical vein ECs (HUVECs). Indeed, it is remarkable that so many concepts in vascular biology have been predicated on the repertoire of umbilical ECs studied under such potentially unphysiologic in vitro conditions; this is especially true considering their derivation from a type of vessel that rarely, if ever, is affected by the most common human vascular disorders. More recently, there has been greater appreciation that EC heterogeneity may contribute both to the maintenance of adaptive processes and to the development of disorders restricted to specific vascular beds.
EC heterogeneity among and within tissues.
Variation in the appearance of capillary endothelium from different vascular sites has long been recognized and appears well suited to postulated differences in function (Fig 2). For example, the brain and retina are lined by continuous ECs connected by tight junctions that help to maintain the blood-brain barrier; the liver, spleen, and bone marrow sinusoids are lined by discontinuous ECs that allow cellular trafficking between intercellular gaps; while the intestinal villi, endocrine glands, and kidneys are lined by fenestrated ECs that facilitate selective permeability required for efficient absorption, secretion, and filtering (see Dejana32 for review). ECs from diverse tissues are also heterogeneous with respect to their surface phenotype and protein expression. For example, von Willebrand factor (vWF), used commonly as a marker for ECs, is not expressed uniformly on cells from all types of vessels,40,41 the expression of tissue type plasminogen activator is limited in vivo to approximately 3% of vascular ECs,42 and the constitutive expression of u-PA is reportedly confined to renal ECs,43,44 which are also uniquely susceptible to injury by verotoxin.45Microvascular ECs also differ in their susceptibility to undergo apoptosis induced by plasma from patients with thrombotic thrombocytopenic purpura.46 The induction of tissue factor after infusion of cytokines or endotoxin is similarly restricted to specific vessels,47 among many other examples of heterogeneity at the level of protein expression.
EC heterogeneity. (A) Electron micrograph showing the junction between two capillary ECs in a guinea pig pancreas (micrographs reprinted with permission from R.F. Bolender, The Journal of Cell Biology, 1974, vol. 61, p. 269). (B) Electron micrograph demonstrating the diversity of ECs from two types of capillaries: (1) vesicular invaginations (arrow) on both luminal and abluminal plasma membrane of a muscle capillary EC; (2) fenestrated capillary from the lamina propria of the colon with thin diaphragms (arrow) covering the plasma membrane pores (micrographs reprinted with permission from E. Weihe, Textbook of Histology, (ed 12), 1994, p. 391, courtesy of Chapman and Hall).
EC heterogeneity. (A) Electron micrograph showing the junction between two capillary ECs in a guinea pig pancreas (micrographs reprinted with permission from R.F. Bolender, The Journal of Cell Biology, 1974, vol. 61, p. 269). (B) Electron micrograph demonstrating the diversity of ECs from two types of capillaries: (1) vesicular invaginations (arrow) on both luminal and abluminal plasma membrane of a muscle capillary EC; (2) fenestrated capillary from the lamina propria of the colon with thin diaphragms (arrow) covering the plasma membrane pores (micrographs reprinted with permission from E. Weihe, Textbook of Histology, (ed 12), 1994, p. 391, courtesy of Chapman and Hall).
One of the clearest examples of EC heterogeneity lies in the expression of homing receptors involved in cell trafficking. In the mouse, Lu-ECAM-1 (lung-specific EC adhesion molecule) is exclusively expressed by pulmonary postcapillary ECs and some splenic venules,48whereas Mad-CAM-1 (mucosal addressin cell adhesion molecule-1) is expressed primarily on high endothelial venules in Peyer's patches of the small intestine.49 Microvascular ECs derived from the bone marrow show an affinity for binding megakaryocytes and CD34+ progenitor cells and constitutively secrete hematopoietic stimulating factors such as Kit-ligand, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, and interleukin-6 (IL-6), which help control trafficking, proliferation, and hematopoietic lineage-specific differentiation.50 Tumor cells may show clear preferential adhesion to the endothelium of specific organs paralleling their in vivo metastatic propensities (see McCarthy et al39 for review).
Microvascular ECs cultured from the brain, liver, and other organs each express distinct patterns of cell surface markers, protein transporters, and intracellular enzymes.51 These tissue-specific phenotypic differences can be maintained for some time under identical tissue culture conditions (eg, Grau et al52). Distinct subsets of ECs often exist within a single organ. In situ studies of adult human liver show two distinct sinusoidal EC phenotypes: hepatic periportal vessels express PECAM-1 and CD34, whereas sinusoidal intrahepatic ECs do not.53During the development of the human liver, ECs progress from a phenotype closely resembling adult hematopoietic sinusoidal bone marrow ECs, which supports fetal intrahepatic hematapoiesis, to one resembling adult hepatic sinusoidal ECs, including the expression of the T-lymphocyte marker CD4.54
Environmental and genetic regulation of EC phenotype.
There is extensive evidence to indicate that heterogeneity develops in part as a result of variation in exposure of EC to environmental stimuli, some of which act only over short distances or even require cell-cell contact to effect change. Numerous exogenous factors affect EC phenotype, including mechanical forces, soluble growth promoters and inhibitors, cytokines, plasma lipids and proteins [eg, thrombin, plasmin, antibodies, Lp(a), etc], and contact with circulating and tissue-based cells (eg, smooth muscle cells and pericytes) and with the ECM, microbes, and their soluble products.
There are numerous examples of how the microenvironment can regulate the endothelial phenotype, a phenomenon that has been referred to as transdifferentiation.2 For example, aortic ECs cultured on extracellular matrix derived from the lung are induced to express Lu-ECAM-1,48 whereas the cells develop fenestrae when cultured on matrix derived from kidney-derived MDCK cells.55 Transplantation studies in the chick-quail system illustrate that ECs can take on the characteristics of the tissue into which they are transplanted in vivo,56 whereas other examples show that ECs acquire a different phenotype ex vivo.57 Studies in transgenic mice expressing the Lac Z reporter gene under control of 2,182 bp of the 5′ flanking sequence and the first exon and intron of the vWF gene suggest that expression is regulated by signals derived from the local microenvironment that influence pathways specific for particular vascular beds.58
EC heterogeneity can thus arise as a consequence of local concentrations of exogenous effectors or due to intrinsic variations in responsiveness (reviewed in McCarthy et al39). ECs grown on extracts of basement membrane from different organs have been observed to develop preferential adhesivity for tumor cells prone to metastasize to that organ.59 ECs derived from saphenous vein have been reported to synthesize less prostaglandin I2(PGI2) than those from the internal mammary artery, a finding that may contribute to the rapidity with which pathogenic changes may develop in venous bypass grafts placed under arterial pressure.60 Possible genetic bases for EC diversity have only recently been considered and have not been studied in depth. Microvascular and macrovascular ECs differ in the fastidiousness of their growth, propsensity to form capillary-like structures, synthesis of PGI2, and expression of adhesion receptors for lymphocytes, among other properties (see Ades et al61 for review). Tissue-specific transcription factors or signal transduction molecules responsible for activating and/or de-repressing transcription apparati in a tissue-specific manner are only beginning to be understood. Identification of these control factors will be important in the design of vectors that will enable expression of EC proteins in a tissue-specific manner.
The effects of cell culture.
Only in the past few years has the technology become available that permits in situ study of EC behavior. These studies indicate that the constitutive phenotype of ECs is unstable and their behavior can change rapidly once explanted. Commonly used culture conditions may activate or otherwise alter the endothelial phenoytpe (eg, Grant et al62). There is, as yet, no model for generating the resting EC in vitro. Thus, all the information described in subsequent sections should be considered in the context of the cell source as well as the ex vivo culture conditions, including passaging, the presence/omission of shear forces, and factors released into blood that alter the behavior of the endothelium from that which occurs in healthy blood vessels in vivo. Thus, much may be gained in the future by a more critical consideration of EC heterogeneity both in terms of understanding homeostasis and vascular pathology, as well as in targeting the delivery of gene therapy, antithrombotic agents, and antitumor agents to an anatomically or functionally distinct endothelial region.63
VASOREGULATION
The endothelium not only provides a structural barrier between the circulation and surrounding tissue, but ECs also secrete mediators that influence vascular hemodynamics in the physiologic state (Table 1). ECs contribute to the regulation of blood pressure and blood flow by releasing vasodilators such as nitric oxide (NO) and prostacyclin (PGI2), as well as vasoconstrictors, including endothelin (ET) and platelet-activating factor (PAF). These chemically diverse compounds are not stored in intracellular granules. Rather, their major biologic effects are regulated by localization of specific receptors on vascular cells, through their rapid metabolism, or at the level of gene transcription. NO is constitutively secreted by ECs, but its production is modulated by a number of exogenous chemical and physical stimuli, whereas the other known mediators (PGI2, ET, and PAF) are synthesized primarily in response to changes in the external environment.
Vasoregulatory Substances Synthesized by the Endothelium
| Substance . | Principal Effect . | Other Effects . | Secretion . | Compound . | Precursor Compound . |
|---|---|---|---|---|---|
| NO (nitric oxide) | Vasodilatation | Maintains basal tone of vessels; inhibits leukocyte adhesion; inhibits platelet adhesion, activation, secretion, and aggregation; promotes platelet disaggregation; inhibits smooth muscle cell migration and proliferation | Paracrine/Constitutive and induced by thrombin, ADP, bradykinin, substance P, muscarinic agonists, shear stress, cyclic strain, cytokines | Heterodiatomic free radical | L-arginine |
| PGI2 (prostacyclin) | Vasodilatation | Retards platelet aggregation and deposition | Paracrine/Induced at sites of vascular perturbation | Eicosanoid | Arachidonic acid |
| PAF (plateletactivating factor) | Vasoconstriction | Promotes leukocyte adhesion at cell surface | Juxtacrine/Induced | Phospholipid | Arachidonic acid |
| ET-1 (endothelin-1) | Vasoconstriction | Mitogen for smooth muscle cells; modulates effect of numerous compounds | Paracrine/Induced by hypoxia, shear stress, and ischemia | 21 Amino acid peptide | Preproendothelin-1 (203 amino acids) |
| Substance . | Principal Effect . | Other Effects . | Secretion . | Compound . | Precursor Compound . |
|---|---|---|---|---|---|
| NO (nitric oxide) | Vasodilatation | Maintains basal tone of vessels; inhibits leukocyte adhesion; inhibits platelet adhesion, activation, secretion, and aggregation; promotes platelet disaggregation; inhibits smooth muscle cell migration and proliferation | Paracrine/Constitutive and induced by thrombin, ADP, bradykinin, substance P, muscarinic agonists, shear stress, cyclic strain, cytokines | Heterodiatomic free radical | L-arginine |
| PGI2 (prostacyclin) | Vasodilatation | Retards platelet aggregation and deposition | Paracrine/Induced at sites of vascular perturbation | Eicosanoid | Arachidonic acid |
| PAF (plateletactivating factor) | Vasoconstriction | Promotes leukocyte adhesion at cell surface | Juxtacrine/Induced | Phospholipid | Arachidonic acid |
| ET-1 (endothelin-1) | Vasoconstriction | Mitogen for smooth muscle cells; modulates effect of numerous compounds | Paracrine/Induced by hypoxia, shear stress, and ischemia | 21 Amino acid peptide | Preproendothelin-1 (203 amino acids) |
Principal regulatory compounds synthesized by the endothelium, their effects on the vasculature and other processes, their mode of secretion, and the nature of their chemical composition and precursor compound.
NO.
ECs elaborate NO, a heterodiatomic free radical product generated through the oxidation of L-arginine to L-citrulline by NO synthases.64 One isoform, eNOS or the Nos3 gene product, is constitutively active in ECs but is stimulated further by receptor-dependent agonists that increase intracellular calcium and perturb plasma membrane phospholipid asymmetry.65Receptor-dependent agonists that stimulate eNOS include thrombin, adenosine 5'-diphosphate, bradykinin, substance P, and muscarinic agonists, in addition to shear stress66 and cyclic strain.67 The increase in eNOS activity evoked by shear stress contributes to the phenomenon of flow-mediated vasodilatation, an important autoregulatory mechanism by which blood flow increases in response to exercise.68 This is in part a result of shear-induced transcriptional activation due to the presence of a shear response consensus sequence, GAGACC, in the promoter of the Nos3 gene.69 In addition to eNOS, cytokines have been shown to stimulate bovine microvascular endothelium in culture to express an inducible isoform of NO synthase, iNOS, or the Nos2 gene product.70 EC-derived NO has several important effects on the vasculature. First, NO maintains basal tone by relaxing vascular smooth muscle cells71 through the binding of NO to the heme prosthetic group of guanylyl cyclase. Endothelial-derived NO also inhibits platelet adhesion, activation, secretion, and aggregation and promotes platelet disaggregation, in part through a cyclic GMP-dependent mechanism.72 PGI2, which does not affect platelet adhesion,73 acts synergistically with NO to inhibit other steps in the platelet activation cascade.74NO also inhibits expression of P-selectin on platelets and, by inhibiting the agonist-dependent increase in intraplatelet calcium,72 suppresses the calcium-sensitive conformational change in the heterodimeric integrin glycoprotein αIIbβ3 (GP IIb-IIIa) required for fibrinogen binding.75 Additionally, NO appears to promote platelet disaggregation indirectly by impairing the activity of phosphoinositide 3-kinase, which normally supports conformational changes in αIIbβIIIa, rendering its association with fibrinogen effectively irreversible.76
In addition to these effects on the vasculature, endothelial-derived NO inhibits leukocyte adhesion to the endothelium77,78 and inhibits smooth muscle cell migration79 and proliferation.80 These latter effects serve to limit neointimal proliferation that occurs after vascular injury and, combined with its stimulatory effect on EC migration and proliferation,81 suggest that NO helps to sustain vascular reparative mechanisms.
ECs also produce a less well-characterized compound known as endothelium-derived hyperpolarizing factor (EDHF) that promotes vascular smooth muscle relaxation (see Garland et al82 for review). Muscarinic agonists stimulate ECs to release EDHF, causing a transient hyperpolarization of the cell membrane. It has been proposed that EDHF exerts its vascular effects by activating ATP-sensitive potassium channels, smooth muscle sodium-potassium ATPase, or both,83 but its role in vascular (patho)physiology requires further study.
ET.
Remarkably, ECs produce not only the potent vasodilator NO, but also synthesize endothelin-1 (ET),84 the most potent vasoconstrictor identified to date. Endothelins comprise a family of 21-amino acid peptides produced by many cell types.84 ET-1 is not stored in granules85 but is formed after transcription of the gene encoding preproendothelin-1, the inactive precursor of ET-1, after stimulation by hypoxia, shear stress, and ischemia. ET-1 released from ECs binds to the abundant G-protein–coupled ET-A receptor expressed on vascular smooth muscle cells, which results in an increased intracellular calcium concentration and, in turn, increases vascular smooth muscle cell tone.86 Of interest, this effect of ET-1 persists after the hormone dissociates from its receptor through longer-lived effects on intracellular calcium. NO shortens the duration of these effects by accelerating the restoration of intracellular calcium to basal levels.87 The interplay between ET-1 and ET-A receptors likely contributes to basal vascular tone as well. ET-1 potentiates the vasoconstrictor actions of catecholamines, which, in turn, potentiate the actions of ET-1. In states of endothelial dysfunction, such as atherosclerosis, in which concentrations of bioactive NO are reduced, the relatively unopposed actions of ET-1 promote vasoconstriction and smooth muscle proliferation.88
Prostacyclin (PGI2) and PAF.
The contribution of ECs to the regulation of vasomotor tone is even more finely regulated as evidenced by the production of additional vasoactive compounds such as prostacyclin (PGI2) and PAF. Prostacyclin and PAF factor provide an interesting contrast. Both are intercellular signaling molecules synthesized by stimulated ECs in vitro and in vivo.89 Both are lipids: PGI2being an eicosanoid and PAF being a phospholipid.90,91 Neither is constitutively present in resting human ECs nor stored within the cell. The synthesis of each is induced rapidly by humoral and mechanical stimuli via discrete, regulated pathways.90,91 Once formed, PGI2 and PAF have relatively short half lives, one of several features that limits the magnitude of their signals and exerts control over their biologic activities.92 93
However, a major difference between the two factors lies in the range over which they exert their effects: PAF acts in a juxtacrine fashion, whereas PGI2 acts as a paracrine signaling molecule. PAF, expressed on the surface of the endothelium, remains cell-associated even in the presence of physiologic concentrations of albumin or other acceptor molecules91 and binds to and activates its receptor on leukocytes,94 fulfilling critical criteria of a juxtacrine signaling molecule. Consistent with this notion, PAF synthesized by cultured human ECs acts in concert with P-selectin (see below) to promote leukocyte adhesion.94
In contrast, PGI2 is rapidly released from ECs,95 although the export mechanism has not been precisely defined. Thus, PAF and PGI2 have spatially differentiated realms of signaling, even though both derive from a common precursor and are synthesized concurrently.91,96 This feature may contribute to differences in their actions at the endothelial interface with the blood: PAF is specialized to signal leukocytes at the cell surface, whereas PGI2 acts primarily in solution to retard platelet aggregation and deposition. Both PGI2 and PAF also elicit autocrine effects on ECs,91 92 which may be important in modulating angiogenesis and controlling the synthesis of EC-derived mediators.
PGI2 was the first endothelial-derived vascular smooth muscle relaxing factor to be identified. PGI2, which was generated locally, and PGI2 or its analogs, which were infused systemically, caused vasodilatation and altered regional blood flow.93 A receptor for PGI2, the IP receptor, is present on vascular smooth muscle as well as on platelets,97 consistent with early experimental observations, indicating that PGI2 acts principally to modulate the function of these two cell types.98 Although IP receptors are present in the arterial vascular wall, PGI2 is not constitutively produced and does not appear to regulate basal systemic vascular tone.99 Rather, PGI2 synthesis is induced at sites of vascular perturbation, where it may regulate vasoconstriction and platelet deposition.66 Because of its effects on blood flow and relevant cell-cell interactions, PGI2 may influence local inflammatory responses as well. An important recent advance has been the identification of prostaglandin H synthase-II (PHS-II), an inducible form of a key enzyme in PGI2 formation providing a mechanism by which the production of PGI2 and other eicosanoids can be sustained in chronic states of inflammation and vascular injury.
The receptor for PAF, the first receptor characterized at a molecular level that recognizes a biologically-active lipid, is a member of the serpentine G-protein–linked family (reviewed in Whatley et al91). Intravascular infusion of PAF causes either vasodilatation or vasoconstriction, depending on the concentration administered, the time, and the specific vascular bed studied.93 Some hemodynamic effects of PAF in vivo are indirect and depend on the generation of eicosanoids or leukotrienes or mediators derived from activated leukocytes or platelets and on cardiac effects.91-93 In shock and other in vivo pathologic states, PAF acts concomitantly or sequentially with other classes of mediators, including leukotrienes and tumor necrosis factor-α (TNF-α).90,91,100 As with PGI2, it is unlikely that PAF is a circulating regulator of blood pressure under basal conditions, despite early studies suggesting that PAF-like activity is released from kidneys.92
THE ROLE OF THE ENDOTHELIUM IN COAGULATION
A crucial physiologic function of the endothelium is to facilitate blood flow by providing an antithrombotic surface that inhibits platelet adhesion and clotting. However, when the endothelium is perturbed by physical forces or by specific chemical factors, the cells undergo programmatic biochemical changes that culminate in their transformation to a prothrombotic surface. A dynamic equilibrium exists between these two states, modulated both at the level of gene transcription and at the level of the intact cell, that often permits the injured endothelium to return to its unperturbed state once the procoagulant stimulus has dissipated (Table2; see Bombeli et al101 for review). Although the fibrin clots formed as a consequence of procoagulant activity may serve a protective organ function by limiting vascular damage induced by trauma, infection, and inflammation, the loss of anticoagulant activity may predispose to several common thrombotic disorders discussed in the sections that follow.
Regulation of Hemostasis and Thrombosis by the Endothelium
| . | Antithrombotic . | Prothrombotic . |
|---|---|---|
| Coagulation protein binding sites | Glycosaminoglycans/ATIII | Binding sites for: fibrin, FIX, IXa, X, Xa, FXII, kallikrein |
| TFPI | Tissue factor | |
| Thrombomodulin | Thrombin receptor | |
| Receptor for protein C/APC | ||
| Products produced and/or stored by platelets | PGI2 NO ADPase | vWF PAF Fibrinogen FV FXI |
| Fibrinolytic factors | t-PA production u-PA expression | PAI-1, PAI-2 PAI-3 (protein C Inhibitor) |
| u-PAR | TAFI activation | |
| Plasminogen binding sites | ||
| Annexin II | ||
| Vasomotor factors | NO PGI2 | TxA2 Endothelin-1 |
| . | Antithrombotic . | Prothrombotic . |
|---|---|---|
| Coagulation protein binding sites | Glycosaminoglycans/ATIII | Binding sites for: fibrin, FIX, IXa, X, Xa, FXII, kallikrein |
| TFPI | Tissue factor | |
| Thrombomodulin | Thrombin receptor | |
| Receptor for protein C/APC | ||
| Products produced and/or stored by platelets | PGI2 NO ADPase | vWF PAF Fibrinogen FV FXI |
| Fibrinolytic factors | t-PA production u-PA expression | PAI-1, PAI-2 PAI-3 (protein C Inhibitor) |
| u-PAR | TAFI activation | |
| Plasminogen binding sites | ||
| Annexin II | ||
| Vasomotor factors | NO PGI2 | TxA2 Endothelin-1 |
Outline of the antithrombotic and prothrombotic properties of ECs detailing the binding sites expressed by ECs, the factors stored and/or secreted by ECs that affect platelet function, factors produced by the EC that influence the fibrinolytic state of the vasculature, and vasomotor substances secreted by the EC.
Abbreviations: ATIII, antithrombin III; PGI2, prostacyclin; TFPI, tissue factor pathway inhibitor; APC, activated protein C; PAF, platelet-activating factor; t-PA, tissue plasminogen activator; u-PA, urokinase plasminogen activator; u-PAR, urokinase plasminogen activator receptor; PAI, plasminogen activator inhibitor; TAFI, thrombin activatable fibrinolysis inhibitor; TxA2, thromboxane A2.
Anticoagulant mechanisms.
Control of thrombin generation is a pivotal step in the balance between the natural antithrombotic and the induced procoagulant activities of the endothelium. Thrombin, a serine protease, serves diverse functions in coagulation, including the activation of platelets, several coagulation enzymes, and cofactors. Thrombin also stimulates procoagulant pathways on the ECs themselves. Therefore, it is not surprising that several highly regulated pathways have evolved to constrain the generation and activity of thrombin (see Rosenberg and Rosenberg102 for review), such that little enzyme activity is found in the plasma of healthy individuals.103 The matrix surrounding the endothelium contains heparan sulfate and related glycosaminoglycans (GAGs) that promote the activity of cell/matrix associated antithrombin III (AT-III)104; the subendothelium contains dermatan sulfate, which promotes the antithrombin activity of heparin cofactor II.105 ECs also prevent thrombin formation through the expression of tissue factor pathway inhibitor (TFPI), which binds to factor Xa within the tissue-factor/VIIa/Xa complex (see Broze106 for review). TFPI is released from its EC stores by heparin. TFPI and AT-III both contribute to physiologic hemostasis and can be depleted in acquired thrombotic states.107 108
The endothelium also helps to contain thrombin activity through the expression of thrombomodulin (see Esmon and Fukudome109 for review). Binding of thrombin to TM facilitates the enzyme's ability to activate the anticoagulant protein C. In turn, the activity of activated protein C (APC) is enhanced by its cofactor protein S, which is synthesized by EC, among other cell types.110 ECs also express receptors for APC111 that regulate the activity of this pathway. APC, in turn, promotes the inactivation of activated factors V and VIII. Binding of thrombin to TM also dampens the enzyme's ability to activate platelets, factor V, factor XIII, and fibrinogen and promotes EC fibrinolytic activity (see below). TM also inhibits prothrombinase activity indirectly by binding factor Xa.112 Thrombin bound to TM is rapidly endocytosed and degraded.113 Various inflammatory cytokines downregulate TM gene transcription and accelerate TM internalization114,115while at the same time promote tissue factor expression (see below). Soluble TM is also shed into plasma and elevated plasma levels have been identified in various disorders associated with EC injury (see Cucurull and Gharavi116 and below).
Procoagulant mechanisms.
The pivotal step in transforming the EC membrane from an anticoagulant to a procoagulant surface is the induction of tissue factor (TF). TF dramatically accelerates factor VIIa-dependent activation of factors X and IX, so it is not surprising that TF is not expressed by unperturbed endothelium, at least in the adult organism.117Interruption of the gene for TF is associated with impaired vascular development and lethal embryonic bleeding,118-120 but the source and function of TF during development have not been elucidated. Synthesis of TF is induced in vitro by diverse agonists, including thrombin, endotoxin, several cytokines, shear, hypoxia, oxidized lipoproteins, and many other provocations (see Rapaport and Rao121 and Nemerson122 for reviews). Procoagulant activity is accelerated by exposure of anionic phospholipids that may occur as a consequence of apoptosis.108 TF is localized primarily beneath and between cultured ECs,123 although some evidence for expression on the cell surface has been presented.124 TF mRNA and protein levels decline despite continued exposure to agonists, a mechanism that may help contain the extent of fibrin formation. Cells in culture also shed microvesicles containing TF,125 and plasma levels of TF are elevated in patients with disseminated intravascular coagulation,126 although the cellular source has not been established. TF expression is rapidly induced after vascular injury,127 and TF is found associated with ECs within atherosclerotic plaque128,129 and in tumor-derived vessels.130 TF may also contribute to the regulation of angiogenesis and tumor metastases through mechanisms independent of coagulation.131,132 Yet, it has been difficult to demonstrate expression of TF by ECs in vivo even in response to potent provocations where expression was expected, another example of the dissociation between the behavior of these cells in culture and that seen in the whole organism.47
Once ECs expressing TF are exposed to plasma, prothrombinase activity is generated and fibrin is formed on the surface of the cells.133 This implies that ECs express binding sites for factors IX, IXa, X, and Xa; thrombin; and fibrin.134 Yet, the identity and location of most of these binding sites is unknown, as is their role in either physiologic hemostasis or in thrombosis. Factor IX has recently been shown to bind type IV collagen in the EC matrix,135 although its cellular association site promoting assembly of the intrinsic FX activation complex has not been identified. Several candidate FX/Xa binding sites have been reported,136,137 whereas others may be induced as a result of exogenous stimuli.138 ECs also express receptors for proteins of the contact factor pathway,139 but their role in hemostasis is uncertain.
The most thoroughly characterized EC binding site for a coagulation protein is the thrombin receptor, also termed the protease-activated receptor-1 (PAR1). The thrombin receptor is a high-affinity G-protein–coupled protein140 that is activated when a fragment derived from the amino terminus of the protein, formed as a result of cleavage by thrombin, binds to the remaining cell-associated receptor fragment. Binding of thrombin leads to a wide array of changes in expression of prothrombotic and antithrombotic molecules by cultured ECs, including TF, PAI-1, NO, PAF, ET, and PGI2, among others (see Kanthou and Benzakour141 for review) and disruption of cell-cell contacts (see Garcia et al142 for review). Thrombin is also mitogenic for ECs, fibroblasts, and smooth muscle cells and is chemotactic for monocytes.
The seemingly normal phenotype of surviving adult mice with targeted disruptions in the thrombin receptor gene143 was unanticipated and raised questions about the physiologic role of this protein as well as other proteinase activated receptors expressed on ECs.144 The subsequent discovery of two additional protease-activated G-protein–coupled receptors, PAR-2 and PAR-3, helped to explain this observation.145 Both PAR-1 and PAR-2 are present on some human ECs, whereas PAR-1, but not PAR-2, is expressed on human platelets. PAR-3 is expressed by human bone marrow and mouse megakaryocytes, but its expression on ECs has not been established. Notable tissue- and species-specific differences in expression and cellular distribution of PARs have been described,145 146 making it difficult at present to relate the phenotype of the various murine knockouts to human physiology.
ECs also express several receptors for fibrin and specific fibrin degradation products,147 including a 130-kD glycoprotein,148 a tissue transglutaminase,149and the αvβ3 integrin, although evidence regarding their expression in vivo is only now emerging.150,151 Binding of fibrin promotes EC adhesion, spreading, proliferation, and migration; cell retraction; leukocyte adhesion; and inhibition of PGI2 synthesis. Cultured ECs also express glycoprotein Ib, which binds vWF secreted constitutively by ECs and, presumably, the ultralarge vWF multimers released from Weibel-Palade bodies in response to a number of agonists (see Wagner and Bonfanti152 for review). Expression of GPIbα by ECs is enhanced by TNF-α,153 but whether this glycoprotein participates in the physiologic or pathophysiologic binding of vWF in vivo requires additional study. The αvβ3integrin also binds vWF in the EC substratum. In vitro, conditions that decrease αvβ3 expression (TNF-α plus interferon-γ [IFN-γ] or arterial shear stress) increase GPIbα expression,154 suggesting that the EC state may affect the availability of adhesion receptors, although these finding require confirmation in vivo.
Undoubtedly, additional receptors for coagulation proteins with distinct functions will be characterized in the future. Genetic and acquired alterations in the structure, expression, and function of these EC receptors may contribute to hitherto unexplained hemorrhagic and thrombotic disorders. However, despite rather extensive study of EC procoagulant function in culture, the extent to which platelet adherence or fibrin formation actually occurs on the surface of the intact endothelium in vivo (as opposed to subendothelial matrix exposed to blood) remains unclear. Perhaps the best indirect evidence comes from animal models of Escherichia coli sepsis and cytokine infusion in which TF- and contact factor-dependent intravascular coagulation and multiorgan ischemic injury occurs in the absence of overt EC disruption.155 Nevertheless, the contribution of endothelium, platelets, monocytes, and other cell types in these models will require further study.
ECs AND FIBRINOLYSIS
Experiments using cultured ECs have yielded a concept that the endothelial surface is profibrinolytic and thus helps maintain blood in its fluid state.156 However, experiments using animal models have shown this conceptually satisfying hypothesis does not accurately reflect the situation in vivo.42 157 It has also become clear that the contribution of ECs to fibrinolysis varies with their metabolic status (ie, quiescent or activated), their vascular derivation, and the concentration of other hemostatically active molecules in the local plasma milieu.
Plasminogen activators.
Studies with ECs cultured from various tissues have led to the widely held inference that t-PA production and secretion is a property of all ECs.156 However, a few studies have gone largely unnoticed in which PA activity, demonstrated by fibrin zymography, was observed only in association with the adventitia and not with lumenal ECs.157 More recent studies using in situ hybridization and immunohistochemistry have demonstrated t-PA antigen and mRNA only in a distinct subset of quiescent microvascular ECs of both primates and mice.42,158 Hence, contrary to assumptions based on work with cultured ECs, t-PA is associated only with a distinct subpopulation of the microvasculature, even after provocation. In both murine brain and lung, the percentage of microvascular ECs producing t-PA increases markedly upon exposure to pertinent stimuli; however, in both cases, t-PA production remains an exclusive property of microvascular ECs.42 Hence, invoking local EC production of t-PA in large vessels as a mechanism of maintaining blood flow may not adequately describe the in vivo situation.
t-PA production by cultured ECs is regulated by a variety of external stimuli at the level of gene transcription and cellular release.159 Measurements of plasma t-PA levels suggest such regulated production/secretion occurs in vivo as well.160Intracellular signaling pathways operative in stimulated t-PA release have been described in vitro.161 The mechanisms that control EC t-PA production in vivo are less well understood, but clearly such regulation occurs.162 Humans exhibit higher plasma levels of t-PA after exercise or venous compression, but the cellular source of this increase has not been established.161
The other mammalian PA, u-PA, appears not to be produced by most quiescent ECs.43 Rather, it is expressed by ECs involved in wound repair or angiogenesis,163 consistent with the hypothesized importance of u-PA in cell migration and tissue remodeling. Yet, u-PA is obviously important to vascular homeostasis, because mice genetically deficient in u-PA develop inflammation induced thrombi164 and manifest thrombotic tissue injury in response to lipopolysaccharide (LPS).165However, the extrarenal source of u-PA in physiologic states has not been established.
PA receptors.
The presence of EC receptors for t-PA has been reported by several groups.166-171 Binding of t-PA to ECs has been reported to promote its fibrinolytic activity166,172 and to stimulate cell proliferation.173 Recently, one such t-PA binding site has been identified as annexin II, which is expressed on ECs174 and binds t-PA in a specific and saturable manner in vitro.172 However, the expression of annexin II on the endothelium in vivo has not yet been demonstrated.
The u-PA receptor (u-PAR) expressed by ECs appears identical to that expressed on other cell types.175,176 u-PAR is a three-domain protein linked to cell surfaces by a glycerophosphatidyl inositol anchor. Single-chain u-PA (the form found in plasma177) bound to cells via u-PAR exhibits increased plasminogen activating efficiency175,178 and is relatively protected from inhibition by PAI-1 and PAI-2.179,180 u-PAR may be expressed primarily on the surface of migrating ECs participating in angiogenesis, rather than on quiescent ECs lining normal vessels.181 Mice genetically lacking u-PAR develop normally and do not exhibit spontaneous vascular occlusion.182 Hence, u-PAR has yet to be shown to participate in maintaining physiologic blood fluidity, although it may be important in vascular repair.
Cells express diverse binding sites for plasminogen, among which are proteins that exhibit a carboxyterminal lysine (see Plow et al35 for review). Plasminogen binds to ECs in vitro with an affinity that would predict receptor occupancy at physiologic plasma concentrations.183 Cell-associated plasmin may be relatively protected from inhibition by α2-plasmin inhibitor.184 However, the exact identity of these EC plasminogen binding sites remains uncertain and their expression in vivo has not been established.185 Lp(a) competes for the binding of plasminogen to ECs,186 which may contribute to the prothrombotic effects of this lipoprotein.187
Plasminogen activator inhibitors (PAIs).
ECs in culture produce abundant PAI-1 that is associated primarily with its extracellular matrix, resulting in stabilization of its activity.188 PAI-1 synthesis is stimulated by numerous agents, including thrombin, endotoxin, various cytokines, Lp(a), and oxidized LDL, among others.189 Yet, experiments in mice have shown that liver is the major source of plasma PAI-1 and that quiescent EC express little or no inhibitor.190 However, after exposure to inflammatory stimuli, ECs in virtually every tissue express PAI-1.190 PAI-2 is found normally in plasma only during pregnancy191 and is not synthesized by ECs to an appreciable extent. However, multiply passaged ECs express PAI-2 in response to some agonists that may point to a local effect in select settings.192 PAI-3 (also known as the protein C inhibitor) has a much lower affinity for u-PA and t-PA than does PAI-1, but it is present in plasma at much higher concentrations.193Production of PAI-3 by ECs has not been reported, but PAI-3 antigen can bind to heparan sulfate proteoglycan on the lumenal surface of ECs, thereby increasing its activity.194
Thrombomodulin.
Binding of thrombin to thrombomodulin (see “The Role of the Endothelium in Coagulation” above) accelerates its capacity to activate a protein known as thrombin-activatable fibrinolysis inhibitor (TAFI).195 TAFI is a procarboxypeptidase-B–like molecule that, when activated, cleaves basic carboxyterminal residues within fibrin and other proteins. This results in the loss of plasminogen/plasmin and t-PA binding sites on fibrin such that fibrinolysis is retarded.195 Thus, through the regulated expression of thrombomodulin, ECs serve as potent templates to decrease the rate of intravascular fibrinolysis.
Although a simple balance between profibrinolytic (PAs) and antifibrinolytic (PAIs) pathways seemed an attractive mechanism to explain the clinical experience that unperturbed endothelium helps maintain blood fluidity, more recent in vivo data have shown that the mechanism may not be quite so straightforward. Indeed, ECs seem to express more antifibrinolytic than profibrinolytic activity in many settings studied to date. Clearly, more work will be required to clarify the contribution of quiescent and activated ECs to fibrinolysis.
Summary.
The first part of this two part series has focused on the development of the vasculature and the physiological functions of the endothelium as a gate-keeper regulating blood flow and hemostasis. Current insights into the generally unappreciated heterogeneity of endothelium from different vascular sites have been noted as potential discrepancies between the quiescent state of the endothelium in vivo and the behavior of these cells in culture. The second part of this review will concentrate on the mechanism by which the endothelium contributes to cell trafficking and the impact of endothelial injury on the development of several common human vascular disorders.
PART II
The endothelium, positioned at the interface between blood and tissue, is equipped to respond quickly to local changes in biological needs caused by trauma or inflammation. In the first part of this review, the capacity of the endothelium to move rapidly between an antithrombotic and prothrombotic state was discussed. In this second part, the mechanisms by which the endothelium regulates the trafficking of the cellular elements of the blood will be considered first, after which the impact of EC dysfunction on the pathogenesis of several common vascular disorders will be reviewed.
INTERACTION BETWEEN ECs AND BLOOD CELLS
In addition to the above-mentioned contribution of the endothelium to regulating blood coagulation, ECs also express cell surface-molecules that orchestrate the trafficking of circulating blood cells. These cell-associated molecules help direct the migration of leukocytes into specific organs under physiologic conditions and accelerate migration towards sites of inflammation, eg, in response to IL-6196or IL-8,197 among many others. Recently, these pathways have also been implicated in the adhesion of platelets and erythrocytes in several common disorders associated with vascular occlusion.
Interactions of platelets and leukocytes with the vessel wall.
Flowing leukocytes and platelets may adhere to specific regions of the endothelium, to exposed subendothelial components, or to each other during the process of immune surveillance as well as in response to tissue injury or infection. These multicellular interactions are essential precursors of physiologic inflammation and hemostasis. Conversely, uncontrolled adhesion of leukocytes and platelets contributes to inflammatory and thrombotic disorders. Under shear forces, both platelets and leukocytes interact with vessel surfaces through a multistep process that includes (1) initial formation of usually reversible attachments; (2) activation of the attached cells; (3) development of stronger, shear-resistant adhesion; and (4) spreading, emigration, and other sequelae (Fig 3).
Physiologic interaction of leukocytes with the endothelium. Leukocyte adhesion and transmigration occurs during inflammation, usually at the postcapillary venules where shear stress is lowest.
Physiologic interaction of leukocytes with the endothelium. Leukocyte adhesion and transmigration occurs during inflammation, usually at the postcapillary venules where shear stress is lowest.
Platelet adhesion during hemostasis.
Circulating platelets normally do not interact with the EC surface (see Schafer198 for review), in part due to the release of PGI2, the release of NO, and the recently described expression of an Ecto-ADPase (CD39).199 However, during hemorrhage, platelets adhere avidly to exposed subendothelial components, where they are rapidly activated. Circulating platelets interact with the adherent platelets, producing a hemostatic plug that promotes thrombin generation and development of a stable fibrin clot. Platelets adhere particularly efficiently to the subendothelium under high shear stress, accounting for the greater number of platelets in fibrin clots within arteries compared with those within veins.200
Under the influence of arterial shear stress, unactivated platelets attach first to the subendothelium through interactions of the platelet glycoprotein (GP) Ib-IX-V complex with immobilized vWF, a large, multimeric protein with binding sites for several other molecules, including subendothelial collagen.201 The GPIb-IX-V complex consists of four proteins, each with one or more leucine-rich repeats: the disulfide-linked GPIbα and GPIbβ and the noncovalently associated GPIX and GPV. The binding site for vWF is located on GPIbα, between the amino-terminal leucine-rich repeats and the membrane-proximal O-glycan–rich domain. The region includes clustered tyrosines that must be sulfated for GPIbα to bind vWF.202GPIbα binds weakly to plasma vWF, but with high activity to immobilized vWF under conditions of high shear stress, which may favor binding by altering the conformation of GPIbα and/or vWF.200,203 Flowing platelets attach transiently to vWF, resulting in continuous movement of the cells along the surface.204 Such cellular interactions require very fast molecular rates for attachment and detachment; the fast dissociation rates are not significantly accelerated by shear stresses for detachment. Under the lower shear stresses found in veins, unactivated platelets use the integrin αIIbβ3 to attach to and immediately arrest on immobilized fibrinogen.204Under low shear conditions, platelets may also use integrins or other molecules to attach to subendothelial matrix components, such as fibronectin, laminin, and thrombospondin.205
Once platelets adhere to either vWF or fibrinogen, they are activated by secreted products such as ADP or epinephrine or by surface molecules, such as collagen, that cross-link the integrin α2β1 and other platelet receptors. The activated platelets spread and adhere more avidly to the subendothelial surface, principally through binding of activated αIIbβ3 to fibrinogen, which recruits additional platelets into aggregates206; platelet αIIbβ3 also binds to a distinct site on vWF.201 Shear-resistant adhesion may be further enhanced by interactions of other integrins or receptors with laminin, fibronectin, and thrombospondin.200 As thrombin is generated, converting bound fibrinogen to fibrin, the aggregated platelets contract to strengthen the clot. Signaling through adhesion receptors, particularly integrins, may regulate the cytoskeletal-protein redistributions required for clot retraction.206 Increased bleeding is observed in patients with inherited defects in molecules that mediate platelet adhesion such as Bernard-Soulier disease (absence/dysfunction of GPIb-IX-V) and Glanzmann's thrombasthenia (absence/dysfunction of αIIbβ3), confirming their physiological function.203
Leukocyte adhesion during inflammation.
During inflammation, leukocytes tether to and roll on the EC surface. The cells then arrest, spread, and finally emigrate between ECs to reach the underlying tissues. Unlike platelets, which typically attach to the subendothelium of arteries under high shear stresses, leukocytes usually attach to the ECs, where shear stresses are lowest, in the lining postcapillary venules.
In most circumstances, interactions with selectins, transmembrane glycoproteins that recognize cell-surface carbohydrate ligands found on leukocytes, initiate and mediate tethering and rolling of leukocytes on the EC surface.207 Selectins constitute a family of three known molecules, each of which has an amino-terminal Ca2+-dependent lectin domain, an EGF domain, a series of short consensus repeats, a transmembrane domain, and a cytoplasmic tail. L-selectin is expressed on most leukocytes and binds to ligands constitutively expressed on high endothelial venules of lymphoid tissues, to ligands induced on endothelium at sites of inflammation, and to ligands exposed on other leukocytes. E-selectin is expressed on activated ECs and leukocytes. P-selectin is rapidly redistributed from secretory granules to the surface of platelets and ECs stimulated with thrombin or other secretagogues. Like E-selectin, P-selectin binds to ligands on leukocytes. Leukocytes adherent to the endothelium can make contact with flowing leukocytes through the L-selectin molecule, resulting in amplification of leukocyte recruitment to sites of inflammation.208 At sites of hemorrhage, leukocytes tether to and roll on adherent platelets.209 Monocytes recruited in this manner may augment fibrin generation, perhaps by elaborating tissue factor after their activation.210 Selectin ligands expressed on high endothelial venules also mediate rolling of activated platelets and enhance accumulation of lymphocytes in lymph nodes.211 Thus, selectins initiate inflammatory, immune, and hemostatic responses by promoting transient multicellular interactions under conditions of shear stress.
The selectins bind weakly to sialylated and fucosylated oligosaccharides, such as sialyl Lewis x, a terminal component of glycans attached to many proteins and lipids on most leukocytes and some ECs. Strikingly, the selectins bind with higher affinity to only a few sialylated and fucosylated glycoproteins on target cells.207 E-selectin binds preferentially to ESL-1, a protein with at most five N-glycans and no described O- glycans. L-selectin and P-selectin bind preferentially to sialomucins whose recognition requires sulfation as well as sialylation and fucosylation. The sulfate esters are attached to O-glycans on GlyCAM-1, a ligand for L-selectin secreted by high endothelial venules.212 In contrast, the sulfate esters are attached to tyrosines near the amino terminus of PSGL-1, a ligand for selectins on leukocytes.213 Construction of some glycans may be restricted to specific sites on the polypeptide backbone of only a few proteins.214 Of the described glycoprotein ligands for selectins, only PSGL-1, a ligand for selectins on leukocytes, has been shown to mediate cell-cell interactions under shear conditions (reviewed in McEver and Cummings215). The α4β1 and α4β7integrins, which are expressed on mononuclear cells and eosinophils, but not on neutrophils, also mediate tethering and rolling and occasionally arrest the flow of leukocytes on ECs by binding to the Ig-ligands VCAM-1 and MAdCAM-1.216,217 Some lymphocytes use CD44 to roll on hyaluronate-bearing surfaces.218
Under hydrodynamic flow, cell tethering and rolling requires bonds with sufficient mechanical strength between adhesion molecules and rapid rates of association and dissociation.219 Interestingly, attachment of leukocytes through selectins requires a threshold hydrodynamic shear force that may prevent leukocyte aggregation in regions of low flow.220 A higher shear threshold for L-selectin may reflect faster dissociation rates of L-selectin ligand bonds220 and/or adhesion-induced shedding of L-selectin from the cell surface.221 Because L-selectin, PSGL-1, and α4 integrins are concentrated on the tips of the leukocyte microvilli, the probability of rapid contact with PSGL-1 is increased and the repulsion is minimized between the charged glycocalyces of apposing cells.222
The slow velocities of rolling leukocytes favor encounters with chemokines or lipid autacoids presented at or near the apical surface of the endothelium. These mediators transduce signals that cooperate with those produced by engagement of L-selectin or PSGL-1 to activate the leukocytes.223 This crucial activation event, coupled with the slow rolling velocities, enables the β2integrins on leukocytes to bind to Ig ligands such as ICAM-1 and ICAM-2 on the EC surfaces.224 Plasma fibrinogen also links leukocytes to the endothelium by binding simultaneously to αMβ2 and ICAM-1,225 two integrins on the vessel wall that provide shear-resistant attachments. Subsequently, leukocytes migrate between ECs into tissues by mechanisms that are not completely understood but are affected by gradients of chemokines with restricted specificities,49β1 and β2 integrins activation states, and homotypic interactions with the Ig-like receptor, PECAM-1.226 This may require disruption of homotypic interaction of cadherins at endothelial tight junctions.227
Leukocyte recruitment to lymphoid tissues or inflammatory sites requires the coordinated expression of specific combinations of adhesion and signaling molecules. Diversity at each step of the multistep cascade ensures that the appropriate leukocytes accumulate for a restricted period in response to a specific challenge.49,224 Absence of P-selectin delays fatty streak formation in mice predisposed to developing atherosclerotic lesions.228 On the other hand, increased numbers of infections are observed in patients who are congenitally deficient in β2 integrins229 or in fucosylated ligands for selectins,230 confirming the physiologic significance of these molecules in immune and inflammatory responses. Increased susceptibility to infection combined with impaired leukocyte accumulation in mice rendered genetically deficient in selectins,231-234 in fucosyltransferases,235 in ICAM-1,236-239 or in α4integrins240 further support the overlapping functions of these molecules.
Endothelium in cell-mediated immunity (CMI).
CMI is defined as the protective set of immune reactions that can be adoptively transferred from a sensitized individual to an unimmunized host by a subset of T lymphocytes but not by antibodies. Vascular ECs may play two important roles in the evolution of CMI reactions: (1) antigen presentation to T cells (reviewed in Pober et al241) and (2) recruitment of inflammatory cells.49 Recall responses such as CMI reactions develop directly in peripheral tissues in which circulating memory T cells are activated by antigen presented on the surface of a resident cell population. This is in contrast to primary immunity that begins in the secondary lymphoid organs such as lymph node or spleen, where naive T cells encounter antigen on the surface of a specialized antigen-presenting cell. Once effector and memory cells develop in a secondary lymphoid organ, they may emigrate to the peripheral site via the blood stream, where reactivation by the antigenic stimulus is possible.
The two cell types that may present antigen to specific T cells in peripheral tissues are macrophages, resident in the tissues, or local microvascular ECs. In vitro, cultured human ECs from a variety of vascular beds constitutively express class I MHC molecules (used to present peptides derived from foreign proteins to CD8+ T cells). IFN-γ can induce ECs to express class II MHC molecules (used to present peptides derived from foreign proteins to CD4+ T cells). There is only limited information on antigen processing by cultured ECs, but indirect evidence (ie, the formation of functional peptide-MHC molecule complexes that can be recognized by cultured T-cell lines) suggests that EC are fully competent to perform this function. In vivo, microvascular ECs constitutively express both class I and class II MHC molecules, although the levels of both molecules can be increased further by cytokines (eg, IFN-γ).
How do ECs compare with tissue macrophages as antigen-presenting cells? This has been a difficult question to address experimentally in humans because the relevant cell populations (ie, ECs, macrophages, and T cells) are not readily isolated from one immunized individual. A commonly used indirect approach is to examine the response of T cells isolated from one donor to cultured ECs and monocytes isolated from a second donor. This allogeneic response of T cells is directed against complexes formed between peptides associated with allogeneic MHC molecules and results from a cross-reaction of T cells that are specific for a foreign peptide associated with a self MHC molecule; it is an excellent model of normal immunity that avoids the requirement for isolating ECs from immunized donors. ECs activate about one fifth as many allogeneic T cells (either CD8+ or CD4+) as monocytes, determined in limiting dilution analyses for production of IL-2. Some of this difference may arise from a larger number of different peptide-MHC molecule complexes displayed on freshly isolated monocytes compared with serially cultured ECs, but a critical contribution to this difference is that monocytes can activate both naive and memory T cells, whereas ECs can only activate memory T cells.242 It has recently been reported that ECs actually make naive T cells unresponsive to stimulation (ie, induce clonal anergy).243 The ability of ECs to activate memory but not naive T cells in vitro is consistent with a role in presentation of antigen as an initiating event in CMI, which is a memory T-cell response.
The differences in ability among cell types to activate resting naive or memory T cells are best explained by differences in expression of cell surface ligands, called costimulators, that provide antigen-independent signals that complement those provided by T-cell antigen-dependent recognition.241 Human ECs primarily provide costimulation to T cells through LFA-3 (CD58), which interacts with T-cell CD2. Monocytes and other professional antigen-presenting cells additionally provide costimulation to T cells through B7.1 (CD80) or B7.2 (CD86), which interact with T-cell CD28. Surprisingly, pig ECs express B7.2 and, more remarkably, pig B7.2 can functionally costimulate human T cells through CD28, a potential problem facing those who wish to use pigs as organ donors in human transplantation.244
The recruitment of inflammatory cells is the second role played by ECs in CMI. Once memory T cells become activated by antigens, they, in turn, can activate a variety of EC functions that contribute to recruitment of inflammatory cells. The signals provided by T cells to activate ECs may involve contact-dependent signals (eg, T-cell CD40 ligand may engage EC CD40)245 or cytokines (eg, T-cell–secreted TNF/LT, IFN-γ, or IL-4 may act on the EC; reviewed in Pober and Cotran246). Several different responses of ECs to these signals contribute to inflammation, including production of vasodilators to increase delivery of leukocytes to the tissue, expression of adhesion molecules that tether and bind circulating leukocytes, synthesis of chemokines that contribute to transendothelial migration, and leakage of plasma proteins that form a provisional matrix in tissues for migration of extravasated leukocytes (reviewed in Pober and Cotran247).
The expression of various adhesion molecules on the endothelial surface changes over time, favoring neutrophil recruitment initially (eg, dependent on E-selectin expression) and recruitment of other leukocytes at later times (eg, dependent on VCAM-1 expression). The identity of chemokines may also change over time from synthesis of neutrophil-activating C-X-C chemokines early on to the subsequent synthesis of C-C chemokines that act on other leukocytes. The net result is that the composition of infiltrates change over time from neutrophil-rich to T-cell–rich and monocyte-rich delayed hypersensitivity (DTH) reactions or to T-cell–rich, eosinophil-rich, and basophil-rich late-phase reactions. The differences between DTH and late-phase inflammation appear to be attributable to local production of IFN-γ versus IL-4 and IL-5, respectively. The T cells recruited in DTH reactions are predominantly Th1-like cells (that mediate DTH), whereas those in late-phase reactions are predominantly Th2-like cells (that mediate late-phase reactions). The selective recruitment of Th1 cells into DTH reactions may be mediated by E-selectins and P-selectins.248 Interestingly, although Th1 and Th2 cells appear to express equivalent levels of PSGL-1, only Th1 cells are able to bind P-selectin.249
Once infiltrates develop, a cytokine-rich milieu is generated that is sustained until the antigen is eliminated. Such chronic cytokine exposure has effects on ECs not seen at early times. For example, over the first few days, adhesion molecules that are initially expressed diffusely on the lumenal surface redistribute to inter-EC junctions.250 The basement membrane becomes enriched in sulfated glycosaminoglycans,251 and the cells assume an altered morphology characteristic of endothelium at sites of high lymphocyte extravasation, such as the high endothelial venules of lymph nodes.252 These features may promote leukocyte extravasation in acute settings. More chronic CMI reactions result in angiogenesis and tissue remodeling.
In general, CMI reactions do not produce endothelial injury, perhaps because T cells efficiently focus the response on the source of antigen, microbe-infected cells. An exception may be instances in which ECs are themselves infected by intracellular microbes (eg, viruses), so that cytolytic T lymphocytes (CTLs) kill the infected endothelium. Endothelial injury may also develop in transplantation, in which the immune system may perceive engrafted cells as self-cells that have been infected by virus. Some of the peptides recognized by graft-rejecting CTLs in association with allogeneic MHC molecules may be EC-specific and not found on leukocytes.253 In these instances, the CTL response may be directed at the endothelium. In addition, endothelium may be killed when CTL or natural killer (NK) cells are overstimulated by cytokines and lose their specificity. The actions of such lymphokine-activated killer (LAK) cells may contribute to the vascular leak syndrome associated with IL-2 or LAK therapy in cancer patients.254 It is increasingly appreciated that ECs may actively resist immune-mediated injury and that several of the resistance mechanisms involve cytokine-inducible genes.255Thus, the CMI response itself may protect ECs if the onset is sufficiently gradual (or if it is delayed by immunosuppression), so that the cells have had adequate time to acquire the resistant phenotype.
Erythrocyte-endothelial adherence.
Interactions between the endothelium and erythrocytes may contribute to the vascular complications of sickle cell anemia (SSA),256infection with Plasmodium falciparum malaria,257and diabetes.258 Red blood cell (RBC) adherence may initiate or promote intravascular sludging and occlusion leading to ischemic tissue and organ damage, retinopathy, dermal ulcers, strokes, and other infarctive pathologies. RBC adherence is dependent on EC surface molecules and is modulated by local hemodynamic factors. Recently, some of the RBC receptors, EC adhesion molecules, cytokines, and other vaso-active substances involved in adherence have been identified.
Sickle cell anemia.
Although the tendency for hemoglobin SS to polymerize at low oxygen tension is assumed to be the dominant factor in the pathogenesis of occlusive pain episodes, morphologic evidence of sickling is not seen immediately after hemoglobin is deoxygenated.259 Rather, adherence of SS-RBCs to vascular endothelium, which retards transit through the microvasculature, may be an important initiating event in this cascade.260,261 Adherence of SS-RBCs in vitro is sufficiently strong to withstand fluid shear forces typical of those seen in postcapillary venules.262 The resultant delay in capillary transit may allow time for sickle cells containing deoxygenated hemoglobin to deform leading to stable vascular obstruction263 and the resultant development of painful crises.
Adherence of SS-RBCs appears to result not only from the intrinsic membrane abnormalities induced in the erythrocytes, but also as a result of specific plasma factors and the state of EC activation (reviewed in Wick and Eckman264). For example, plasma from patients with SSA promotes RBC adherence in excess of that seen with normal plasma,265,266 and plasma collected during painful crises promotes adherence to an even greater extent.265Fibrinogen,265 fibronectin,262vWF,262 and thrombospondin267 268 have all been identified as factors in plasma that modulate SS-RBC adhesion.
Several adherence pathways have been described in vitro, including (1) bridging of GPIb-like molecules on sickle cells with their cognate receptors on ECs by unusually large vWF multimers released from activated platelets or by the stimulated endothelium itself262; (2) bridging by thrombospondin via CD36 on sickle reticulocytes269 and the αvβ3 integrin on large vessel ECs267 or αvβ3 and CD36 on microvascular endothelium268; (3) binding of sickle reticulocytes via α4β1receptors269,270 to VCAM-1 expressed on ECs stimulated by cytokines270,271 or by double-stranded RNA272; (4) binding of sickle reticulocyte via α4β1activated by phorbol ester or IL-8 to EC-associated fibronectin273; and (5) binding of SS-RBCs to E-selectin expressed on ECs stimulated by IL-1β.274 The expression of VLA-4 and CD36 on reticulocytes from sickle cell patients is reduced by treatment with hydroxyurea.275 However, the precise contribution of each of these or additional pathways to SS-RBC adherence in vivo requires further study.
In vitro, adherence of sickled RBCs to large venous vessels differs both qualitatively and quantitatively from adherence to microvascular endothelium.276 High molecular weight vWF multimers promote greater adherence to venous than to microvascular endothelium.276 Autologous plasma promotes greater adherence of sickle RBCs to microvascular endothelium than does plasma from individuals without SS disease.276 Sickle cell adherence is localized to postcapillary venules in ex vivo tissue perfusion studies, with no adherence observed in either capillaries or arterioles.263 These differences are likely due, at least in part, to variation in the expression of adhesion molecules and their receptors on the vasculature (see, eg, Swerlick et al277).
Sickle cell adherence is also dependent on local hemodynamic conditions (reviewed in Wick and Eckman264). Under static conditions, the dense SS-RBCs adhere most avidly, possibly due to intrinsic membrane alterations.261 In contrast, the least-dense sickle cells and reticulocytes are most adherent to the endothelium in vitro270,273,274 and ex vivo under flow conditions.230 Sickle cell adherence under flow is also more tenacious than under static conditions.278 Thus, adherence of reticulocytes expressing adhesion receptors may dominate in vivo in situations in which flow is maintained.264Adherence and trapping of membrane-damaged sickled erythrocytes may follow once flow has been further impeded, ultimately leading to complete vascular occlusion.279
Painful crises frequently accompany ischemia, infarction, infection, or inflammation, situations in which the coagulation cascade may be activated as well.256 Activation of leukocytes and/or platelets may result in the generation and release of cytokines, adhesive proteins, or other factors that modulate endothelial or sickle cell adhesivity. Observations that activation of sickle cells273 and ECs with cytokines,270virus,272 or thrombogenic plasma proteins262,265,267,268 promotes RBC adherence suggests a mechanism by which infection and inflammation may initiate or propagate vaso-occlusion and episodic pain. Presumably, sickle cell adherence in vivo is most extensive at sites where the relevant adherence molecules are expressed most highly and shear stresses are sufficiently low to permit binding.264 Adherence of sickle cells may alter their metabolism,280 promote leukocyte adhesion,281 and contribute to their desquemation.281
Malaria.
Plasmodium falciparum causes cerebral manifestations, perhaps the most serious complication of malaria. Maturation of the parasite within host RBCs induces membrane changes which promote adherence to cerebral microvascular endothelium in vitro and may lead to vascular congestion and hypoxia in vivo.257 It has been proposed that cytoadherence provides selective advantage to the invading parasites by facilitating their growth under the conditions of reduced oxygen tension found in the cerebral microcirculation and by enabling parasitized RBCs to avoid splenic filtration.257
Parasitized RBCs bind to cell surface molecules on ECs, including CD36,257 ICAM-1,257 VCAM-1,282 and E-selectin.282 Additionally, thrombospondin allows bridging between parasitized RBCs and CD36 receptors on ECs.257 The various endothelial receptors act synergistically to slow and arrest parasitized RBCs under flow conditions and at shear stresses in the physiological range.283,284 Additional binding sites for parasitized RBCs may be induced as a consequence of the inflammatory response to Plasmodium falciparum infection257 due to leukocyte activation,285 cytokine release,286 and EC activation.285
Binding to the endothelium occurs through knobs on RBCs induced by the parasite. These knobs contain Plasmodium falciparum erythrocyte membrane protein 1 that appears to participate in this process.257 Cytoadherence is inhibited by peptide fragments of the erythrocyte band 3 protein287 and by monoclonal antibodies that recognize band 3 on RBCs infected with mature parasites, suggesting involvement of cryptic regions of the protein exposed or altered during the course of infection.288 It has also been reported that some field isolates of Plasmodium falciparum promote adherence to chondroitin sulfate A.289 These data provide additional opportunities for antimalarial therapy based on inhibition of interactions between parasitized RBCs and ECs.
Diabetes mellitus.
Erythrocytes from patients with diabetes mellitus are more adherent to normal ECs than are RBCs from healthy donors.290 The extent of adhesion correlates with the severity of vascular complications290; greater adherence is observed in the absence of plasma,266 suggesting a defect intrinsic to the RBC. Adhesion is augmented further by plasma from diabetic patients as well as by fibrinogen,258 suggesting a mechanism by which acute-phase reactants may modulate vascular obstruction. Persistent exposure to hyperglycemia induces the formation of advanced glycation end products (AGEs) that modify structures on erythrocyte. AGE modification of erythrocytes allows them to engage a specific receptor, RAGE, (receptor for advanced glycation endproducts), which has been identified immunohistochemically and by in situ hybridization in the vasculature in vivo.258 Exposure of RBCs harvested from patients with diabetes to cultured endothelium results in increased adherence compared with those from euglycemic individuals due to erythrocyte-associated AGEs binding to endothelial RAGE.258Furthermore, it has been hypothesized that interaction of diabetic erythrocytes with endothelium through an AGE/RAGE linkage may promote oxidant stress leading to EC activation (see below).
EC PERTURBATION AND VASCULAR DISEASE
It is currently believed that endothelium must remain in a resting or unperturbed state to optimize expression of anticoagulant activities which prevent thrombus formation (see “The Role of the Endothelium in Coagulation” above). However, the endothelium is a dynamic organ that responds to an array of agonists and environmental challenges by undergoing an activation process not unlike that of platelets, which eventuates in the loss of anticoagulant properties and/or acquisition of procoagulant function. Although the role of the endothelium in the pathogenesis of thrombosis in vivo remains unproven, accumulating evidence points towards dysregulation of EC function as pivotal in the development of several important thrombotic disorders. Plasma factors such as antibodies or lipoproteins that perturb EC function in vitro have been identified. It is likely that genetic differences in EC responsiveness to environmental pressures will be uncovered as contributors to the development of other common vascular diseases.
The endothelium in atherosclerosis.
Atherosclerosis is the most prevalent vascular disease in developed countries. The concept that atherosclerosis arises in response to endothelial injury was first proposed more than 20 years ago, when it was appreciated that irregularities in EC organization are often found overlying early fatty streaks, whereas overt endothelial denudation is seen only in the late stages of the disease (see Ross and Glomset291 for review; Fig 4). There is now extensive evidence that this morphologically abnormal endothelium is also dysfunctional and actually contributes to the propagation of lesions (see Ross292 and McGorisk and Treasure293 for reviews). These findings not only provide insight into the pathogenesis of atherosclerosis, but also suggest means to monitor the progression of lesions and effectiveness of treatment.
Very early development of atherosclerosis in a nonhuman primate. The focal origin of atherosclerosis is apparent beneath an intact endothelium. Scanning electron microscopy of ECs outlined by silver deposition in a thoracic aorta (photograph courtesy of Peter F. Davies, PhD, Institute for Medicine and Engineering, University of Pennsylvania, Philadelphia, PA).
Very early development of atherosclerosis in a nonhuman primate. The focal origin of atherosclerosis is apparent beneath an intact endothelium. Scanning electron microscopy of ECs outlined by silver deposition in a thoracic aorta (photograph courtesy of Peter F. Davies, PhD, Institute for Medicine and Engineering, University of Pennsylvania, Philadelphia, PA).
Atherosclerosis is a multifactorial disease with numerous predisposing factors, including smoking, diabetes, hyperlipidemia, hypertension, mechanical stress, and inflammation. Such diverse and complex processes may perturb EC function through a common pathway. Alternatively, the endothelium may react to diverse stimuli with a limited repertoire of reparative, but ultimately dysfunctional, responses.
Oxidant stress.
Oxidant stress has been proposed as a mechanism common to diverse injuries such as unsaturated lipids that can be converted to cytotoxic lipid peroxidation products, various chemicals, radiation, and reactive oxygen metabolites released by leukocytes that migrate into the vasculature in response to infection and autoimmune injury. The pathways involved in the initiation and control of oxidant injury are receiving considerable study. Oxidized LDL and its peroxide derivative lysophosphatidylcholine stimulate protein kinase C activity, phosphoinositide turnover, and release of internal calcium; impair EC replication and angiogenesis; and induce apoptosis (reviewed in Henry294). Cytokines, such as TNF-α, can both induce reactive oxygen species in ECs and stimulate the ubiquitous transcription factor NF-κB, resulting in a transcriptional activation of other proatherogenic molecules such as VCAM-1 (reviewed in Larrick and Wright295). Oxidation reactions also promote the formation of AGEs that contribute to diabetic vasculopathy (reviewed in Schmidt et al296) and initiate transcriptional activation of VCAM-1297 and monocyte chemotactic protein-1 (MCP-1), which promotes monocyte entry into the vessel wall (reviewed in Gimbrone298).
Shear stress.
ECs are exposed continuously to fluid shear stresses that lead to a dynamic interaction between the cell and the substratum via focal contact sites. Shear-induced changes in transduced biomechanical forces can cause not only cytoskeletal rearrangement and altered morphology but changes in endothelial gene expression299 300(Fig 5). Most studies have examined primarily changes that occur within hours of initiating flow, which may best reflect the situation in vascular beds exposed to newly flowing blood such as postangioplasty, but the adaptive response of endothelium to shear forces is less well characterized.
EC alignment by directional steady flow in vitro (photographs courtesy of Peter F. Davies, PhD, Institute for Medicine and Engineering, University of Pennsylvania, Philadelphia, PA). (A) Before exposure to shear stress (no flow). (B) Twenty-four hours after exposure to flow (shear stress, 10 dynes/cm2).
EC alignment by directional steady flow in vitro (photographs courtesy of Peter F. Davies, PhD, Institute for Medicine and Engineering, University of Pennsylvania, Philadelphia, PA). (A) Before exposure to shear stress (no flow). (B) Twenty-four hours after exposure to flow (shear stress, 10 dynes/cm2).
An effect of shear on vascular biology is suggested by the observation, eg, of decreased vasodilator function at coronary branch points that have a predilection for atherosclerosis.301 Consistent with this notion, a number of genes relevant to the development of atherosclerosis expressed by ECs have shear stress response elements that coordinate their induction. Shear modulates EC production of products regulating vasoconstriction (NO, endothelin-1), vessel growth (βFGF, platelet derived growth factor [PDGF]-A and -B, and TGF-β), fibrinolysis (t-PA), and cell adhesion (MCP-1, VCAM-1, and ICAM-1) (see Malek and Izumo,300 Ando and Kamiya,302 and Tsao et al303 for reviews). Shear has been reported to modulate the expression of thrombomodulin in a reversible manner299 and abrogate cytokine-induced EC tissue factor expression.304
The mechanism(s) responsible for the modulation of gene expression by shear is under study. At least part of these shear-induced effects are mediated through modulation of gene transcription. A number of genes, such as PDGF-B, contain one or more shear stress responsive elements (SSREs) that include an NF-κB–responsive GAGACC promoter sequence305 in the 5′ upstream region. However, the induction of TGF-β and MCP-1 appear to occur through alternative sites, eg, a TRE/AP-1–responsive element.306 Levels of transcription factors NF-κB and AP-1 are increased in sheared EC.307 How shear-related transcription factors affect immediate and persistent gene transcription and how this inductive pathway differs from other injury-related responses remain to be elucidated.
Homocysteine.
Homocysteine is a sulfhydryl amino acid formed during the conversion of methionine to cysteine. Elevated plasma levels of homocysteine may result from deficiencies of cystathionine-β-synthase, deficiencies of enzymes involved in the folate-dependent pathway of homocysteine remethylation, or deficiencies of folate or vitamin B12 themselves (see Guba et al308 and Rees and Rodgers309 for reviews). Homozygous deficiency of cystathionine β-synthase leads to markedly elevated plasma concentrations of homocysteine and is associated with premature atherosclerosis and arterial thrombosis. The results of several recent large prospective and case-controlled studies suggest that even modestly elevated levels of homocysteine may pose a risk factor for atherosclerosis as well as for arterial and venous thrombosis (reviewed in Mayer et al310), although it is not clear that all pathways leading to hyperhomocysteinemia pose comparable risks. Several large prospective studies are underway to assess the magnitude of this risk.
These observations have led to the identification of several pathways by which homocysteine may affect the anticoagulant and procoagulant functions of cultured ECs (Table 2).311 It is important to consider that most studies to date have used cultured cells exposed for brief periods to concentrations of homocysteine that exceed that which is observed in vivo. Clearly, such concentrations of homocysteine induce formation of hydrogen peroxide312 and oxidized LDL313 and may be directly cytotoxic for ECs; NO is at least partially protective.314 However, induction of TF,315 activation of factor V,316 decreased binding of t-PA to annexin-II,317 inhibition of thrombomodulin,318 and reduced expression of heparan sulfate319 and possibly PGI2,320among many other changes, have all been observed in ECs exposed to high concentrations of homocysteine. Homocysteine is also mitogenic for rat aortic smooth muscle cells.321 That similar effects may occur in vivo is suggested by vascular dysfunction in monkeys with diet-induced hyperhomocysteinemia322 and elevated levels of thrombomodulin and vWF in the plasma of homocysteinemic patients with peripheral arterial disease. Therapy with pyridoxine and folic acid led to rapid reductions in the levels of both markers.323Studies in animal models in which moderately elevated levels of homocysteine is sustained may provide additional insight into the role of the endothelium and other pathogenic mechanisms of thrombosis and atherosclerosis.
Consequences of EC injury on endothelial-derived vasoactive factors.
Healthy human epicardial coronary arteries dilate when acetylcholine is infused,324 whereas atherosclerotic arteries constrict325 due to impaired release of NO (see “Vasoregulation” above) and PGI2 from the endothelium.326 Loss of vasodilitation in the absence of overt stenosis has been observed in patients with hypercholesterolemia,327 increased Lp(a),328diabetes,329 homocystinuria,330 and possibly hypertension (reviewed in Woodman331 and Heistad et al332). Similar effects have been observed with advancing age,333 exposure to cigarette smoke,330,334 and sedentary life style.335 Endothelial dysfunction is almost universal 12 to 24 months after cardiac transplantation.336Indeed, abnormal vasodilator function may be a more sensitive marker of coronary artery disease in some settings than is angiography.337
The reduction in NO is attributable in part to lowered levels of eNOS in atherosclerotic vessels,338 but the reason for this is uncertain. One clue may be that impaired vasodilatation is especially marked at coronary branch points where flow and shear stress have been shown to stimulate NO production in healthy vessels.301 In addition, free oxygen radicals and hydrogen peroxide generated by EC exposed to high levels of LDL may inactivate NO.339
Inhibition of NO activity accelerates atherosclerosis in animal models,340 whereas supplementation with L-arginine, the precursor of NO, diminishes lesion formation (reviewed in Cooke341) and reverses endothelial dysfunction in otherwise healthy young humans with hypercholesterolemia.342 The loss of NO may impact on multiple steps in the atherogenic process.341 NO may also counteract the proatherogenic effects of endothelin (reviewed in Mathew et al343). Oxidized LDL binds to a newly described receptor on ECs344and increases the production and secretion of ET in cultured ECs and in intact blood vessels.345 ET is also released by dysfunctional coronary arteries that constrict in response to acetylcholine.346 Plasma concentrations of ET are elevated in asymptomatic patients with hypercholesterolemia and increased plasma levels of both Lp(a)347 and ET have been reported to correlate with the severity of atherosclerosis.346
Monitoring the reversal of EC dysfunction.
A significant reduction in cardiac events has been reported as a result of lipid lowering therapy, but the mechanism responsible for this benefit has not been elucidated. Little reduction in the cross-sectional area of preexisting lesions is seen, although progression of the lesions may be slowed348 and incidence of plaque rupture may be lessened. What is clear is that there is an improvement in various indirect measures of EC function in vivo possibly as a result of a more favorable profile of vasoactive agents that are produced at critical locations.349 Similar beneficial effects on surrogate markers of EC function have been seen with the use of antioxidants,350 whereas in other studies the acute administration of vitamin C has been associated with improved EC-dependent vasodilatation in chronic smokers351 and patients with coronary artery disease.352 Similar beneficial effects of angiotensin converting enzyme inhibitors have been confirmed in some353 but not in all354animal models (reviewed in Lonn et al355).
Thus, with the advent of potential means to treat atherosclerosis, the need for reliable, noninvasive surrogate markers of risk and vascular function have become apparent. Several candidate molecules have emerged. Abnormalities in serotonin-induced arterial vasodilatation, a process dependent on NO, precede the development of clinical disease and resolve within 12 weeks of the institution of cholesterol lowering therapy.356 Elevated plasma levels of PAI-1 and thrombomodulin revert towards normal as well.357 In contrast, little change has been seen in the elevated plasma levels of E-selectin, VCAM-1, and ICAM-1.358 There is also interest in measuring levels of 8-epi PGF2α, an isoprostane with potent vasoconstrictor activity in the pulmonary and renal circulations generated through free radical catalyzed peroxidation of arachidonic acid by ECs and other vascular cells. Levels of 8-epi PGF2α in the urine have been used as a marker of oxidant stress in vivo (reviewed in Morrow and Roberts359). Levels in smokers are elevated and fall with cessation of smoking or treatment with vitamin C but not with vitamin E or aspirin therapy.360
Perspective.
Healthy ECs contribute to the prevention of atherosclerosis in medium to large arteries by inhibiting platelet activation, limiting the entry of cells and lipids into the vessel wall, maintaining a nonproliferative and biochemically quiescent intima,361 and secreting products under appropriate stimuli that limit potentially injurious responses that occur as a byproduct of host response to injury. These self-protective mechanisms are impaired as a result of oxidant, chemical, and shear stress, while at the same time the biochemical profile of the endothelium changes in a way that promotes inflammatory and fibroproliferative responses. The role of ECs in preventing or limiting the effects of plaque rupture and terminal thrombosis is little understood. The injurious processes that initiate atherosclerosis appear to persist throughout life in most individuals. Studies are now being conducted to determine the extent to which the biochemical and functional changes in the vessel wall can be reversed at different stages of the disease.
Endothelial perturbation and vascular dysfunction in diabetes.
Vascular dysfunction is a contributing factor in the etiology of several clinically important secondary complications of diabetes mellitus including retinopathy, accelerated atherosclerosis, microvascular disease, nephropathy, neuropathy, and impaired wound healing.362,363 The effects of hyperglycemia on EC function can be imparted through several pathways: (1) production of reactive oxygen intermediates; (2) direct activation of protein kinase C; (3) activation of the aldose reductase pathway resulting in an accumulation of sorbitol and diminished levels of myo-inositol; and (4) nonenyzmatic glycoxidation of long-lived macromolecules.362,363 Because glycoxidation of proteins and lipids occurs ubiquitously in patients with diabetes and is irreversible, its consequences are especially relevant to long-term vascular dysfunction. Initially, exposure of free amino groups to reducing sugars, such as glucose, results in the formation of early glycation products, Schiff bases, and Amadori products. These are reversibly modified species, such as hemoglobin A1c, used for long-term monitoring of blood sugar in diabetic patients. Further molecular rearrangements occur, in part due to oxidation, resulting in irreversible AGEs (Fig 6). The latter have pathophysiologic relevance in that AGE-modified proteins may not function normally and/or may perturb cellular properties in a manner distinct from that of the native molecule. This occurs when the AGE form of the molecule binds to cellular receptors which recognize AGEs, including RAGE364,365 and the macrophage scavenger receptor.366 RAGE is expressed by endothelium, monocytes, and smooth muscle cells and is likely to play a major role in the development of vascular disease in diabetics.365
A two-hit model of vascular perturbation. Stage 1 shows the interaction between AGEs, modified biochemical species and their receptors (RAGEs), transmembrane protein receptors of the Ig family present at low levels on a range of cells including ECs in addition to macrophages (Mfs), smooth muscle cells, and neurons. AGEs may perturb the normal physiologic function of the modified species and thus alter the EC's normal vascular functions. Stage 2 details subsequent perturbations resulting from the superimposed stimuli of accumulated lipoprotiens as seen in atherosclerotic lesions, foreign materials that may be seen in wound repair, and bacterial infection that may be seen in periodontal disease.
A two-hit model of vascular perturbation. Stage 1 shows the interaction between AGEs, modified biochemical species and their receptors (RAGEs), transmembrane protein receptors of the Ig family present at low levels on a range of cells including ECs in addition to macrophages (Mfs), smooth muscle cells, and neurons. AGEs may perturb the normal physiologic function of the modified species and thus alter the EC's normal vascular functions. Stage 2 details subsequent perturbations resulting from the superimposed stimuli of accumulated lipoprotiens as seen in atherosclerotic lesions, foreign materials that may be seen in wound repair, and bacterial infection that may be seen in periodontal disease.
RAGE is a member of the Ig superfamily of cell surface molecules. It is composed of an extracellular domain with one V-type, followed by two C-type regions.364,365 There is a single transmembrane spanning domain and a short, highly charged cytosolic tail that likely transmits the signal of ligand occupancy by interacting cytosolic transduction molecules. The single RAGE gene is located on chromosome 6 in the major histocompatibility complex between genes for class II and class III molecules. This proximity of RAGE to genes contributing to the host response is in keeping with expression of the receptor. In mature animals, RAGE is present at low levels in a range of cells (endothelium, smooth muscle cells, mononuclear phagocytes, and neurons), but after perturbation, as in diabetes, immune/inflammatory disorders, or Alzheimer's disease, RAGE expression is dramatically upregulated. In diabetes, AGE-modified proteins appear to act as ligands for RAGE modulating a number of secondary messenger pathways. For example, AGE interaction with RAGE results in cellular oxidant stress eventuating in activation of p21ras, MAP kinases (erk's 1 and 2), and the transcription factor NF-κB.367 Such activation of NF-κB results from binding of p50/p65 heterodimers to DNA binding motifs, as in the gene for vascular cell adhesion molecule-1 (VCAM-1). RAGE-dependent enhanced VCAM-1 expression is observed both in cultured ECs and in vivo in mice infused with AGEs.368 Increased VCAM-1 levels are also observed in diabetic vasculature upon immunohistological analysis. In parallel with expression of VCAM-1 on the cell surface, ECs release a soluble form of VCAM-1 (sVCAM-1) into culture supernatants, potentially providing a means of monitoring cellular stress in vivo. Patients with diabetes and microalbuminuria, the latter considered a harbinger of impending future vascular complications, display higher plasma sVCAM-1 than those without microalbuminuria.369
AGE-RAGE interaction likely underlies vascular hyperpermeability, another salient feature of diabetic vasculopathy. Such hyperpermeability is blocked by anti-RAGE IgG or by preventing AGE binding by infusion of a soluble form of the extracellular domain of RAGE (the latter is termed sRAGE). Vascular leakage of solutes in diabetic animals can be largely blocked by infusion of sRAGE. These data identify a reversible component of diabetic vascular dysfunction and suggest that AGE-RAGE-induced cellular perturbation may be a contributor. The principal insights to be gained from analysis of RAGE binding to nonenzymatically glycated ligands is probably in the setting of chronic vascular perturbation.365 Because AGE modification of proteins is irreversible, AGEs accumulated in the vessel wall are present for extended periods of time. Thus, the diabetic vascular milieu has properties that distinguish it from that in euglycemic subjects. A two-hit model can be envisioned (Fig6) in which tissue and blood AGEs interacting with RAGE provide a baseline state of vascular activation for the first-hit. The second stage comprises a superimposed stimulus, such as accumulated lipoproteins in atherosclerotic lesions, foreign material in wounds, and bacterial infection in periodontal disease. AGE-RAGE interaction provides a backdrop of chronic inflammation, with increased expression of proinflammatory cytokines, thrombogenic factors, cell adherence molecules, and vascular permeability, which aggravates and probably accelerates the development of vascular lesions.
Antibody-mediated EC injury: Solid organ transplantation.
Transplantation of vascularized organs, such as kidney, heart, lung, and liver, has become the treatment of choice for end-stage organ failure. The key limitations on clinical transplantation today are rejection of allografts posttransplantation and the shortage of available donor organs. The EC lining of graft vessels plays a prominent role in both of these clinical problems.
Despite the enormous advances in clinical immunosuppression of transplant recipients that have been made in the last 50 years of practice, the principal cause of graft failure is still rejection, ie, immunological reactions of the host against graft cell alloantigens that injure and destroy the graft. ECs play three crucial roles in the process of graft rejection: (1) ECs stimulate the host immune system by presenting alloantigens in an immunogenic form to host lymphocytes, thereby helping to initiate graft rejection; (2) ECs respond to host stimuli, eg, inflammatory cytokines, to promote intragraft inflammation and thrombosis that contribute to graft injury; and (3) ECs lining graft vessels are primary cellular targets of the host antigraft response. In addition, graft ECs are sensors and mediators of antigen-independent injury to which the graft is subjected during harvest, transport, and implantation, a dramatic instance of ischemia reperfusion. The mechanisms by which ECs present antigens to lymphocytes, promote inflammation in response to cytokines, are injured by the immune response, and respond to oxidant injury have been discussed in previous sections of this review and will not be duplicated in this section. We will focus here on specific issues related to immunologic allograft rejection.
Rejection reactions are commonly classified according to the time when they occur after surgical transplantation and by their histopathologic features.370 Hyperacute rejection occurs within minutes to hours of perfusion of the graft by host blood, ie, in the perioperative period. It is characterized by extensive intravascular thrombosis of graft vessels and consequent graft ischemic infarction. Hyperacute rejection is mediated by host antigraft antibodies, usually IgM antibodies reactive with graft endothelial carbohydrate epitopes such as ABO blood groups, and by complement activation that is initiated by the antibodies bound to graft ECs. Thrombosis results from EC lysis and desquamation, exposing thrombogenic subendothelial basement membrane, or, in cases of sublytic quantities of complement depositon, by loss of endothelial antithrombotic mechanisms (eg, shedding of cell surface anticoagulant heparan sulfate) combined with activation of endothelial prothrombotic mechanisms (eg, release of stored high molecular weight vWF, release of lipid procoagulants, and possibly induction of TF).371 Matching of donors and recipients for ABO blood groups has significantly reduced the incidence of hyperacute rejection in allografts.
Acute rejection reactions usually develop between the first and second week after transplantation. Although a variety of effector mechanisms may be involved, recent studies of human allograft biopsies have emphasized the primary role of cytolytic cells, principally CTLs.372,373 Some host CTLs recovered from rejecting grafts have shown specificity for graft ECs over graft leukocytes.374 Because the specificity of CTL is determined by the antigens involved in their induction (ie, the same antigen receptor mediates initial differentiation and subsequent effector function of CTL), graft ECs must have played a role in stimulating host CTL development by presenting antigen to precursor cells, ie, to resting CD8+ T cells recruited from the circulation into the graft. Lysis of microvascular ECs is a prominent and early component of acute cell-mediated rejection.375 More severe rejection reactions typically involve injury of larger graft vessels as well. Such vascular rejection is believed to start as a host CTL reaction against graft arterial or arteriolar ECs (called endothelialitis or intimal arteritis at this early stage373) and may progress to severe necrotizing, transmural vasculitis. The most severe vascular rejection reactions appear to involve host antigraft antibodies (both IgG and IgM) as well as cytolytic lymphocytes. Presensitized hosts (eg, resulting from a prior transplantaton procedure) who have expanded numbers of memory T or B cells reactive with the donor may show accelerated acute rejection during the first few days after transplantation370). These accelerated rejection reactions are similar to experimental second set rejection seen in animal models of retransplantation. The use of tissue typing (for renal transplantation) and of improved immunosupression (especially since the introduction of cyclosporin A) have reduced the incidence of graft loss due to acute and/or accelerated rejection to fewer than 10% of organs. ECs are considered prime targets for further improvements in immunosupression, eg, by targeting adhesion molecules such as ICAM-1 to reduce posttransplant ischemia-reperfusion, inflammation, and CTL effector functions.
With current advances in controlling acute rejection, the major cause of graft loss has become chronic rejection.376 Chronic rejection appears in biopsies as replacement fibrosis of graft parenchyma, developing over months to years. These changes are widely thought, at least in cardiac and renal transplantation, to be secondary to graft ischemia caused by progressive occlusion of the lumen of graft arteries (called graft arteriosclerosis). Graft arteriosclerosis is characterized by concentric, diffuse intimal hyperplasia of large, medium, and small graft arteries. These changes are accelerated compared with atherosclerosis in that they can develop into clinically significant lesions as early as 6 months to a few years posttransplantation. Despite all of the advances in immunosuppresion for acute rejection, the incidence of allograft failure to chronic rejection has remained at about 10% of grafts per year, with no evidence of improvement.
Graft arteriosclerosis is restricted to graft vessels, ie, it completely spares the host's vessels. Involved graft arteries contain increased numbers of intimal smooth muscle cells and deposition of extracellular matrix accompanied (or preceeded) by a sparse subendothelial infiltrate of host T cells and macrophages.377 Cytolytic effector cells are markedly fewer than in acute vascular rejection,378 and the endothelium shows only rare apoptotic cells.379 The major theories of pathogenesis are that graft arteriosclerosis results from chronic, low-level endothelial injury (ie, persistent endothelialitis caused by CTLs and/or alloantibodies), followed by fibroproliferative repair, or that graft arteriosclerosis results from a conversion of an acute cytolytic immune response to a chronic delayed-type hypersensitivity reaction in response to persistent immune stimulation by graft ECs. There is currently no therapy for graft arteriosclerosis except retransplantation. Future therapy may be targeted at preventing the intimal smooth muscle fibroplastic reaction rather than further increases in immunosuppression.
The next horizon in transplantation is to address the donor organ shortage by xenotransplantation of animal organs (eg, pigs) into human recipients.380 The use of pig organs has raised concerns about introducing new infectious agents into the human population, but the major practical problem is a very high incidence of hyperacute rejection. Two factors contribute to development of severe, uncontrollable hyperacute rejection of pig xenografts by human or old world monkey recipients. First, all mammals, except humans and old world monkeys, express a galactose α-1,3 galactose carbohydrate epitope instead of ABO blood groups on their ECs.381Moreover, virtually all humans have high levels of circulating natural IgM antibodies reactive with this alternative epitope, so that ABO matching cannot be used to evade hyperacute rejection in xenotransplantation. Second, the problem of abundant natural antibody is compounded by the fact that pig ECs express complement regulatory proteins, eg, DAF and CD59, that are unable to control the human complement system, ie, they are species specific for pig complement proteins.382 The combination of high levels of complement-activating IgM antibodies and limited EC resistance to human (or primates) complement proteins invariably leads to rapid and overwhelming intravascular graft thrombosis.
A major current effort is underway to produce transgenic pigs that will have reduced levels of galactose α-1,3 galactose and/or express human complement regulatory proteins. If successful, transplanters will still need to address later phases of the human-antipig rejection reaction (eg, potentially strong acute cellular rejection due to expression B7.2 costimulator molecules on pig ECs383 or to strong NK reactions384 due to possibly antibody binding and absence of self class I MHC molecules on pig ECs). Some of these problems may be partly ameliorated by the failure of pigs to respond to certain human cytokines (eg, to IFN-γ385), but additional studies will be needed to determine the significance of these differences.
Systemic lupus erythematosus (SLE) and the antiphospholipid antibody syndrome (aPS).
Immune-mediated endothelial dysfunction may contribute to the development of thrombosis in patients with SLE and the aPS (see McCrae and Cines386 for review). One mechanism by which endothelial damage and/or activation may occur is through the effects of EC-reactive antibodies. Several groups have demonstrated anti-EC antibodies (AECA) in sera of patients with SLE387and in patients with primary and secondary aPS (see McCrae et al,388 among others). Controversy remains as to whether antiphospholipid antibodies per se comprise the biologically important subpopulation of AECA (see Cines389 for review), although recent studies suggest that they may activate ECs through an effect on the plasma protein β2-glycoprotein I.390 AECA have been shown to alter the anticoagulant and procoagulant activities of cultured ECs in a number of ways. However, the in vivo importance of these antibodies in the pathogenesis of thrombosis remains unknown. Indeed, in only some studies has the presence of AECA correlated with thrombotic events or disease activity.388 Nevertheless, the fact that such antibodies induce the secretion of markers of EC injury/activation such as vWF from cultured ECs,388 when considered in light of reports demonstrating elevated levels of vWF in the plasma of patients with SLE,391 suggests that at least some of these in vitro effects may reflect processes that occur in vivo.
Heparin-induced thrombocytopenia and thrombosis (HITT).
Approximately 1% to 3% of patients who receive heparin develop severe thrombocytopenia, approximately 20% of whom also develop venous and/or arterial thrombi (see Arepally and Cines392and McCrae and Cines393 for reviews). Plasma from approximately 90% of patients with HITT contain antibodies that bind to complexes of heparin and platelet factor 4 (PF4).394 PF4 released from activated platelets may form complexes with heparin on the surface of activated platelets, targeting them for FcγRIIA-dependent activation by antiheparin-PF4 antibodies. However, the remarkable propensity of patients with HITT to develop thrombosis may also be promoted by the capacity of these antibodies to recognize PF4 bound to EC-heparan sulfate proteoglycans, which stimulate ECs to express TF and to bind platelets.395 396
EC-reactive antibodies in other vasculitic disorders.
AECAs have been described in several other disorders in which their role in pathogenesis is even less certain. PR-3, a common target of antineutrophil cytoplasmic antibodies (ANCA) is expressed on activated ECs in vitro397 and has been implicated in vascular injury in an animal model of vasculitis.398 However, EC-specific antibodies, apparently distinct from ANCA, have also been described in many patients with Wegener's granulomatosis.399 Plasma from children with the hemolytic uremic syndrome contain lytic AECA that recognize an unidentified EC surface protein that is suppressed by IFN-γ in vitro.400 AECA have also been implicated in mixed connective tissue disease, rheumatoid arthritis, and scleroderma,401 in which EC apoptosis has been implicated,402 as well as in atherosclerosis,403 Kawaski's disease,404Bechet's disease,405 and various forms of vasculitis.406 In each of these conditions, elevated levels of EC-derived proteins have also been described in the plasma of affected patients implying endothelial activation or injury.407 However, a pathogenic role of AECA or immune complexes in human vasculitis has not been proven. It is also pertinent to note that complex changes in antigen expression occur when ECs have been activated by cytokines or other agonists in vitro (see Favaloro408 for review) and in vivo.409 This activation promotes cell mediated immunity, promotes leukocyte/platelet adhesion (see “EC Pertubation and Vascular Disease” above) and exposes cryptic autoantigens. Fcγ receptors capable of binding circulating immune complexes may exist410 or can be induced on ECs411 at certain vascular sites.
Complement-mediated EC activation.
The vascular endothelium is exposed to activated complement components as a consequence of antigen-antibody interactions that occur naturally in plasma and within the vessel wall in certain pathologic conditions.387 ECs also constitutively express several complement proteins412 and can be induced to synthesize others by various cytokines,413 at least in vitro. Complement deposition on the vasculature is controlled, in part, by concerted actions of the regulatory proteins C1-esterase inhibitor, which is secreted by ECs,414 and by decay-accelerating factor, membrane cofactor protein of complement, and homologous restriction factor, which are expressed on the cell surface.415-419 Complement that deposits on the endothelium when these containment mechanisms are exceeded can increase vascular permeability, stimulate procoagulant pathways, and recruit ECs to become active participants in the inflammatory processes.
For example, multiple components of the complement cascade act in concert to augment the recruitment of leukocytes to sites of vascular inflammation. Binding of C1q to specific EC receptors420augments expression of E-selectin and possibly ICAM-1 and VCAM-1,421 C5a upregulates the expression of P-selectin,422 and the terminal C5b-9 membrane attack complex (MAC) acts synergistically with TNF-α to stimulate expression of E-selectin and ICAM-1.423 Sublytic concentrations of MAC also activate the NF-κB pathway leading to secretion of IL-8 and MCP-1.424
MAC also stimulates cultured ECs to express TF,425,426 to release large vWF multimers,427 and to release procoagulant microvesicles containing anionic phospholipids and binding sites for factor Va that accelerate prothrombinase activity.428 On the other hand, MAC also stimulates production of PGI2429 and the complement components C7 and C9 provide sites for the binding and activation of plasminogen.430 However, as with other areas of research in vascular biology, studies have been confined largely to cultured cells and additional studies are required to elucidate the relative contribution of complement-dependent responses in vivo.
Thrombotic disorders of uncertain cause: Thrombotic thrombocytopenic purpura (TTP) and the hemolytic uremic syndrome (HUS).
TTP and HUS are related-disorders characterized pathologically by the development of platelet microthrombi that occlude small arterioles and capillaries and clinically by microangiopathic hemolytic anemia and thrombocytopenia. Endothelial dysfunction plays a prominent role in the pathogenesis of both disorders (see Moake431and Heild432). Approximately 90% of cases of HUS occur in early childhood, often after an episode of bloody diarrhea caused by enteropathic strains of Escherichia coli that release an exotoxin, designated verotoxin-1 (VT-1), which is similar to the 70-kD Shiga toxin 18. VT-1 binds with high affinity to globotriosylceramide (Gb3) receptors expressed at the highest density on renal glomerular ECs.431 VT-1 is directly cytotoxic to ECs. In addition, VT-1 promotes neutrophil-mediated EC injury433,434 and induces the production of TNF-α by monocytes435 and cells within the kidney.436 In turn, TNF-α, in concert with IL-1, increases Gb3 expression and exacerbates the sensitivity of the endothelium to toxin-mediated437 and antibody-mediated404 cytotoxicity, promotes vWF release,438 and impairs fibrinolytic activity.44 In accord with this putative pathogenic mechanism, elevated plasma levels of PAI-1 have been reported to be a sign of a poor prognosis in childhood HUS.439 EC injury may also contribute to the pathogenesis of the microangiopathic syndromes that may follow the use of certain chemotherapeutic agents,440 cyclosporin,441 quinine/quinidine (see Gottschall et al442 for review), or after bone transplantation.440
There is considerable evidence to suggest that EC injury plays a role in the pathogenesis of TTP. The most exhaustively studied protein in this regard is plasma vWF, which circulates in plasma as oligomers that range in size from 1 to 15 × 106 kD. The so-called unusually large vWF multimers (ULvWF) are normally found in subendothelial matrix, in the supernatant of cultured ECs,431 and in platelet releasates, but are not normally detected in plasma.431 Platelet microthrombi in TTP contain abundant vWF but little fibrinogen, in contrast to those seen in DIC. A subgroup of patients has been identified who suffer from chronic, relapsing TTP and whose plasma continues to contain elevated levels of ULvWF between relapses.443 Furthermore, plasma from patients with sporadic or isolated episodes of TTP often contains either ULvWF or decreased amounts of larger vWF multimers during periods of active disease. ULvWFs may exacerbate microvascular thrombosis through their ability to aggregate platelets at high levels of shear stress. The secretion of ULvWF by cultured ECs is stimulated by many agonists including Shiga toxin.431 However, elevated levels of vWF occur in other thrombotic microangiopathies, and their exact role in TTP/HUS requires further study. Reports of elevated levels of thrombomodulin,444 tissue-type plasminogen activator (t-PA), plasminogen activator inhibitor type 1 (PAI-1),445 ELAM-1,446 and decreased levels of PGI2447 and TFPI448 in plasma from patients with TTP provides additional support for the notion that endothelial damage plays a pivotal role in the pathogenesis of the disease.
The events that initiate TTP remain unknown. AECA have been described in TTP and HUS, but their role is uncertain. More recently, plasma from patients with TTP and HUS has been reported to induce apoptosis in microvascular ECs; it is of great interest that cells from dermal, renal, and cerebral origin were most susceptible, whereas pulmonary and coronary arterial cells were not.46 The plasma factors responsible for these changes remain to be identified.
Pregnancy-induced hypertension.
Pregnancy-induced hypertension (or preeclampsia) is the most common medical disorder of pregnancy, affecting 5% to 13% of all primaras. Although the clinical manifestations of preeclampsia are generally not evident until the third trimester, the pathogenesis of this disorder may involve a deficiency in placentation,449 the process in which fetal trophoblast cells remodel the maternal uterine spiral arteries early in pregnancy. Incomplete remodeling of the spiral arteries leads to compromised placental perfusion. Alterations in EC morphology occur within the placenta450 and in the glomerular capillaries (glomerular endotheliosis), the latter being characterized by EC swelling and lipid accumulation (see Ferris451 for review). Fibrin deposition in microvasculature is common. Affected women show increased responsiveness to the pressor effects of angiotensin II,314increased amounts of thromboxane A2 relative to PGI2 in their urine,452 elevated plasma levels of endothelin,453 and their umbilical vessels demonstrate less PGI2 synthesis and decreased NO release in response to bradykinin (see Ferris451 for review). Additional evidence suggesting endothelial damage is the reported findings of elevated plasma levels of EC-derived fibronectin,454,455vWF,455 and PAI-1455 in affected women. Importantly, increased levels of vWF456 and cellular fibronectin454 may be detected before the onset of clinical manifestations.
The pathophysiology of EC damage in preeclampsia remains a mystery. Some,457,458 but not all groups,459,460 have reported that plasma from affected women is cytotoxic for cultured ECs. Preeclamptic sera have also been reported to impair EC proliferation,461 stimulate fibronectin release,462 increase triglyceride accumulation,463 and increase PDGF synthesis464; variable effects on PGI2synthesis have been reported.463,465 A role for abnormalities of lipid peroxidation,466 for immunologic factors, and for underlying, but otherwise inapparent, maternal vascular disease (see Ness and Roberts467 for review) have also been advanced. Yet, it must be noted that all of these studies remain largely unconfirmed, the putative injurious plasma factor(s) has yet to be identified, and the pathophysiology and significance of EC injury outside of the terminal cases of eclampsia remains enigmatic.
CONCLUSION
The endothelium can no longer be viewed as a static physical barrier that simply separates blood from tissue. Rather, it is now clear that the endothelium helps to coordinate functions of differentiated tissues in a way that meets the requirements of the organism as a whole. In part, this is accomplished by the location of the endothelium at the interface with the blood and the capacity of these specialized cells to receive and transmit biochemical and physical information bidirectionally. Information sensed on the lumenal surface of the endothelium can be transmitted either by direct permeation or active transport of soluble mediators through the capillaries to deeper tissues or indirectly through the capacity of ECs to modulate the behavior of smooth muscle cells and other components of the vessel wall. In turn, physiologic and pathophysiologic events in tissue alter EC interactions with soluble and cellular blood components.
The endothelium, as with all cell types, displays an immediate and prototypic response to diverse agonists that is modulated in complex ways by subsequent events. In the case of the endothelium, this first response appears designed to prevent physical disruption of the vessel wall by trauma, microbial organisms, toxins, or other threats to the maintenance of intravascular volume and oxygen delivery. This protective response is accomplished by the rapid transformation of the endothelium to a procoagulant, vasoconstrictive, and proinflammatory state that has multiple effects on of its structure and behavior.
Several ramifications of this reflexive, adaptive response of the endothelium have now become evident. First, it is clear that ECs rapidly undergo some of these same biochemical and phenotypic changes soon after being placed in culture, as a consequence of which the behavior of the unperturbed endothelium cannot be reliable inferred from currently available in vitro techniques.
Second, an extensive experimental literature has emerged supporting the notion that several common human vascular diseases are in part a consequence of the same responses of the endothelium to stress; ie, that prolonged or exaggerated endothelial activation leads to dysfunction that is an early, often preclinical component of vascular disease. Unfortunately, it is generally impossible to access vascular tissue directly and sequentially during these preclinical stages of disease development; without such tissue, the EC contribution to disease development can only be inferred. As a consequence, most research in vascular biology continues to (1) focus on the footprints of disease by analyzing damaged vessels, generally at the endstage of the process; (2) link putative circulatory factors to disorders through their effect on cultured ECs, often derived from unaffected tissue; and (3) develop animal models that may simulate human diseases.
Third, it is now clear that the endothelium is not a homogeneous organ. ECs from different vascular beds show highly differentiated functions as a consequence of genetic diversity and the impact of specialized surroundings. These biochemical and phenotypic differences extend to their susceptibility to injury and effect function of the vasculature as a whole.
Fourth, there is remarkably little information on the potential contribution of genetic differences in EC behavior among individuals with respect to bleeding disorders, thrombosis, atherosclerosis, and vasculitis. Without such information, our current approach to studying these major vascular disorders can be compared with the study of anemia without appreciating the existence of hemoglobinopathies or the study of bleeding disorders without appreciating the contribution of genetic abnormalities in platelet function. It is hoped that future research will enable the direct study of EC behavior and thereby enhance our understanding of the contribution of the endothelium to vascular biology.
EC injury in a case of antiphospholipid antibody syndrome (photographs courtesy of Emma E. Furth, MD, Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia, PA). EC injury seen in a duodenal biopsy from a 40-year-old woman who presented with profuse intestinal bleeding and was found to have a lupus anticoagulant and a markedly positive anticardiolipin antibody. (A) Elastic stain (original magnification × 200) highlighting a fresh thrombus (right) with the beginning stages of organization and EC ingrowth, an older organized thrombus with fibroblast proliferation (center), within a vessel showing vacuolated, injured, and disrupted ECs. (B) A trichrome stain (original magnification × 400) of the same field highlighting the thrombus material (red acellular material on the right) with early stages of organization. (C) A hematoxylin and eosin (H and E) stain (original magnification × 400) showing fibrinoid intimal necrosis (right) in the absence of an inflammatory reaction within a small vessel in the same duodenal biopsy as shown in (A) and (B). The ECs show marked vacuolization (left). The surrounding eosinophilic vascular cells are smooth muscle cells surrounded by fibroblasts. (D) A higher power view (original magnification × 600) of the same biopsy showing four capillaries with grossly vacuolated ECs and luminal effacement.
EC injury in a case of antiphospholipid antibody syndrome (photographs courtesy of Emma E. Furth, MD, Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia, PA). EC injury seen in a duodenal biopsy from a 40-year-old woman who presented with profuse intestinal bleeding and was found to have a lupus anticoagulant and a markedly positive anticardiolipin antibody. (A) Elastic stain (original magnification × 200) highlighting a fresh thrombus (right) with the beginning stages of organization and EC ingrowth, an older organized thrombus with fibroblast proliferation (center), within a vessel showing vacuolated, injured, and disrupted ECs. (B) A trichrome stain (original magnification × 400) of the same field highlighting the thrombus material (red acellular material on the right) with early stages of organization. (C) A hematoxylin and eosin (H and E) stain (original magnification × 400) showing fibrinoid intimal necrosis (right) in the absence of an inflammatory reaction within a small vessel in the same duodenal biopsy as shown in (A) and (B). The ECs show marked vacuolization (left). The surrounding eosinophilic vascular cells are smooth muscle cells surrounded by fibroblasts. (D) A higher power view (original magnification × 600) of the same biopsy showing four capillaries with grossly vacuolated ECs and luminal effacement.
Address reprint requests to Douglas B. Cines, MD, Department of Pathology and Laboratory Medicine, University of Pennsylvania, 513A Stellar-Chance, 422 Curie Blvd, Philadelphia, PA 19104; e-mail:dcines@mail.med.upenn.edu.

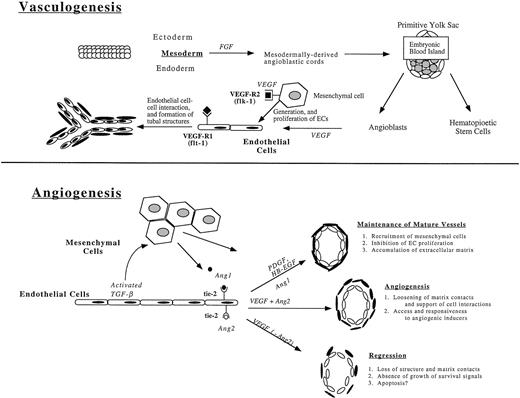
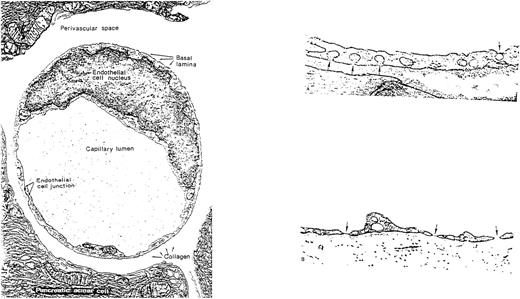
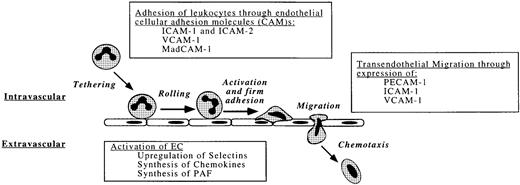
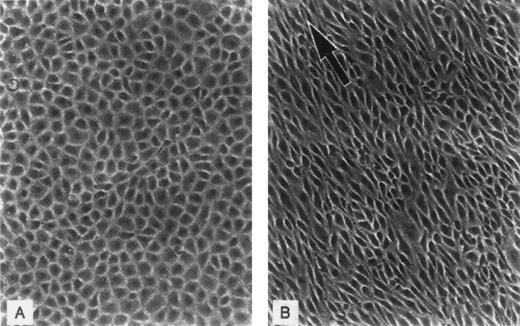
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal